Abstract
Background
ING5 is the last member of the Inhibitor of Growth (ING) candidate tumor suppressor family that has been implicated in multiple cellular functions, including cell cycle regulation, apoptosis, and chromatin remodeling. Our previous study showed that ING5 overexpression inhibits lung cancer aggressiveness and epithelial–mesenchymal transition (EMT), with unknown mechanisms.
Methods
Western blotting was used to detect total and phosphorylated levels of β‐catenin and EMT‐related proteins. Immunofluorescent staining was used to observe E‐cadherin expression. Proliferation and colony formation, wound healing, and Transwell migration and invasion assays were performed to study the proliferative and invasive abilities of cancer cells.
Results
ING5 overexpression promotes phosphorylation of β‐catenin at Ser33/37, leading to a decreased β‐catenin protein level. Small hairpin RNA‐mediated ING5 knockdown significantly increased the β‐catenin level and inhibited phosphorylation of β‐catenin S33/37. Treatment with the WNT/β‐catenin inhibitor XAV939 inhibited ING5‐knockdown promoted proliferation, colony formation, migration, and invasion of lung cancer A549 cells, with increased phosphorylation of β‐catenin S33/37 and a decreased β‐catenin level. XAV939 also impaired ING5‐knockdown‐induced EMT, as indicated by upregulated expression of the EMT marker E‐cadherin, an epithelial marker; and decreased expression of N‐cadherin, a mesenchymal marker, and EMT‐related transcription factors, including Snail, Slug, Twist, and Smad3. Furthermore, XAV939 could inhibit the activation of both IL‐6/STAT3 and PI3K/Akt signaling pathways.
Conclusion
ING5 inhibits lung cancer invasion and EMT by inhibiting the WNT/β‐catenin pathway.
Keywords: Epithelial–mesenchymal transition (EMT), ING5, lung cancer, phosphorylation, WNT/β‐catenin
Introduction
Lung cancer remains the leading cause of cancer‐related death worldwide. The progression of cancer cells into a metastatic phenotype contributes to more than 90% of cancer deaths.1 Metastasis is a multistep process in which epithelial–mesenchymal transition (EMT) is a key event for cancer progression and metastasis in the early stage.1, 2, 3 EMT is characterized by a loss of intercellular adhesion, downregulation of epithelial markers, and upregulation of mesenchymal markers,4, 5 which could be regulated and reversed as a target of anti‐metastasis therapy.3
ING5 belongs to the Inhibitor of Growth (ING) candidate tumor suppressor family, which is involved in multiple cellular functions, including regulation of the cell cycle, apoptosis, differentiation, DNA damage repair, and chromatin remodeling.6, 7, 8, 9, 10 Recently, the results of our studies and those of others have revealed that ING5 inhibits tumor progression and metastasis in lung,11 gastric,12 and breast cancers13 by inhibiting EMT. Using protein array, we have screened and confirmed that activation of both IL‐6/STAT3 and EGFR/PI3K/Akt signaling pathways is involved in ING5 knockdown‐promoted lung cancer invasiveness and EMT.14
Apart from PI3K/Akt and STAT3 oncogenic signaling pathways, hyperactivation of Wnt/β‐catenin signaling is widely implicated in many types of cancers.15, 16 Our previous protein array results showed an increased β‐catenin level in ING5 knockdown A549 cells,14 suggesting that ING5 may also affect the β‐catenin signaling pathway. β‐catenin plays a key role in the Wnt signaling cascade. In the absence of Wnt stimulation, cytosolic β‐catenin interacts with the scaffolding protein Axin, casein kinase 1 (CK1), glycogen synthase kinase 3β (GSK‐3β), and tumor suppressor APC to form a large complex that promotes the phosphorylation of the N terminus of β‐catenin by GSK‐3β at its consensus phosphorylation‐sites (S33, 37, 45, and T41).17, 18 Phosphorylated β‐catenin is then targeted for ubiquitination and proteasome degradation. Thus, β‐catenin phosphorylation controls the β‐catenin protein level and Wnt signaling.17
In the current study, for the first time, we show that ING5 overexpression inhibits lung cancer EMT and invasiveness by promoting β‐catenin phosphorylation and degradation. The Wnt/β‐catenin inhibitor XAV939 could partly reverse EMT and the invasiveness promoted by ING5 knockdown.
