Background
Immunotherapy has considerably changed the treatment of lung cancer. As immunotherapy has a special mechanism of action, the disease remission that it can induce is unique. Recently, Immune Response Evaluation Criteria in Solid Tumors (iRECIST), which focus on assessing the apparent curative effect of immunotherapy, have become widely accepted. Based on iRECIST criteria, if the response to immunotherapy is determined to be immunity‐confirmed progressive disease or immunity‐unconfirmed progressive disease, and the Eastern Cooperative Oncology Group score is worse than before treatment, immunotherapy should be discontinued. We report two immunity‐confirmed progressive disease cases after pembrolizumab treatment and one immunity‐unconfirmed progressive disease case after nivolumab treatment. All three patients benefited from continued immunotherapy, which indicates that the iRECIST criteria may have limitations in assessing the efficacy of immunotherapy for non‐small cell lung cancer patients.
Keywords: Immunotherapy, iRECIST criteria, non‐small cell lung cancer, pseudoprogression
Introduction
Lung cancer has the highest morbidity and mortality of all malignancies in China. In 2015, 733 300 new lung cancer cases were diagnosed, and 610 200 people died as a result of lung cancer.1 In recent years, cancer immunotherapy has ushered in a new era of cancer treatment. Clinical studies have shown that immunotherapy is effective for the treatment of advanced cancer, increasing survival time and improving quality of life.2, 3, 4 Different from traditional treatment methods, however, some patients administered immunotherapy will have a special treatment response. Recently, Immune Response Evaluation Criteria in Solid Tumors (iRECIST), which focus on assessing the apparent immunotherapy curative effect, have been widely accepted. Based on iRECIST, if the response to immunotherapy is determined to be immunity‐confirmed progressive disease (iCPD) or immunity‐unconfirmed progressive disease (iUPD), and the Eastern Cooperative Oncology Group (ECOG) score is worse than before treatment, immunotherapy should be discontinued.5 In our clinical experience we observed two cases of non‐small cell lung cancer (NSCLC) in which the response was determined as iCPD after pembrolizumab treatment, and one assessed as iUPD after nivolumab therapy. The patient who received nivolumab had a worse ECOG score after treatment. However, all three patients clinically benefited from the continued use of immunotherapy.
Case 1
A 65‐year old Chinese man with a 30‐year history of smoking initially presented with new‐onset wheezing. His physical examination was unremarkable and his ECOG score was 3 (confined to bed > 50% of the time and only walked around in the bedroom). Chest computed tomography (CT) showed an irregular mass (30.5 mm × 38 mm × 52.5 mm) in the left hilum and associated pleural effusion (Fig 1a). Bronchoscopic examination and a biopsy of the mass were performed; pathologic examination revealed moderately differentiated squamous cell carcinoma. Genetic testing for EGFR, EML4‐ALK, ROS‐1, and c‐MET mutations via next generation sequencing was negative. The abdominal CT scan showed metastatic nodules in the left adrenal gland, and the patient was staged as cT3N0M1b (IVb). After pemetrexed plus carboplatin were administered, the patient has disease progression with pembrolizumab 200 mg every three weeks. One month after commencing treatment, a chest CT scan showed an increase in the left hilar mass (45 mm × 43 mm × 60.5 mm) and pleural effusion, as well as left lower lobe swelling (Fig 1b). The treatment response was assessed as iUPD according to iRECIST. However, the patient perceived a beneficial result from treatment; his ECOG score improved to 1. A chest CT scan taken six months after initial presentation and after four courses of pembrolizumab showed no obvious disease progression. At this point, we determined that the response was immune stable disease (iSD), and pembrolizumab was continued. A chest CT scan taken 10 weeks later showed left lung consolidation with unclear left hilar mass boundaries and increased left pleural effusion (Fig 1c). The treatment response was therefore downgraded to iUPD. In contrast to his imaging examination, the patient reported significant improvement in physical strength, and he could work continuously and exercise lightly for 20 minutes. Consequently, he strongly wished to continue treatment with pembrolizumab. A chest CT scan taken six weeks later showed increased pulmonary dilatation and left pleural effusion (Fig 1d). An iCPD response was then determined. However, the patient once more reported considerable subjective improvement in his physical condition. He reported lightly exercising ≥ 30 minutes daily for the last month, and his ECOG score was 0. The patient has continued to use pembrolizumab.
Figure 1.
