Abstract
Long follow up is needed in prospective cohort study evaluation of plasma biomarkers for incident peripheral arterial disease (PAD) Middle-aged PAD-free individuals from the cardiovascular cohort of the Malmö Diet and Cancer study (n = 5550; 1991–94) were followed prospectively for a median time of 23.4 years. The plasma biomarkers lipoprotein-associated phospholipase A2 (Lp-PLA2) activity and mass, proneurotensin, and CRP, were studied in relation to incidence of PAD until December 31st, 2016. The diagnosis of PAD could be validated and confirmed in 98%. Cox regression was used to calculate hazard ratios (HR) per 1 standard deviation increment of each respective log transformed plasma biomarker. Cumulative incidence of PAD was 4.4% (men 5.9%, women 3.3%). Adjusting for age, gender, smoking, body mass index, hypertension, diabetes mellitus, Lp-PLA2 activity (HR 1.33; 95% CI 1.17–1.52), Lp-PLA2 mass (HR 1.20; 95% CI 1.05–1.37) and CRP (HR 1.55; 95% CI 1.36–1.76) remained independently associated with incident PAD. The plasma biomarkers Lp-PLA2 activity and mass, and CRP were markers of PAD risk, implying that they might be useful biomarkers for subclinical atherosclerosis and atherosclerotic disease.
Introduction
Atherosclerosis is a systemic disease, often affecting the lower extremity arteries first1. Peripheral arterial disease (PAD), defined as occlusive atherosclerosis of the lower extremity arteries2 or the arteries distal to the aortic bifurcation, is highly associated with concomitant coronary, carotid and cerebral artery disease, an increased cardiovascular and overall mortality, despite secondary preventive efforts such as smoking cessation, antiplatelet and statin therapy3. Presence of PAD is associated with an extensive burden of atherosclerosis throughout the body and high, 29%, rate of silent myocardial infarctions1. Almost a fifth of elderly individuals have some stage of PAD and women are more afflicted than men4. The total number of individuals with PAD is peaking, with a 23% increase in the last decade due to increase in total population, global ageing, increased incidence of diabetes mellitus worldwide and smoking5.
Plasma biomarkers measured in healthy individuals may be useful to detect individuals at increased risk for developing PAD, and may also serve to identify factors enhancing or halting development of the disease. While C-reactive protein (CRP) is a well-established plasma biomarker for atherosclerosis6 and a predictor of worse outcome in patients with PAD7, several plasma biomarkers have been introduced in vascular research. The enzyme lipoprotein-associated phospholipase A2 (Lp-PLA2) is considered to be a biomarker for atherogenesis8. Lp-PLA2 levels have been associated with incident cardiovascular disease including coronary artery disease and ischaemic stroke9. The plasma biomarker proneurotensin10, a satiety regulator and marker of fat and digestion metabolism, has been found to be a associated with risk of cardiometabolic disease. Data on associations, however, between these biomarkers and incident PAD is scarce.
The main aim of this longitudinal cohort study was to evaluate Lp-PLA2 and proneurotensin in relation to an established risk marker such as CRP and known confounders for incident PAD risk at long term follow up.
Material and Methods
Study sample
The population-based Malmö Diet and Cancer study (MDCS)11–13 included 30 447 middle-aged individuals from Malmö, Sweden. Participants attended baseline examinations between 1991–1996. Current smoking was defined as self-reported regular smoking or smoking cessation within the last year. Diabetes mellitus was defined as self-reported physician’s diagnosis, use of anti-diabetic medication, or fasting blood glucose >6.0 mmol/L. Hypertension was defined as use of antihypertensive medication or blood pressure ≥140/90 mmHg. From this cohort, a random sample, examined between November 1991 and February 1994 was included in the MDCS cardiovascular cohort14, of whom 5550 individuals underwent blood sampling under standardized fasting conditions15 (Fig. 1). Informed consent was obtained from all participants and long-term follow-up of study participants for incident cardiovascular disease. The Regional ethical review board in Lund, Sweden (Dnr LU 51/90; 2013/566) approved the study. All research was performed in accordance with relevant guidelines/regulations.