Methods
Cell culture and regents
Human lung cancer cell lines (A549, H1299) and a human colorectal cancer cell line (HCT116) were purchased from Type Culture Collection (Chinese Academy of Sciences, Shanghai, China). These cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 10 mg/mL antibiotics (penicillin and streptomycin), and 2 mmol/L L‐glutamine at 37°C under 5% CO2 and saturated moisture. The establishment of cell lines with ING5 stable overexpression or knockdown has been described previously.11 XAV939, ZSTK474, and Niclosamide were purchased from Selleck Chemicals LLC (Houston, TX, USA).
Proliferation assay
Cells were seeded in triplicate in 96‐well culture plates at a density of 5 × 104 cells/200μL/well. Methyl thiazolyl tetrazolium (MTT) assay was performed as described previously.14 Experiments were performed in triplicate and results were analyzed by paired t‐test.
Colony formation assay
Cells were seeded in six‐well plates at a density of 3 × 102 cells/2 mL/well and incubated until the colonies were visible, which usually occurs after 15 days. Crystal violet staining was performed and the number of colonies was counted.
Wound‐healing assay
Cells were seeded in six‐well plates at a density of 4 × 105 cells/well. Once the cells reached 90% confluence, a wound area was carefully created by scraping the cell monolayer with a sterile 200 μL pipette tip from one end of the well to the other. The detached cells were removed by washing with phosphate buffered saline. The cells that had migrated to the wounded region were observed using an Olympus CK‐2 inverted microscope (Olympus Corporation, Tokyo, Japan) and photographed (100× magnification) at 0, 8, 16, 20, and 24 hours. The experiments were performed in triplicate.
Transwell migration and invasion assay
For the migration assay, 5 × 104 cells were suspended in serum‐free medium and plated on chambers (Corning Costar, New York, NY, USA) that were not coated with Matrigel. For the invasion assay, the upper chamber was precoated with Matrigel (BD Bioscience, San Jose, CA, USA) according to the manufacturer's protocols before 5 × 104 cells in serum‐free DMEM were added to the chamber. The assays were performed as previously described.11
Western blot
Cells were lysed in lysis buffer and protein concentrations of the lysates were determined using the Bradford protein assay system (Bio‐Rad, Hercules, CA, USA). Equal amounts (30μg protein each lane) of total cellular protein were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis. The membrane was blocked and then incubated with primary antibody overnight at 4°C. Primary antibodies used included antibodies for ING5 and SMAD3 (Proteintech Group, Inc., Rosemont, IL, USA); IL‐6 (Bioworld Technology Co., Ltd., Nanjing, China); pAKT (Ser473/Thr308) and STAT3 (Cell Signaling
Technology, Danvers, MA, USA); p‐STAT3 (Y705), AKT, β‐catenin, p‐β‐catenin (Ser33/S37), E‐cadherin, N‐cadherin, Snail, Slug, Twist, EGFR, and CEACAM6 (Abcam, Cambridge, MA, USA); and β‐actin (Actin) (Sigma‐Aldrich, St. Louis, MO, USA). The membrane was incubated with corresponding secondary antibody conjugated with horseradish peroxidase (1:5000, Sigma‐Aldrich) at room temperature for one hour. The blots were developed using an enhanced chemiluminescence Western blot detection system (Amersham Bioscience, Buckinghamshire, UK).
Immunofluorescent staining
Cells were seeded on sterile coverslips placed in 24‐well culture plates at 3 × 104/600 μL/well and treated with 20 μM XAV939 or dimethyl sulfoxide as a vehicle for eight hours. Cells were then fixed with 4% paraformaldehyde for 15 minutes at room temperature, and permeabilized with 0.5% Triton X‐100 for 10 minutes. After blocking with 5% bovine serum albumin for one hour at room temperature, cells were incubated with the primary antibody (mouse monoclonal antibody against E‐cadherin 1:100 dilution or rabbit monoclonal antibody against β‐catenin 1:100 dilution) at 4°C overnight. Cells were then incubated with conjugated goat anti‐rabbit secondary antibody at 1:1000 dilution for one hour at room temperature in the dark. For nuclear counterstaining, cells were incubated with 4′,6‐diamidino‐2‐phenylindole
(1:50 dilution) for five minutes. Coverslips were then mounted with Fluoromount‐G (Thermo Fisher Scientific, Waltham, MA, USA). Cells were visualized using a Zeiss LSM510 Meta Confocal Microscope (Carl Zeiss Microscopy GmbH, Baden‐Wuerttemberg, Germany). Images were acquired at 200× total magnification using Zeiss Zen 2009 software.
