Abstract
Background
This prospective study investigated the association between tooth loss and upper gastrointestinal (UGI) cancer mortality in the Linxian Dysplasia Nutrition Intervention Trial Cohort.
Methods
Subjects were categorized into three groups according to age at baseline. No missing teeth and less or greater than median tooth loss in each group was defined as none, moderate, and severe, respectively. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were estimated using the Cox proportional hazard model.
Results
Through 30 September 2015, 541 esophageal squamous cell carcinoma (ESCC), 284 gastric cardia carcinoma (GCC), and 77 gastric non‐cardia carcinoma (GNCC) deaths occurred. In the six‐year follow‐up, severe tooth loss was associated with an increased risk of GCC mortality (HR 1.55, 95% CI 1.06–2.18). In the 15‐year follow‐up, moderate tooth loss increased the ESCC mortality risk by 58% (HR 1.58, 95% CI 1.06–2.35), while severe loss increased the GCC mortality risk by 30% (HR 1.30, 95% CI 1.03–1.64). In the 30‐year follow‐up, moderate tooth loss increased the risk of ESCC mortality (HR 1.34, 95% CI 1.01–1.76). In subjects aged < 55 at baseline and men, moderate tooth loss had 53% and 52% higher risks of ESCC mortality (HR<55 years 1.53, 95% CI 1.06–2.05; HRmen 1.52, 95% CI 1.01–2.28). No significant association was observed for GNCC in any subjects or subgroups.
Conclusion
Moderate tooth loss increased the risk of ESCC mortality, particularly in younger subjects and men. Severe tooth loss increased the risk of GCC mortality. Future studies are needed to confirm these findings.
Keywords: Cohort study, dysplasia, tooth loss, upper gastrointestinal cancer
Introduction
Tooth loss significantly impacts mastication, diet, nutrition intake, aesthetics, and food choice.1 Oral health goals recommended by the World Health Organization for 2020 have stated that there should be an increase in the number of individuals aged 35–44 and 65–74 years with functional dentitions (≥ 21 natural teeth).2 Tooth loss is the result of a complex interaction of factors, and is said to vary by age, gender, race, education, income, and geographic region.3 It is considered to impact health‐related quality of life4 aggravate people with severe mental illness;5 and increase the risk of cancer in several sites, including oral cavities,6 the esophagus,7 stomach,8, 9 and pancreas,10 as well as esophageal dysplasia11 and cardiovascular disease.12
Upper gastrointestinal (UGI) cancer, a significant cause of morbidity and mortality, has become a major concern worldwide.13 Esophageal cancer ranked as the sixth cause of cancer mortality in 2018, causing an estimated 572 000 new cases and 508 000 deaths.14 Despite decreased incidence and mortality rates of gastric cancer over the last three decades, this disease remains the second leading cause of cancer‐related mortality, with approximately 1033,000 new cases and 782 000 deaths recorded worldwide in 2018.14 Approximately 54% of esophageal cancer and 44% of gastric cancer cases occur in China.15 It is widely believed that UGI cancer is an etiological and pathogenic disease and a number of studies in physics, chemistry, and genetics have been conducted to determine the risk factors,16, 17, 18 Associations between oral hygiene and UGI cancers have previously been reported.19 , 20
Linxian, a rural county located in north central China, has some of the highest rates of esophageal and gastric cancer.21 The mortality rate of esophageal cancer in Linxian exceeded the Chinese average by 10‐fold and the American average (for white men) by 100‐fold.22, 23 The Linxian Nutrition Intervention Trial (NIT) including a dysplasia‐based cohort (1985–1991, 3318 participants) and a general population‐based cohort (1986–1991, 29 584 participants) was the first randomized, double blind, placebo‐controlled trial to report a reduction in total and cancer mortality following supplementation.24 A number of etiologic studies have been conducted using data from the Linxian NIT, which indicated a strong intrinsic association between tooth loss and UGI cancer mortality in the general NIT population.19, 25 However, little prospective data of an association between tooth loss and UGI cancer is available in the dysplasia population.
We examined the associations between tooth loss and risk of esophageal squamous cell carcinoma (ESCC), gastric cardia carcinoma (GCC), and gastric non‐cardia carcinoma (GNCC) mortality in a dysplasia population‐based cohort over a 30‐year period.
Methods
Study population
A detailed description of the Linxian Dysplasia NIT has been reported in previous studies. Briefly, a total of 3318 individuals with a previous cytological diagnosis of esophageal squamous dysplasia were recruited into the Linxian Dysplasia NIT cohort on 30 April 1985 and randomized to receive either a vitamin‐mineral combination or a placebo for six years until 30 April 1991, according to the trial design. Potential participants were eligible if they were aged between 40 and 69 years, lived in one of four northern Linxian communes (Yaocun, Rencun, Hengshui, and Donggang), signed informed consent, and had a diagnosis of esophageal squamous dysplasia based on a balloon cytology examination. Individuals were excluded if they were taking any vitamin or mineral regularly, or had a history of malignancy or other chronic disease. This study was based on an analysis of the Dysplasia population‐based NIT cohort to explore the association between tooth loss and UGI cancer mortality risk over a 30‐year period, ending on 30 September 2015.
