Abstract
Background
Primary malignant melanoma of the esophagus (PMME) is rare. Patients with advanced melanoma of esophageal origin tend to have lower response rates to traditional therapies than those with other melanomas. We report our experience of 12 patients with PMME administered PD‐1 inhibitors.
Methods
This is a retrospective analysis of the clinical data of 76 patients with PMME who attended Peking University Cancer Hospital between January 2008 and September 2017. Objective response rates (ORRs) and progression‐free survival (PFS) were assessed.
Results
The 76 PMMEs were classified as unresectable or metastatic. The patients were allocated to three cohorts according to their treatment: chemotherapy (C: 46 patients), targeted therapy (T: 2 patients), and PD‐1 inhibitors (IT: 12 patients). The PFS in the C cohort was three months with a limited ORR of 10.9%. In the IT cohort, seven patients (75.0%) achieved a partial response and three had stable disease for 4+ months. The median PFS in the IT cohort was not reached and the mean was 15.6 months, which was much longer than in cohort C (P < 0.001).
Conclusion
Although this cohort of patients was small, it is the largest series investigated thus far. To the best of our knowledge, this is the first report of the outcomes of advanced PMMEs treated with PD‐1 inhibitors. Dramatic responses can occur in patients with advanced PMMEs.
Keywords: Adjuvant therapy, immunotherapy, melanoma of esophagus
Introduction
Primary malignant melanoma of the esophagus (PMME) is extraordinarily rare, comprising only 0.1%–0.2% of all malignant esophageal tumors and 0.5% of all non‐cutaneous melanomas, with an estimated incidence of 0.0036 million/year.1, 2, 3, 4, 5 Although Pava reported a case of PMME as early as 1963,6 only 339 cases had been reported worldwide by 2016, most as individual case reports.7, 8, 9 PMME behaves aggressively and has a poor prognosis, with a reported five‐year overall survival (OS) rate of < 5%.4 The clinicopathological characteristics of PMME have only rarely been reported and no comprehensive treatment strategy has been established because of the lack of cases and strong evidence.
In the present study, we reviewed 76 patients with PMME and sought to analyze the epidemiology, stage at presentation, and effects of therapy on survival.
Methods
Patient selection and follow‐up
The data of 76 patients with PMME who had attended Peking University Cancer Hospital between January 2008 and September 2017 were extracted from the hospital's database. Relevant personal and clinical characteristics (gender, age at diagnosis, and Eastern Cooperative Oncology Group performance status), and the histopathological features of tumors were investigated. Pathological diagnosis and staging of the primary tumors was made in accordance with the Tumor Node Metastasis (TNM) classification of the American Joint Commission on Cancer staging (AJCC) for the esophagus.10 Tumor responses were measured radiographically using Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1.
Follow‐up assessments were conducted by telephone or at the outpatient clinic. Patients were followed up until 1 July 2018. Follow‐up details, including survival status and cause of death, were obtained. The clinical features, treatment procedures, and outcomes of the 76 patients were analyzed. The Ethics Committee of Peking University Cancer Hospital and Institute approved this study.
DNA preparation and mutation screening
Genomic DNA was extracted from formalin‐fixed paraffin‐embedded sections using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany).11 To detect hotspot mutations, BRAF (exon 15), NRAS (exons 1 and 2), and C‐kit (exons 9, 11, 13, 17, and 18) were amplified by PCR in at least two separate preparations of genomic DNA.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0. Numerical variables are expressed as the mean ± standard deviation. Survival time was calculated from the date of diagnosis until the date of last contact or death. The Kaplan–Meier method was used to assess associations between TNM staging and survival outcome. Univariate and multivariate analyses were performed using Cox regression. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The log‐rank test was used to compare survival curves. P < 0.05 was considered to denote significance.
Results
Symptoms and clinical characteristics
Patient age ranged from 27 to 83 years (mean 56.5 years, median 57.0 years); 22% of the patients (n = 18) were aged < 50 at diagnosis. Only 24 (31.6%) patients were female, with a male/female ratio of 2.17:1.
