Abstract
Frontal electroencephalographic (EEG) alpha asymmetry is widely researched in studies of emotion, motivation, and psychopathology, yet it is a metric that has been quantified and analyzed using diverse procedures, and diversity in procedures muddles cross-study interpretation. The aim of this article is to provide an updated tutorial for EEG alpha asymmetry recording, processing, analysis, and interpretation, with an eye towards improving consistency of results across studies. First, a brief background in alpha asymmetry findings is provided. Then, some guidelines for recording, processing, and analyzing alpha asymmetry are presented with an emphasis on the creation of asymmetry scores, referencing choices, and artifact removal. Processing steps are explained in detail, and references to MATLAB-based toolboxes that are helpful for creating and investigating alpha asymmetry are noted. Then, conceptual challenges and interpretative issues are reviewed, including a discussion of alpha asymmetry as a mediator/moderator of emotion and psychopathology. Finally, the effects of two automated component-based artifact correction algorithms—MARA and ADJUST—on frontal alpha asymmetry are evaluated.
The Utility of Frontal Asymmetry: An overview
The difference between left and right alpha activity over the frontal regions of the brain during electroencephalographic (EEG) recording is termed frontal EEG asymmetry, a phenomenon that researchers first linked to patterns of emotion processing decades ago (e.g., Davidson, Schwartz, Saron, Bennett, & Goleman, 1979; Ahern & Schwartz, 1985; Davidson, Schaffer & Saron, 1985; Tucker, 1981). Frontal EEG asymmetry is now employed by scientists worldwide to study constructs such as temperament and personality, various types of psychopathology, motivation, emotion, and cognitive control (for reviews, see Allen & Reznik, 2015; Coan & Allen, 2003, 2004, Harmon-Jones, 2003, and Harmon-Jones, Gable & Peterson, 2010).
Frontal EEG asymmetry has been recorded during resting state and task conditions. Although used inconsistently in the literature when describing frontal EEG asymmetry, the term “activity” refers to data recorded during some period of time, such as while a participant is in a resting state, and “activation” refers to a change in activity due to some task or state change. Stated differently, activity reflects level and activation captures change in activity. One dominant perspective suggests that lateralized activity (i.e., resting-state frontal EEG asymmetry) reflects the tendency or predisposition of an individual to engage in certain types of emotional (positive versus negative) and/or motivational (appetitive versus avoidant) responses, whereas activation is thought to reflect state motivational and/or emotional responding. Frontal EEG asymmetry has most frequently been studied in relation to these emotional/motivational states and traits, and the relationship of frontal EEG asymmetry with emotional/motivational variables is the primary theoretical framework of the authors and of this review. A growing literature also indicates that relatively greater left than right frontal activity/activation characterizes approach-oriented situations (e.g., jealousy: Harmon-Jones, Peterson, & Harris, 2009; state anger: Harmon-Jones & Sigelman, 2001; self-control: Schmeichel, Crowell, & Harmon-Jones, in press) and/or individuals (high dispositional anger: Harmon-Jones & Allen, 1998; high trait optimism: De Pascalis, Cozzuto, Caprara, & Alessandri, 2013), whereas greater right than left frontal activity/activation is thought to reflect withdrawal-related motivational traits and states (e.g., sadness and fear: Coan, Allen, & Harmon-Jones, 2001; empathy: Tullett, Harmon-Jones, & Inzlicht, 2012) or internalizing personality traits (depression: Thibodeau, Jorgensen, & Kim, 2006; anxiety: Mathersul, Williams, Hopkinson, & Kemp, 2008). In addition to emotional/motivational states and traits, several reports have also linked frontal EEG alpha asymmetry to other variables like executive functions (Ambrosini & Vallesi, 2016; Çiçek, & Nalçaci, 2001), worry (Heller, Nitschke, Etienne, & Miller, 1997; Smith, Zambrano-Vazquez, & Allen, 2016), and verbal fluency (Hoptman, & Davidson, 1998).