Baseline questionnaire and examination
At the time of study recruitment, subjects completed a questionnaire and received a brief physical examination. The baseline questionnaire collected detailed information regarding age, gender, tooth loss status, smoking, alcohol consumption, body mass index (BMI), education, family history of tumors, and dietary habits. Ever tobacco users were defined as individuals who had smoked cigarettes, or used hookah or a pipe at least weekly for at least six months, and use of alcohol was dichotomized into no alcohol or any alcohol consumed in the previous 12 months. A family history of tumors was considered positive if cancer was reported in at least one first‐degree relative, including parents, siblings, or offspring. Dietary variables collected from the baseline questionnaire included the intake frequency of persimmon bread, moldy bread, foods cooked in oil, meats, eggs, fruit, and vegetables. To avoid the bias caused by seasonal effect, we calculated the frequency of fresh fruit and vegetable consumption in winter/spring and summer/autumn seasons, respectively.
As part of the baseline oral cavity examination, subjects underwent an oral exam to determine if they had lost any permanent teeth. Trained medical personnel then counted the number of remaining teeth in all those who reported missing teeth, and recorded the age of first adult tooth loss and information of oral hygiene habits. Teeth were considered missing if they were observed as missing on examination or indicated for extraction, such as root stumps, grossly destructed teeth, mobile teeth, and in the presence of a fixed or removable prosthesis. Supernumerary teeth and bilateral maxillary and mandibular third molars were excluded. Those who reported no missing teeth were assumed to have 32 teeth.
Classification of tooth loss status
For the purpose of the present analysis, tooth loss status was coded as a three‐level indicator variable: none, moderate, and severe. The classification of the degree of tooth loss was as follows: the study population was categorized into three age groups: 40–49 years, 50–59 years, and 60–69 years. We calculated the median numbers of tooth loss in the three groups, which were 4, 13, and 21, respectively. Therefore, in each age group, those with no lost teeth were included in the “none” group as the referent category, while 1~median and median~32 were divided into moderate and severe tooth loss groups, respectively. That is, 1~4, 1~13, and 1~21 missing teeth were considered as moderate tooth loss in the corresponding three age groups, and 5~32, 13~32, and 21~32 as the severe tooth loss accordingly, respectively.
Follow‐up of cancer
The main outcomes of our study were ESCC, GCC, and GNCC mortality. The international diagnostic team, a joint panel of Chinese and American cytology pathologists and radiologists, finally determined terminal cases. This panel reviewed and confirmed 85% of the cancer diagnoses based on pathology, cytology, and endoscopy. All esophageal cancers were ESCC. Cancers in the most proximal 3 cm of the stomach were defined as GCC and those originating elsewhere in the stomach were defined as GNCC.22
The institutional review boards of the US National Cancer Institute and the Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS) approved the study. Each commune was considered as a unit for registration and record keeping. Town hospitals and their affiliated village clinics participated in follow‐up care, assisted by the Bureau of Health and the Cancer Prevention and Control Institute in Linxian. Data of cancer mortality were dutifully collected, entered into the registry, classified, and reported to CHCAMS.
Statistical analysis
This study concluded on 30 September 2015. Survival duration was calculated by determining the number of months from 30 April 1985 to the date of death from UGI cancer or other causes, or the observation end point. Differences in baseline demographic and health‐related characteristics within groups were examined with nonparametric Kruskal–Wallis and chi‐squared (χ2) tests. Cox proportional hazards regression models were used to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for 6‐year, 15‐year, and 30‐year effects of UGI cancer mortality. Potential confounders included age at baseline (continuous variable), gender (men/women), smoking (yes/no), alcohol consumption (yes/no), BMI (continuous variable), family history of tumors (yes/no), education (none or < primary education or ≥ primary education or unknown), and consumption of fresh fruit (continuous variable). Stratification analysis models were performed by age at baseline (< 55 and ≥ 55 years) and gender (men/women). Kaplan–Meier estimates were used to compare cumulative mortality rates among the three groups, followed by the log‐rank test to assess the significance between survival curves. Statistical significance was assessed using two‐tailed tests with a significant level of 0.05. Analyses were conducted using SPSS version 22.0.
Results
We excluded 28 subjects with missing data of tooth loss at baseline and four subjects who were lost to follow‐up; a total of 3286 individuals were included in the final analysis. During the 30‐year follow‐up, 2578 deaths occurred, including 541 ESCC, 284 GCC, and 77 GNCC deaths. The baseline characteristics of the study participants are shown in Table 1. Compared to subjects without tooth loss, those with severe tooth loss were younger, more often male, had a relatively lower BMI, were more likely to have a lower level of education, lower consumption of fresh fruit, and more commonly non‐smokers and non‐drinkers (P < 0.005).
Table 1.