Statistical analysis
Two groups of data were analyzed by t‐test. All of the statistical tests were two‐sided. All data were analyzed using SPSS version 17.0. P < 0.05 was regarded as statistically significant.
Results
ING5 overexpression promotes β‐catenin phosphorylation and degradation
Our previous study using protein array showed an increase in the β‐catenin level in ING5 knockdown A549 cells.14 We confirmed a decreased β‐catenin protein level by Western blotting in ING5 knockdown A549 and H1299 cells (Fig 1a). However, the β‐catenin messenger RNA level was not affected in ING5 knockdown cells (Fig 1b). We further found downregulated phosphorylation of β‐catenin at Serine S33 and S37 by ING5 knockdown (Fig 1a). In addition, we confirmed that the β‐catenin level was downregulated in ING5 overexpressed A549, H1299, and HCT116 cells, with increased p‐β‐catenin (Fig 1c). The WNT/β‐catenin target gene c‐Myc was upregulated in ING5 knockdown cells and downregulated with ING5 overexpression (Fig 1d). These results indicate that ING5 inhibits the WNT/β‐catenin signal by promoting phosphorylation‐dependent degradation of β‐catenin.
Figure 1.
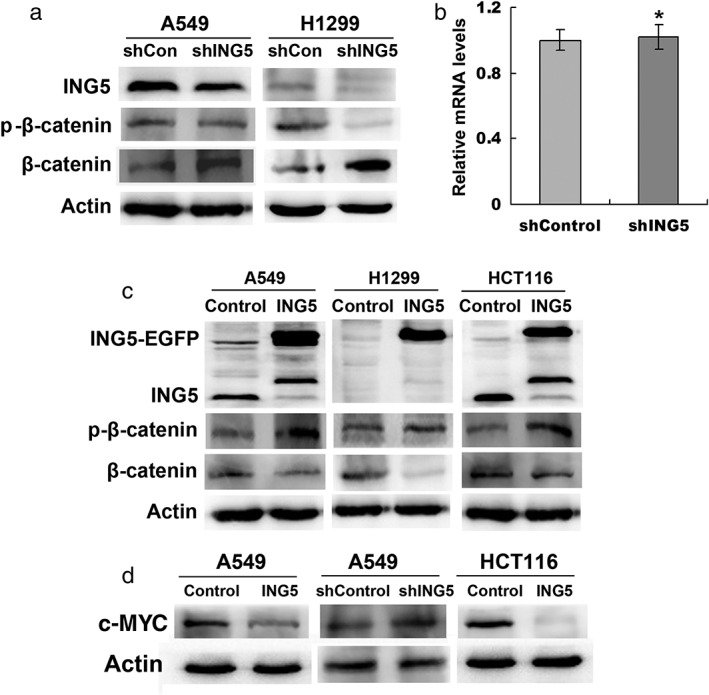
ING5 overexpression promotes β‐catenin phosphorylation and degradation. (a) Effects of ING5 knockdown on β‐catenin protein level in lung cancer A549 and H1299 cells. (b) Effects of ING5 knockdown on β‐catenin messenger RNA (mRNA) level by quantitative reverse transcription‐PCR. Data are shown as mean plus standard error of three independent experiments. *P < 0.05 compared to control. (c) Effects of ING5 overexpression on β‐catenin protein level. (d) Effects of ING5 overexpression or knockdown on c‐MYC level. ShCon, small hairpin control.
Inhibition of the Wnt/β‐catenin pathway impaired ING5 knockdown‐induced invasion of lung cancer cells
To investigate whether ING5 inhibited cancer cell invasiveness by affecting the Wnt/β‐catenin signaling pathway, we treated A549 small hairpin (sh)Control and shING5 cells with the Wnt/β‐catenin inhibitor, XAV939, which inhibits tankyrase and thus increases Axin stability, leading to β‐catenin degradation.19 Immunofluorescent staining (Fig 2a) and Western blotting (Fig 2b) revealed that XAV939 significantly decreased the β‐catenin level in both shControl and shING5 A549 cells and increased β‐catenin phosphorylation (Fig 2b). XAV939 inhibited cell proliferation and colony formation of A549 shControl and shING5 cells (Fig 2c,d). Furthermore, XAV939 inhibited the migration of A549 shControl and shING5 cells assessed by wound healing and Transwell migration assays (Fig 2e,f). In addition, XAV939 significantly prevented A549 shControl and shING5 cells from invading through Matrigel‐coated polycarbonate filter in the transwell chamber (Fig 2g). These results show that the Wnt/β‐catenin signaling pathway is involved in the ING5 knockdown‐promoted invasive abilities of lung cancer cells, which could partly be reversed by XAV939.