Figure 1.
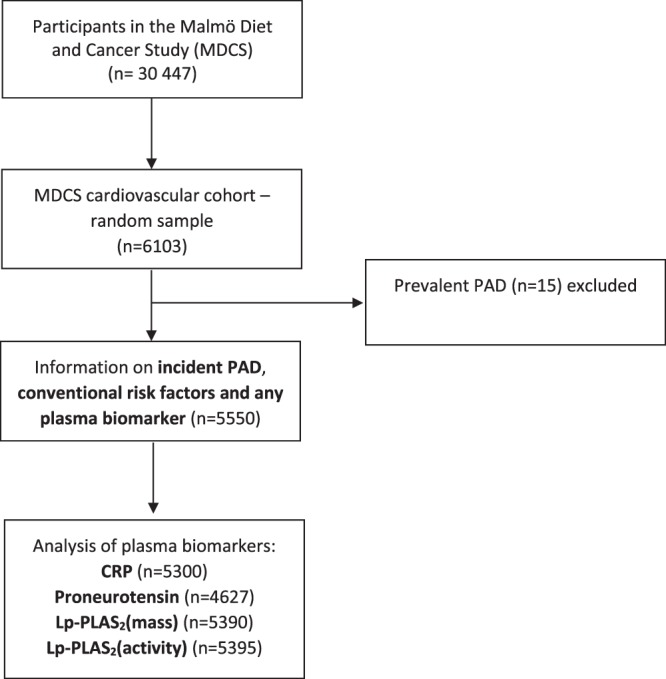
Descriptive flow diagram of study participants and plasma biomarker data. PAD; peripheral arterial disease, CRP; C-reactive protein, Lp-PLA2 (activity and mass); lipoprotein-associated phospholipase A2.
Endpoint ascertainment
Individuals from the MDCS with a first registered diagnosis of PAD were identified from Swedish national registers16 (the Inpatient17 and Outpatient Register and the Cause of Death Register) by linkage of the ten-digit personal identification number unique to each Swedish resident. Follow-up extended until December 31st, 2016. In both the Inpatient and Cause of Death registers, diagnoses are coded using a Swedish revision of the International Classification of Disease (ICD), version 8 (443,90; 443,99; 440,20; 445,00; 445,98; 445,99), 9 (443×; 440 C), 10 (I73.9 [all sub codes except I73.9 A]; I70.2 [all sub codes]). Codes for embolism to the lower extremity (444,20, 444; I74.3, respectively) were excluded. Surgical procedures are coded using a Swedish classification system (Op6: 8884; 8885; 8815; 8816; 8817; 8818; 8886; 8887; 8825; 8826; 8827; 8828; 8861; 8862; 8865; 8866. KKÅ97: PDH 10; PDH 20; PDH 21; PDH 22; PDH 24; PDH 30; PDH 35; PDP 10; PDP 30; PDQ 10; PDQ 30; PDU 82; PDU 83; PDU 84; PDU 99; PEF (all sub codes); PEH (all sub codes); PEN (all sub codes), PEQ (all sub codes), PEU 82, PEU 83, PEU 84, PEU 99, PFH (all sub codes), PFN (all sub codes), PFP (all sub codes), PFQ (all sub codes), PFU 82; PFU 83; PFU 84; PFU 99; PGH 20; PGH 21; PGH 22; PGH 23; PGH 30; PGH 31; PGH 40.
Validation of PAD diagnosis
One hundred patients with diagnosis of PAD were randomly selected for the validation procedure using patient record data. Among 100 patients, 69 had chronic limb-threatening limb ischemia, 13 had acute limb ischemia, 15 had claudication, and one had asymptomatic PAD. Among the 13 patients with acute limb ischemia, twelve had acute thrombotic occlusion and one had an embolic occlusion. Two patients had venous insufficiency and were misdiagnosed. PAD could therefore be confirmed in 98% of cases and symptomatic PAD in 97% of cases.