Although some researchers have embraced the use of state emotion manipulations to examine relationships between motivation/action and patterns of hemispheric EEG activation, the majority of frontal asymmetry studies of psychopathology over the past three decades have examined differences in left versus right hemisphere activity while individuals are seated in a resting state. These latter studies have been predictive of withdrawal-related psychopathology in some, but not all cases, with inconsistencies in findings for depression and anxiety symptom ratings measured dimensionally as well as for Major Depressive Disorder (Quinn, Rennie, Harris, & Kemp, 2014; Stewart, Bismark, Coan, Towers, & Allen, 2010) and Posttraumatic Stress Disorder (see Meyer et al., 2015 for a review). In addition to sample-specific characteristics, several important methodological factors may, at least in part, account for such conflicting results. Some key issues include: a) choice of EEG reference (Hagemann, 2004; Hagemann, Naumann, & Thayer, 2001; Stewart et al., 2010); b) EEG recording length (Towers & Allen, 2009); c) the reliability/stability of EEG asymmetry within and across sessions (Allen, Coan, & Nazarian, 2004; Allen, Urry, Hitt & Coan, 2004; Hagemann, Naumann, Thayer, & Bartussek, 2002); d) disorder comorbidity/heterogeneity (Davidson, 1998; Miller et al., 2002; Meyer et al., 2015); e) sex differences (Miller et al., 2002; Stewart et al., 2010); and, f) seasonal and temporal variations interacting with individual differences in waking preference (Velo, Stewart, Hasler, Towers, & Allen, 2012). Issues a-c will be discussed in greater detail below. With respect to disorder heterogeneity, Nusslock, Walden, and Harmon-Jones (2015) argue that instead of attempting to link frontal asymmetry with diagnostic criteria for a particular disorder, researchers should relate left versus right frontal activity to psychological symptom clusters (cf. Reid, Duke, & Allen, 1998) such as anhedonia, mania, and anxious apprehension across clinical groups to increase power and better inform assessment and intervention strategies.
In addition to attending to these concerns, approaches have also focused on increasing the signal to noise ratio to enhance the magnitude of correlations between EEG profiles and emotional responding, by moving beyond simple resting state recordings or analysis. The resting state is far from static, with dynamic shifts in asymmetry within subject across time during a typical several-minute resting session (cf. Allen & Cohen, 2010). One strategy can be to parse the relatively long resting state into moments that capture the most signal, either by looking for microstates of asymmetry bursts (Allen & Cohen, 2010) or identifying moments within a recording with the greatest indication that a target emotional or motivational state is present (e.g., facial expressions of emotion: Davidson, Ekman, Saron, Senulis, & Friesen, 1990). The other strategy is to provide a challenge so that engages relevant motivational systems during a longer period of recording (Coan, Allen, & McKnight, 2006). In their capability model of individual differences in brain asymmetry, Coan et al. (2006) argue that utilizing motivationally relevant challenges may produce more powerful individual differences than just recording frontal asymmetry during a resting state, wherein uncontrolled subject factors might reduce power to find meaningful relationships between brain activity and subjective reports of trait/state responding (Coan & Allen, 2003). Indeed, a comparison of activity at rest and activity during a motivationally-relevant elicitation (directed facial action task) recorded in the same sample showed that task-related activity (during happy, angry, sad, and fearful directed facial expressions; Stewart, Coan, Towers, & Allen 2011) differentiated depressed from never-depressed men and women more strongly than resting activity when examining average, Cz, and linked-mastoid referenced data (Stewart et al., 2014); by contrast, current-source density (CSD) transformed data for resting and task activity both differentiated depressed from never-depressed groups. These results suggest that motivationally-salient challenges, and EEG transformations such as the CSD transformation that highlight frontal neural sources (see below for details), may produce more predictive and reliable estimates of motivational states and traits.
The literature on EEG asymmetry is sizable, and continues to grow, and yet reflects great diversity in terms of “the conditions under which data were recorded, … the manner in which data were reduced and … the manner in which data were subsequently analyzed” (Allen, Coan, & Nazarian, 2004, p. 214). This review thus details important data acquisition and analysis procedures in the hopes that consistency in data collection and processing practices will lead to greater reliability across studies in linking frontal EEG asymmetry to emotion, motivation, and psychopathology.
Obtaining the best estimates of Frontal Asymmetry: Procedures for Data Collection, Transformation, and Reduction
Recording Considerations
Guidelines for recording EEG asymmetry are much the same as for recording other EEG signals in terms of subject preparation and laboratory procedures. A special consideration for working with EEG asymmetry, given that it can function as both a trait individual difference and also vary with state manipulations, is to avoid unintentionally inducing a state manipulation before or during EEG recording. Subject preparation should be professional, efficient, emotionally equanimous, and mindful of the reaction of the participant, guidelines that are likely part of most laboratory preparation protocols. These considerations assume special importance for EEG asymmetry research, as emotional responses to recording preparation have been shown to predict frontal EEG asymmetry scores (Blackhart, Kline, Donohue, LaRowe, & Joiner, 2002), as have specific experimenter characteristics such as attractiveness (Wacker, Mueller, Pizzagalli, Hennig, & Stemmler, 2013). Moreover, experimenters conducting multiple studies within the same subject should also be mindful of carryover effects of emotional stimuli from one experiment to another.