Figure 2.
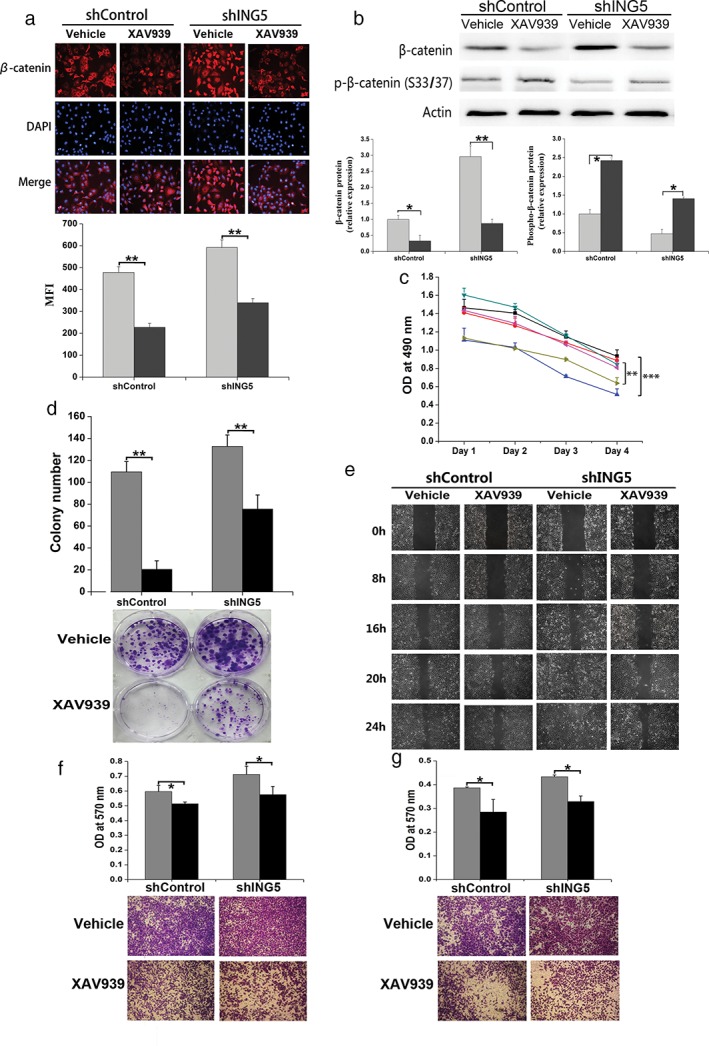
Inhibition of Wnt/β‐catenin pathway impaired ING5 knockdown‐induced invasion of lung cancer cells. (a) Immunofluorescent staining showed that XAV939 significantly decreased the β‐catenin level in both small hairpin (sh)Control and shING5 A549 cells. The mean fluorescence intensity (MFI) values were compared. **P < 0.01, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. ( ) Vehicle, and (
) Vehicle, and ( ) XAV939. (b) Western blotting showed that XAV939 significantly decreased the β‐catenin level, with increased p‐β‐catenin. The density of bands was quantified and analyzed. *P < 0.05 and **P < 0.01, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. (
) XAV939. (b) Western blotting showed that XAV939 significantly decreased the β‐catenin level, with increased p‐β‐catenin. The density of bands was quantified and analyzed. *P < 0.05 and **P < 0.01, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. ( ) Vehicle, and (
) Vehicle, and ( ) XAV939. (c) Effects of XAV939 on proliferation of shControl and shING5 A549 cells. **P < 0.001, XAV939 group compared to the vehicle control group of shControl A549 cells. **P < 0.01, XAV939 group compared to the vehicle control group of shING5 A549 cells. (
) XAV939. (c) Effects of XAV939 on proliferation of shControl and shING5 A549 cells. **P < 0.001, XAV939 group compared to the vehicle control group of shControl A549 cells. **P < 0.01, XAV939 group compared to the vehicle control group of shING5 A549 cells. ( ) shControl+0μMXAV939, (
) shControl+0μMXAV939, ( ) shControl+10μMXAV939, (
) shControl+10μMXAV939, ( ) shControl+20μMXAV939, (
) shControl+20μMXAV939, ( ) shING5+0μMXAV939, (
) shING5+0μMXAV939, ( ) shING5+10μMXAV939, and (
) shING5+10μMXAV939, and ( ) shING5+20μMXAV939 (d) The effects of XAV939 on the colony formation abilities of shControl and shING5 A549 cells. **P < 0.01, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. (
) shING5+20μMXAV939 (d) The effects of XAV939 on the colony formation abilities of shControl and shING5 A549 cells. **P < 0.01, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. ( ) Vehicle, and (
) Vehicle, and ( ) XAV939. (e) The effects of XAV939 on migration of A549 shControl and shING5 cells by wound‐healing assay. A scratch wound was made on cell surface and cells were photographed at 0, 8, 16, 20, and 24 hours. (f) Effects of XAV939 on Transwell migration of A549 shControl and shING5 cells. The migrated cells were photographed (100 × magnification). *P < 0.05, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. (
) XAV939. (e) The effects of XAV939 on migration of A549 shControl and shING5 cells by wound‐healing assay. A scratch wound was made on cell surface and cells were photographed at 0, 8, 16, 20, and 24 hours. (f) Effects of XAV939 on Transwell migration of A549 shControl and shING5 cells. The migrated cells were photographed (100 × magnification). *P < 0.05, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. ( ) Vehicle, and (
