Abstract
Background
Tumor spread through alveolar spaces (STAS) is a recently described invasive pattern associated with the prognosis and recurrence of lung adenocarcinoma. This study was performed to determine whether the presence and distance of STAS can be predicted by the immunohistochemical intensity of SLX, a well‐known cell adhesion protein.
Methods
In total, 245 patients with pathological stage I lung adenocarcinoma who underwent lobectomy with radical mediastinal lymph node dissection were identified from 1998 to 2012. Recurrence‐free survival (RFS) was compared between patients stratified by STAS and the immunohistochemical intensity of SLX in the main tumor. Patients were divided into three groups based on the intensity of SLX staining: high (n = 108), moderate (n = 48), and low (n = 89).
Results
STAS was observed in 71 patients (29.0%). Patients with STAS had significantly poorer five‐year RFS (67.1%) than those without STAS (84.8%). Although no relationship was observed between the existence of STAS and SLX intensity, the distance between STAS cells and the main tumor was significantly shorter in the moderate group (median 0.9 mm, range: 0.2–1.2 mm) than in the other two groups (median 1.2 mm, range: 0.4–5.0 mm). The five‐year RFS rates in the high, moderate, and low groups were 80.0%, 96.0%, and 75.8%, respectively. Multivariate analysis revealed that pathological stage, lymphatic/vascular invasion, and SLX intensity were independent predictors of recurrence.
Conclusion
SLX staining cannot predict the presence of STAS; however, it can predict the distance between STAS and the main tumor in stage I lung adenocarcinoma.
Keywords: Lung adenocarcinoma, tumor spread through alveolar spaces, SLX
Introduction
With the technical advances in thoracic imaging and the use of computed tomography (CT) in screening programs, thoracic surgeons will likely encounter a significant increase in the number of small peripheral lesions of lung cancer.1 Recent studies have shown that lung cancers detected by CT are mostly classified as stage I and their prognosis is acceptable.2 The treatment for stage I lung cancer has primarily been surgery. Recently, limited reduction surgery, such as anatomical segmentectomy, has also been performed. However, approximately 20% of patients with stage I lung adenocarcinoma develop disease recurrence after curative resection.3 Several researchers have investigated the effects of clinicopathologic factors on postoperative recurrence in patients with complete resection of stage I lung cancer.4, 5, 6, 7, 8, 9 Other than physiological factors, such as old age and male gender, histological factors including solid/micropapillary‐predominant tumors, poorly differentiated tumors, lymphatic/vascular invasion, and pleural invasion have been reported.4, 5, 6, 7, 8, 9 On the other hand, although it seems that there is no seeding macroscopically, sowing is sometimes microscopically separated from the main lesion, and the invasion pattern of this lung cancer is called tumor spread through alveolar spaces (STAS).10 STAS is defined as the extension of tumor cells into the pulmonary parenchymal alveolar space beyond the margin of the main tumor.11 Several reports have indicated that the appearance of STAS is a predictive factor for recurrence and a poor prognosis,12 even for stage I lung cancer.13 The existence of STAS can be a problem, especially when treating stage I lung adenocarcinoma with limited resection. If the existence of STAS can be predicted before surgery, unnecessary limited resection can be avoided.
SLX is a cell adhesion protein in the selectin family that adheres to vascular endothelial cells, particularly in target organs of cancer cells.14 A relationship between serum SLX and lung cancer prognosis has been reported; in tumors with high SLX intensity, the frequency of blood vessel invasion is significantly higher and overall survival is decreased.15, 16 However, the relationship between SLX staining and the existence of STAS is unclear. We hypothesized that higher SLX staining of cancer cells predicts the higher the existence of STAS. This study was performed to determine whether the immunohistochemical intensity of the SLX antigen in the main tumor can predict STAS‐related factors, presence, or distance in patients with stage I lung adenocarcinoma who have undergone lobectomy.