Baseline characteristics of study participants according to tooth loss status
| Baseline characteristics | Status of tooth loss | P * | ||
|---|---|---|---|---|
| None | Moderate | Severe | ||
| Age (n, %) | 0.000 | |||
| < 55 years | 423 (81.2%) | 521 (45.6%) | 827 (51.0%) | |
| ≥ 55 years | 98 (18.8%) | 622 (54.4%) | 795 (49.0%) | |
| Gender (n, %) | 0.000 | |||
| Men | 239 (45.9%) | 505 (44.2%) | 1104 (68.1%) | |
| Women | 282 (54.1%) | 638 (55.8%) | 518 (31.9%) | |
| BMI (n, %) | 0.000 | |||
| (Mean ± SD, kg/m2) | 20.82 ± 2.36 | 20.41 ± 2.25 | 20.15 ± 2.28 | |
| Education (n, %) | 0.000 | |||
| Non | 127 (24.4%) | 458 (40.1%) | 816 (50.3%) | |
| < Primary education | 193 (37.0%) | 375 (32.8%) | 408 (25.2%) | |
| ≥ Primary education | 141 (27.1%) | 145 (12.7%) | 139 (8.6%) | |
| Unknown | 60 (11.5%) | 165 (14.4%) | 259 (16.0%) | |
| Smoking (n, %) | 0.000 | |||
| Yes | 165 (31.7%) | 412 (36.0%) | 374 (23.1%) | |
| No | 356 (68.3%) | 731 (64.0%) | 1248 (76.9%) | |
| Alcohol consumption (n, %) | 0.000 | |||
| Yes | 122 (23.4%) | 247 (21.6%) | 243 (15.0%) | |
| No | 399 (76.6%) | 896 (78.4%) | 1379 (85.0%) | |
| Family history of tumors (n, %) | 0.816 | |||
| Yes | 223 (42.8%) | 508 (44.4%) | 716 (44.1%) | |
| No | 298 (57.2%) | 635 (55.6%) | 906 (55.9%) | |
| Consumption of fresh vegetables | 0.895 | |||
| (Mean ± SD, times/week) | 11.65 ± 4.48 | 11.73 ± 4.58 | 11.68 ± 4.40 | |
| Consumption of fresh fruit | 0.000 | |||
| (Mean ± SD, times/week) | 0.26 ± 0.55 | 0.23 ± 0.72 | 0.19 ± 0.57 | |
P value derived from χ2 or nonparametric Kruskal–Wallis tests, as appropriate, for categorical and continuous variables. BMI, body mass index; SD, standard deviation.
In multivariable Cox regression analyses, crude and fully adjusted HRs and 95% CIs for the associations between tooth loss and the risk of ESCC, GCC, and GNCC mortality are shown in Table 2. In the six‐year follow‐up analysis, severe tooth loss was associated with a significantly increased risk of GCC mortality (HR 1.79, 95% CI 1.20–2.66). Moreover, this association remained significant after full adjustment (HR 1.55, 95% CI 1.06–2.18). In the 15‐year follow‐up analysis, moderate tooth loss increased the risk of ESCC mortality by 80% (HR 1.80, 95% CI 1.22–2.65), and the HR remained statistically significant after extended adjustment to the model (HR 1.58, 95% CI 1.06–2.35). Severe tooth loss increased the GCC mortality risk by 30% (HR 1.30, 95% CI 1.03–1.64). During the 30‐year follow‐up period, our results indicated that moderate tooth loss in dysplasia patients also had a significant effect on ESCC mortality, as the risk increased 34% (HR 1.34, 95% CI 1.01–1.76) after adjusting for potential confounders. No associations were observed for tooth loss and risk of GNCC mortality in during the study period.
Table 2.