) Vehicle, and ( ) XAV939 (g) The effects of XAV939 on the invasive abilities of A549 shControl and shING5 cells. The invaded cells were photographed (100× magnification). *P < 0.05, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. (
) XAV939 (g) The effects of XAV939 on the invasive abilities of A549 shControl and shING5 cells. The invaded cells were photographed (100× magnification). *P < 0.05, XAV939 group compared to the vehicle control group of shControl A549 cells and shING5 A549 cells, respectively. ( ) Vehicle, and (
) Vehicle, and ( ) XAV939. Representative pictures are shown. Data are shown as mean plus standard error of three independent experiments. DAPI, 4′,6‐diamidino‐2‐phenylindole; OD, optical density.
) XAV939. Representative pictures are shown. Data are shown as mean plus standard error of three independent experiments. DAPI, 4′,6‐diamidino‐2‐phenylindole; OD, optical density.
Inhibition of Wnt/β‐catenin pathway impairs ING5 knockdown‐induced epithelial–mesenchymal transition of lung cancer cells
To investigate whether ING5 knockdown‐induced EMT through an elevated Wnt/β‐catenin signaling pathway, we treated ING5 knockdown lung cancer cells with XAV939 and investigated changes in EMT‐related markers and transcription factors. Immunofluorescent staining and Western blotting revealed that XAV939 treatment could increase epithelial marker E‐cadherin expression in shControl and ING5‐knockdown A549 cells (Fig 3a,b); and downregulate the mesenchymal marker N‐cadherin, EMT‐related transcription factors (Snail, Slug, Smad3, Twist), and EMT‐inducing protein CEACAM6 (Fig 3b). These results suggest that the Wnt/β‐catenin signaling pathway plays an important role in ING5 knockdown‐induced EMT, which could partly be reversed by the Wnt/β‐catenin inhibitor XAV939.
Figure 3.
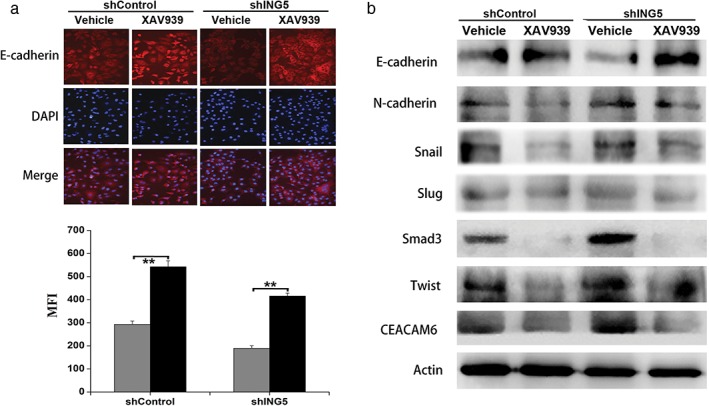
Inhibition of the Wnt/β‐catenin pathway impairs ING5 knockdown‐induced epithelial–mesenchymal transition (EMT). (a) Immunofluorescent staining revealed the effects of XAV939 on the expression of E‐cadherin in small hairpin (sh)Control and shING5 A549 cells. Representative pictures are shown (200× magnification). Data are shown as mean plus standard error of at least three independent experiments. The mean fluorescence intensity (MFI) values were compared. **P < 0.01 compared to the corresponding vehicle control. ( ) Vehicle, and (
) Vehicle, and ( ) XAV939. (b) Western blotting revealed the effects of XAV939 on the protein expression of EMT markers and EMT‐related proteins. Actin was used as an internal loading control. DAPI, 4′,6‐diamidino‐2‐phenylindole.