When planning a recording session, an investigator must balance the need to obtain a sufficient quantity of data to provide reliable estimates of frontal EEG asymmetry while minimizing participant burden; with longer sessions, emotional state can change within session, contributing heterogeneous sources to the single metric that typically summarizes the session. Because the recording session is segmented into short epochs and a power spectrum is derived from each epoch (see below), the power spectrum from any single epoch will reflect both frequencies that are common across epochs as well as those idiosyncratic to any given epoch. Yet averaging together spectra across epochs can allow those frequencies to emerge that are present in a reasonably large proportion of epochs while attenuating the influence of infrequent or irregular signals that might be considered noise (Nunez, 1981). As shown by Towers and Allen (2009), excellent reliability of frontal EEG asymmetry may be obtained with as few as 100 epochs, which corresponds to one to three minutes (depending on epoch overlap) of artifact-free recorded data. For resting assessments, this would likely be a continuous recording, but for state-elicited asymmetry scores, this could reflect pooling across multiple shorter segments where the investigator has good reason to believe the emotion is similarly elicited during each segment (e.g., film segments, picture viewing, emotional manipulation; cf. Coan et al., 2001). Depending on how many epochs are rejected due to artifacts (see below for methods of retaining epochs using correction instead of rejection), an investigator may need to plan to record 2–3 times as long as the target of 1–3 minutes, as rejection of epochs for blinks and other artifacts can often result in data loss approximating 50% (Allen, Urry, Hitt, & Coan, 2004).
The Impact of Reference and Reference-free Transformations
Interpretation of frontal EEG asymmetry data assumes that measures of spectral power from a given recording site reflect activity at that site rather than activity at the reference lead or elsewhere. For this purpose, researchers may seek a relatively inactive reference, and have often used averaged ears or mastoids, or an average reference comprising mean activity at all recorded sites (Reid, Duke, & Allen, 1998; Tomarken, Dichter, Garber, & Simien, 2004). With a sufficiently large array of electrodes arranged in a sphere, the average reference will nicely approximate an inactive reference; activity generated from dipoles will be revealed as positivity at one site and negativity at a site 180 degrees opposite this site, and thus the sum across sites will approach zero with adequate representation of the entire sphere. Limited recording montages, with few electrodes that do not provide adequate coverage of the sphere will have more residual activity in the average reference (e.g., potentially mirroring alpha power from distal occipital sources onto frontal electrodes; Hagemann, 2001).
Especially troubling in terms of isolating activity to recorded leads is the Cz reference, which has been utilized frequently in the literature (Coan & Allen, 2004; Thibodeau, Jorgensen, & Kim, 2006). The Cz reference may potentially under- or over-estimate activity at the target site (Hagemann et al., 2001), and empirical comparisons find that asymmetry scores using Cz-referenced data possess lower correlations to scores from other reference schemes (e.g., Hagemann et al., 2001; Reid et al., 1998).
Despite providing low-activity or relatively inactive references, averaged mastoids and the average reference montages create a localization difficulty so that power from distant intracranial sources is apparent at an unrepresentative scalp electrode (see Figure 1). Most vexing is alpha “mirroring” whereby frontal alpha power is contaminated by recording the opposite polarity of an oscillating occipital alpha dipole (Hagemann et al., 2001). Whereas in the time domain, a dipole will produce a positivity at one location and a negativity 180 degrees opposite, spectral power simply summarizes the magnitude of oscillations (without regard to polarity), resulting in power from that dipole emerging in both locations (see Figure 1). An alternative is to use a spatial filter, such as the reference-free CSD transformation (Kayser & Tenke, 2006; Perrin, Bertrand, Giard, & Pernier, 1990; Perrin, Pernier, Bertrand, & Echallier, 1989), which computes the second spatial derivative of voltage between nearby electrode sites, providing a spatially-enhanced signal representation that increases the contribution of local electrical activities and attenuates those from distal volume-conducted sources.
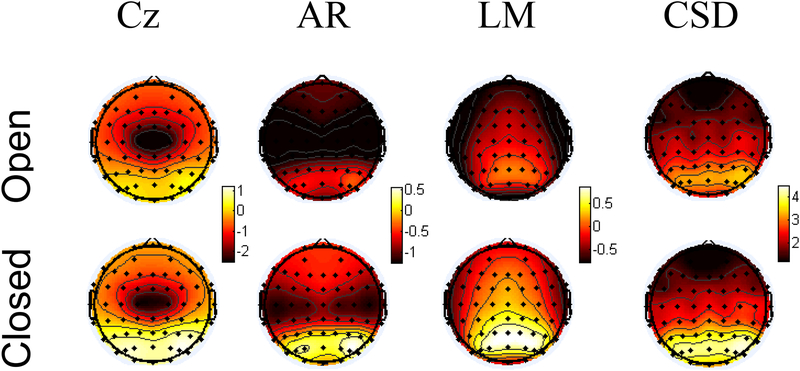
Figure 1.