HRs and 95% CIs for the association between tooth loss status and UGI cancer mortality in the Dysplasia Population Trial Cohort, Linxian
| Study period | ESCC | GNCC | GCC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | HR (95% CI)† | HR (95% CI)‡ | N | HR (95% CI)† | HR (95% CI)‡ | N | HR (95% CI)† | HR (95% CI)‡ | |
| 6‐year trial period (baseline–1991) | |||||||||
| None | 9 | 1.00 | 1.00 | 2 | 1.00 | 1.00 | 7 | 1.00 | 1.00 |
| Moderate | 57 | 1.82 (1.01–2.74) | 1.77 (0.87–3.57) | 3 | 0.90 (0.15–5.38) | 0.87 (0.12–6.16) | 34 | 1.87 (0.83–4.22) | 1.69 (0.72–2.97) |
| Severe | 45 | 1.43 (0.70–2.93) | 1.28 (0.61–2.67) | 9 | 1.92 (0.42–8.89) | 1.47 (0.19–11.63) | 34 | 1.79 (1.20–2.66) | 1.55 (1.06–2.18) |
| 15‐year trial period (baseline–2000) | |||||||||
| None | 32 | 1.00 | 1.00 | 4 | 1.00 | 1.00 | 25 | 1.00 | 1.00 |
| Moderate | 126 | 1.80 (1.22–2.65) | 1.58 (1.06–2.35) | 13 | 1.96 (0.64–6.01) | 1.12 (0.30–4.18) | 82 | 1.77 (0.87–3.57) | 1.49 (0.65–3.42) |
| Severe | 160 | 1.57 (1.08–2.30) | 1.49 (0.94–2.16) | 20 | 2.51 (0.86–7.35) | 1.28 (0.37–4.43) | 99 | 1.67 (1.06–2.63) | 1.30 (1.03–1.64) |
| 30‐year trial period (baseline–2015) | |||||||||
| None | 72 | 1.00 | 1.00 | 14 | 1.00 | 1.00 | 37 | 1.00 | 1.00 |
| Moderate | 202 | 1.51 (1.16–1.98) | 1.34 (1.01–1.76) | 25 | 1.51 (0.77–2.97) | 1.10 (0.53–2.29) | 107 | 1.44 (0.98–2.11) | 1.09 (0.73–1.64) |
| Severe | 262 | 1.33 (1.02–1.73) | 1.28 (0.97–1.68) | 36 | 1.89 (0.99–3.59) | 1.35 (0.66–2.74) | 139 | 1.26 (0.87–1.83) | 1.08 (0.72–1.62) |
Not adjusted for age at baseline, gender, smoking, alcohol consumption, body mass index (BMI), family history of tumors, education, or consumption of fresh fruit.
Adjusted for age at baseline, gender, smoking, alcohol consumption, BMI, family history of tumor, education, and consumption of fresh fruit. Bold text indicates statistical significance. “N” represents the number of deaths from esophageal squamous cell carcinoma (ESCC), gastric non‐cardia carcinoma (GNCC), or gastric cardia carcinoma (GCC). CI, confidence interval, HR, hazard ratio; UGI, upper gastrointestinal.
Table 3 shows the associations stratified by age and gender during the 30‐year follow‐up. Overall, positive associations were observed for moderate tooth loss and UGI cancer mortality among patients aged < 55 years (HR 1.52, 95% CI 1.13–2.04) and men (HR 1.31, 95% CI 1.00–1.71), with statistically significant interactions for tooth loss, age, and gender (P < 55 years = 0.005; Pmen = 0.048). Furthermore, stronger associations were detected for the risk of ESCC mortality in younger and male groups (HR < 55 years 1.53, 95% CI 1.06–2.05; HRmen 1.52, 95% CI 1.01–2.28). However, no significant associations were observed for GCC and GNCC mortality in subgroups.
Table 3.
HRs and 95% CIs of the 30‐year analysis of the association between tooth loss and UGI cancer mortality stratified by age and gender in the dysplasia population trial cohort, Linxian
| Characteristic | UGI cancer† | ESCC | GNCC | GCC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR (95% CI) | P interaction | N | HR (95% CI) | P interaction | N | HR (95% CI) | P interaction | N | HR (95% CI) | P interaction | |||
| Age | < 55 years | Moderate | 151 | 1.52 (1.13–2.04) | 0.005 | 95 | 1.53 (1.06–2.05) | 0.021 | 11 | 1.212 (0.442–3.328) | 0.709 | 45 | 1.35 (0.78–2.36) | 0.284 |
| Severe | 215 | 1.26 (0.98–1.63) | 0.071 | 135 | 1.24 (0.90–1.71) | 0.184 | 18 | 3.078 (0.075–8.423) | 0.229 | 62 | 1.26 (0.70–2.24) | 0.443 | ||
| ≥ 55 years | Moderate | 183 | 1.25 (0.84–1.84) | 0.270 | 107 | 1.75 (0.98–3.13) | 0.058 | 14 | 0.451 (0.086–2.378) | 0.348 | 62 | 0.79 (0.43–1.46) | 0.458 | |
| Severe | 222 | 1.17 (0.79–1.74) | 0.437 | 127 | 1.52 (0.84–2.75) | 0.167 | 18 | 0.404 (0.069–2.359) | 0.314 | 77 | 0.83 (0.45–1.53) | 0.556 | ||
| Gender | Men | Moderate | 207 | 1.31 (1.00–1.71) | 0.048 | 117 | 1.52 (1.01–2.28) | 0.044 | 14 | 1.440 (0.511–4.056) | 0.490 | 76 | 1.21 (0.76–1.95) | 0.423 |
| Severe | 168 | 1.24 (0.94–1.63) | 0.129 | 92 | 1.29 (0.89–1.88) | 0.185 | 14 | 1.353 (0.430–4.254) | 0.605 | 62 | 1.37 (0.84–2.23) | 0.209 | ||
| Women | Moderate | 127 | 1.09 (0.75–1.59) | 0.639 | 85 | 1.28 (0.80–2.04) | 0.299 | 11 | 0.549 (0.154–1.958) | 0.355 | 31 | 0.72 (0.31–1.66) | 0.437 | |
| Severe | 269 | 1.07 (0.76–1.51) | 0.690 | 170 | 1.22 (0.79–1.89) | 0.364 | 22 | 1.022 (0.385–2.718) | 0.964 | 77 | 0.62 (0.29–1.33) | 0.222 | ||
| Smoking | Yes | Moderate | 366 | 1.27 (0.90–1.80) | 0.172 | 74 | 1.54 (0.95–2.51) | 0.082 | 5 | 0.500 (0.082–3.048) | 0.452 | 50 | 0.91 (0.51–1.63) | 0.758 |
| Severe | 330 | 1.28 (0.90–1.81) | 0.170 | 67 | 1.46 (0.89–2.40) | 0.138 | 9 | 3.648 (0.750–17.744) | 0.109 | 52 | 1.02 (0.58–1.80) | 0.945 | ||
| No | Moderate | 580 | 1.16 (0.88–1.54) | 0.300 | 128 | 1.20 (0.84–1.70) | 0.320 | 20 | 1.017 (0.407–2.541) | 0.972 | 57 | 1.15 (0.64–2.07) | 0.635 | |
| Severe | 937 | 1.10 (0.84–1.44) | 0.492 | 195 | 1.13 (0.80–1.59) | 0.487 | 27 | 1.141 (0.489–2.661) | 0.760 | 87 | 1.01 (0.55–1.84) | 0.980 | ||
Includes esophageal squamous cell carcinoma (ESCC), gastric non‐cardia carcinoma (GNCC), and gastric cardia carcinoma (GCC).