Fifty‐five (69.7%) patients presented with a history of months of dysphagia, the most common complaint. The other 16 patients presented with retrosternal pain or acid regurgitation. Seven (9.2%) patients were asymptomatic; their lesions were detected during routine examination.
Forty‐four tumors (57.9%) were located in the lower thoracic portion of the esophagus, 26 (34.2%) in the middle esophagus, and 6 (7.9%) in the upper portion.
At the time of diagnosis, 14 (18.4%) patients had metastatic disease, 28 (36.8%) had node positive disease, and 34 (44.7%) had localized disease. Only 46 patients had analyzable tumor thickness data; the tumor had invaded the mucosa or submucosa in more than half of them (25/54.3%). The depth of tumor invasion was not correlated with the presence of peri‐esophageal lymph nodal metastasis. Nine (11.8%) patients harbored C‐Kit mutations, the most common occurring in exon 18:L862L in three patients, followed by exon 11:V560D in two patients. Other mutations included exon 11:L576P, exon 13:G658R, exon 17:N822K, and exon 9:K484K. Five (6.6%) were NRAS with five different mutation types: exon 1:G12S, exon 1:G13D, exon 1:G13R, exon 2:Q61H, and exon 2:Q61K. Five patients (6.6%) had BRAF mutations, most of which were V600E; only one was D594N. Further details regarding patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | Total number of patients (%) |
|---|---|
| Gender | |
| Male | 42 (68.4%) |
| Female | 24 (31.6%) |
| Extent of disease | |
| Local | 34 (44.7%) |
| Lymphonodus positive | 28 (36.8%) |
| Metastatic | 14 (18.4%) |
| Thickness | |
| Mucosae or submucosa | 25/46 (54.3%) |
| Muscularis propria | 18/46 (39.1%) |
| Adventitia | 3/46 (6.5%) |
| Location | |
| Up | 6 (7.9%) |
| Middle | 26 (34.2%) |
| Lower | 44 (57.9%) |
| Mutations | |
| C‐KIT | 9 (11.8%) |
| NRAS | 5 (6.6%) |
| BRAF | 5 (6.6%) |
Treatment
Surgery and adjuvant therapy
Fifty‐nine (77.6%) patients underwent subtotal esophagectomy or esophagogastrostomy plus systematic mediastinal or abdominal lymph node dissection. The reasons for not performing surgery were as follows: 2 patients were in extremely poor condition, 1 had an unresectable T4 tumor, and 14 had distant metastases (Table 2).
Table 2.
Surgery and adjuvant therapy
| Therapy | Number of patients by disease extent (%) | ||
|---|---|---|---|
| Total | N+ | M1 | |
| Esophagectomy | |||
| Yes | 59/76 (77.6%) | 24(40.7%) | 3(5.1%) |
| No | 17/76 (22.4%) | 8(4.7%) | 10(58.9%) |
| Adjuvant therapy | |||
| Yes | 37/59 (62.7%) | 15(37.8%) | 1(2.7%) |
| No | 22/59 (37.3%) | 10(45.5%) | 2(9.1%) |
Thirty‐seven patients (62.7%) underwent adjuvant therapy after surgery (Table 2): 32 were administered adjuvant temozolomide/dacarbazine‐based chemotherapy (TMZ/DTIC) and only 5 were administered adjuvant high‐dose‐interferon. At the time of last data collection in January 2018, recurrences had occurred in 52/58 (89.7%) of the patients whose initial excision had been considered complete. Fourteen of these patients had local recurrences and 44 had distant metastases; the median overall recurrence‐free survival (RFS) was 4.5 months. In comparison, an observation cohort of 22 patients had shorter RFS of just 2 months (P = 0.001). The RFS in patients administered adjuvant chemotherapy was 6 months, which represents a significantly lower risk of recurrence than in the control cohort (HR 0.56; P = 0.006) (Fig 1).
Figure 1.
