Abstract
The zinc finger protein (ZFP) transcription factor family plays an important role in regulating plant growth, development, and response to abiotic stress. In this study, we aimed to determine the role of GmZAT4, a C2H2-type transcription factor, in abiotic stress tolerance. The complete coding sequence of the GmZAT4 gene was isolated from soybean root RNA, which shows highest expression level compared with leaf, flower and other tissues. Using multiple sequence alignment and conserved domain analysis, we showed that GmZAT4 is a typical C2H2-type transcription factor which is comprised of two C2H2 domains, including a highly conserved QALGGH motif, and implied the regulation of abiotic stress tolerance in plant. A phylogenetic tree revealed that the soybean GmZAT4 gene clustered with ZAT4 from Glycine soja and AZF1, AZF2, and AZF3 from Arabidopsis thaliana. The mRNA expression levels of GmZAT4 were determined in two soybean cultivars by quantitative reverse transcription (qRT)-PCR and compared. The results showed higher expression (up to 60, 25 and 4 times, respectively) in the drought-tolerant type (Jinda 74) compared to the drought-sensitive soybean cultivar (Jinda 53) following treatment with 18% PEG, 150 mM NaCl, or 100 µM abscisic acid (ABA). GmZAT4 was ectopically over-expressed in A. thaliana to determine its role in abiotic stress tolerance. GmZAT4 overexpression enhanced the tolerance of A. thaliana to treatment with 20% PEG and 150 mM NaCl, and improved the germination rate following treatment with 1 µM or 2 µM ABA. The expression profiles of marker genes in the ABA signaling pathway, such as RD29A, RD29B, ABI, and RAD, indicated that GmZAT4 enhanced the abiotic stress tolerance of Arabidopsis. These results suggest that the C2H2-type ZFP encoded by GmZAT4 plays an important role in PEG and NaCl stress tolerance and ABA responses in soybean and A. thaliana.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1673-0) contains supplementary material, which is available to authorized users.
Keywords: Soybean, GmZAT4, Zinc finger protein, Abiotic stress, ABA response
Introduction
Soybean [Glycine max (L.) Merrill] is one of the most important agricultural commodities and economic crops in the world (Clemente and Cahoon 2009). Drought and NaCl stress is considered one of the main causes of soybean crop yield loss in many countries. Therefore, a better understanding of abiotic stress response mechanisms is expected to drive the breeding and development of drought- and NaCl-tolerant soybean cultivars, which will be crucial in maintaining soybean yields and countering the threat of global warming and loss of soil fertility (Cutforth et al. 2007; Phang et al. 2008).
The zinc finger domain is one of the most important structural motifs involved in protein–DNA interactions, and it is also known to be involved in protein–protein interactions in plants. The classical zinc finger domain is small, consisting of ~ 30 amino acids with a consensus sequence of CX2–4CX3FX5LX2HX3–5H (X: any amino acid, subscript: the number of amino acid). Zinc finger domains are composed of one zinc ion interaction motif (ZII), as well as two cysteine (Cys) and two histidine (His) residues located upstream and downstream of the ZII motif. In combination with Zn2+, these generate a stable three-dimensional conformation of the DNA-binding region, resulting in a transcription factor capable of binding the promoters of target genes and regulate their expression (Min et al. 1989).
ZFPs are a large family which is classified into groups according to the number and location of Cys and His residues, including C2H2, C2HC5, C2C2, CCCH, C3HC4, C4, C4HC3, C6, and C8 (Moore and Ullman 2003). The C2H2-type ZFPs, also known as TFIIIA-type ZFPs, represent the family of eukaryotic transcription factors. In Arabidopsis thaliana, a total of 176 proteins have been reported to contain one or more zinc finger domains, and 99 and 47 C2H2-type ZFPs have been identified in rice and wheat, respectively (Agarwal et al. 2007; Huang et al. 2004), making the ZFP family one of the largest families of putative transcriptional regulators (Englbrecht et al. 2004). C2H2-type ZFPs in plants typically contain a QALGGH motif within the zinc finger domain, which is involved in recognizing cis-regulatory elements in the promoters of downstream target genes (Takatsuji 1999). In vitro analysis revealed that a specific domain, especially the conserved QALGGH motif in plants, conferred that zinc finger proteins (ZFPs) as a regulator by recognizing the target sequences and regulating expression level of target gene in a plant-specific manner (Yu et al. 2014).
With 176 members in Arabidopsis, the C2H2-type zinc finger proteins, constitute a large multi-function transcriptional regulator in plants (Ciftci-Yilmaz and Mittler 2008). Several C2H2-type ZFPs have been reported to be related to abiotic stress, including AZF1, AZF2, and AZF3 (Kodaira et al. 2011; Sakamoto et al. 2004). The C2H2-type ZFP182 in rice has been shown to be involved in abscisic acid (ABA)-induced antioxidant defense (Zhang et al. 2012). Moreover, the ectopic expression of VvZFP11, containing two C2H2-type zinc finger motifs, in A. thaliana was found to increase resistance to Golovinomyces cichoracearum (Yu et al. 2016). In soybean, two C2H2-type ZFP genes, GmZF1 and GmZFP1, were shown to enhance tolerance to cold and drought stress in Arabidopsis (Huang et al. 2006; Yu et al. 2014). Expression of the GmZFP3 gene reduces drought stress and increases the response to polyethylene glycol (PEG) and ABA in Arabidopsis (Zhang et al. 2016). These results have improved our understanding of the functional diversity of C2H2-type ZFPs and their role in combating abiotic and biotic stress in plants.