Bold text indicates statistical significance. “N” represents the number of deaths from ESCC, GNCC, GCC, or upper gastrointestinal (UGI) cancer. CI, confidence interval; HR, hazard ratio.
Cumulative mortality rates caused by ESCC, GNCC, GCC, and UGI cancer during the 30‐year follow‐up period are presented in Figure 1. Survival curves showed that during most of the 30 years of follow‐up, the cumulative mortality rates of patients with moderate and severe tooth loss were higher than those in patients with no tooth loss, which indicated that tooth loss could increase the long‐term risk of UGI cancer mortality. The log‐rank test results suggested that the cumulative mortality rates of dysplasia patients with moderate and severe tooth loss who died from UGI cancer were both significantly different from patients who did not lose any teeth (P < 0.050).
Figure 1.
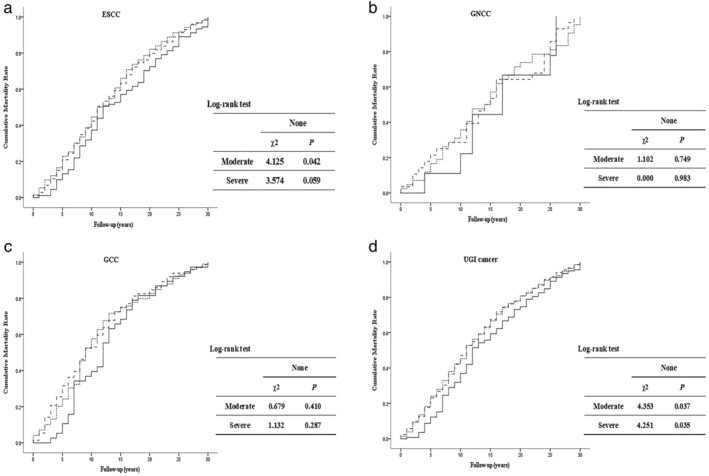
Effect of tooth loss on 30‐year cumulative mortality caused by upper gastrointestinal (UGI) cancer. Comparison of cumulative mortality rates of (a) esophageal squamous cell carcinoma (ESCC), (b) gastric non‐cardia carcinoma (GNCC), (c) gastric cardia (GCC), and (d) UGI cancer in groups divided by tooth loss status. Solid lines represent participants who had no tooth loss; dotted lines represent participants with moderate tooth loss; dashed lines represent participants with severe tooth loss. ( ) None, (
) None, ( ) Moderate, and (
) Moderate, and ( ) Severe.
) Severe.
Discussion
This is the first prospective study to examine the association between tooth loss and the risk of UGI cancer mortality among an esophageal squamous dysplasia population in a nutritionally deficient area in China. Overall, we found that subjects with moderate tooth loss had a 34% higher risk of ESCC mortality, especially in younger and male groups. The risk of GCC mortality was also increased in patients with severe tooth loss. No associations with GNCC were observed in any subjects or subgroups. Our study results make up for the lack of information on risk factors of UGI cancer mortality in a dysplasia cohort, aiming to promote health management and provide a scientific basis for developing an effective preventive strategy according to the heterogeneity between dysplasia and general populations.
In several previous studies, evidence of an association between tooth loss and UGI cancer has been inconsistently reported. Michaud et al. reported a significant association between tooth loss and esophageal cancer in a large prospective cohort of male health professionals, but not between tooth loss and stomach cancer.26 However, two studies from Japan and Iran found an association between tooth loss and gastric cancer.9, 27 One of the possible reasons underlying the different outcomes could be differences in the criteria used for grouping tooth loss. In most cases, a two28 or three‐category29 variable was used and the bound values for each category were not standardized. In this study, we stratified participants according to age at baseline and graded tooth loss as moderate or severe by taking the median number of missing teeth in each age group as a boundary point, considering the disparities in nutritional status and oral cavity function at different age stages; thus our results basically represent an accurate burden of UGI cancer mortality as the severity of tooth loss increased.