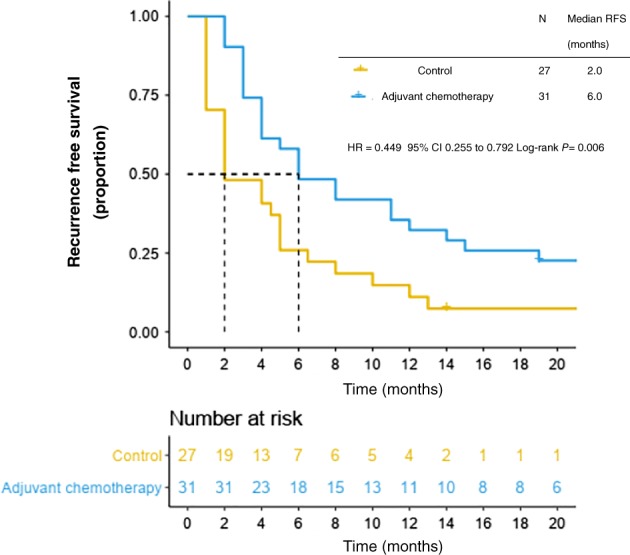
Recurrence‐free survival (RFS) in adjuvant chemotherapy and control cohorts.
Systemic therapy
The data of 69 patients with stage IV or unresected stage III disease were analyzed according to prior systemic treatment. Nine patients did not receive any treatment, for various reasons. The remaining patients were allocated to three cohorts according to the type of systemic treatment they received: chemotherapy (C), targeted therapy (TT), and immunotherapy (IT).
Cohort C: 46 (60.5%) of 76 patients were administered chemotherapy as first‐line treatment: 27 DTIC or TMZ and 19 paclitaxel (PTX) + carboplatin (CAR). Twelve patients in cohort C harbored gene mutations: four C‐Kit, three BRAF, and five NRAS. PFS was three months in cohort C: three and four months for the DTIC/TMZ and PTX + CAR groups, respectively (P = 0.529). ORR was 11.1% for DTIC/TMZ (3/27), 10.5% for PTX + CAR (2/19, 1 complete response [CR]), and 10.9% for cohort C overall (5/46).
Cohort TT: Two patients were administered imatinib because they had a C‐Kit mutation in exon 11. One (exon 11:V560D) achieved an eight month partial response (PR) and the other short‐term stable disease (SD: 2 months).
Cohort IT: Twelve patients were administered PD‐1 checkpoint inhibitors (2 with C‐Kit mutations). Nine patients (75.0%) achieved PR; the median response duration was 11.4 months, the longest being 21.3+ months. The remaining three patients had SD for at least four months. The median PFS for the IT cohort was not reached and the mean was 15.6 months, which is much longer than in cohort C (P < 0.001) (Fig 2). At the last follow up, eight patients were still receiving anti‐PD‐1 treatment (Table 3). Adverse events of any cause were reported in 91.7% of the patients in cohort IT, while grade 3 or 4 adverse events related to the trial drug occurred in 8.3%. During the treatment, there were no adverse events of any grade that resulted in the drug discontinuation or death. In general, no unexpected toxicity was observed.
Figure 2.
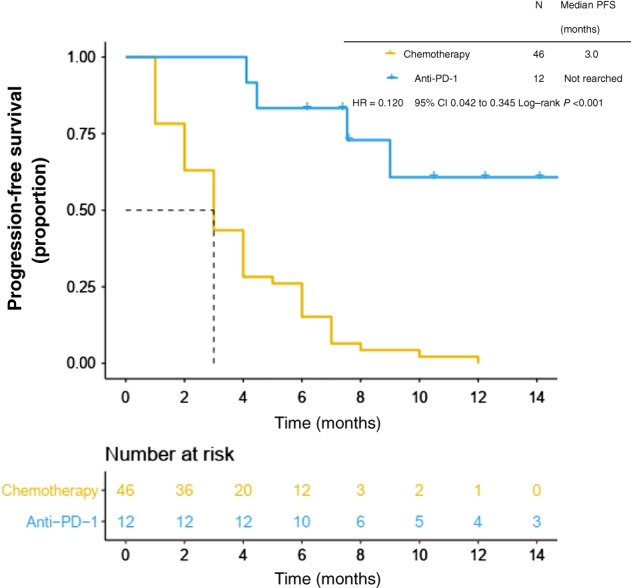
Progression‐free survival (PFS) in anti‐PD‐1 and chemotherapy cohorts.