Methods
Patients
Of 1037 patients who underwent lobectomy and mediastinal lymph node dissection (ND2a) for primary lung cancer at our hospital from January 1998 to December 2012, we retrospectively investigated the records of 245 patients with adenocarcinomas without lymph node metastasis (pathological stage I), according to the eighth edition of the Tumor Node Metastasis (TNM) Classification.2, 3 Patients who underwent segmentectomy or partial resection and those who did not undergo radical mediastinal lymph node dissection were excluded. All patients underwent postoperative follow‐up at an outpatient clinic. The mean follow‐up duration was 71 (range: 4–194) months. Follow‐up evaluation included physical examination, chest X‐ray, and blood examination, including for tumor markers. Chest and abdominal CT were performed at 4–12 month intervals. Whenever any symptoms or signs of recurrence were detected, brain magnetic resonance imaging and positron emission tomography scans was performed. All recurrences were confirmed by clinical, radiological, or pathological assessment and were classified into locoregional or distant recurrence. Locoregional recurrence was defined as evidence of a tumor in another ipsilateral lobe and in the ipsilateral hilar or mediastinal lymph nodes. Distant recurrence was defined as evidence of a tumor in the contralateral lung, in the contralateral hilar or supraclavicular lymph nodes, or outside the hemithorax.
Informed consent was obtained from all patients before surgery for the use of their clinical data for research purposes. The ethics committee of Osaka City University approved this study (No. 3391).
Histopathologic evaluation
Two pathologists performed comprehensive histologic subtyping using a multi‐head microscope and the findings were discussed until consensus was achieved. The tumors were divided into six distinctive subtypes according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) classification: lepidic, acinar, papillary, micropapillary, solid, and mucinous adenocarcinoma.17 The invasion/tumor ratio (ITR) was defined as the ratio of the invasion size to the maximum tumor size as the total tumor size. We categorized the tumors into three groups according to the IASLC/ATS/ERS classification: low‐grade, intermediate‐grade, and high‐grade.18
Tumor spread through alveolar spaces (STAS) evaluation and immunohistochemical intensity of SLX
STAS was defined as the presence of tumor cells within air spaces in the lung parenchyma beyond the edge of the main tumor that exhibited three morphological patterns: scattered, discohesive single cells; micropapillary structures without central fibrovascular cores, which occasionally formed ring‐like structures within air spaces; and solid nests or tumor islands filling air spaces.12 The distance between the tumor margin and the furthest STAS was measured with a ruler.
The tumor tissues were stained using an immunohistochemical method to detect SLX intensity. The mouse monoclonal antibody FH6 was kindly provided by Prof. S. Hakomori (University of Washington, Seattle, WA, USA). The avidin‐biotinylated horseradish peroxidase complex (ABC) method of immunoperoxidase histochemistry was performed using ABC kits (Vector Laboratory, Burlingame, CA, USA). Serial 4 μm thick sections were cut and stained with the monoclonal antibody FH6 (1:400). SLX staining was graded as follows: low, one‐third of cells had membranous staining; moderate, one‐third to two‐thirds of cells were stained; and high, ≥ two‐thirds of cells were stained.
Statistical analysis
Statistical analysis was performed using JMP version 10 (SAS Institute Inc., Cary, NC, USA). The Mann–Whitney U test was used to analyze continuous variables and χ2 tests were used to analyze categorical variables. Recurrence‐free survival (RFS) was calculated using the Kaplan–Meier method; differences were assessed using the log‐rank test. Independent risk factors associated with recurrence were calculated using a Cox proportional hazard model. A two‐sided P value of < 0.05 was considered significant.
Results
Patient characteristics stratified by STAS
The 245 patients comprised 122 men and 123 women with a median age of 67 (range: 20–93) years. In accordance with the classification, the papillary pattern was the most common (149 cases, 60.8%). STAS was detected in 71 (29.0%) patients, and the median distance between the tumor surface and the farthest STAS measured was 1.1 mm (range: 0.2–5.0 mm). STAS was more likely to be observed in patients with high‐grade histologic adenocarcinoma (3.4% with lepidic, 41.2% with acinar, 26.2% with papillary, 38.1% with mucinous, 70.0% with micropapillary, and 50.0% with solid; P < 0.001). Additionally, a high‐grade tumor (G2 or G3), ITR ≥ 50%, the presence of lymphatic/vascular invasion, and advanced pathological stage were significantly more frequent in patients with than without STAS. There were no differences in age, gender, smoking history, serum SLX level, or serum CEA level between patients with and without STAS. Recurrence and disease progression occurred in 51 patients (20.8%) during follow‐up. The incidence of recurrence was 16.1% (28/174) among patients without STAS and 32.4% (23/71) among those with STAS (P < 0.001). The RFS was higher in patients without than with STAS (P < 0.001); the five‐year RFS rates were 84.8% and 67.1%, respectively.