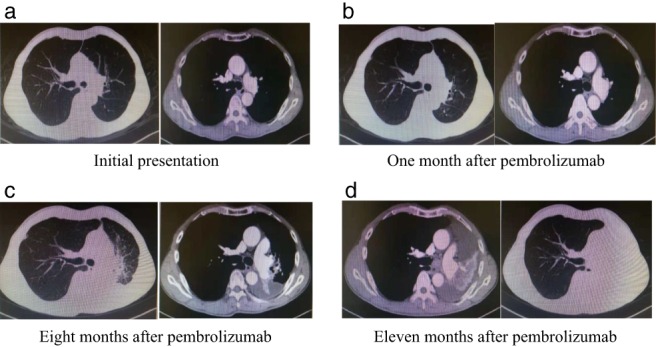
Computed tomography (CT) evolution of the tumor (Case 1). (a) CT at initial presentation revealed a primary tumor in the left hilum. (b) After one month of pembrolizumab, the patient's disease progressed. The CT scan showed an increase in the left hilar mass and a slight increase in pleural effusion. (c) After eight months of pembrolizumab treatment, the patient's disease showed a second progression. The CT scan showed an increase in the left hilar mass, and the pleural effusion had increased.
Case 2
A 62‐year old Chinese woman with a 40‐year history of smoking initially presented with a decline in activity endurance without an obvious cause. Her physical examination was unremarkable. Her ECOG score was 1. Chest CT suggested an irregular soft tissue mass in the left hilum of the lung (4.0 cm × 5.3 cm) adjacent to the left pulmonary artery and multiple enlarged lymph nodes in the right hilum and mediastinum (Fig 2a). Bronchoscopy showed that the left superior lobe orifice was completely blocked and the left lingual lobe orifice was stenotic. Pathologic examination of the biopsy specimen revealed a moderate to low‐grade squamous carcinoma. EGFR mutation testing via the amplification refractory mutation system was negative, as was the fluorescence in situ hybridization EML4‐ALK fusion gene test. There were no abnormalities in the magnetic resonance imaging scan of the brain or the whole body nuclear medicine bone scan. The patient was staged as cT4N2M0 (IIIB). Pembrolizumab 200 mg every three weeks was initiated two weeks after presentation. After two doses, a follow‐up chest CT scan showed no significant change in the left hilar mass. An iSD response was determined, and pembrolizumab was continued. Four and a half months after presentation, chest CT showed that the left hilar mass had reduced (2.5 cm × 1.5 cm) (Fig 2b). The patient was assessed as having an immune partial response (iPR). However, despite continuing pembrolizumab, a chest CT scan taken four months later showed that the left hilar mass had increased in size (3 cm × 2.2 cm), and the area of obstructive pneumonia had increased significantly (Fig 2c). The tumor markers CEA, progastrin‐releasing peptide, neuron‐specific enolase, and cytokeratin‐19 fragment (21‐1) were significantly elevated. An iUPD response was determined. The lesion was evaluated again four weeks later and the chest CT scan showed that the left hilar mass had progressed in size (3.5 cm × 3 cm) (Fig 2d). The response was subsequently determined as iCPD, as the patient did not complain of any symptoms and had an ECOG score of 0. She requested continued pembrolizumab treatment. After a further four months, a repeat chest CT scan showed no significant changes in the left hilum (Fig 2e). The patient was assessed as having iSD. The patient had no obvious discomfort, with an ECOG score of 0. She has continued to use pembrolizumab. Another CT scan taken six months later also showed iSD (Fig 2f).
Figure 2.
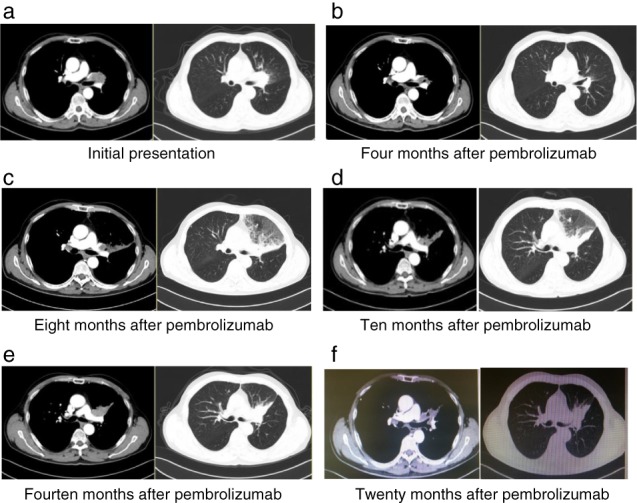
Computed tomography (CT) evolution of the tumor (Case 2). (a) CT at initial presentation revealed a primary tumor in the left hilum. (b) CT after four months of pembrolizumab therapy showed that the tumor had significantly reduced. (c) CT after eight months of pembrolizumab treatment showed the first radiographic disease progression, with enlargement of the left hilar mass and new obstructive pneumonia. (d) After 10 months of pembrolizumab treatment, the patient's disease showed a second radiologic progression. (e,f) CT scans at 14 and 20 months after commencing pembrolizumab treatment, respectively; the CT scan showed stable left hilar lesions.