Table 3.
Therapy with checkpoint inhibitors
| No. | Therapy | Gender | Age | LDH (norm < 240U/l) | Stage | Response | Therapy duration (months) |
|---|---|---|---|---|---|---|---|
| 1 | Anti‐PD‐1 | M | 62.0 | 240.0 | IV | PR | 6.2+ |
| 2 | Anti‐PD‐1 | F | 52.0 | 578.0 | IV (Liver) | SD | 4.0 |
| 3 | Anti‐PD‐1 | F | 63.0 | 289.0 | IV | PR | 21.3+ |
| 4 | Anti‐PD‐1 | F | 58.0 | 284.0 | IV | PR | 10.5+ |
| 5 | Anti‐PD‐1 | M | 65.0 | 195.0 | IV | PR | 14.1+ |
| 6 | Anti‐PD‐1 | F | 66.0 | 190.0 | IV | PR | 12.3+ |
| 7 | Anti‐PD‐1 | M | 61.0 | 171.0 | IV | PR | 7.6+ |
| 8 | Anti‐PD‐1 | M | 54.0 | 195.0 | IV | PR | 16.0+ |
| 9 | Anti‐PD‐1 | M | 51.0 | 172.0 | IV | PR | 9.0 |
| 10 | Anti‐PD‐1 | M | 56.0 | 202.0 | IV | SD | 7.5 |
| 11 | Anti‐PD‐1 | F | 66.0 | 146.0 | IV | SD | 4.5 |
| 12 | Anti‐PD‐1 | M | 54.0 | 138.0 | III | PR | 7.5+ |
LDH, lactate dehydrogenase; PR, partial response; SD, stable disease.
Disease progression
By the time of the last data collection in January 2018, 51/72 (70.8%) patients had died of melanoma and four had been lost to follow‐up. The median follow‐up duration was 19.4 months (range: 3.0–120.0). The median OS was 22.3 months (95% CI 16.4–28.2) in the entire cohort and 19.5 months in patients with stage IV and unresected stage III disease. The one and two‐year OS was 79.2% and 38.9%, respectively. Patients with metastases at the time of diagnosis had a median survival rate of 15.8 months, whereas those who developed metastases later or had unresected stage III disease had an average survival rate of 22.8 months from the date of first diagnosis (P = 0.032). The median OS from the first diagnosis was 18.5 months in cohort C (n = 45) and was not reached in cohort IT (n = 12). Cohort IT had significantly longer OS than cohort C in patients with stage IV or unresected stage III PMME (P = 0.013) (Fig 3).
Figure 3.
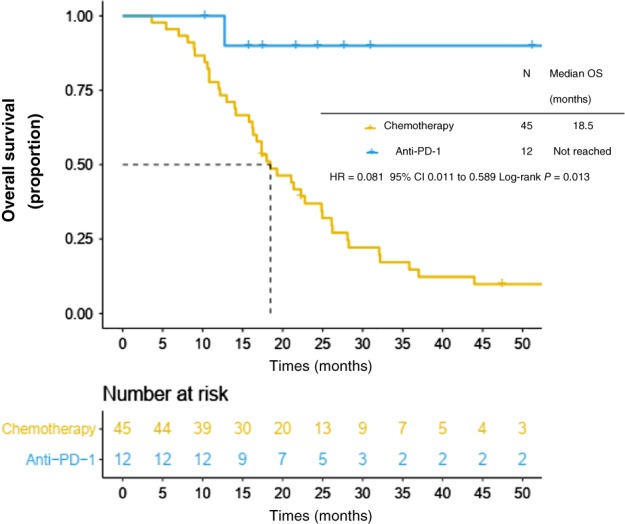
Overall survival (OS) in anti‐PD‐1 and chemotherapy cohorts.