Laboratory measurements
Plasma biomarkers were measured from fasting plasma samples that had been frozen at −80 °C immediately after collection15. Proneurotensin was measured using a chemiluminometric sandwich immunoassay to detect a proneurotensin fragment10. CRP was measured by a high-sensitivity Tina-quant® latex assay (Roche Diagnostics, Basel, Switzerland). The average coefficient of variation (CV) was 4.59%18. Lp-PLA2 was expressed as enzymatic activity and mass (quantity)19. Lp-PLA2 activity was measured in duplicate using [3 H]-platelet activating factor as a substrate19. The range of detection was 8–150 nmol/min/ml. Samples were retested if the replicate CV was >20%. The average CV was 5.78%18. Lp-PLA2 mass measurements were performed using the second generation PLAQTM test (diaDexus Inc., South San Francisco, CA, USA) commercially available enzyme-linked immunosorbent assay (ELISA) kit19. All samples were analyzed in duplicate, and if a duplicate showed a CV of more than 20%, the sample was reanalyzed. The average CV was 4.62% on random of 50 first participants in the MDCS18. Plasma-EDTA samples are stable for Lp-PLA2 activity and mass measurements within 7 days of collection for refrigerated samples and for more than 10 years from collection when stored at −70 °C19.
Statistical analysis
Quantitative normal and skewed distributed variables are presented as mean with standard deviation and median with interquartile range (IQR), respectively. Dichotomous variables are presented as count and proportion. Individuals with a diagnosis of PAD at baseline were excluded from the current study and prospective analyses included only incident PAD. Plasma biomarkers and confounders for incident PAD were assessed using Cox regression models, and hazard ratios (HRs) were expressed per one standard deviation (SD) increment of each respective log transformed plasma biomarker (skewed distributed) in the Cox regression models. Cumulative incidence of PAD was analyzed using Kaplan-Meier method. Log-rank test was used in the comparison of quartiles for Lp-PLA2 activity. Analyses were performed using SPSS for Windows, version 23.0 (SPSS Inc, Chicago, IL). A p-value less than 0.05 was considered significant.
Results
Baseline conventional risk factor assessment
Fifteen individuals with known PAD at baseline were excluded. The cumulative incidence of PAD was 4.4% (244/5550), 5.9% (137/2307) for men versus 3.3% (107/3243) for women (p < 0.001), during a median follow up period of 23.4 years (IQR 19.4–24.3). Baseline risk factor characteristics for individuals with or without PAD in the cohort are shown in Table 1. When including the conventional risk markers listed in Table 1 into a Cox regression analysis, age at baseline (p < 0.001), male gender (p < 0.001), current smoking (p < 0.001), diabetes mellitus (p < 0.001) and hypertension (p = 0.001) were independently associated with incident PAD, whereas BMI (p = 0.74) were not.
Table 1.
Baseline characteristics in participants with and without later development of PAD.
| Characteristic | No PAD (n = 5306) | PAD (n = 244) |
|---|---|---|
| Baseline age, years, mean (SD) | 57.5 (5.9) | 59.3 (5.6) |
| Male sex, % | 40.9 (n = 2170) | 56.1 (n = 137) |
| Body mass index, kg/m2, mean (SD) | 25.7 (3.9) | 26.0 (4.0; n = 243) |
| History of hypertension, % | 63.5 (3365/5302) | 78.2 (190/243) |
| History of diabetes, % | 2.8 (121/4358) | 13.3 (27/203) |
| Current smoking, % | 27.4 (1453/5302) | 53.9 (131/243) |
| Total cholesterol, mmol/L, median (IQR) | 6.1 (5.4–6.8; n = 5237) | 6.3 (5.7–7.1; n = 239) |
| Triglycerides, mmol/L, median (IQR) | 1.2 (0.9–1.6; n = 5236) | 1.4 (1.0–2.0; n = 239) |
| Haemoglobin A1c, %, median (IQR) | 4.8 (4.5–5.1; n = 5231) | 5.0 (4.7–5.5; n = 240) |
| Plasma inflammatory biomarkers, median (IQR) | ||
| CRP (mg/L) | 1.4 (0.7–2.8) (n = 5071) | 2.1 (1.0–4.6) (n = 229) |
| Proneurotensin (pmol/L) | 104.6 (75.7–148.0) (n = 4420) | 108.0 (77.7–156.5) (n = 207) |
| Lp-PLAS2 (mass) (ng/ml) | 255.0 (214.0–316.5) (n = 5154) | 279.2 (224.9–345.1) (n = 236) |
| Lp-PLAS2 (activity) (nmol/min/ml) | 44.0 (36.2–52.7) (n = 5158) | 49.4 (40.6–59.7) (n = 237) |
PAD; peripheral artery disease, CRP; C-reactive protein, Lp-PLA2 (activity and mass); lipoprotein-associated phospholipase A2, SD; standard deviation, IQR; interquartile range.