) XAV939. (b) Western blotting revealed the effects of XAV939 on the protein expression of EMT markers and EMT‐related proteins. Actin was used as an internal loading control. DAPI, 4′,6‐diamidino‐2‐phenylindole.
Inhibition of Wnt/β‐catenin pathway suppresses IL‐6/STAT3 and EGFR/PI3K/AKT signaling pathways
There is crosstalk between Wnt/β‐catenin, IL‐6/STAT3, and PI3K/AKT signaling pathways in cancer progression. Our previous work showed that ING5 knockdown activates both IL‐6/STAT3 and PI3K/AKT signaling pathways.14 In the current study, the effects of XAV939 on the IL‐6/STAT3 and PI3K/AKT signaling pathways of lung cancer cell A549 were observed. Western blot results showed that XAV939 treatment decreased Akt phosphorylation at S473/T308 and STAT3 phosphorylation at Y705, which indicated inactivation of the IL‐6/STAT3 and PI3K/AKT pathways. EGFR and IL‐6, which are known activators of the PI3K/Akt and STAT3 signaling pathways, respectively, were downregulated in XAV939‐treated ING5 knockdown cells (Fig 4a). PI3K inhibitor ZSTK474 or STAT3 inhibitor Niclosamide also decreased the β‐catenin level in ING5 knockdown A549 cells, with increased p‐β‐catenin (Fig 4b,c).
Figure 4.
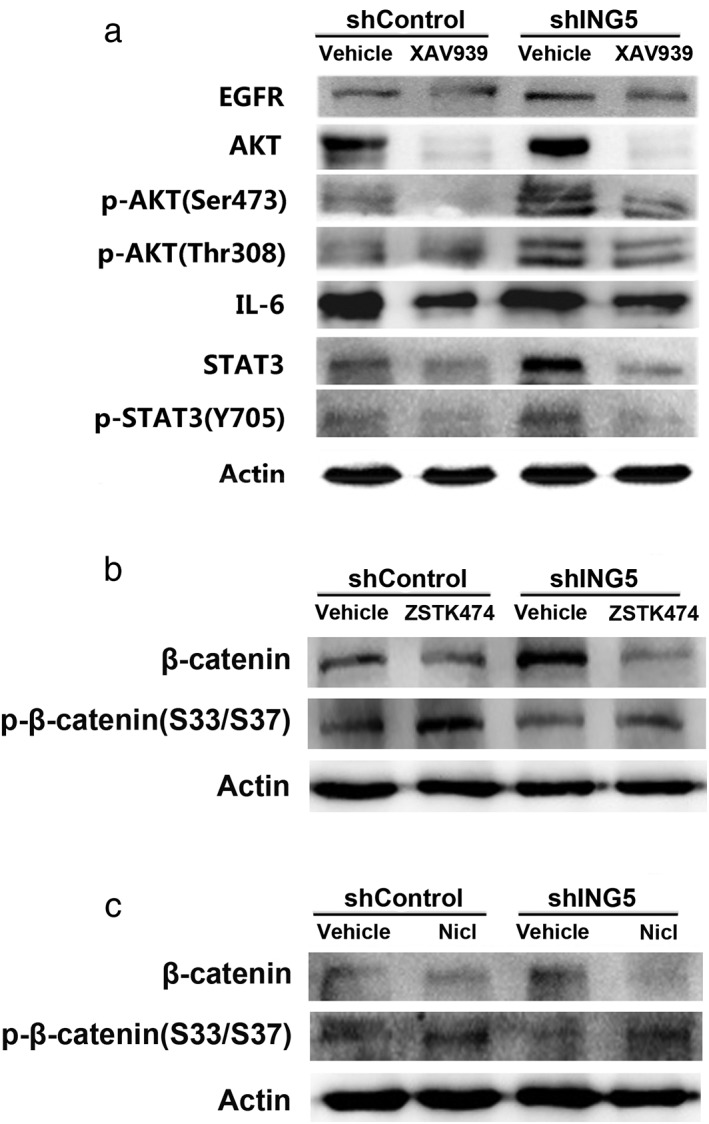
Inhibition of the Wnt/β‐catenin pathway suppresses IL‐6/STAT3 and EGFR/PI3K/AKT signaling pathways. (a) The effects of XAV939 on the expression of proteins involved in the IL‐6/STAT3 and PI3K/AKT signaling pathways. Western blotting revealed the effects of (b) PI3K inhibitor ZSTK474 and (c) STAT3 inhibitor Niclosamide (Nicl) on β‐catenin level. Actin was used as an internal loading control.