Prognostic factors for overall and recurrence‐free survival
According to univariate analysis, patients with stage I or II disease at first diagnosis had significantly greater OS and RFS than patients with stages III to IV at first diagnosis. Adjuvant chemotherapy was associated with significantly superior recurrence‐free survival (P = 0.005); however, this was not true for OS (P = 0.056). Prior IT systemic treatment was correlated with a significant OS benefit (P < 0.001) (Table 4).
Table 4.
Univariate analysis of prognostic factors (Cox proportional hazard model)
| Overall survival | HR | 95% CI | P | |
|---|---|---|---|---|
| Stage at initial diagnosis | I + II versus III + IV | 0.478 | 0.267–0,855 | 0.013 |
| Prior systemic immunotherapy | Yes or No | 0.078 | 0.011–0.569 | 0.012 |
| Recurrence‐free survival | HR | 95% CI | P | |
| Stage at initial diagnosis | I + II versus III + IV | 0.577 | 0.328–0.995 | 0.041 |
| Adjuvant chemotherapy | Yes or No | 0.449 | 0.255–0.792 | 0.006 |
CI, confidence interval; HR, hazard ratio.
Univariate hazard analysis showed the expected impact of known prognostic variables for melanoma, such as thickness, location of the primary tumor, mutations, and age. However, none of these variables had independent prognostic significance for RFS and OS.
As expected, multivariate analysis showed a highly significant effect of adjuvant chemotherapy on RFS (P = 0.008), as well as statistically significant associations between tumor stage at time of the initial diagnosis and prior IT systemic treatment with OS (P = 0.022 and P = 0.006, respectively).
Discussion
In this study, we evaluated the data of 76 patients with PMME who had attended Peking University Cancer Hospital over a period of 10 years. To the best of our knowledge, this is the largest study to exhaustively investigate the clinical and histological features of PMMEs.
Similar to previous reports, in our cohort, PMME occurred more commonly in men, with a male‐to‐female ratio of 2.17:1. Most reported patients are symptomatic on diagnosis, dysphagia being the most common major symptom of PMME. Concerning the locations of the tumors in our series, 92.1% were in the middle and lower portion of the esophagus, similar to findings by Sabanathan et al. 12 In our series, there was a high percentage of thick melanomas. Half of the tumors had invaded the muscularis propria or further. Consistent with the results of other studies, the incidence of peri‐esophageal lymph node metastasis did not correlate with the depth of tumor invasion.12
There is currently no standard TNM staging system for PMME. We found that the initial TNM stage of PMME according to the AJCC classification for esophageal cancer was significantly related to both RFS and OS, likely indicating that this staging system may accurately discriminate the prognosis of patients with PMME. However, in some previous studies, esophageal TNM stage was found to be an independent prognostic factor for OS. Further larger studies are needed to determine a standard staging system for PMME.13, 14
Surgery is the most commonly reported treatment method. In the present study, 95% of patients with limited stage PMME underwent surgery; however, as reported by others, the interval between primary surgery and recurrence was only 4.5 months. The risk of recurrence is extremely high after an initial staging operation, which likely reflects the aggressive characteristics of PMME and the important role of adjuvant therapy. Adjuvant therapy has been shown to increase RFS and to have varying effects on OS in patients with cutaneous melanoma.15, 16 Our previous trial suggested that TMZ‐based adjuvant chemotherapy can improve both RFS and OS in patients with mucosal melanoma.17 However, because of its rarity, the optimal adjuvant therapies for PMME have not yet been established. Our data suggest that postoperative adjuvant chemotherapy may be considered for patients with PMME because it significantly improves RFS. However, even with adjuvant chemotherapy, RFS is still much lower than in other subtypes of mucosal melanoma (6 vs. 20.8 months)17 and this advantage does not extend to improvements in OS. Randomized controlled trials of adjuvant therapy with chemotherapy for patients with PMME are required. In addition, other more dynamic agents, such as ipilimumab or pembrolizumab, should be tested for their efficacy as adjuvant and neoadjuvant therapy in the future.