Plasma biomarkers
Plasma biomarkers at baseline in individuals with and without incident PAD are shown in Table 1. When Lp-PLA2 activity was stratified to an ordinal variable in quartiles, an increased risk in cumulative incidence of PAD over the quartiles was found (p < 0.001). Cumulative incidence of PAD in the highest quartile was 2.6 times higher compared to the lowest quartile (Fig. 2). In the multi-variable adjusted analysis, Lp-PLA2 activity (HR 1.33; 95% CI 1.17–1.52), Lp-PLA2 mass (HR 1.20; 95% CI 1.05–1.37), and CRP (HR 1.55; 95% CI 1.36–1.76) were all independently associated with incident PAD (Table 2). When CRP was added as a covariate together with Lp-PLA2 (activity or mass), besides age at study entry, sex, BMI, current smoking, diabetes mellitus, hypertension, in the extended multi-variable analysis, Lp-PLA2 activity (HR 1.33; 95% CI 1.17–1.52; p < 0.001), Lp-PLA2 mass (HR 1.16; 95% CI 1.01–1.32; p = 0.038) and CRP (HR 1.36; 95% CI 1.18–1.58; p < 0.001) remained associated with incident PAD.
Figure 2.
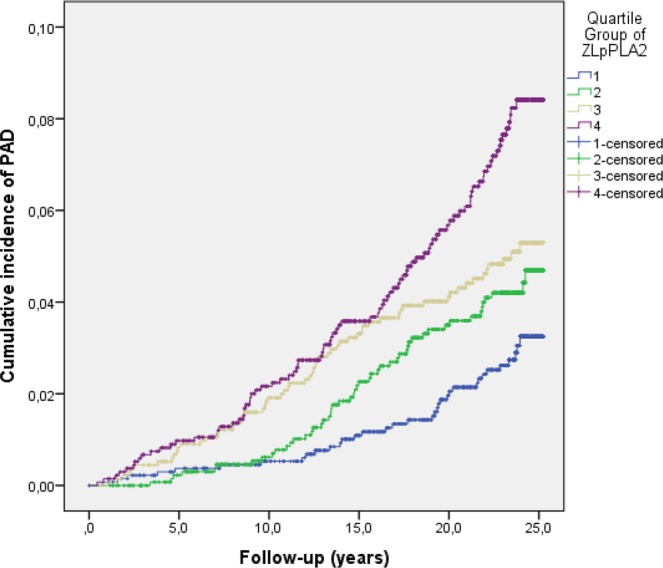
Cumulative incidence of PAD in relation to Lp-PLA2 activity stratified in quartiles.
Table 2.
Multi-variable adjusted hazards ratios for incident PAD in relation to plasma biomarkers.
| Variable | PAD | p |
|---|---|---|
| N = 244 | ||
| HR* (95% CI) | ||
| Plasma inflammatory biomarkers | ||
| CRP (n = 5300) | 1.55 (1.36–1.76) | <0.001 |
| Proneurotensin (n = 4627) | 0.94 (0.80–1.09) | 0.41 |
| Lp-PLAS2 (mass) (n = 5390) | 1.20 (1.05–1.37) | 0.008 |
| Lp-PLAS2 (activity) (n = 5395) | 1.33 (1.17–1.52) | <0.001 |
The following variables were entered in the multivariable analysis besides each respective plasma biomarker:
Age at study entry, sex, body mass index, current smoking, diabetes mellitus, hypertension.