Topography of alpha power under eyes open (top) and eyes closed (bottom) conditions as a function of transformation (Cz, average (AR), or linked mastoid (LM) reference or current source density (CSD) transformation) from a sample of over 2400 recordings. Power values at each site represent natural-log transformed values; thus negative numbers represent mean power values less than one. Each transformation is scaled independently, but within each transformation, eyes open and closed data are plotted on the same scale. Only the CSD transformation contains occipital alpha to occipital leads, whereas the other three montages show reflected alpha at frontal regions, visible most clearly by a comparison of frontal leads under eyes closed compared to eyes open recordings.
Recent work suggests that the CSD transformation may provide a better index of individual differences in frontal asymmetry and reduce the contributions of non-frontal sources to frontal asymmetry (Stewart et al., 2010; Stewart, Coan, Towers, & Allen, 2014; Velo et al., 2012; also see a special issue of International Journal of Psychophysiology 97, 3, September 2015 for an in-depth review of the CSD montage).
Stewart et al. (2010) found that only CSD-transformed frontal asymmetry – but not average, nor averaged mastoids, nor Cz-referenced asymmetry – differed as a function of lifetime history of depression during the resting state for 306 participants. The impact of these different surface potential transformations is depicted in Figure 1. Occipital alpha power increases when participants close their eyes (Berger, 1929); this pattern is clearly illustrated in Figure 1, and only the CSD transformation contains occipital alpha to occipital sites. Apparent with the averaged mastoids reference and average references is considerable alpha at frontal sites, especially during eyes closed, when alpha power should predominate at occipital sites. The figure illustrates how the CSD transform attenuates the contributions of distal sources to surface leads, and thus may be the preferred approach for assessing the relationship of frontal EEG asymmetry to depression, and for analyzing the asymmetrical activity of frontal neural sources.
There are some caveats to using the CSD approach. CSD accentuates differences between neighboring electrodes, and when electrodes are spaced widely apart small active patches of cortex between sensors may be filtered-out. This is akin to aliasing in the time domain: low spatial sampling rates will not resolve high-frequency spatial signals. In short, CSD transformations poorly represent activity at the scalp in montages with few channels. Similarly, effects at the edges of the cap (where the scalp is under-sampled) should also be interpreted cautiously. Finally, this may also be a problem when an electrode is systematically interpolated within a condition: activity at an interpolated electrode will have low-spatial frequency and may be attenuated with a CSD reference (interpolation sites do not covary with experimental conditions, thus this effect equates to random noise in most cases). Despite the name, CSD is not a source localization algorithm in the typical sense, CSD is a representation of local sources and sinks at the level of the dura (fewer assumptions regarding dural energy sources avoids the inverse problem). Whereas sources with high spatial-frequency and radial dipoles are showcased with a CSD montage, deep distributed sources tangential to the scalp may be attenuated or invisible. In the experience of the writers, CSD does a good job of capturing EEG alpha asymmetry with a 60 channel cap. As an alternative, other spatial filters can also be used such as independent components or source estimation (e.g., LORETA). Of note to researchers: when in doubt, compare and contrast how different montages influence results, and consider reporting results for multiple montages in publications.
Transforming Raw Signals into Asymmetry Scores
Figure 2 depicts the basic steps involved in transforming time-domain EEG signals into spectral power at a given site (also see Allen, Coan, et al., 2004). The signal collected in the time-domain (Panel A, left side) is converted into a frequency-domain representation in the form of a power spectrum (Panel A, right side). Whether data are collected from a continuous resting period, or from distinct relatively short segments (such as during emotion elicitation), short epochs are created (Panel B) and converted to frequency spectra. These power spectra are then averaged across many epochs. For resting data, a large data segment is first epoched into smaller 1–2 second epochs (although other durations are possible, such as longer epochs if time-frequency approaches will subsequently be used). For EEG acquired in the context of elicited emotional expressions or experiences, the data segment might still need to be epoched into a few smaller epochs, and data across these similar expressions or experiences would then be aggregated.