The role of systematic therapy remains unclear because most published studies are only single case reports. In our study and in previous studies, traditional cytotoxic chemotherapies have displayed very little efficacy against advanced stage PMME. The overall response rate (ORR) of chemotherapy in our cohort was only 10.9% with a short PFS of three months. Other studies have also shown unsatisfactory results of chemotherapy. Weiner et al. reported OS of chemotherapy of only 7.7 months and three‐year OS of 0 in eight patients.18
Treatment options for patients with metastatic melanoma have improved dramatically in the past five years with the development of targeted therapies and immunotherapies. Up to 25% of cutaneous melanomas have BRAF V600 mutations,19 resulting in aberrant signaling and cell growth through the MAPK pathway. Selective inhibition of the mutated RAF kinase with vemurafenib or dabrafenib improves survival of patients with stage IV disease. However, BRAF mutations occur at a lower frequency in mucosal than in cutaneous melanoma;20, 21, 22 BRAF mutations were detected in only 6.6% of tumors in our study, indicating that targeted therapy to the MAPK pathway is frequently unsuitable for patients with PMME. Anti‐PD‐1 antibodies have been demonstrated to be effective in patients with advanced stage melanoma and some data are available on the efficacy in treatment of mucosal melanoma. However, the potential role of immunotherapy in PMME has not yet been investigated. In our series, PD‐1 checkpoint inhibitors demonstrated activity against PMME. Initial results suggest a response rate of 75% with 11.4 months median duration of response. Additionally, multivariate survival analysis showed that IT systemic treatment was correlated with a significant OS benefit. Previous studies have concluded that PD‐1 checkpoint inhibitor monotherapy achieves unsatisfactory clinical outcomes in patients with mucosal melanoma,23 particularly those with visceral metastases (ORR 17.6%).24 However the PMME subtype of mucosal melanoma has a dramatically high response rate. More basic research, including whole genome sequencing is required to determine the mechanism of, and biomarkers for, immunotherapy for mucosal melanoma.
In conclusion, PD‐1 inhibitors appear to be a viable option for patients with advanced PMMEs. More evidence is required and the mechanism of action needs to be determined in future clinical trials and research.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank Dr. Trish Reynolds, MBBS, FRACP, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Contributor Information
Lu Si, Email: silu15_silu@126.com.
Jun Guo, Email: guoj307@126.com.
References
- 1. Bisceglia M, Perri F, Tucci A et al. Primary malignant melanoma of the esophagus: A clinicopathologic study of a case with comprehensive literature review. Adv Anat Pathol 2011; 18: 235–52. [DOI] [PubMed] [Google Scholar]
- 2. Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016; 41: 88–95. [DOI] [PubMed] [Google Scholar]
- 3. Archer HA, Owen WJ. Primary malignant melanoma of the esophagus. Dis Esophagus 2000; 13: 320–3. [DOI] [PubMed] [Google Scholar]
- 4. Kido T, Morishima H, Nakahara M et al. Early stage primary malignant melanoma of the esophagus. Gastrointest Endosc 2000; 51: 90–1. [DOI] [PubMed] [Google Scholar]
- 5. Caldwell CB, Bains MS, Burt M. Unusual malignant neoplasms of the esophagus: Oat cell carcinoma, melanoma, and sarcoma. J Thorac Cardiovasc Surg 1991; 101: 100–7. [PubMed] [Google Scholar]