*Hazard ratios (HR) were expressed per one SD increment of each respective log transformed plasma biomarker.
PAD; peripheral artery disease, CRP; C-reactive protein, Lp-PLA2 (activity and mass); lipoprotein-associated phospholipase A2, HR; Hazard Ratio, CI; Confidence interval.
Discussion
The present longitudinal prospective study suggests that elevation of plasma Lp-PLA2 (activity), Lp-PLA2 (mass), and CRP, are all markers of subclinical disease susceptibility long time before diagnosis of PAD. This finding suggests that these biomarkers indicate subclinical PAD prior to onset of symptoms due to PAD. The association between Lp-PLA2 and incident PAD has previously been evaluated in three different cohorts, the Cardiovascular Health Study20, Multi-Ethnic Study of Atherosclerosis (MESA)21 and Atherosclerosis Risk in Communities (ARIC) study22. The Cardiovascular Health and ARIC study showed an association between Lp-PLA2 and incident PAD, whereas the MESA study showed no association. The negative findings in the MESA study21 were probably related to the fact that PAD was exclusively defined with repeated measurements of ankle-brachial index (ABI), resulting in inclusion of mainly asymptomatic PAD patients, whereas the other cohort studies used clinical endpoint data retrieved from hospital registries. Hence, it is likely that individuals that later develop symptomatic PAD as opposed to asymptomatic PAD may have been exposed to larger amounts of pathophysiological stimulators such as Lp-PLA2, promoting development of PAD. Plasma Lp-PLA2 (activity) and Lp-PLA2 (mass) were both strong predictors for incident PAD in the present study, since these biomarkers remained independently associated with incident PAD after adjusting for confounders in the extended multivariable analysis including the well established risk marker CRP6,7. The elevated Lp-PLA2 levels themselves, however, seem unlikely to be a primary causal factor for atherosclerotic disease according to phase III randomized controlled trials of inhibitors of Lp-PLA2, varespladib and darapladib23, and also to genetic studies using Mendelian randomization24. Instead, Lp-PLA2 levels appears to reflect an ongoing inflammatory process.
CRP is an established marker of systemic inflammation, vascular disease, and increased risk in PAD patients6,7 and the present study data showed that CRP, after adjusting for relevant confounders, was elevated already at baseline in patients developing symptomatic PAD, probably reflecting presence of subclinical atherosclerotic disease. Results from an interventional trial evaluating rosuvastatin (JUPITER)25 suggest that CRP may assist in risk stratifying healthy participants without hyperlipidemia for rosuvastatin treatment in primary prevention of atherosclerosis.
Increased plasma proneurotensin levels has been shown to be strongly associated with obesity26 and a predictor of cardiovascular disease in both genders and diabetes mellitus in women in two separate prospective cohorts10,27. The absence of association between proneurotensin and incident PAD, may be attributed to the finding that body mass index not was associated with incident PAD in the present study.
The MDCS cohort was originally designed to investigate the effects of diet on cancer risk28. A limitation of the study was that ABI not was measured at baseline to classify and exclude asymptomatic PAD individuals. Re-invitation of study participant survivors for detection of asymptomatic PAD by measurement of ABI would have been interesting to increase the total number of incident PAD individuals, perform subgroup analysis of factors associated with symptomatic and asymptomatic incident PAD, and increase power in the overall statistical analysis. A major strength of the present longitudinal study design, on the other hand, is the inclusion of healthy middle-aged individuals followed up for a median time of 23.4 years. Re-invitation would also have captured changes in risk factor status among study participants during this long period. During the last decade, the declining prevalence of smoking and the improved pharmacological treatment among individuals with cardiovascular disease might well have impacted the cumulative incidence of, above all, symptomatic PAD29. Another strength of this study was the high validity of symptomatic PAD, we could reliably confirm the PAD diagnosis in 98% of cases.