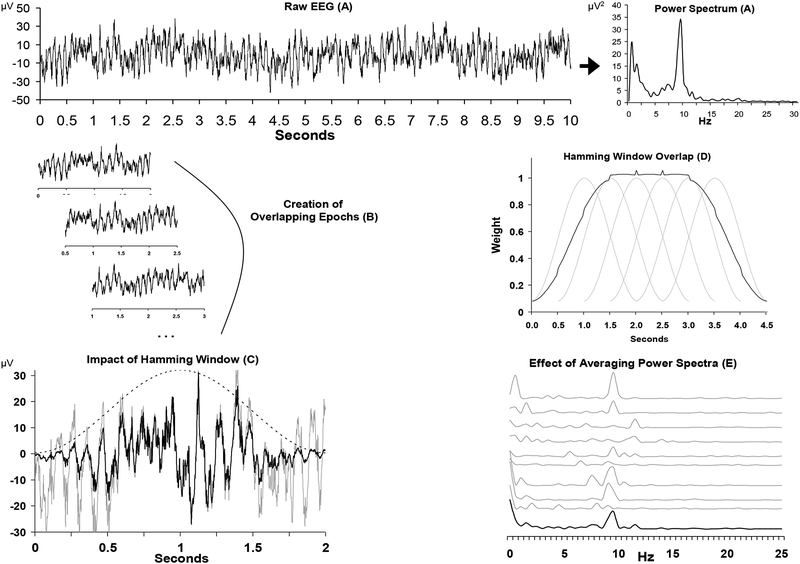
Figure 2.
Overview of converting time-domain signals to power spectra for EEG asymmetry research. Panel A depicts a 10-second segment of raw data from a single channel on the left, and the spectral representation of this epoch on the right. Panel B illustrates the process of epoching the longer segment into shorter overlapping two-second epochs. Panel C shows the impact of the Hamming window (dotted bell curve) on a single epoch, with the grey line representing the raw signal and the black line representing the signal after the application of the window. Note that a discontinuity would result if a copy of the raw (grey) signal were concatenated following this signal, but no such discontinuity would result for a similarly concatenated windowed (black) signal. Panel D displays the net weighting (black line, scaled to fit graph) of overlapping hamming windows (grey lines) for two-second epochs. Panel E illustrates the impact of averaging power spectra. The top 9 grey lines are the spectral representation of 9 two-second epochs, and the lower black line is the average spectrum. Note that alpha power (8–13 Hz) is somewhat variable from epoch to epoch, but that the average spectrum reveals a distinct alpha peak. Vertical axis in Panel E is power in microvolts-squared. Figure after Allen, Coan, and Nazarian (2004).
Creating epochs that are relatively short (1–2 seconds) better matches an assumption of the Fourier transform, the predominant approach to derive power spectra from time-domain signals. Fourier analyses assume that each signal is periodic (the stationarity assumption) and that any periodic signal can be decomposed into a series of sine and cosine waveforms at many frequencies, with the beginning of each waveform having its own particular phase. A periodic signal is one that repeats, and does so at uniformly spaced intervals of time. Strictly speaking, EEG signals are not periodic because repetition of features is not precisely spaced at uniform intervals. Short epochs, however, provide small segments of data with features that repeat in a highly similar fashion at other points in the waveform. Often epochs are overlapped (Panel B) because weighting functions applied to the time-domain signal in the process of “windowing” (described below) will greatly attenuate the signal at the distal regions of each epoch, and only the signal near the central portion of each epoch will receive considerable weight (Panel C). By overlapping epochs, all portions of the signal receive high weighting in some epoch.
Windowing of the time-domain signal will minimize the creation of artifactual frequencies in the resultant power spectra. Because the Fourier approach assumes a periodic signal repeating infinitely both forward and backward in time, without the windowing function that reduces the signal to near-zero at the ends of each epoch, discontinuities would arise if one placed a copy of the epoch immediately before or after itself. With such discontinuities, Fourier methods introduce spurious frequencies to reconstruct such a signal. Windowing avoids the discontinuity (Panel C), but greatly attenuates the contribution of data near the end of the epoch to the power spectrum. Overlapping epochs (Panel D) solve this problem; data receiving minimal weight near the end of epoch n will be weighted more heavily in epoch n+1.
The Fast Fourier Transform (FFT; Cooley & Tukey, 1965) is a considerably faster and computationally less complex instantiation of the Discrete Fourier transform (DFT). To derive power spectra using the FFT, epochs must have 2n data points, which can be accomplished by selecting epochs with precisely a power of two data points (e.g., 2.048 seconds at 500 Hz) or upsampling data to obtain a power of two (e.g., 2.0 seconds at 500 Hz is upsampled to 512 Hz). Alternatively, an epoch can be padded with zeros on either end to obtain a length that is a power of two (e.g. 2.0 seconds at 500 Hz is padded with 12 data zeros, 6 at the start and 6 at the end of the epoch). Some commercial software packages may handle this internally if epochs are not a power of two, and Matlab’s fft function1 can specify padding to any epoch length. The length of the epoch (T) will also have consequences for the spectral precision. The power spectrum reflects power in the signal at each frequency from direct current (DC) to the Nyquist frequency (half the sampling rate), with a spectral value every 1/T points. Thus with one-second epochs, one would have a spectral power value at integer frequencies (1,2,3,…), whereas with a two second epoch, one would have greater precision with values ever 0.5 Hz (0.5, 1.0, 1.5 …).