Immunohistological intensity of SLX in main tumor and STAS
We first analyzed whether the SLX intensity of the main tumor could predict the presence of STAS. The intensity of SLX was primarily observed in the cell membrane of the tumor tissue. The SLX intensity in the main tumor is represented in Figure 1. The patients were divided into three groups based on the intensity of SLX staining: high (108 patients, 44.1%); moderate (48 patients, 19.6%); and low (89 patients, 36.3%) (Table 1). There were no significant differences in age, gender, smoking history, pathological stage, histological grade, lymphatic/vascular invasion, or serum SLX and CEA levels among the three groups. With respect to the predominant histologic subtype, lepidic adenocarcinoma was more common in patients with high SLX intensity (15/29, 51.7%) than in those with moderate or low intensity. Conversely, more patients in the low‐intensity group had acinar (10/17, 58.8%), mucinous (13/21, 61.9%), micropapillary (5/9, 55.6%), and solid (9/18, 50.0%) adenocarcinoma. Notably, there was no significant difference between the presence or absence of STAS and the intensity of SLX staining. The SLX intensity of the STAS cell membrane itself, not the main tumor, showed high intensity in 57 patients (80.3%).
Figure 1.
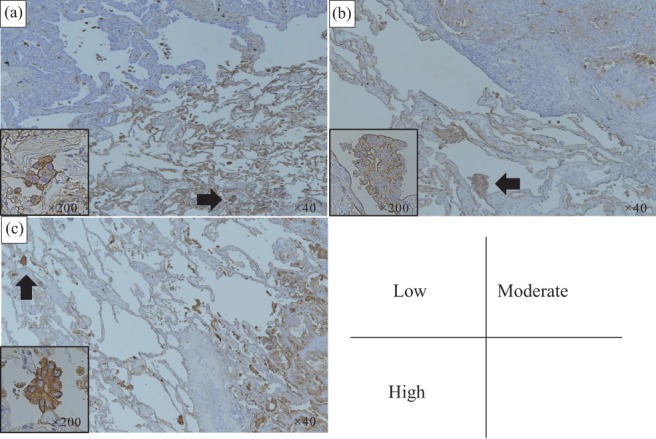
Immunohistochemical staining of SLX in tumor tissue. The staining was graded as follows: low, one‐third of cells had membranous staining; moderate, one‐third to two‐thirds of cells were stained; and high, ≥ two‐thirds of cells were stained. (a) Low, (b) moderate, and (c) high staining (original magnification ×40). The black arrow indicates tumor spread through alveolar spaces (original magnification ×200).
Table 1.