Lp-PLA2 mass and activity, but not CRP, have previously been found to be predictors of incident abdominal aortic aneurysm (AAA)30 in the same cohort during shorter follow up. CRP was the strongest predictor for incident PAD in the present study, and this discrepancy compared to the findings concerning AAA prediction may be interpreted as suggesting that PAD is a more inflammation-driven disease than AAA. Whereas the two diseases shared some similar risk factors in the two cohort studies, diabetes mellitus and hypertension were associated with incident PAD, but not with incident AAA. Actually, diabetes mellitus is a protective factor and reduces the incidence of AAA31. These differences in premorbid plasma biomarker profile highlight that PAD and AAA are different diseases with different pathophysiologies which should be analyzed and discussed separately.
In conclusion, plasma biomarkers Lp-PLA2 activity and mass were found to be useful markers of PAD risk during long-term follow-up together with the established biomarker CRP, implying that they might also be used as predictors for subclinical atherosclerosis and atherosclerotic disease.
Acknowledgements
The authors wish to thank all participants in the Malmö Diet and Cancer Study for making this study possible. The Malmö Diet and Cancer study was made possible by grants from the Swedish Cancer Society, the Swedish Medical Research Council, the Swedish Dairy Association, the Albert Påhlsson and Gunnar Nilsson Foundations and the Malmö city council. G.E. was supported by the Swedish Heart-Lung Foundation (2016-0315) and the Medical Faculty of Lund University. SA and AG were supported by grants from Research Funds at Skåne University Hospital, Region Skåne (430751), the Hulda Ahlmroth Foundation, and from the Swedish Government under the LUA/ALF agreement.
Author Contributions
The corresponding author had full access to all data, takes responsibility for its integrity and the accuracy of the data analyses, and had final responsibility for the decision to submit for publication. All authors have read, critically revised the manuscript and agree to the manuscript as written. Study design: S.F., A.G., M.Z., G.E., O.M., M.P., S.A. Data collection: S.F., S.A. Data analysis: S.F., S.A. Writing: S.F., A.G., M.Z., G.E., O.M., M.P., S.A.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weir-McCall J, et al. Whole body cardiovascular magnetic resonance imaging to stratify symptomatic and asymptomatic atherosclerotic burden in patients with isolated cardiovascular disease. BMC Medical Imaging. 2016;16:18. doi: 10.1186/s12880-016-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiatt WR, et al. Atherosclerotic Peripheral Vascular Disease Symposium II. Nomenclature for Vascular Diseases. Circulation. 2008;118:2826–2829. doi: 10.1161/CIRCULATIONAHA.108.191171. [DOI] [PubMed] [Google Scholar]
- 3.Tern, P. J. W. et al. Site and burden of lower limb atherosclerosis predicts long-term mortality in a cohort of patients with peripheral arterial disease. Eur J. Vasc Endovasc Surg. Oct1, 10.1016/j.ejvs.2018.07.020 (2018). [DOI] [PubMed]
- 4.Sigvant B, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J. Vasc Surg. 2007;45:1185–91. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Fowkes FG, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Singh TP, Morris DR, Smith S, Moxon JV, Golledge J. Systematic Review and Meta-Analysis of the Association Between C-Reactive Protein and Major Cardiovascular Events in Patients with Peripheral Artery Disease. Eur J. Vasc Endovasc Surg. 2017;54:220–233. doi: 10.1016/j.ejvs.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Charniot JC, et al. Interpretation of lipoprotein-associated phospholipase A2 levels is influenced by cardiac disease, comorbidities, extension of atherosclerosis and treatments. Int J. Cardiol. 2013;168:132–138. doi: 10.1016/j.ijcard.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Lp-PLA2 Studies Collaboration, T. A. et al. Lipoprotein-associated phospholipase A (2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–44. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melander O, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308:1469–1475. doi: 10.1001/jama.2012.12998. [DOI] [PubMed] [Google Scholar]
- 11.Manjer J, et al. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J. Cancer Prev. 2001;10:489–99. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Manjer J, Elmståhl S, Janzon L, Berglund G. Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scand J. Public Health. 2002;30:103–12. doi: 10.1177/14034948020300020401. [DOI] [PubMed] [Google Scholar]
- 13.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17:299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosvall M, Janzon L, Berglund G, Engström G, Hedblad B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis. 2005;179:325–31. doi: 10.1016/j.atherosclerosis.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Melander O, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.socialstyrelsen.se/register/halsodataregister/inenglish. Accessed Sept 11 (2018).