Because the FFT converts each time-domain epoch to a power spectrum, the average of these power spectra is ultimately taken as the basis for analysis (Panel E). Alpha power, either total (μV2 by summing all spectral points in the frequency range) or density (μV2/Hz by summing all spectral points in the frequency range and dividing by the range in Hz), is most often examined. Alpha power is typically operationalized as between 8 and 13 Hz in adults, sometimes divided into lower and upper alpha (e.g., 8.0–10.5 Hz, and 11–13 Hz), and lower frequencies have been examined in children, as such lower frequencies in the developing brain are assumed to be equivalent to adult alpha (e.g. Fox & Davidson, 1987). Alpha oscillations are believed to functionally inhibit neural activity (Mathewson et al., 2011) and play an important role in synchronizing large-scale networks (Laufs et al., 2006; Scheeringa, Petersson, Kleinschmidt, Jensen, & Bastiaansen, 2012). The relationship between alpha and cortical activity may also be moderated by activity in other frequency bands (especially theta and beta; Ambrosini & Vallesi, 2016; Laufs et al., 2006; Ota, Toyoshima, & Yamauchi, 1996). Although the idea that frequency bands interact in terms of functional significance is interesting, using cross-frequency ratios (i.e., the ratio of 8–13Hz to 15–30Hz activity) makes interpretation of results across studies less tenable. At present, there simply is not much information on the functional significance of cross-frequency interactions, or how cross-frequency ratios relate to traditional asymmetry measures.
To calculate alpha asymmetry scores, alpha power at any given site is first natural log transformed, as untransformed power values tend to be positively skewed (Allen, Coan, et al., 2004). Then, a difference score (ln[right]-ln[left] alpha power) can then summarize the relative activity at homologous right and left leads. Higher asymmetry scores calculated this way putatively reflect relatively greater left frontal activity (assuming that alpha is inversely related to cortical network activity; Allen, Coan, et al., 2004). Additionally, this log-difference score provides some degree of correction for overall alpha power (Allen, Coan, et al., 2004), such as those due to individual differences in skull thickness that influence signal amplitude (Eshel, Witman, Rosenfeld, & Abboud, 1995; Leissner, Lindholm, & Petersen, 1970; Pfefferbaum, 1990).
The asymmetry difference score indicates the relative difference in alpha power between the hemispheres, but does not indicate whether a higher asymmetry score results from less left alpha power, more right alpha power, or some combination of both. Thus, asymmetry scores reveal little in terms of absolute frontal laterality. Some investigators have entered alpha power at homologous left and right electrodes with hemisphere as a factor into an analysis of variance to address this question, but the power of this approach is hampered by large differences between subjects in alpha power unrelated to the asymmetry score: uncorrected frontal alpha power is affected by nuisance variables like overall alpha power and skull thickness. Such individual differences in overall power are especially problematic for examining correlations between criterion variables and single site alpha power, as the resultant correlations will reflect relationships primarily with overall power rather than site-specific power, as overall differences in global power are considerably larger than site-specific differences within subjects.
Researchers interested in examining alpha power at an individual site (i.e., F7) instead of the difference in hemispheres have at least two options: one option is to correct alpha power using a residualization/regression procedure, and another option is to correct for overall alpha power using a topographical normalization. A convenient method to adjust for overall power is to residualize the single site power on whole head power, and then examine correlations between the residualized site power and the criterion variable; this method was described in detail by Allen and colleagues (2004). Another approach is to first divide alpha power at a single electrode by the summed alpha power across all electrodes, correcting for overall power. Then, this relative alpha power metric is transformed to a within-subject Z-score by normalizing over all electrodes. This topographically normalized metric can improve the localization of alpha effects, mitigating the effects of nuisance variables like skull thickness and overall alpha power, and revealing which hemisphere drives an asymmetry score (i.e., correlation between topographically normalized alpha at F7 with an F8/F7 asymmetry score; also see Allen, Coan, et al.,, 2004). Either of these approaches provide single site alpha power metrics that are suitable for correlation with criterion variables, as they reduce the irrelevant variance that may disproportionately influence such correlations.