Characteristics of patients stratified by SLX staining
| Moderate | Low | P | ||
|---|---|---|---|---|
| Characteristics | High | (n = 108) | (n = 48) | (n = 89) |
| Gender (male/female) | 51/57 | 21/27 | 50/39 | 0.295 |
| Age (years) | 67 (39–85) | 69 (27–93) | 68 (20–86) | 0.394 |
| Smoking (never/ever) | 47/61 | 29/19 | 40/49 | 0.126 |
| p‐stage (UICC8) | ||||
| IA1/IA2/IA3/IB | 13/33/32/30 | 6/13/15/14 | 5/30/21/33 | 0.480 |
| Grade (G1/G2, G3) | 39/69 | 23/25 | 33/56 | 0.352 |
| Invasion/tumor ratio | ||||
| ≤ 50%/> 50% | 16/92 | 6/42 | 8/81 | 0.452 |
| Lymphatic/vascular invasion never/ever | 70/38 | 31/17 | 67/22 | 0.225 |
| Predominant histologic subtype† | ||||
| Lepidic | 15 (1) | 6 (0) | 8 (0) | 0.059 |
| Acinar | 6 (3) | 1 (0) | 10 (4) | |
| Papillary | 74 (22) | 31 (6) | 44 (11) | |
| Mucinous | 4 (1) | 4 (2) | 13 (5) | |
| Micropapillary | 3 (2) | 2 (2) | 5 (3) | |
| Solid | 5 (3) | 4 (2) | 9 (4) | |
| STAS (absent/present) | 76/32 | 36/12 | 62/27 | 0.786 |
| Serum SLX (U/ml) | 23.0 (10.0–85.4) | 19.7 (10.6–51.7) | 21.1 (11.5–48.6) | 0.330 |
| Serum CEA (ng/ml) | 3.6 (0.7–65.8) | 3.4 (0.5–80.5) | 3.0 (0.6–122) | 0.943 |
Number of patients with spread through alveolar spaces (STAS) is shown in parentheses. Values are shown as N or median (range), except for the predominant histologic subtype category of the table.
UICC8, Union for International Cancer Control, 8th Edition.
Distance of STAS from edge of main tumor and recurrence stratified by SLX intensity
We next analyzed whether the distance of STAS could be predicted by SLX intensity. In all cases the distance of STAS from the edge of the main tumor was < 5 mm. The distance between STAS cells and the main tumor was significantly shorter in the moderate SLX group than in the other two groups, as shown in Figure 2a. The median distance between STAS cells and the main tumor was 0.9 mm (range: 0.2–1.2 mm) in the moderate group and 1.2 mm (range: 0.4–5.0 mm) in the other two groups. The five‐year RFS rates of patients in the high, moderate, and low SLX intensity groups were 74.2%, 95.6%, and 77.8%, respectively (Fig 2b). Although 6 cases of locoregional and 16 cases of distant metastasis occurred in the low group and 7 cases of locoregional and 20 cases of distant metastasis in the high group, only 1 case of locoregional and 1 case of distant metastasis occurred in the moderate group. There was no significant difference in the metastatic pattern among the three groups. We performed multivariate analysis to determine which covariates were predictors of recurrence. Pathological stage, lymphatic or vascular invasion, and SLX intensity were identified as independent predictive factors for RFS (high or low vs. moderate SLX intensity: hazard ratio 6.65; 95% confidence interval 2.05–40.8) (Table 2).
Figure 2.
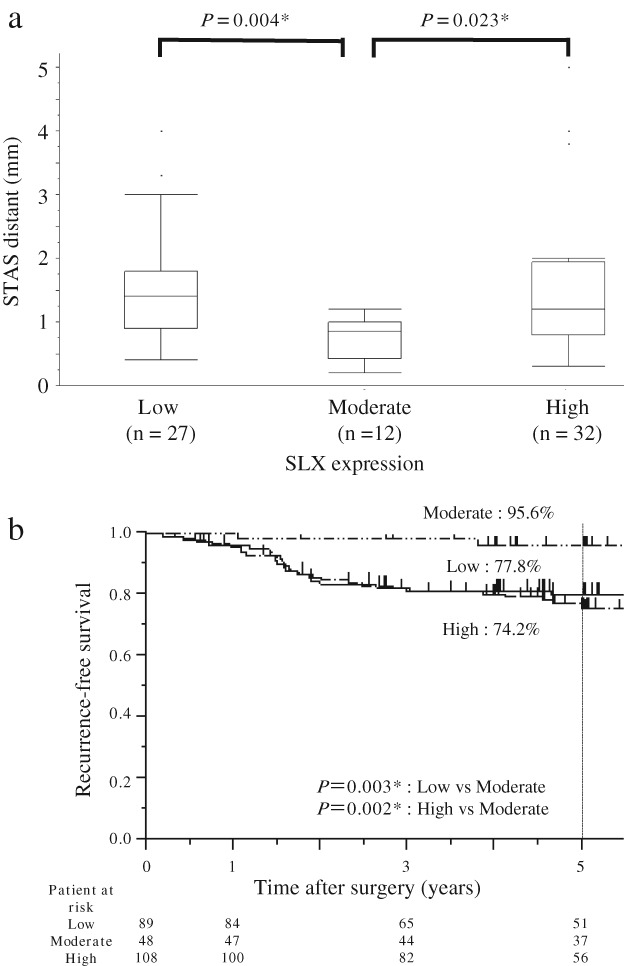
(a) Relationship between the distance of tumor spread through alveolar spaces (STAS) and SLX staining in the main tumor. (b) Recurrence‐free survival in patients with adenocarcinoma stratified by the intensity of SLX staining.