Christian et al. previously reported that, in the Linxian general population trial, individuals with tooth loss had a statistically significant 13% increased risk of total mortality, a 35% increased risk of UGI cancer mortality, and a 28% increased risk of heart disease mortality during a five‐year intervention and 10‐year follow‐up period (1986–2001).25 In our study, we compared the results at three follow‐up periods (6, 15, and 30 years), and the longitudinal trends of UGI cancer mortality risk were detected. Although tooth loss significantly increased the risk of both ESCC and GCC mortality, there were differences in the period in which it played a significant role. For ESCC, no significant increase in mortality risk was observed in the first six years (1985–1991), while the 15‐year follow‐up analysis showed that the risk in subjects with moderate tooth loss was 58% higher than in those without tooth loss, and the risk remained high until 2015 (34%). Thus, the effect of ESCC mortality was characterized by a lag delay. In GCC, the role played by time was the opposite to ESCC, with significant effects in the first six and 15 years, gradually weakening to insignificance after 30 years of follow‐up. These results clearly illustrate that the effect of tooth loss on the risk of GCC mortality cannot be long‐term maintenance.
Further analysis by age demonstrated that the effect of tooth loss on UGI cancer, particularly on ESCC mortality risk, was primarily confined to younger patients but there was a less clear association in older participants. Christian et al. observed the same strong associations in younger people,19, 25 while Abnet et al., in a complete model using all oral health indicators, reported that patients aged < 40 who lost teeth earlier had a higher risk of ESCC.30 All findings for UGI cancer mortality suggested that there was a more significant risk prior to extensive tooth loss, when chewing and digestive functions declined significantly. Several explanations may have contributed to this effect. Firstly, this may represent a birth cohort effect with changes in diet, water supply, and other environment factors, followed by different risks associated with tooth loss. Secondly, in the dysplasia population, the age difference may have caused different sensitivity to exposure to malignant transformation of tumors in the upper digestive tract. Furthermore, in our study, 93.0% of the older group had lost at least one tooth, which may to some extent limit the power to detect the risk of UGI cancer mortality in this subgroup. However, a population‐based case‐control study conducted in Taixing City in eastern China reported that an increased risk of ESCC associated with tooth loss was more pronounced in older subjects (age ≥ 70 years).31 This may be related to the overall level of oral health in Taixing in the 2010s, which was much better than in Linxian in the 1990s. In our < 55 year age group, approximately 75.3% of subjects had lost ≥ 1 tooth, compared to 37% in the < 50 year age group in the Taixing study.
Previous studies and reviews have discussed the potential causal mechanisms that might explain associations between tooth loss and esophageal and gastric cancer. Authors have hypothesized that tooth loss would cause individuals to swallow large, poorly chewed boluses of food that might irritate the esophagus.32 In recent years, a strong hypothesis has posited that the progression of tooth loss destroys the normal periodontal tissue, allowing oral microorganisms to accumulate deep in the oral tissue, thereby facilitating their growth. It has been postulated that poor oral hygiene and tooth loss mediate a bacterial load and “overgrowth” of microorganisms on teeth,33 which can transform nitrates into nitrites and then combine with amines to form carcinogenic nitrosamines, some of which may be gastrointestinal organ‐specific carcinogens.34, 35 In a study by Nair et al., individuals with poor oral hygiene and tooth loss had an eight‐fold increase in the potential to form nitrosamine in the oral cavity,36 and intra‐oral nitrate‐reducing activity may contribute to the majority of overall nitrosamine exposure in humans. It is also not difficult to envisage that during normal physiological behavior, such as swallowing and drinking, oral microorganisms, as well as the produced “nitrosamine,” are passed into the esophagus and stomach from the oral cavity along with food and drinks. Therefore, an association between tooth loss and UGI cancer seems plausible, although more detailed surveys of the associations between tooth loss, oral bacteria, carcinogen formation, and UGI cancer are warranted.
The present study exhibits several strengths. First of all, the data demonstrated excellent compliance and 30‐year follow‐up with virtually complete ascertainment of cases in a well‐defined population. The large sample size and sufficient power to examine the questions of interest were further strengths. Moreover, the cohort used trained medical personnel to conduct interviews and tooth counts were ascertained by physical examination. This measurement is objective, and because it was gathered prospectively, it was not influenced by subsequent outcomes. Furthermore, we collected extensive information on potential confounders.