Artifact Reduction Approaches
The major sources of artifact in frontal EEG asymmetry research include ocular movements, blinks, muscle bursts, and signal discontinuities. Spectral decomposition of signals including these non-cerebral contributions may artifactually influence alpha power at the sites where the artifacts are present.
The eyes, being dipoles with a positive charge at the cornea relative to the retina, create electrical fields that, with movement or blinks, produce artifacts in the EEG that are as large or larger than the EEG signals. The majority of the power of ocular artifacts is in the delta and theta range (Gasser, Sroka, & Mocks, 1985; Hagemann & Naumann, 2001), outside the alpha band typically examined in EEG asymmetry research. Nonetheless, some ocular noise still exists in the alpha band. Because of the concern that ocular artifacts may contribute to alpha power in EEG signals, investigators often reject epochs containing blinks or other ocular artifact. Moreover, because of the concern that the electrooculographic (EOG) signals recorded using two leads adjacent to the eye may contain some EEG signal, including alpha activity (cf. Iacono & Lykken, 1981), regression-based approaches that subtract a portion of the time-domain EOG signal from the time-domain EEG signal have been avoided, as such a procedure might subtract alpha activity of neural origin. Further considerations of the contribution of ocular signals can be found in Hagemann and Naumann (2001).
In addition to EOG signals, muscle bursts and signal discontinuities produce spectral power across a broad range of frequencies. These bursts and discontinuities reduce the signal-to-noise ratio; thus epochs with these artifacts are typically rejected.
Decomposing scalp data to sources to identify artifacts.
An alternative to epoch rejection involves identifying artifacts using blind source-separation techniques to isolate artifacts and remove them from the scalp-recorded EEG. Independent component analysis (ICA) is one such technique, which decomposes EEG signals into independent components (ICs) that are identified by statistical criteria. Each IC is a time-series, and all ICs sum to create the observed EEG. ICA creates components that represent maximally independent time series. Each component time-series can be conceptualized as a source (not necessarily an intracranial source) that contributes to scalp-recorded EEG signals; each scalp EEG signal is thus a weighted composite of ICs. Changes in voltage that co-occur in time will be identified as components (i.e., have high temporal kurtosis), especially when the spatial-temporal relationships are stereotyped (i.e., similar spatial-temporal patterns that co-occur, such as occipital alpha bursting, or frontally-prominent ocular activity due to blinks). The number of ICs is determined by the rank of the data, which is in turn determined by the number of data vectors that are linearly independent of one another. In most cases, the number of independent data vectors is the number of electrodes, and in the case of an average reference, the number of electrodes minus one. One important distinction is that ICA is a so-called “blind” source separation: the user neither specifies the number of ICs, nor the orientation of IC axes.
ICA has been especially useful for separating neural and artifact signals in the EEG. After ICA demixing, artifact components can be discarded, resulting in ‘clean’ EEG (Makeig, Bell, Jung, & Sejnowski, 1996; Jung et al., 1998a, 1998b, 2000). An open-source infomax ICA approach is available with EEGlab (pop_runica function; Delorme & Makeig, 2004; Makeig, Debener, Onton, & Delorme, 2004), and is frequently used for EEG artifact correction (Chaumon, Bishop, & Busch, 2015; Delorme, Sejnowski, & Makeig, 2007; McMenamin, et al., 2010; Mognon, Jovovich, Bruzzone, & Buiatti, 2011; Jung et al., 2000; Winkler, Haufe, & Tangermann, 2011; Winkler, et al., 2014) and dimensionality reduction (Makeig et al., 2004; Onton, Westerfield, Townsend, & Makeig, 2006). ICA assumes: 1) delays in propagation between electrodes are negligible; 2) sources are stationary in terms of topography;3) the time courses of sources are independent; and 4) the number of sources is the same as the number of sensors. ICA assumptions are likely not entirely tenable, especially in the cases of 2, 3 and 4. In the case of 2, it is well known that EEG phenomena may not be spatially stationary (see Onton & Makeig, 2006 for review), especially when using longer epochs (>10s, see Jung et al., 1998b) or when calculating ICs with multiple-subject averages. For 3, eye blinks are known to vary with the P300 (i.e., blinking in response to unexpected or surprising stimuli), and bursts of alpha power may also covary with eye blinks (i.e., the closing of the eyes; Berger, 1929)); yet ICA has demonstrated that it can adequately separate these signals (Jung et al., 2000). The case of number 4 is impossible to determine, but it seems to be that in many cases, researchers have more channels than sources (there may be as few as 15 reliable sources in typical EEG recordings; Artoni, Menicucci, Delorme, Makeig, & Micera, 2014). One other consideration when working with ICA: ICA alters the time series of the data, including EEG phase. For example, removal of an eye blink IC would affect the EEG phase in the ~1–5Hz range (eye blinks are typically around 1–5Hz in the spectral domain). Researchers interested in phase-based metrics may want to consider procedures that do not alter the phase of their time-series, because IC removal could ostensibly increase or decrease the phase-based metrics. Because ICA assumptions are rarely perfectly satisfied, signals are rarely perfectly demixed. Some suggestions have been made by researchers to improve ICA performance prior to ICA calculation:
Aggressive zero-phase-shift finite impulse response (FIR) high-pass filtering (1–2Hz) of the continuous data (eegfiltnew in EEGlab; also see Cook & Miller, 1992) may improve stationarity and ICA decomposition.