Table 2.
Cox proportional hazards regression model for recurrence‐free survival
| Variables | Univariate | HR | 95% CI | Multivariate | HR | 95% CI |
|---|---|---|---|---|---|---|
| Age (> 70 years) | 0.145 | 1.51 | 0.87–2.62 | — | ||
| Gender (male) | 0.033 | 1.83 | 1.05–3.26 | 0.602 | 1.19 | 0.63–2.29 |
| Smoking | 0.029 | 1.87 | 1.06–3.39 | 0.676 | 1.15 | 0.60–2.25 |
| p‐Stage (IB vs. IA) | < 0.001 | 3.94 | 2.27–6.98 | 0.001 | 2.78 | 1.53–5.11 |
| Lymphatic or vascular invasion | < 0.001 | 2.78 | 1.59–4.85 | 0.040 | 1.89 | 1.03–3.46 |
| Grade (G1/G2, G3) | 0.004 | 2.44 | 1.32–4.89 | 0.169 | 1.58 | 0.83–3.24 |
| Invasion/tumor ratio (≤ 50%/> 50%) | 0.003 | 7.87 | 1.73–139.2 | 0.066 | 4.45 | 0.93–79.9 |
| Predominant histologic subtype (high vs. intermediate) | ||||||
| 0.492 | 1.38 | 0.57–2.87 | — | |||
| STAS (+) | 0.006 | 2.21 | 1.26–3.83 | 0.506 | 1.22 | 0.67–2.20 |
| SLX expression (high/low, moderate) | <0.001 | 6.78 | 2.11–41.5 | < 0.001 | 6.65 | 2.05–40.8 |
CI, confidence interval; HR, hazard ratio; STAS, spread through alveolar spaces.
Discussion
Our findings indicate that the presence or absence of STAS itself cannot be predicted by the intensity of SLX staining in the main tumor; however, the distance between STAS and the main tumor was greater in association with high or low SLX intensity. The distance was significantly shorter in the moderate group than in the other two groups. This suggests that moderate SLX staining intensity is associated with a significant extension of RFS compared to low or high intensity, even in patients with stage I lung adenocarcinoma that have undergone lobectomy with radical mediastinal lymph node dissection.
Travis et al. proposed STAS as a new type of lung cancer invasion.10, 11 The existence of STAS itself is reportedly a risk factor for recurrence and poor prognosis.11, 12 Warth et al. showed that STAS was detected at high rates in men and in patients with wild‐type EGFR status, lymph node positivity, distant metastasis, and advanced‐stage lung cancer.18 Our data are consistent with these results and show that high‐grade cancer, a high ITR, lymphatic/vascular invasion, and the predominant histologic subtype were significantly associated with STAS. Considering these findings, predicting STAS before surgery is greatly useful for determining the optimal surgical procedure. When we perform SLX staining of the main tumor tissue obtained preoperatively by bronchoscopy or CT‐guided biopsy, there is a possibility that the STAS cells are located far from the main tumor if the SLX intensity rate of the main tumor is high or low. Considering the margin of the tumor, limited lung resection, such as segmentectomy, should be avoided. In this study, the maximum distance between STAS cells and the main tumor was 5 mm in collapsed lung tissue. Considering other literature as well, we suggest that 20 mm of distance is necessary as a surgical margin.19 In fact, the local recurrence rate reportedly worsens when performing limited lung resection for lung adenocarcinoma that is associated with STAS.6
In this study, we could not predict the presence of STAS by SLX staining. However, STAS was rarely present in well‐differentiated predominant histologic subtypes, such as lepidic adenocarcinoma, and it tended to appear more frequently in poorly differentiated predominant subtypes, such as micropapillary and solid adenocarcinoma. As a preoperative method to predict STAS using tumor markers, the serum CEA level was also not useful to predict STAS;20 we previously found that a high serum SLX level was associated with N2 disease21 and was a postoperative risk factor for recurrence of resected non‐small cell lung cancer,22 even in stage I,23 but serum SLX was not useful for predicting STAS in the present study. Although there is no association between serum SLX and the staining intensity of SLX in the main tumor, the moderate SLX intensity in abundant normal alveolar cells and SLX secreted from these normal cells might explain the discrepancy between SLX staining of the main tumor and serum SLX elevation.