Case 3
A 58‐year old Chinese man with a 20‐year history of smoking presented with pain in the right shoulder area. Physical examination showed a hard lymph node near the right supra‐clavicle of 2 cm in size. His ECOG score was 1. Chest CT showed an irregular soft tissue mass in the anterior segment of the right upper lobe (2.5 cm × 1.5 cm) invading the left pulmonary artery; multiple enlarged lymph nodes were visualized near the vena cava, under the carina, and in the right hilum (Fig 3a). Bronchoscopic biopsy revealed a moderate to low‐grade adenocarcinoma. EGFR via the amplification refractory mutation system method and ALK via fluorescence in situ hybridization testing was negative; c‐MET amplification testing was positive. A diagnosis of low‐grade lung adenocarcinoma, stage cT4N3M1a (IVa) was made. Two cycles of pemetrexed plus carboplatin were administered, resulting in significant reduction in the patient's physical strength and appetite. One month after completing chemotherapy, a chest CT scan showed that the right lung mass had enlarged (6 cm × 8 cm), and multiple enlarged and merged lymph nodes (postcaval vein, subcarinal, and right hilar) (Fig 3b). Disease progression was clear. Because of the presence of c‐MET amplification in tissue genetic testing, the patient was given oral crizotinib 250 mg twice a day. One month later, a chest CT scan revealed that the right hilar mass had reduced (4 cm × 4.5 cm), which suggested that crizotinib was effective (Fig 3c). A follow‐up chest CT scan taken two months later revealed soft tissue density of the right hilar mass (6 cm × 7 cm) (Fig 3d). Considering crizotinib resistance, we recommended changing the treatment to nivolumab 300 mg every three weeks, which was initiated one month later. Two months after commencing nivolumab, a chest CT scan showed that the right pleural effusion and pericardial effusion had increased significantly (Fig 3e). At this time, the patient felt chest tightness and nausea, and lost the ability to work; his ECOG score was 3. According to iRECIST, immunotherapy should have been stopped; however, the patient refused chemotherapy. He did not consent to drainage of the pleural effusion, was given intermittent oxygen, and continued immunotherapy with nivolumab. A follow‐up chest CT scan taken two months later showed that the right pleural effusion and pericardial effusion had significantly reduced, and the right hilar mass was significantly smaller (1.9 cm × 3.2 cm) (Fig 3f). We assessed treatment response as iPR (Fig 2). The patient's activity tolerance and appetite increased, and he continued nivolumab therapy.
Figure 3.
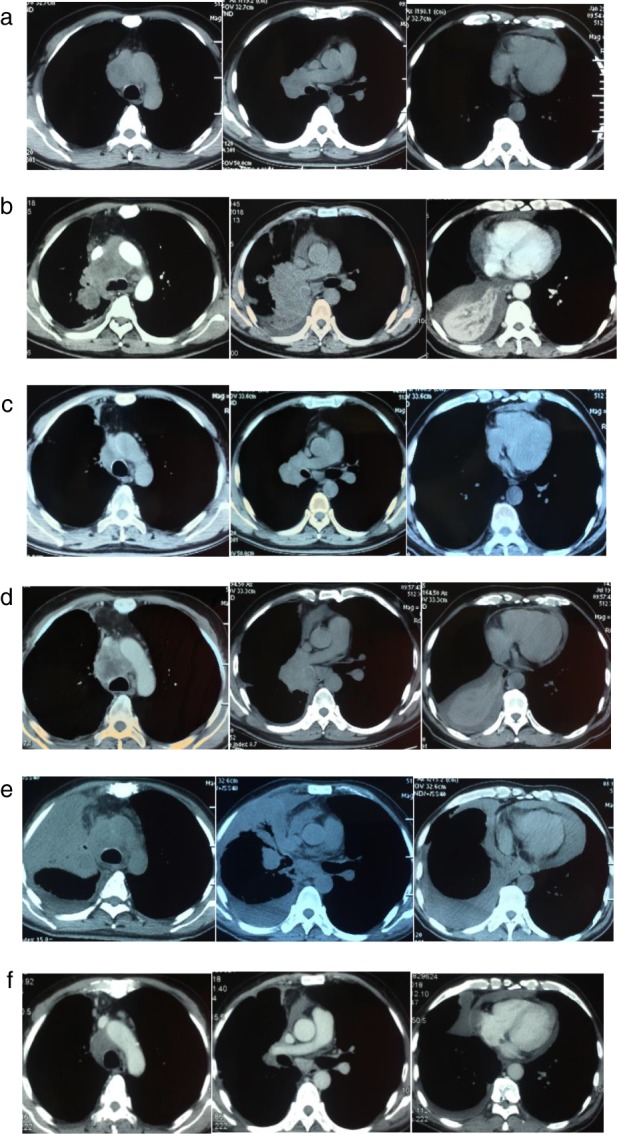
Computed tomography (CT) evolution of the target lesion and non‐target lesions (Case 3). (a) CT at presentation revealed the primary tumor in the left hilum. (b) The CT scan showed an increase in right hilar mass and lymph node enlargement after two cycles of chemotherapy. (c) After one month of crizotinib treatment, the right hilar mass and the mediastinal lymph nodes were smaller than before, and the pleural effusion was reduced. (d), Drug resistance occurred after three months of treatment with crizotinib, with radiographic progression visualized on chest CT. (e) One month after commencing nivolumab treatment, the CT scan showed that the right lung mass and lung tissues were unclear; the pleural effusion and pericardial effusion had significantly increased. (f) After three months of nivolumab treatment, the pleural effusion and pericardial effusion were significantly reduced and the right hilar mass was significantly smaller than before.
Discussion