In this study, a C2H2-type transcription factor named GmZAT4 was cloned from the soybean root, and the nucleotide and protein sequences were analyzed using bioinformatics tools. The expression patterns of GmZAT4 were investigated in various tissues of two soybean cultivars. Ectopic expression of GmZAT4 in Arabidopsis revealed its role in combating PEG- and NaCl-induced stress and responding to ABA treatment. These results demonstrate that GmZAT4 is an important factor that broadly enhances the tolerance to PEG and NaCl stress.
Materials and methods
Plant materials
Soybean seeds (Glycine max, cultivars ‘Jinda74’ and ‘Jinda53’) were planted in pots with soil and sand (1:1) in a greenhouse maintained at a temperature of 25 °C (night)–30 °C (day), irradiance of 200 µmol m− 2 s− 1, and photoperiod of 16/8 h (day/night). These two cultivars through the national certification have the different cross backgrounds. Base on the previously study, they have significant drought resistance (Hou et al. 2014). Mature soybean plant, nearly 90 DAPS (days after plant), were divided into roots, stems, leaves, flowers, and pods, and frozen in liquid nitrogen for gene expression analysis. Arabidopsis seedlings were grown in a chamber at a temperature of 20 °C (night)–22 °C (day), irradiance of 37 µmol m− 2 s− 1, and photoperiod of 16/8 h (day/night).
Stress treatments
For soybean stress treatment, when the fifth true leaves of the soybean plants were fully expanded, the whole plant was transferred from a pot to a hydroponic box wrapped in aluminum foil. Plants were subsequently treated with Hoagland solution containing 18% PEG-6000, 150 mM NaCl, or 100 µM ABA for 0, 4, 8, 12, or 24 h. Finally, roots were collected from three different soybean plants, mixed, and frozen at − 80 °C for RNA isolation.
For Arabidopsis stress treatment, WT (Col-0) and transformed T3 generation seeds (OE1 and OE3) were plated in 1/2 MS medium with 20% PEG, 150 mM NaCl, or 1–2 µM ABA.
RNA isolation and cDNA synthesis
Total RNA was isolated from plant tissue samples using a TRIzol® Reagent RNA kit (Invitrogen, USA), according to the manufacturer’s specifications. First-strand cDNA was synthesized from 1 µg total RNA using Oligo d(T)18 and 200 U PrimeScript II RTase (TaKaRa, Japan).
Gene cloning and sequence analysis
The coding sequence of the GmZAT4 gene was cloned into the pMD18-T vector and amplified by RT-PCR using ZFP4GF (5′-GATCCTTATCGAAATTCAATGG-3′) and ZFP4GR (5′-TCACCTTTGGCTATGAGATAAG-3′) primers. PCR was conducted on a Bio-Rad c1000 PCR system (Bio-Rad Laboratories, Inc., USA) with each 25-µL RT-PCR reaction containing 1 µL cDNA template (50 ng µL− 1), 2.5 µL 10 × PCR reaction buffer (plus Mg2+), 2.0 µL dNTP mixture (2.5 mM each), 1.0 µL each forward primer (10 µM) and reverse primer (10 µM), and 1.5 U ExTaq polymerase (TaKaRa), with H2O added to the final volume. Target gene clones were sequenced by Shanghai Sheng Gong Co. (China) and submitted to the NCBI database (GenBank ID: KC967125). The GmZAT4 sequence was analyzed by DNAstar Lasergene v7.1 software. BLAST searches for homologous proteins and conserved ZFP domains were performed on the National Center for Biotechnology Information (NCBI) server. Phylogenetic tree analysis was performed using MEGA 6.0 software based on the maximum likelihood method (Tamura et al. 2013).
qRT-PCR analysis
All qRT-PCR reactions were carried out with a Bio-Rad CFX96 Touch™ Deep Well Real-Time PCR Detection System with SYBR Green. Each 50 µL PCR reaction contained 1 µL cDNA template (50 ng µL− 1), 25 µL 2 × SYBR Green Mix (TaKaRa), 1.0 µL each sense primer (10 µM) and anti-sense primer (10 µM), and 22 µL H2O. Ct values were normalized to those of the soybean or Arabidopsis actin gene as a reference and calculated using the 2−△△Ct method (Hou et al. 2015). All assays were repeated three times. The sequences of the qRT-PCR primers used in this study are provided in Table S1.
Generation of transgenic A. thaliana
PCR-amplified ZFP4BF (5′-CGCGGATCCGGGGGGTCACATGAGGTCTC-3′) and ZFP4SR (5′-GCGAGCTCCTATGAGATAAGGCCTACCAGTGC-3′) primers were generated from the BamHI–SacI sites flanking the full-length coding sequence of GmZAT4 and subcloned into the pBI121 vector. The clones of this gene were sequenced for verification prior to further analysis. The p35S:GmZAT4 vector was transformed into Agrobacterium tumefaciens strain EHA105 by electroporation and then transformed into Arabidopsis ecotype Col-0 using the floral-dip transformation method (Zhang et al. 2006).
Phenotype analysis
Two independents transgenic T3 generation Arabidopsis seedlings (OE1 and OE3) were treated with 20% PEG, 150 mM NaCl, or 1 and 2 µM ABA in MS solid medium. Seedlings were then photographed and root length was calculated after growth for 2 weeks. Values were compared with those of the WT (Col-0) control. All experiments were carried out with three independent biological replicates. Data were analyzed using analysis of variance (ANOVA) and Duncan’s multiple-range test with SPSS statistical software, with statistical significance determined at p < 0.05.
Statistical analysis
All the assays described above were repeated at least three times on three biological replicates. The data were subjected to SPSS to discover significant differences, and the level of significance was determined using the Duncan’s multiple-range test at p < 0.05.