However, several potential limitations of this study should be considered. Firstly, there is no international unification index or diagnostic criteria to evaluate tooth loss. The majority of studies use different methods to assess tooth loss, such as clinical physical examination, questionnaires, and self‐reporting. Therefore, our results should be considered with caution because of exposure misclassification when comparing findings from other research. Secondly, although we adjusted for multiple potential confounding factors, the existence of some unknown confounders in this population or residual confounding could not be completely excluded. For example, earlier studies have suggested that low socioeconomic status (SES) may be associated with poor oral health,37 which has consistently been linked to ESCC risk,38 but we could not obtain information on SES for this study. However, education level is known to be an indispensable indicator of SES,39, 40 thus we included education level as a confounder into the final model, and our results were not materially altered. Further studies to clarify this point are warranted. Thirdly, competing risk bias inevitably existed in the follow‐up process. The outcome of this study was UGI cancer mortality, which accounted for 35% (902/2578) as some subjects died from other diseases (e.g. subjects who died of cardiovascular disease will no longer die from UGI cancer during the follow‐up period). This may lead to false estimates of the effects of tooth loss on UGI cancer mortality. How to reasonably explain the results of this study on the basis of competing risk bias should be further verified. Fourthly, as the present study has only dealt with baseline tooth loss, but not with the later management of tooth loss, such as removable denture restoration, fixed bypass repair, and dental implants, subsequent research should take follow‐up dental management into consideration.
In summary, in this prospective cohort study, we found that tooth loss in esophageal squamous dysplasia patients significantly increased the risk of UGI cancer mortality, especially among younger subjects and men. It is of primary importance to determine to what extent tooth loss actually affects UGI cancer, as this will facilitate the development of clinical policy‐making in public health and provide appropriate guidance for oral health care. Further studies are warranted to verify these findings and to explore more accurate mechanisms of the associations with tooth loss.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank the participants and staff of the Dysplasia Trial Cohort Study for their valuable contributions. Participants did not receive compensation and staff were not compensated outside of their salaries.
References
- 1. Adegboye AR, Twetman S, Christensen LB, Heitmann BL. Intake of dairy calcium and tooth loss among adult Danish men and women. Nutrition 2012; 28 (7–8): 779–84. [DOI] [PubMed] [Google Scholar]
- 2. Hobdell M, Petersen PE, Clarkson J, Johnson N. Global goals for oral health 2020. Int Dent J 2011; 53 (5): 285–8. [DOI] [PubMed] [Google Scholar]
- 3. Joseph WJ, Ann WC, Jeffry CH, Stephen HJ, Marc LS, Andrew KR. Tooth loss in the very old: 13–15‐year incidence among elderly Iowans. Community Dent Oral Epidemiol 2010; 30 (1): 29–37. [DOI] [PubMed] [Google Scholar]
- 4. Bortoluzzi MC, Traebert J, Lasta R, TND R, Capella DL, Presta AA. Tooth loss, chewing ability and quality of life. Contemp Clin Dent 2012; 3 (4): 393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kisely S, Quek LH, Pais J, Lalloo R, Johnson NW, Lawrence D. Advanced dental disease in people with severe mental illness: Systematic review and meta‐analysis. Br J Psychiatry 2011; 199 (3): 187–93. [DOI] [PubMed] [Google Scholar]
- 6. Zuo C, Zhu Y, Wang X, Zeng X, Huang C. Tooth loss and risk of oral squamous cell carcinoma in Chinese Han population. Int J Clin Exp Med 2015; 8 (11): 21893–7. [PMC free article] [PubMed] [Google Scholar]
- 7. Chen H, Nie S, Zhu Y, Lu M. Teeth loss, teeth brushing and esophageal carcinoma: A systematic review and meta‐analysis. Sci Rep 2015; 5: 15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin XH, Wang YD, Luo H et al. Association between tooth loss and gastric cancer: A meta‐analysis of observational studies. PLoS One 2016; 11 (3): e0149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shakeri R, Malekzadeh R, Etemadi A et al. Association of tooth loss and oral hygiene with risk of gastricadenocarcinoma. Cancer Prev Res 2013; 6 (5): 477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang J, Roosaar A, Axéll T, Ye W. A prospective cohort study on poor oral hygiene and pancreatic cancer risk. Int J Cancer 2016; 138 (2): 340–7. [DOI] [PubMed] [Google Scholar]
- 11. Wei WQ, Abnet CC, Lu N et al. Risk factors for oesophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut 2005; 54 (6): 759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joshipura KJ, Douglass CW, Willett WC. Possible explanations for the tooth loss and cardiovascular disease relationship. Ann Periodontol 2017; 3 (3): 175–83. [DOI] [PubMed] [Google Scholar]
- 13. Wickremesekera SK, Seo HB, Trimber MA, Bann S, Tse K. A morbidity/mortality analysis of a tertiary level upper gastrointestinal/hepatopancreaticobiliary surgical unit. N Z Med J 2016; 129 (1444): 68–78. [PubMed] [Google Scholar]
- 14. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (6): 394–424. [DOI] [PubMed] [Google Scholar]
- 15. Globocan 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018 Available from URL: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx [accessed on 15 February 2019].