Discussion
ING5 functions as a tumor suppressor via multiple mechanisms. Previously, we revealed for the first time that ING5 inhibits lung cancer aggressiveness by preventing EMT11. Subsequently, we reported that the EGFR/PI3K/Akt and IL‐6/STAT3 oncogenic signaling pathways are involved in promoted cancer aggressiveness and EMT by ING5 knockdown.14 In the current study, we further show that ING5 overexpression promotes phosphorylation‐dependent degradation of β‐catenin, leading to downregulated Wnt/β‐catenin signaling and inhibition of lung cancer invasion and EMT.
Wnt/β‐catenin signaling plays a pivotal role in regulating cell proliferation, migration, and invasion. Aberrant activation of Wnt/β‐catenin signaling plays a crucial role in cancer progression and metastasis by activating the β‐catenin/T‐cell factor (TCF) complex and subsequent regulation of target genes with TCF‐binding elements.17, 18 The Wnt/β‐catenin pathway is regulated through degradation of β‐catenin via phosphorylation of its N terminus by GSK‐3β in a complex with CKI, APC, and Axin.17 Our data show that β‐catenin is inversely regulated by ING5 at the protein level but not at the messenger RNA level. Furthermore, phosphorylation of the N terminus of β‐catenin at GSK‐3β target sites Ser33/37 is upregulated by ING5 overexpression, demonstrating that ING5 promotes β‐catenin phosphorylation and subsequent proteasomal degradation. When ING5 was knocked down, phosphorylation of β‐catenin by GSK‐3β was inhibited, leading to the accumulation of β‐catenin and subsequent transcription of its target genes, such as c‐MYC, which may promote lung cancer invasion and progression. GSK‐3β is one of the key kinases in the multiprotein complex that mediates β‐catenin phosphorylation and degradation. In the absence of Wnt signals, GSK‐3β is unphosphorylated and active to phosphorylate β‐catenin.20 Upon Wnt stimulation, GSK‐3β activity is inhibited through phosphorylation at Serine 9 by oncogenic processes, such as PI3K/Akt activation.21 Nevertheless, modulation of GSK‐3β activity may occur by other upstream processes. The mechanisms by which ING5 promotes GSK‐3β‐mediated β‐catenin phosphorylation require further investigation.
The WNT/β‐catenin pathway inhibitor XAV939 functions by inhibiting the poly‐ADP‐ribosylating enzymes tankyrase 1 and 2 and stabilizing Axin, thus stimulating β‐catenin degradation through the ubiquitin‐proteasome pathway.19 ING5 knockdown‐promoted dephosphorylation and accumulation of β‐catenin was partly reversed by XAV939 treatment. The β‐catenin pathway is one of the major signaling pathways implicated in different factor‐induced EMT and the invasive abilities of lung cancer cells.22 Our data show that treatment with XAV939 caused a reversal of EMT and the invasive phenotype induced by ING5 knockdown.
XAV939 also prevents activation of both PI3K/Akt and IL‐6/STAT3 signals, further confirming the crosstalk between the three pathways in lung cancer EMT and progression. Activation of these oncogenic signaling pathways is involved in ING5 knockdown‐promoted EMT and invasion of lung cancer. However, which is the major pathway regulated by ING5 and the underlying mechanisms need to be elucidated.
In conclusion, our results show for the first time that ING5 overexpression inhibits EMT and lung cancer invasiveness by promoting phosphorylation‐dependent degradation of β‐catenin, leading to downregulation of WNT/β‐catenin signaling. The mechanism by which ING5 promotes β‐catenin phosphorylation warrants further investigation.