Notably, the moderate‐intensity SLX group showed significantly better RFS than the low and high‐intensity SLX groups. Two main reasons for this finding should be discussed. First, lung adenocarcinoma itself is a heterogeneous cancer. In terms of the predominant subtype, more patients in the high‐intensity SLX group had the lepidic and papillary subtypes. In the low‐intensity SLX group, more patients had the acinar and mucinous subtypes. This deviation indicates that the intensity of SLX might have a different clinical implication according to the histological subtype. Second, SLX staining is also high in normal tissue cells (alveoli).24 Compared to the SLX intensity in normal and cancer tissues in the present study, most normal alveolar cells were classified into the moderate group (Fig 1). Thus, the cancer cells in the moderate group might maintain physiological characteristics similar to those of normal cells. The intensity in the moderate SLX group was almost equivalent to the SLX staining intensity of normal alveolar tissue cells. In the high‐intensity SLX group, staining of the adhesion molecules was strongly expressed, thus the metastatic potential may be high. On the other hand, the low‐intensity SLX group was easy to separate from the main tumor because of a low adherence junction. These hypotheses will need to be proven with further experimental conditions in future research.
This study has some limitations. First, some potential biases, such as selection and performance bias, were inevitable because of the retrospective nature of the study. Second, this clinical research was carried out in a single medical center. Third, molecular alterations such as EGFR and BRAF mutation status were unknown. In some studies, STAS was associated with lower rates of EGFR mutation but higher rates of BRAF mutation.18, 20 Fourthly, STAS is easily dismissed as an artifact when cells within air spaces are regarded as floaters or carry over as the result of contamination during processing. If the pathologist overlooks this as manipulation of the tumor, the data may be insufficient. Importantly, the ratio of SLX staining of STAS (80.3%) was higher than in the main tumors in this study. Even in the SLX negative stain of the main tumor, STAS was stained SLX‐positive, thus SLX staining might be a good method to distinguish STAS from contamination. Finally, lobectomy was performed in all patients and accurate information on locoregional recurrence could not be obtained; however, the accurate maximum distance between STAS and the main tumor could be measured in all patients because we excluded those who had undergone anatomical lobectomy.
In summary, these results provide preliminary evidence that the distance between the main tumor and STAS can be predicted by SLX staining, especially in stage I lung adenocarcinoma.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank Professor S. Hakomori (The Biomembrane Institute and University of Washington, Seattle, WA, USA) for his kind supply of monoclonal antibody FH6.