- 6. Mikami T, Fukuda S, Shimoyama T et al. A case of early‐stage primary malignant melanoma of the esophagus. Gastrointest Endosc 2001; 53: 365–7. [DOI] [PubMed] [Google Scholar]
- 7. Lwanuma Y, Tomita N, Amano T et al. Current status of primary malignant melanoma of the esophagus: Clinical features, pathology, management and prognosis. J Gastroenterol 2012; 47: 21–8. [DOI] [PubMed] [Google Scholar]
- 8. Spencer KR, Mehnert JM. Mucosal melanoma: Epidemiology, biology and treatment. Cancer Treat Res 2016; 167: 295–320. [DOI] [PubMed] [Google Scholar]
- 9. Jiang W, Zou Z, Liu B. Primary malignant melanoma of the esophagus: A case report and review of the literature. Oncol Lett 2015; 9: 2036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou YB, Yuan Y, Hu B, Che GW. Primary multifocal malignant melanoma of esophagus co‐occurs with esophagogastric junction adenocarcinoma. Am J Gastroenterol 2016; 111: 312. [DOI] [PubMed] [Google Scholar]
- 11. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: Esophagus and esophagogastric junction. Ann Surg Oncol 2010; 17: 1721–4. [DOI] [PubMed] [Google Scholar]
- 12. Sabanathan S, Eng J. Primary malignant melanoma of the esophagus. Scand J Thorac Cardiovasc Surg 1990; 24: 83–5. [DOI] [PubMed] [Google Scholar]
- 13. Ahn JY, Hwang HS, Park YS et al. Endoscopic and pathologic findings associated with clinical outcomes of melanoma in the upper gastrointestinal tract. Ann Surg Oncol 2014; 21: 2532–9. [DOI] [PubMed] [Google Scholar]
- 14. Gao S, Li J, Feng X, Shi S, He J. Characteristics and surgical outcomes for primary malignant melanoma of the esophagus. Sci Rep 2016; 6: 23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weber J, Mandala M, Del Vecchio M et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377: 1824–35. [DOI] [PubMed] [Google Scholar]
- 16. Long GV, Hauschild A, Santinami M et al. Adjuvant dabrafenib plus trametinib in stage III BRAF‐mutated melanoma. N Engl J Med 2017; 377: 1813–23. [DOI] [PubMed] [Google Scholar]
- 17. Lian B, Si L, Cui C et al. Phase II randomized trial comparing high‐dose IFN‐α2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma. Clin Cancer Res 2013; 19: 4488–98. [DOI] [PubMed] [Google Scholar]
- 18. Weiner JP, Shao M, Schwartz D, Wong A, Schreiber D. Patterns of care and survival outcomes in the treatment of esophageal melanoma. Dis Esophagus 2017; 30: 1–6. [DOI] [PubMed] [Google Scholar]
- 19. Si L, Kong Y, Xu X et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: Large scale analysis of BRAF and NRAS mutations in a 432‐case cohort. Eur J Cancer 2012; 48: 94–100. [DOI] [PubMed] [Google Scholar]
- 20. Langer R, Becker K, Feith M, Friess H, Höfler H, Keller G. Genetic aberrations in primary esophageal melanomas: Molecular analysis of c‐KIT, PDGFR, KRAS, NRAS and BRAF in a series of 10 cases. Mod Pathol 2011; 24 (4): 495–501. [DOI] [PubMed] [Google Scholar]
- 21. Wong CW, Fan YS, Chan TL et al. BRAF and NRAS mutations are uncommon in melanomas arising in diverse internal organs. J Clin Pathol 2005; 58 (6): 640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheung MC, Perez EA, Molina MA et al. Defining the role of surgery for primary gastrointestinal tract melanoma. J Gastrointest Surg 2008; 12 (4): 731–8. [DOI] [PubMed] [Google Scholar]
- 23. D'Angelo SP, Larkin J, Sosman JA et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: A pooled analysis. J Clin Oncol 2017; 35: 226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maeda T, Yoshino K, Nagai K et al. Efficacy of nivolumab monotherapy against acral lentiginous melanoma and mucosal melanomain Asian patients. Br J Dermatol 2018; 17 10.1111/bjd.17434. [DOI] [PubMed] [Google Scholar]