Disclosure
No authors report any conflict of interests.
Acknowledgments
This study was sponsored by National Natural Science Foundation of China (No. 81672269), Natural Science Foundation of Shaanxi Province (No. 2017JM8034) and a grant from the No. 309 Hospital of PLA (No. 2016MS‐016).
Contributor Information
Guan‐Ren Zhao, Email: yanliqun79@163.com.
Tao Zhang, Email: zhangft@fmmu.edu.cn.
References
- 1. Mittal V. Epithelial mesenchymal transition in aggressive lung cancers. Adv Exp Med Biol 2016; 890: 37–56. [DOI] [PubMed] [Google Scholar]
- 2. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial‐mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15: 178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev 2016; 35: 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serrano‐Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial‐mesenchymal transition through epigenetic and post‐translational modifications. Mol Cancer 2016; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JY, Kong G. Roles and epigenetic regulation of epithelial‐mesenchymal transition and its transcription factors in cancer initiation and progression. Cell Mol Life Sci 2016; 73: 4643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiseki M, Nagashima M, Pedeux RM et al. p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res 2003; 63: 2373–8. [PubMed] [Google Scholar]
- 7. Doyon Y, Cayrou C, Ullah M et al. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell 2006; 21: 51–64. [DOI] [PubMed] [Google Scholar]
- 8. Zhang F, Baumer N, Rode M et al. The inhibitor of growth protein 5 (ING5) depends on INCA1 as a co‐factor for its antiproliferative effects. PLoS One 2011; 6: e21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mulder KW, Wang X, Escriu C et al. Diverse epigenetic strategies interact to control epidermal differentiation. Nat Cell Biol 2012; 14: 753–63. [DOI] [PubMed] [Google Scholar]
- 10. Liu N, Wang J, Wang J et al. ING5 is a Tip60 cofactor that acetylates p53 in response to DNA damage. Cancer Res 2013; 73: 3749–60. [DOI] [PubMed] [Google Scholar]
- 11. Zhang F, Zhang X, Meng J et al. ING5 inhibits cancer aggressiveness via preventing EMT and is a potential prognostic biomarker for lung cancer. Oncotarget 2015; 6: 16239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gou WF, Shen DF, Yang XF et al. ING5 suppresses proliferation, apoptosis, migration and invasion, and induces autophagy and differentiation of gastric cancer cells: A good marker for carcinogenesis and subsequent progression. Oncotarget 2015; 6: 19552–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao QY, Ju F, Wang ZH, Ma XZ, Zhao H. ING5 inhibits epithelial‐mesenchymal transition in breast cancer by suppressing PI3K/Akt pathway. Int J Clin Exp Med 2015; 8: 15498–505. [PMC free article] [PubMed] [Google Scholar]
- 14. Liu XL, Zhang XT, Meng J et al. ING5 knockdown enhances migration and invasion of lung cancer cells by inducing EMT via EGFR/PI3K/Akt and IL‐6/STAT3 signaling pathways. Oncotarget 2017; 8: 54265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polakis P. Wnt signaling and cancer. Genes Dev 2000; 14: 1837–51. [PubMed] [Google Scholar]
- 16. Valenta T, Hausmann G, Basler K. The many faces and functions of beta‐catenin. EMBO J 2012; 31: 2714–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C, Li Y, Semenov M et al. Control of beta‐catenin phosphorylation/degradation by a dual‐kinase mechanism. Cell 2002; 108: 837–47. [DOI] [PubMed] [Google Scholar]
- 18. Tai D, Wells K, Arcaroli J et al. Targeting the WNT signaling pathway in cancer therapeutics. Oncologist 2015; 20: 1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang SM, Mishina YM, Liu S et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009; 461: 614–20. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura T, Hamada F, Ishidate T et al. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta‐catenin, GSK‐3beta and APC and reduces the beta‐catenin level. Genes Cells 1998; 3: 395–403. [DOI] [PubMed] [Google Scholar]
- 21. Desbois‐Mouthon C, Cadoret A, Blivet‐Van Eggelpoel MJ et al. Insulin and IGF‐1 stimulate the beta‐catenin pathway through two signalling cascades involving GSK‐3beta inhibition and Ras activation. Oncogene 2001; 20: 252–9. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial‐mesenchymal transition. Sci Signal 2014; 7: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