We also thank Angela Morben, DVM, and ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
References
- 1. Sihoe AD, Van Schil P. Non‐small cell lung cancer: When to offer sublobar resection. Lung Cancer 2014; 86: 115–20. [DOI] [PubMed] [Google Scholar]
- 2. Rami‐Porta R, Bolejack V, Giroux DJ et al. The IASLC Lung Cancer Staging Project: The new database to inform the eighth edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2014; 9: 1618–24. [DOI] [PubMed] [Google Scholar]
- 3. Rami‐Porta R, Ball D, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2007; 2: 593–602. [DOI] [PubMed] [Google Scholar]
- 4. Ujiie H, Kadota K, Chaft JE et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol 2015; 33: 2877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hung JJ, Jeng WJ, Chou TY et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Lung Adenocarcinoma Classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013; 258: 1079–86. [DOI] [PubMed] [Google Scholar]
- 6. Shimada Y, Saji H, Yoshida K et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non‐small cell lung cancer. Chest 2013; 143: 1626–34. [DOI] [PubMed] [Google Scholar]
- 7. Song IH, Yeom SW, Heo S et al. Prognostic factors for post‐recurrence survival in patients with completely resected stage I non‐small‐cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 262–7. [DOI] [PubMed] [Google Scholar]
- 8. Hung JJ, Jeng WJ, Hsu WH et al. Prognostic factors of postrecurrence survival in completely resected stage I non‐small cell lung cancer with distant metastasis. Thorax 2010; 65: 241–5. [DOI] [PubMed] [Google Scholar]
- 9. Okada S, Mizuguchi S, Izumi N et al. Prognostic value of the frequency of vascular invasion in stage I non‐small cell lung cancer. Gen Thorac Cardiovasc Surg 2017; 65: 32–9. [DOI] [PubMed] [Google Scholar]
- 10. Travis WD, Brambilla E, Nicholson AG et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 11. Kadota K, Nitadori J, Sima CS et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015; 10: 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morimoto J, Nakajima T, Suzuki H et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016; 152: 64–72. [DOI] [PubMed] [Google Scholar]
- 13. Uruga H, Fujii T, Fujimori S, Kohno T, Kishi K. Semiquantitative assessment of tumor spread through air spaces (STAS) in early‐stage lung adenocarcinomas. J Thorac Oncol 2017; 12: 1046–51. [DOI] [PubMed] [Google Scholar]
- 14. Sawada R, Tsuboi S, Fukuda M. Differential E‐selectin‐dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 1994; 269: 1425–31. [PubMed] [Google Scholar]
- 15. Ogawa JI, Inoue H, Koide S. Alpha‐2, 3‐Sialyltransferase type 3N and alpha‐1, 3‐fucosyltransferase type VII are related to sialyl Lewis(x) synthesis and patient survival from lung carcinoma. Cancer 1997; 79: 1678–85. [DOI] [PubMed] [Google Scholar]
- 16. Rami‐Porta R, Bolejack V, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015; 10: 990–1003. [DOI] [PubMed] [Google Scholar]
- 17. Travis WD, Brambilla E, Noguchi M et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warth A, Muley T, Kossakowski CA et al. Prognostic impact of intra‐alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol 2015; 39: 793–801. [DOI] [PubMed] [Google Scholar]
- 19. Dai C, Xie H, Su H et al. Tumor spread through air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma > 2 to 3 cm. J Thorac Oncol 2017; 12: 1052–60. [DOI] [PubMed] [Google Scholar]
- 20. Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016; 23: 567–72. [DOI] [PubMed] [Google Scholar]
- 21. Mizuguchi S, Inoue K, Iwata T et al. High serum concentrations of Sialyl Lewisx predict multilevel N2 disease in non‐small‐cell lung cancer. Ann Surg Oncol 2006; 13: 1010–8. [DOI] [PubMed] [Google Scholar]
- 22. Mizuguchi S, Nishiyama N, Iwata T et al. Clinical value of serum cytokeratin 19 fragment and sialyl‐Lewis x in non‐small cell lung cancer. Ann Thorac Surg 2007; 83: 216–21. [DOI] [PubMed] [Google Scholar]
- 23. Mizuguchi S, Nishiyama N, Iwata T et al. Serum Sialyl Lewis x and cytokeratin 19 fragment as predictive factors for recurrence in patients with stage I non‐small cell lung cancer. Lung Cancer 2007; 58: 369–75. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu T, Yonezawa S, Tanaka S, Sato E. Expression of Lewis X‐related antigens in adenocarcinomas of lung. Histopathology 1993; 22: 549–55. [DOI] [PubMed] [Google Scholar]