- 17.Ludvigsson JF, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persson M, Berglund G, Nelson J, Hedblad B. Lp-PLA2 activity and mass are associated with increased incidence of ischemic stroke. A population-based cohort study from Malmö, Sweden. Atherosclerosis. 2008;200:191–98. doi: 10.1016/j.atherosclerosis.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Persson M, Nilsson J-Å, Nelson J, Hedblad B, Berglund G. The epidemiology of Lp-PLA2: Distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis. 2007;190:388–96. doi: 10.1016/j.atherosclerosis.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Garg PK, et al. Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease in older adults: The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2016;36:750–756. doi: 10.1161/ATVBAHA.115.306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg PK, et al. Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease in a multi-ethnic cohort: The Multi-Ethnic Study of Atherosclerosis. Vasc Med. 2017;22:5–12. doi: 10.1177/1358863X16671424. [DOI] [PubMed] [Google Scholar]
- 22.Garg PK, et al. Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease: Findings from The Atherosclerosis Risk in Communities study (ARIC) Atherosclerosis. 2018;268:12–18. doi: 10.1016/j.atherosclerosis.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talmud PJ, Holmes MV. Deciphering the causal role of sPLA2 and Lp-PLA2 in coronary heart disease. Arterioscler Thromb Vasc Biol. 2015;35:2281–9. doi: 10.1161/ATVBAHA.115.305234. [DOI] [PubMed] [Google Scholar]
- 24.Orho-Melander M. Genetics of coronary heart disease: towards causal mechanisms, novel drug targets and more personalized prevention. J. Intern Med. 2015;278:433–446. doi: 10.1111/joim.12407. [DOI] [PubMed] [Google Scholar]
- 25.Ridker, P. M. et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New Engl J. Med. 359, 2195–207 (2008). [DOI] [PubMed]
- 26.Barchetta I, et al. Increased plasma proneurotensin levels identify NAFLD in adults with and without type 2 Diabetes. J. Clin Endocrinol Metab. 2018;103:2253–2260. doi: 10.1210/jc.2017-02751. [DOI] [PubMed] [Google Scholar]
- 27.Fawad A, et al. Proneurotensin predicts cardiovascular disease in an elderly population. J. Clin Endocrinol Metab. 2018;103:1940–1947. doi: 10.1210/jc.2017-02424. [DOI] [PubMed] [Google Scholar]
- 28.Berglund G, Elmstahl S, Janzon L, Larsson SA. Design and feasibility. The Malmo Diet and Cancer Study. J. Intern Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 29.Alhadad A, Wictorsson C, Alhadad H, Lindblad B, Gottsäter A. Medical risk factor in peripheral arterial disease Need for further improvement. Int Angiol. 2013;32:332–8. [PubMed] [Google Scholar]
- 30.Acosta S, et al. Lp-PLA2 activity and mass for prediction of incident abdominal aortic aneurysms: A prospective longitudinal cohort study. Atherosclerosis. 2017;262:14–18. doi: 10.1016/j.atherosclerosis.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Avdic T. et al. Reduced long-term risk of aortic aneurysm and aortic dissection among individuals with type 2 diabetes mellitus: A nationwide observational study. J Am Heart Assoc e007618, 10.1161/JAHA.117.007618 (2018). [DOI] [PMC free article] [PubMed]