- 16. Ajani JA, D'Amico TA, Almhanna K et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015; 13 (2): 194–227. [DOI] [PubMed] [Google Scholar]
- 17. Lin SW, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. Prospective study of self‐reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemioly Biomarkers Prev 2011; 20 (5): 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vogtmann E, Corley DA, Almers LM, Cardwell CR, Murray LJ, Abnet CC. Oral bisphosphonate exposure and the risk of upper gastrointestinal cancers. PLoS One 2015; 10 (10): e0140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR, Dawsey SM. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 2001; 12 (9): 847–54. [DOI] [PubMed] [Google Scholar]
- 20. Divaris K, Olshan AF, Smith J et al. Oral health and risk for head and neck squamous cell carcinoma: The Carolina Head and Neck Cancer Study. Cancer Causes Control 2010; 21 (4): 567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang JB, Fan JH, Dawsey SM et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep 2016; 6: 22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murphy G, Fan JH, Mark SD et al. Prospective study of serum cysteine levels and oesophageal and gastric cancers in China. Gut 2011; 60 (5): 618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blot WJ, Li JY. Some considerations in the design of a nutrition intervention trial in Linxian, People's Republic of China. Natl Cancer Inst Monogr 1985; 69 (1): 29–34. [PubMed] [Google Scholar]
- 24. Wang SM, Taylor PR, Fan JH et al. Effects of nutrition intervention on total and cancer mortality: 25‐year post‐trial follow‐up of the 5.25‐year Linxian Nutrition Intervention Trial. J Natl Cancer Inst 2018; 110 (11): 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population‐based cohort. Int J Epidemiol 2005; 34 (2): 467–74. [DOI] [PubMed] [Google Scholar]
- 26. Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol 2008; 9 (6): 550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ansai T, Takata Y, Yoshida A et al. Association between tooth loss and orodigestive cancer mortality in an 80‐year‐old community‐dwelling Japanese population: A 12‐year prospective study. BMC Public Health 2013; 13 (1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicopouloukarayianni K, Tzoutzoukos P, Mitsea A et al. Tooth loss and osteoporosis: The OSTEODENT Study. J Clin Periodontol 2009; 36 (3): 190–7. [DOI] [PubMed] [Google Scholar]
- 29. Momenheravi F, Babic A, Tworoger SS et al. Periodontal disease, tooth loss, and colorectal cancer risk: Results from the Nurses’ Health Study. Int J Cancer 2017; 140 (3): 646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abnet CC, Kamangar F, Islami F et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008; 17 (11): 3062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen X, Yuan Z, Lu M, Zhang Y, Jin L, Ye W. Poor oral health is associated with an increased risk of esophageal squamous cell carcinoma: A population‐based case‐control study in China. Int J Cancer 2017; 140 (3): 626–35. [DOI] [PubMed] [Google Scholar]
- 32. Yang CS. Research on esophageal cancer in China: A review. Cancer Res 1980; 40 (1): 2633–44. [PubMed] [Google Scholar]
- 33. Peterson LA. Formation, repair, and genotoxic properties of bulky DNA adducts formed from tobacco‐specific nitrosamines. J Nucleic Acids 2010; 2010 (14): 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zetterquist W, Marteus H, Kalm‐Stephens P et al. Oral bacteria: The missing link to ambiguous findings of exhaled nitrogen oxides in cystic fibrosis. Respir Med 2009; 103 (2): 187–93. [DOI] [PubMed] [Google Scholar]
- 35. Sánchez GA, Miozza VA, Delgado A, Busch L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide 2014; 36: 31–5. [DOI] [PubMed] [Google Scholar]
- 36. Nair J, Ohshima H, Nair UJ, Bartsch H. Endogenous formation of nitrosamines and oxidative DNA‐damaging agents in tobacco users. Crit Rev Toxicol 1996; 26 (2): 149–61. [DOI] [PubMed] [Google Scholar]
- 37. Ghorbani Z, Ahmady AE, Lando HA, Yazdani S, Amiri Z. Development of a socioeconomic status index to interpret inequalities in oral health in developing countries. Oral Health Prev Dent 2013; 11 (1): 9–15. [DOI] [PubMed] [Google Scholar]
- 38. Zhang L, Cheng H, Zhou Y, Yuan Z, Chen T, Chen X, Lü M [Association between socioeconomic status and esophageal squamous cell carcinoma in the population of Taixing area, Jiangsu province]. Zhonghua Liu Xing Bing Xue Za Zhi 2014;35(2):147–50. (In Chinese.) [PubMed] [Google Scholar]
- 39. Carozza SE, Puumala SE, Chow EJ et al. Parental educational attainment as an indicator of socioeconomic status and risk of childhood cancers. Br J Cancer 2010; 103 (1): 136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cundiff JM, Uchino BN, Smith TW, Birmingham W. Socioeconomic status and health: education and income are independent and joint predictors of ambulatory blood pressure. Journal of behavioral medicine 2015; 38 (1): 9–16. [DOI] [PubMed] [Google Scholar]