The mechanism of immunotherapy causes a unique type of remission, as a result of immune memory effects. Traditional RECIST cannot accurately evaluate a patient's response to tumor immunotherapy. In 2017, the RECIST working group formally proposed iRECIST,5 which proposed new terminology for efficacy evaluation; the type of response is assigned with the prefix “i” (immune). These criteria introduced two breakthrough concepts: iUPD and iCPD. Progressive disease as defined by the previous RECIST version 1.1 was temporarily regarded as iUPD; treatment was continued according to the patient's tumor type, disease stage, and clinical condition; and re‐evaluation was performed after four to six weeks to confirm iCPD.
Case 1 presented here is the first case in which NSCLC showed radiographic progression after immunotherapy, despite the patient subjectively noting a significant improvement in his quality of life. Consequently, we had no reason to perform thoracentesis to evacuate the pleural effusion and analyze the fluid. However, based upon the patient's physical condition at the time, we speculate that the pleural effusion was not malignant in nature. Inflammatory infiltrating cells may have been the cause, because a higher proportion of PD‐1+, Tim‐3+, and CTLA‐4+ cells than CD4 and CD8+ T cells can cause a strong immune response when using PD‐1/PD‐L1 inhibitors, resulting in increased fluid accumulation. Such cases with incongruent imaging and clinical manifestations are rare in NSCLC patients after immunotherapy. If possible, pleural fluid should be retained before and after treatment for comparison in order to identify the possible causes and guide further clinical treatment. This case suggests that the clinical manifestations of NSCLC patients are a very important reference factor for clinicians to determine the efficacy of NSCLC immunotherapy.
Case 2 had radiographic progression, and according to iRECIST for iCPD, immunotherapy should have been stopped. However, because of the clinical benefit we observed, PD‐1 inhibitor treatment was continued and the patient benefited. Her clinical condition remained good, and the tumor decreased in size. At the 2017 American Society of Clinical Oncology conference, the OAK research was reported.6 The results of treatment beyond disease progression (TBP) with atrizumab for advanced NSCLC showed that 7% of 168 patients had target lesion regression and 49% had a stable target lesions; median overall survival was 12.7 months (95% confidence interval 9.0–14.9). The risk of continuing treatment did not increase compared to chemotherapy, and patients tolerated continued therapy well. However, it is still unclear whether tumor shrinkage after TBP is caused by the function of TBP itself or a follow‐up effect of immunotherapy. The risk of follow‐up treatment (e.g. immune‐related side effects) should be adequately assessed to balance the benefits and risks. In addition, it is necessary to identify characteristics and specific biomarkers to determine which patients could benefit from TBP; further randomized clinical trials are needed. This case suggests that it may be necessary to extend the follow‐up time to evaluate treatment response again after iCPD has been determined.
Case 3 presented symptoms of disease progression and poor results on imaging. According to iRECIST, immunotherapy should have been discontinued. However, the patient refused chemotherapy, and received significant clinical benefit from continued immunotherapy. After investigating previous reports, we found a common point: the accompanying symptoms of disease progression are likely associated with serous effusion (Table 1).7, 8 We suggest that patients receiving immunotherapy who have radiographic progression accompanied by symptoms associated with serous effusion should continue immunotherapy while treating the effusion.