Results
Sequence characterization of GmZAT4
The cDNA of a ZFP gene was cloned and sequenced from soybean root, revealing a sequence length of 1710 bp encoding 549 amino acid residues. By comparing the cDNA sequence with the soybean genome sequence, we confirmed that this was an intronless gene. According to computational predictions, the molecular weight of the protein is 60590.57 Da, and the isoelectric point is 7.298. The protein consists of 73 strongly basic (+) amino acids (K, R), 74 strongly acidic (−) (D, E), 133 hydrophobic (A, I, L, F, W, V), and 191 polar amino acids (N, C, Q, S, T, Y). Based on conserved domain analysis, the protein contains two C2H2-type zinc finger domains, one containing the typical conserved QALGGH motif at amino acid positions 381–386, and the other containing a variant motif of RALGGH at amino acid positions 483–488 (Fig. 1a). Computer analysis showed that the 116 amino acid residues at positions 367–484 are partially mapped to the ZFP ZIC1 in Mus musculus, which contains two C2H2-type domains in two α-helical structures (Fig. 1b). BLAST analysis of the predicted amino acid sequence against the non-redundant protein database (Nr) revealed that the protein is 86% similar to the ZFP ZAT4 from Glycine soja. Hence, we designated this protein as GmZAT4. Phylogenetic tree analysis of GmZAT4 and ZFPs from other plant species, including Medicago truncatula, Prunus mume, Theobroma cacao, Vitis vinifera, Vigna radiata var. radiata, G. soja, Cajanus cajan, Lupinus angustifolius, and A. thaliana, showed that the ZFPs clustered into two groups (groups 1 and 2). Fourteen of the proteins were included in group 1 along with GmZAT4, including AZF1, AZF2, and AZF3 from A. thaliana. GmZFP3 clustered in group 2, suggesting that the proteins in this cluster possess a different function from those in group 1 (Fig. 1c).
Fig. 1.
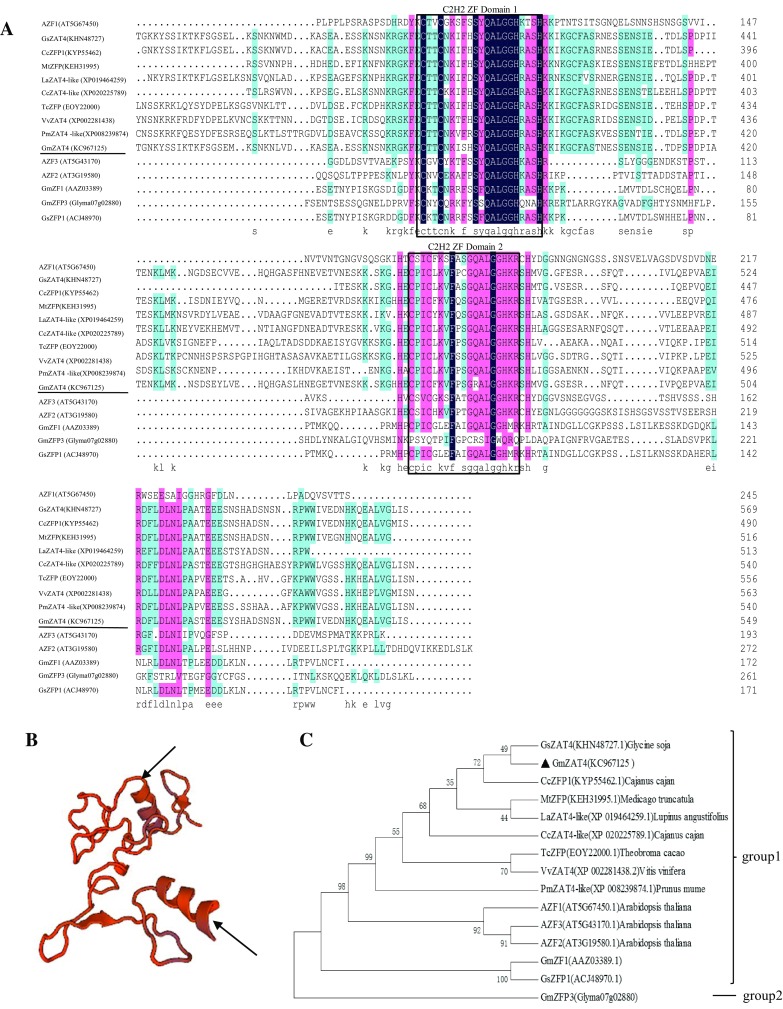
Protein sequence characteristics and phylogenetic analysis of GmZAT4. a Conserved motif analysis of GmZAT4 protein. Black rectangles indicate the C2H2-type motifs. b Three-dimensional structure of the C2H2-type domain. The arrows indicate the C2H2 domains. c Phylogenetic tree of GmZAT4 and other C2H2-type proteins from Arabidopsis thaliana, Glycine max, Nicotiana tomentosiformis, and Gossypium hirsutum. The filled triangle indicates the GmZAT4 protein cloned in this paper
Expression patterns of GmZAT4 in soybean
We investigated the expression patterns of GmZAT4 in the various tissues and organs of mature soybean plants using quantitative reverse transcription (qRT)-PCR and observed the highest expression of GmZAT4 transcripts in the roots, with an expression level 97.6-fold higher than that in the leaves (Fig. 2a). Osmotic stress treatment with 18% PEG resulted in higher expression of GmZAT4 in the ‘Jinda74’ (a drought-tolerant cultivar) than in the ‘Jinda53’ cultivar after 8 and 12 h of treatment. Moreover, GmZAT4 expression gradually decreased in ‘Jinda53’ from 4 to 24 h after the initiation of treatment (Fig. 2b). Following treatment with 150 mM NaCl, transcript levels of GmZAT4 gradually increased in ‘Jinda74’ from 4 to 8 h, then decreased from 12 to 24 h. This was in contrast with the pattern in ‘Jinda53’, in which expression gradually increased throughout the entire treatment period (Fig. 2c). In addition, treatment with 100 µM ABA led to increases in GmZAT4 expression from 4 to 24 h in both cultivars; however, at 12 h, transcript levels were higher in ‘Jinda74’ than in ‘Jinda53’ (Fig. 2d). Previous reports showed that ‘Jinda74’ is a drought-tolerant cultivar, exhibiting higher drought tolerance than ‘Jinda53’ (Hou et al. 2014). Although the expression patterns differed somewhat in the two cultivars, our results strongly suggest that GmZAT4 expression is rapidly induced in soybean following treatment with 18% PEG, 150 mM NaCl, or 100 µM ABA.
Fig. 2.
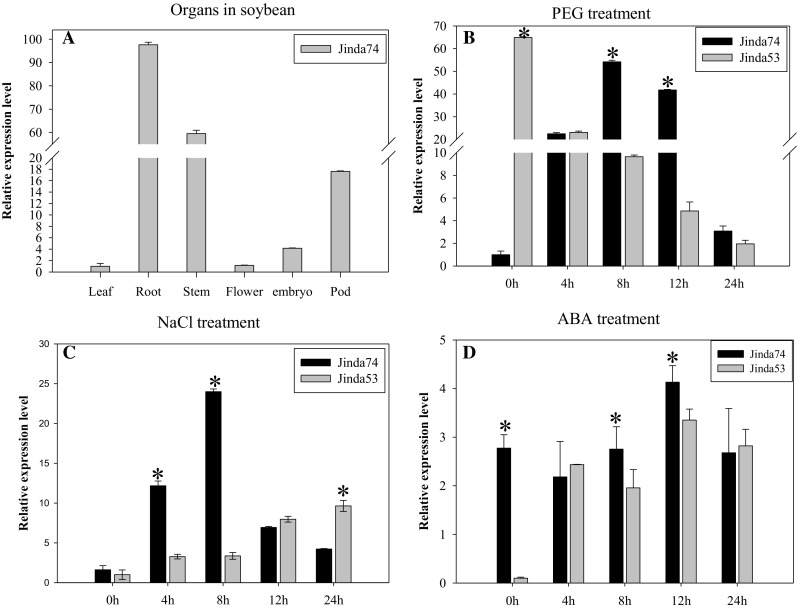
Spatiotemporal patterns of GmZAT4 gene expression in soybean. a GmZAT4 gene expression levels were assessed in various organs in ‘Jinda74’. GmZAT4 gene expression levels were assessed in ‘Jinda74’ and ‘Jinda53’ after treatment with b 18% PEG, c 150 µM NaCl, or d 100 µM ABA for 0, 4, 8, 12, and 24 h. Asterisk above the columns indicate a statistical significance with Duncan’s multiple-range test (p < 0.05)
Abiotic stress enhances GmZFP4 expression in transgenic Arabidopsis
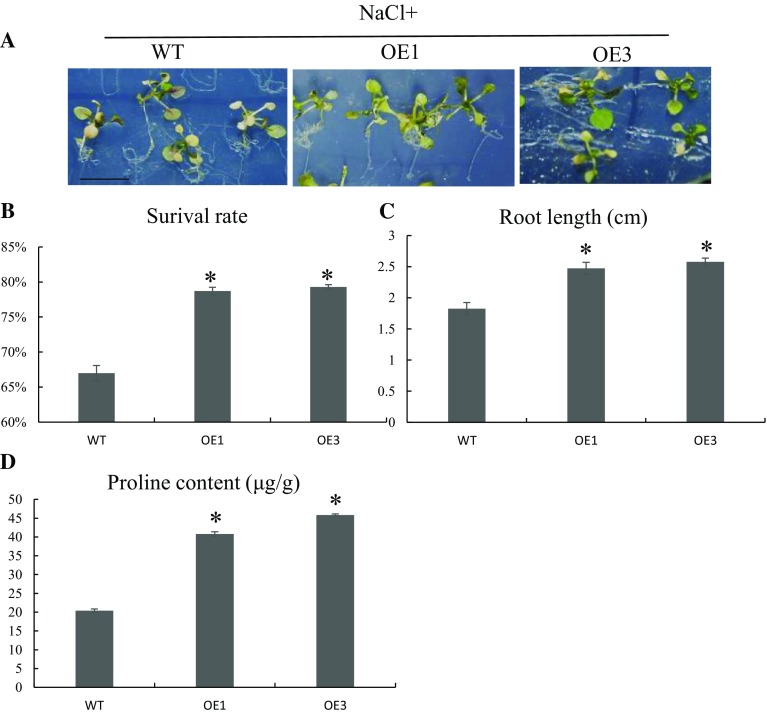
Two independent A. thaliana lines were transformed with the GmZAT4 gene driven by the 35S promoter to study GmZAT4 gene function in response to abiotic stress (Supplementary Figure S1). RT-PCR was performed with T3 generation transformants and wild-type (WT) plants (Col-0) to determine their stress tolerance. In response to stress treatment with 150 mM NaCl, 2-week-old seedlings from the two transgenic lines (OE1 and OE3) exhibited greener leaves and stronger roots than those of WT plants (Fig. 3a). The survival rate, relative root lengths, and proline contents were determined to reflect their NaCl tolerance (Fig. 3b–d). The survival rates of the OE1 and OE3 lines were significantly higher than that of WT plants, with values of, 78.19%, 78.43% and 66.74%, respectively. The root lengths of OE1 and OE3 were 35% and 42% longer, respectively, than those of WT, with values of 1.8 cm, 2.43 cm, and 2.56 cm, respectively. Salinity stress is a type of osmotic stress that can lead plants to synthesize compatible organic solutes, such as proline, in the cytosol (Gharsallah et al. 2016). The proline contents of OE1 and OE3 were significantly higher than that of WT, with values of 40.79 µg/g and 45.85 µg/g vs. 20.06 µg/g, respectively.