Table 1.
Case reports of symptomatic deterioration associated with serous effusion
| Author (year) | Gender | Age | Pathology | PD‐1/PD‐L type | PD‐L1 expression | Initial symptom deterioration time | Serous effusion | Response time |
|---|---|---|---|---|---|---|---|---|
| Hochmair et al. (2017)7 | Male | 63 | Adenocarcinoma | Pembrolizumab | 90% | Eight weeks | Pleural effusion | Six weeks |
| Hochmair et al. (2017) 7 | Female | 63 | Adenocarcinoma | Pembrolizumab | 90% | Five weeks | Pleural effusion | Four weeks |
| Kolla & Patel (2016)8 | Male | 46 | Small cell carcinoma | Nivolumab | NR | Eight weeks | Pleural effusion, pericardial effusion | Eight weeks |
| Kolla & Patel (2016)8 | Female | 54 | Adenocarcinoma | Nivolumab | NR | Seven weeks | Pleural effusion, pericardial effusion | Two weeks |
NR, no response.
All three patients presented here can be included in the scope of “pseudoprogressive” immunotherapy,9 but do not represent the typical pseudoprogressive model. Distinguishing between pseudoprogression and true progression is a considerable clinical challenge. The incidence of pseudoprogression in NSCLC after immunotherapy has been reported as 0.6–5.8%.9 The second biopsy after immunotherapy treatment is the most powerful evidence to identify pseudoprogression. However, for various reasons, many patients are unable to undergo a biopsy. Previous studies have investigated other methods to determine if pseudoprogression is present. Lee et al. suggested that circulating tumor DNA downregulation can be used in melanoma patients to identify pseudoprogression, with a sensitivity of 90% and a specificity of 100%.10 Tanizaki et al. reported that measurement of serum tumor markers may help to determine whether to cease immunological checkpoint inhibitor treatment when the treatment response is assessed as iPD.11 The mechanism may be a result of tumor cell lysis, resulting in the release of a large number of tumor‐associated antigens, including tumor antigens and tumor cell lysate, into the blood circulation. Curioni et al. suggested that assessing the metabolic activity of lesions by positron emission tomography‐CT may help to identify false progression;12 however, further clinical trials are needed.
In conclusion, these three cases indicate that iRECIST may have a “blind area” for assessing the efficacy of lung cancer immunotherapy. Recently, Stephen et al. proposed imRECIST: when patients are treated with PD‐1 inhibitor monotherapy and there is no rapid progression of disease or severe adverse events, the study determined that the patient received clinical benefit from continuing treatment. imRECIST is a completion of iRECIST with stronger clinical application value.13 iRECIST should not be used as the sole criteria for determining the efficacy of immunotherapy but should be combined with pathological biopsy, clinical status changes, positron emission tomography‐CT metabolic activity, and other indicators to determine the appropriateness of immunotherapy. The iRECiST standard may have limitations in determining the efficacy of lung cancer immunotherapy.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank the patients and their families for providing their information for this study.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer: A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 5. Seymour L, Bogaerts J, Perrone A et al iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. RittmeyerI A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochmair MJ, Schwab S, Burghuber OC, Krenbek D, Prosch H. Symptomatic pseudo‐progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer 2017; 113: 4–6. [DOI] [PubMed] [Google Scholar]
- 8. Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy: A report of two cases. J Immunother Cancer 2016; 4: 80. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishino M, Ramaiya NH, Chambers ES et al Immune‐related response assessment during PD‐1 inhibitor therapy in advanced non‐small‐cell lung cancer patients. J Immunother Cancer 2016; 4: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JH, Long GV, Menzies AM et al Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti‐programmed cell death 1 antibodies. JAMA Oncol 2018; 4: 717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanizaki J, Hayashi H, Kimura M et al Report of two cases of pseudoprogression in patients with non‐small cell lung cancer treated with nivolumab‐including histological analysis of one case after tumor regression. Lung Cancer 2016; 102: 44–8. [DOI] [PubMed] [Google Scholar]
- 12. Curioni‐Fontecedro A, Ickenberg C, Franzen D et al Diffuse pseudoprogression in a patient with metastatic non‐small‐cell lung cancer treated with nivolumab. Ann Oncol 2017; 28: 2040–1. [DOI] [PubMed] [Google Scholar]
- 13. Hodi FS, Ballinger M, Lyons B et al Immune‐modified response evaluation criteria in solid tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018; 36: 850–8. [DOI] [PubMed] [Google Scholar]