Fig. 3.
Phenotypic and physiological analyses of transgenic plants treated with 150 mM NaCl. a WT (Col-0) and transgenic seedings germinated in MS medium with 150 mM NaCl for 2 weeks. b Root lengths, c proline contents, and d survival rates were measured after 2 weeks. Asterisk indicates significant differences (p < 0.05) according to Duncan’s multiple-range test
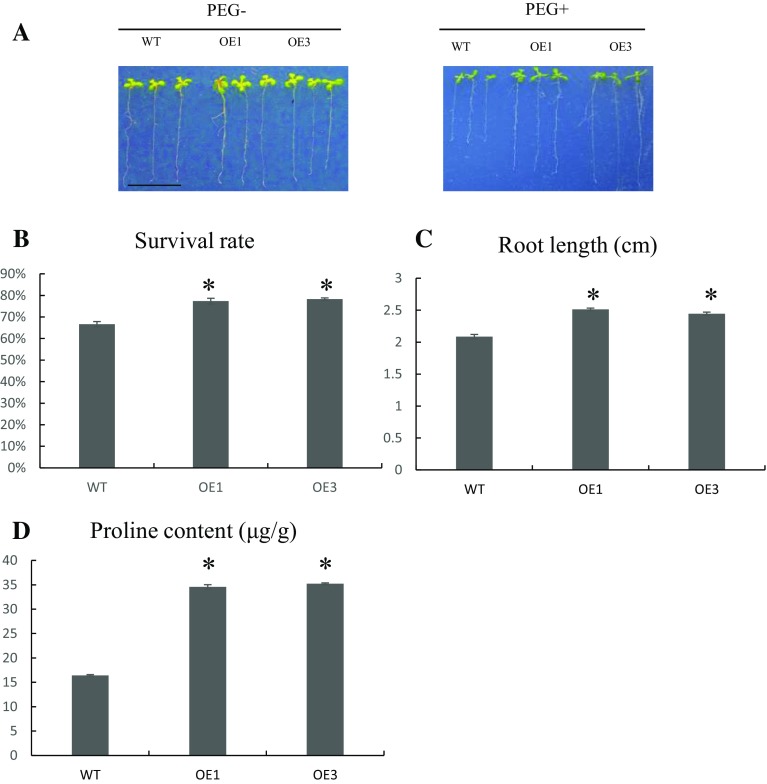
PEG-6000 was used to simulate drought stress in Arabidopsis (Fig. 4a) and the survival rate, root length, and proline content of each line were determined to assess the difference between the transgenic and WT lines. The survival rates of OE1 and OE3 were 6–8% higher than that of WT (Fig. 4b). In addition, the root lengths of OE1 and OE3 were significant longer than that of WT (Fig. 4c), and the proline contents (Fig. 4d) were 34.24–35.16 µg/g, higher than that of WT (16.7 µg/g). These results revealed that GmZFP4 expression enhances salt and drought tolerance, indicating that it may regulate the abiotic stress response in plants.
Fig. 4.
Phenotypic and physiological analyses of transgenic plants treated with 20% PEG. a WT and transgenic seedlings were germinated in MS medium with 20% PEG for 1 week before measuring b root length and c proline content. Asterisk indicated the significant difference (p < 0.05) according to Duncan’s multiple-range test
Overexpression of GmZFP4 in response to ABA treatment
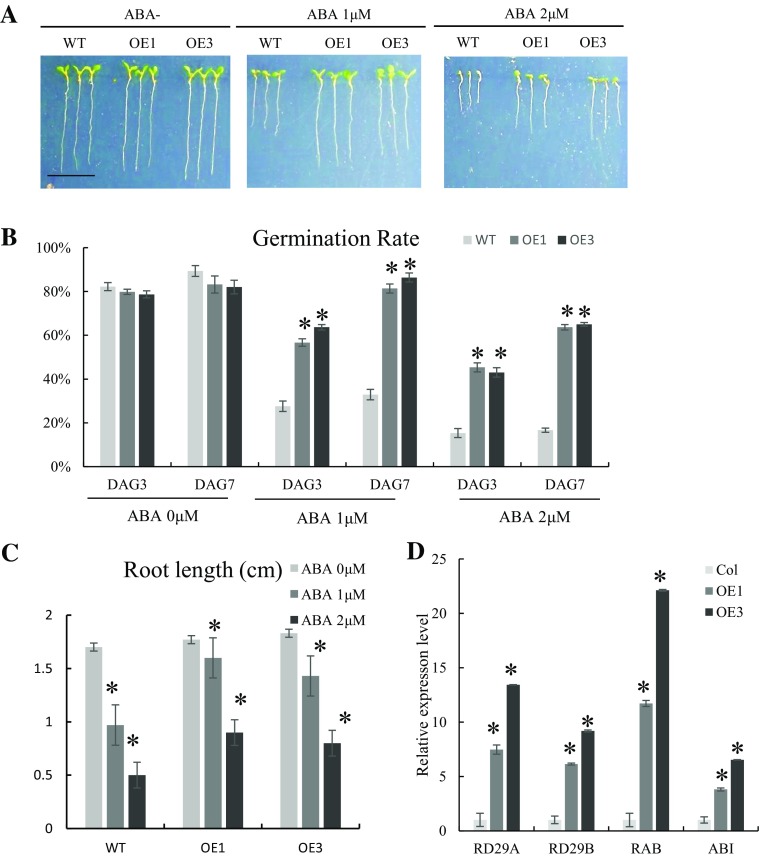
Based on these findings, we further examined the response of the OE1, OE3, and WT lines to 1 µM and 2 µM ABA treatment. The results of this experiment showed that the OE1 and OE3 lines exhibited increases in root length and growth vigor over those of the WT (Fig. 5a). And the root length did not dramatically shorten in OE1 and OE3 compared with WT with ABA treatment after 7 days. Especially with ABA 1 µM, the root length of OE1 and OE3 maintains 1.72 cm compared with 1.89 cm of WT. It showed significance longer than WT (0.95 cm) at the same condition (Fig. 5c). The germination rates were calculated after 3 days and 7 days. Treatment with the lower ABA concentration (1 µM) resulted in a higher germination rate than treatment with 2 µM ABA. The OE1 and OE3 lines exhibited strong germination abilities under both 1 µM and 2 µM ABA treatment. This was particularly true in the OE3 line, in which the germination rate reached 91.43% and 77.14% after 7 days in 1 µM and 2 µM ABA, respectively (Fig. 5b). Finally, we assessed the expression levels of ABA-synthesis and stress-responsive marker genes RD29A, RD29B, RAB, and ABI in the three lines following ABA treatment. All four genes were upregulated in the OE1 and OE3 lines, especially RAB, which was expressed at levels from 11.7- to 22.1-fold higher in the OE1 and OE3 lines than in the WT, respectively (Fig. 5d). These results suggest that GmZAT4-overexpressing plants are insensitive to exABA and that GmZAT4 expression may be positively regulated by the ABA signaling pathway in transgenic Arabidopsis plants.
Fig. 5.
Phenotypic and physiological analyses of transgenic plants treated with 0, 1, or 2 µM ABA. a Wild-type and transgenic seedings germinated in MS medium with 0, 1, 2 µM L− 1 ABA for 1 week. Germination rate (b) and ABA marker gene expression level (c) of these plants were measured and statistical analysis after 1 week. Asterisk indicated the significant difference (p < 0.05) according to Duncan’s multiple-range test
Discussion
Soybean is an important economic crop worldwide but requires intensive water use (Clemente and Cahoon 2009). It is, therefore, important to study its mechanisms of resistance to abiotic stress to improve the ability of this crop to grow in more arid environments. Many key transcription factors related to abiotic stress have been reported in plants, such as bZIPs (Gao et al. 2011), NACs (Huang et al. 2013), WRKYs (Vives-Peris et al. 2018), DREBs (Lata and Prasad 2011), MYBs (Katiyar et al. 2012), and ERFs (Zhao et al. 2017), but only a few C2H2-type ZFPs (Yu et al. 2014; Luo et al. 2012a) have been cloned and their functions studied in relation to abiotic stress tolerance in soybean and other plants. Previously, GmZF1, which contains two conserved QALGGH motifs, has been shown to enhance the tolerance of Arabidopsis to cold stress (Yu et al. 2014). The transformation of Arabidopsis with GsZFP1 lacking the typical QALGGH motif revealed that the gene still enhanced the plant’s ability to withstand cold and drought stresses, suggesting that the QALGGH motif in GsZFP1 is not necessary for adaptation to abiotic stress in wild soybean (Huang et al. 2006). Furthermore, GsZFP1 overexpression in Arabidopsis reduced its sensitivity to ABA and decreased its stomata size under ABA treatment (Luo et al. 2012a, b). Another study showed that overexpressing GsZFP1 in alfalfa resulted in enhanced salt tolerance, demonstrating the positive regulation of stress-responsive marker gene expression. In addition, an investigation into the function of GmZFP3, which has a typical QALGGH motif, suggested that this protein has a negative effect on drought tolerance in transgenic Arabidopsis (Zhang et al. 2016). Finally, in Arabidopsis, while AZF1 and AZF2 are induced by osmotic stress and ABA, transgenic plants overexpressing these genes exhibit severe impairments in plant growth and viability, and the repression of downstream genes that are negatively regulated by osmotic stress and ABA treatment (Kodaira et al. 2011; Sakamoto et al. 2004). These results suggest that C2H2-type transcription factors expressed in soybean and other plants exhibit different functions in response to stress with or without the QALGGH conserved motif.
In soybean, 321 C2H2-type ZFPs are described as key transcription factors in the Plant Transcription Factor Database (Jin et al. 2017), but the functions of these ZFPs in the abiotic stress response are largely unknown due to a lack of experimental data from soybean and other plants. In this study, a novel C2H2 zinc finger TF, GmZFP4, was isolated and characterized. The expression levels of GmZAT4, which encodes a conserved QALGGH motif comparing with other species, were 5- and 1.25-fold higher in the drought-tolerant soybean cultivar ‘Jinda74’ compared with those in the drought-sensitive soybean cultivar ‘Jinda53’ after 12 h of PEG and NaCl stress, respectively. The similar results are shown in GmZF1 and GsZFP1; it increases the ability of cold tolerance and drought stress that involved in an ABA-dependent pathway (Yu et al. 2014; Luo et al. 2012a, b).
It is well known that ABA as a signaling has broad functions in plant growth and development, and its mains function is regulated plant osmotic stress (Xiong et al. 2002; Lee and Luan 2012). Lots of studies showed that some osmotic stress-responsive genes are induced completely independent of ABA, some are fully dependent on ABA and others are only partially ABA dependent (Zhu 2002). So far, to C2H2 transcript factor, it was reported that some C2H2-type zinc finger genes were induced by ABA treatment related to dehydration and cold stress, and others do not respond to exogenous ABA (Zhang et al. 2016). To Arabidopsis, AZF1 and AZF2 are ABA-dependent type because the gene expressions were induced by osmotic stress and ABA treatments (Kodaira et al. 2011). To soybean, GmZF1, GmZFP1 and GmZFP3 were both involved in plant responses to osmotic stress through an ABA-dependent signal transduction pathway since these gene expressions in soybean seedlings were clearly induced by ABA (Yu et al. 2014; Zhang et al. 2016). In our study, GmZAT4 showed two distinct gene expression patterns in soybean mature plants of two cultivars with different drought stress tolerance ability. The evidence also indicated the response time of GmZAT4 gene expression in ‘Jinda74’ was earlier than in ‘Jinda53’. These results suggested that the GmZAT4 as a transcript factor was a ‘early response gene’ and it could be regulated those ‘delayed response gene’ expression under osmotic stress and ABA treatment. Then, GmZAT4 was transformed into transgenic Arabidopsis lines; transformants also exhibited enhanced PEG and NaCl stress tolerance. These results suggested that GmZAT4 plays an important function role in plant cellular dehydration tolerance. Moreover, the transgenic lines also exhibited reduced ABA response. Taken together, it demonstrates that the GmZAT4 function as a transcript factor is completely dependent on ABA signal pathway to regulate these ABA-responsive marker gene expressions which endowed dehydration tolerance ability in soybean and Arabidopsis.
In summary, GmZAT4 is a C2H2-type zinc finger transcription factor that positively responds to PEG and NaCl stresses in soybean. The overexpression of GmZAT4 enhances PEG and NaCl tolerances and reduces ABA sensitivity in Arabidopsis. These results may prove useful for the development of genetic engineering technologies to improve the tolerance of soybean and other plants to abiotic stress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1. Transgenic GmZAT4-transformed lines identified by gDNA-PCR and RT-PCR. (A) WT, OE1, and OE2 at 4 weeks. (B) CDS fragments of GmZAT4 amplified from vector (control, CK), transgenic lines (lanes 1–6), WT (lane 7), and negative control (lane 8) using gDNA-PCR. (C) RT-PCR of GmZAT4 gene expression in WT and transgenic lines (lanes 1–6). Lines #1 and # 3 (designated OE1 and OE3, respectively) were selected from the six transgenic lines and used for further analysis. (PDF 96 KB)
Acknowledgements
We would like to thank Professor Donald Grierson, University of Nottingham, UK, for discussion and helping with the manuscript. We also thank Prof. Guiquan Li for donating the plant material and Prof. Yuguo Wang for giving advice for the experiments. We would like to thank Science and Technology Project of Shanxi Province (20150311007-1) and Research Project Supported by Shanxi Scholarship Council of China (2017069).
Author contributions
ZS, SH, HL and YH designed the experiments and drafted the manuscript. ZS and KH collected plant materials and cloned the gene. RL, BG and LW transformed the Arabidopsis and analysis the data. YH modified the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Zhaoxia Sun, Email: 18636071356@163.com.
Ronghua Liu, Email: 1356787046@qq.com.
Bin Guo, Email: 170230241@qq.com.
Kesheng Huang, Email: 469620010@qq.com.
Li Wang, Email: 175981836@qq.com.
Yuanhuai Han, Email: swgctd@163.com.
Hongying Li, Email: Hongylswgctd@163.com.
Siyu Hou, Email: 18635068055@163.com.
References
- Agarwal P, Arora R, Ray S, Singh AV, Takatsuji H, Kapoor S, Tyagi AK. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol Biol. 2007;65(4):467–485. doi: 10.1007/s11103-007-9199-y. [DOI] [PubMed] [Google Scholar]
- Ciftci-Yilmaz S, Mittler R. The zinc finger network of plants. Cell Mol Life Sci. 2008;65:1150–1160. doi: 10.1007/s00018-007-7473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente TE, Cahoon EB. Soybean oil: genetic approaches for modification of functionality and total content. Plant Phys. 2009;151(3):1030–1040. doi: 10.1104/pp.109.146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutforth HW, Mcginn SM, Mcphee KE, Miller PR. Adaptation of pulse crops to the changing climate of the northern great plains. Agron J. 2007;99(6):1684–1699. doi: 10.2134/agronj2006.0310s. [DOI] [Google Scholar]
- Englbrecht CC, Schoof H, Bohm S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004;5(1):39. doi: 10.1186/1471-2164-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SQ, Chen M, Xu ZS, Zhao CP, Li L, Xu HJ, Tang YM, Zhao X, Ma YZ. The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol Biol. 2011;75(6):537–553. doi: 10.1007/s11103-011-9738-4. [DOI] [PubMed] [Google Scholar]
- Gharsallah C, Fakhfakh H, Grubb D, Gorsane F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in Tomato cultivars. AoB Plants. 2016;8:plw055. doi: 10.1093/aobpla/plw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SY, Sun ZX, Guo B, Wang YG, Li GQ, Han YH. Cloning and expression analysis of two C2H2 transcription factors in soybean. Plant Physiol J. 2014;50(5):665–674. [Google Scholar]
- Hou SY, Sun ZX, Linghu B, Wang YG, Huang KS, Xu DM, Han YH. Regeneration of buckwheat plantlets from hypocotyl and the influence of exogenous hormones on rutin content and rutin biosynthetic gene expression in vitro. Plant Cell Tissue Organ Cult. 2015;120(3):1159–1167. doi: 10.1007/s11240-014-0671-5. [DOI] [Google Scholar]
- Huang J, Wang JF, Zhang HS. Structure and function of plant C2H2 zinc finger protein. Yichuan. 2004;26(3):414–418. [PubMed] [Google Scholar]
- Huang F, Chi Y, Meng Q, Gai J, Yu D. GmZFP1 encoding a single zinc finger protein is expressed with enhancement in reproductive organs and late seed development in soybean (Glycine max) Mol biol Rep. 2006;33(4):279–285. doi: 10.1007/s11033-006-9012-z. [DOI] [PubMed] [Google Scholar]
- Huang GQ, Li W, Zhou W, Zhang JM, Li DD. Seven cotton genes encoding putative NAC domain proteins are preferentially expressed in roots and in responses to abiotic stress during root development. Plant Growth Regul. 2013;71(2):101–112. doi: 10.1007/s10725-013-9811-x. [DOI] [Google Scholar]
- Jin JP, Tian F, Yang DC, Meng YQ, Kong L, Luo JC, Gao G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45:1040–1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar A, Smita S, Lenka SK, Rajwanshi R, Chinnusamy V, Bansal KC. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genom. 2012;13(1):544. doi: 10.1186/1471-2164-13-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira KS, Qin F, Tran LS, Maruyama K, Kidokoro S, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 2011;157(2):742–756. doi: 10.1104/pp.111.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata C, Prasad M. Role of DREBs in regulation of abiotic stress responses in plants. J Exp Bot. 2011;62(14):4731–4738. doi: 10.1093/jxb/err210. [DOI] [PubMed] [Google Scholar]
- Lee SC, Luan S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012;35(1):53–60. doi: 10.1111/j.1365-3040.2011.02426.x. [DOI] [PubMed] [Google Scholar]
- Luo X, Bai X, Zhu D, Li Y, Ji W, Cai H, Wu J, Liu B, Zhu Y. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta. 2012;235(6):1141–1155. doi: 10.1007/s00425-011-1563-0. [DOI] [PubMed] [Google Scholar]
- Luo X, Cui N, Zhu Y, Cao L, Zhai H, Cai H, Ji W, Wang X, Zhu D, Li Y, Bai X. Over-expression of GsZFP1, an ABA-responsive C2H2-type zinc finger protein lacking a QALGGH motif, reduces ABA sensitivity and decreases stomata size. J Plant Physiol. 2012;169(12):1192–1202. doi: 10.1016/j.jplph.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Min SL, Gippert GP, Soman KV, Case DA, Wright PE. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989;245(4918):635–637. doi: 10.1126/science.2503871. [DOI] [PubMed] [Google Scholar]
- Moore M, Ullman C. Recent developments in the engineering of zinc finger proteins. Brief Funct Genom Protemic. 2003;1(4):342–355. doi: 10.1093/bfgp/1.4.342. [DOI] [PubMed] [Google Scholar]
- Phang TH, Shao GH, Lam HM. Salt tolerance in soybean. J Integr Plant Biol. 2008;50(10):1196–1212. doi: 10.1111/j.1744-7909.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004;136(1):2734–2746. doi: 10.1104/pp.104.046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuji H. Zinc-finger proteins: the classical zinc finger emerges in contemporary plant science. Plant Mol Biol. 1999;39(6):1073–1078. doi: 10.1023/A:1006184519697. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Peris V, Marmaneu D, Gómez-Cadenas A, Pere-Clemente RM. Characterization of Citrus, WRKY transcription factors and their responses to phytohormones and abiotic stresses. Biol Plant. 2018;62(1):33–44. doi: 10.1007/s10535-017-0737-4. [DOI] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:165–183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GH, Jiang LL, Ma XF, Xu ZS, Liu MM, Shan SG, Cheng XG. A soybean C2H2-type zinc finger gene GmZF1 enhanced cold tolerance in transgenic Arabidopsis. PLoS One. 2014;9(10):e109399. doi: 10.1371/journal.pone.0109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YH, Li XZ, Wu ZJ, Chen DX, Li GR, Li XQ, Zhang GH. VvZFP11, a Cys2His2-type zinc finger transcription factor, is involved in defense responses in Vitis vinifera. Biol Plant. 2016;60(2):292–298. doi: 10.1007/s10535-016-0598-2. [DOI] [Google Scholar]
- Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ni L, Liu Y, Wang Y, Zhang A, Tan M, Jiang M. The C2H2-type zinc finger protein ZFP182 is involved in abscisic acid-induced antioxidant defense in rice. J Integr Plant Biol. 2012;54(7):500–510. doi: 10.1111/j.1744-7909.2012.01135.x. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Tong JF, Xu ZL, Wei PP, Xu L, Wan Q, Huang YH, He XL, Yang JY, Shao HB, Ma HX. Soybean C2H2-type zinc finger protein GmZFP3 with conserved QALGGH motif negatively regulates drought responses in transgenic arabidopsis. Front Plant Sci. 2016;7:325. doi: 10.3389/fpls.2016.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chang X, Qi D, Dong L, Wang G, Fan S, Jiang L, Cheng Q, Chen X, Han D, Xu P, Zhang S. A Novel soybean ERF transcription factor, GmERF113, increases resistance to Phytophthora sojae infection in soybean. Front Plant Sci. 2017;8(47):299. doi: 10.3389/fpls.2017.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Transgenic GmZAT4-transformed lines identified by gDNA-PCR and RT-PCR. (A) WT, OE1, and OE2 at 4 weeks. (B) CDS fragments of GmZAT4 amplified from vector (control, CK), transgenic lines (lanes 1–6), WT (lane 7), and negative control (lane 8) using gDNA-PCR. (C) RT-PCR of GmZAT4 gene expression in WT and transgenic lines (lanes 1–6). Lines #1 and # 3 (designated OE1 and OE3, respectively) were selected from the six transgenic lines and used for further analysis. (PDF 96 KB)