Similarly, removal of slow-drifts or DC-offsets in the data by subtraction of the mean from an entire epoch improves demixing. Researchers are discouraged from using short (100–200 millisecond) baseline corrections for event-related designs (see Groppe, Makeig, & Kutas, 2009).
Removal of gross paroxysmal artifacts (artifacts that are large, sparse, and have varying spatial topography) prior to ICA demixing may improve performance; for example, body and electrode movements that have time courses with non-stationary spatial distributions are “split” by the ICA into many different ICs.
The more data points used for the ICA decomposition the better, as the quality of the ICA is a function of the number of data points and the number of electrodes (at least 20 samples per number of sensors-squared has been recommended; Onton & Makeig, 2006; Groppe et al., 2009). ICA decompositions for montages with more electrodes will benefit from using more data points for ICA (Onton & Makeig, 2006; Groppe et al., 2009).
When sources with platykurtic distributions are apparent in the data (e.g., line noise), the ‘extended’ parameter of the runica/pop_runica function in EEGlab may improve demixing (Jung, et al., 1998a), or line noise can be removed prior to ICA using a notch filter, or the cleanline plugin for EEGlab (Bigdely-Shamlo, Mullen, Kothe, Su, & Robbins, 2015; Mullen, 2012) can also be used to remove 50/60Hz noise.
It is critical that the rank of the data is accurately determined for ICA decomposition, and Matlab does not always compute rank accurately automatically. The rank of the data is reduced when using an average reference (rank is reduced by 1), or when channels are interpolated prior to ICA (rank is reduced by number of interpolated channels). In the case of reduced rank, researchers should exclude channels prior to ICA, so that the number of channels used for ICA is equal to the rank of the data (any channel(s) will do). Alternatively, researchers can use the pca argument for the pop-runica function to reduce the dimensionality of the data to the correct rank prior to ICA computation.
Dozens of blind source separation approaches are available (see Delorme, Palmer, Onton, Oostenveld, & Makeig, 2012 for a comparison of 21 source separation approaches), and it may be the case that some approaches are better for certain experimental designs (Dien, Khoe, & Mangun, 2007; Dien, 2010), or for different varieties of signals (Fitzgibbon, Powers, Pope, & Clark, 2007).
The garbage-in, garbage-out principle also applies to ICA, and it would be ill-conceived to believe that ICA is a panacea for fixing poor-quality recordings.
Altogether, ICA can demix signals adequately, but rarely perfectly. Demixing performance may improve following quick preprocessing of data as noted above, with careful consideration of ICA assumptions and data dimensionality, or from use of an alternative blind-source separation implementation.
ICA has been especially useful for mitigation of stereotyped EEG artifacts like eye blinks, eye movements, and muscle activity (artifacts with near invariant spatial distributions). For example, assume a signal is composed of power in the 8–13 Hz band overlaid on a slower wave (1–3Hz) resulting from an eye blink. The EEG in this case is a mixture of a neural signal (8–13 Hz activity) and an artifact (eye blink). Insofar as the eye blink may be captured adequately as an IC, this component can be subtracted from the EEG resulting in eye blink-free EEG (but the 8–13 Hz activity remains in the EEG). Although eye blinks and 8–13Hz activity may co-occur in some epochs, it is likely that they do not co-occur during every epoch, and their distribution across electrodes will differ; thus, they are separable via ICA (i.e., timecourses are independent). In fact, even eye blinks that are frequently coincident with the oddball P300, are also separable with ICA (Jung et al., 2000). Thus, ICA can be used to demix neural and artifact components, and researchers can isolate and remove artifact while keeping neural data that overlaps in time. In fact, artifact removal from good ICA decompositions result in EEG data free from eye blinks, eye movements, and muscle artifact with almost no reduction in neural activity (Jung et al., 2000; McMenamin et al., 2010). In cases of poor ICA demixing, however, overlapping neural and artifactual data may also be discarded.
Because ICA rarely demixes signals perfectly, ICs may include mixed artifact and neural contributions, and researchers or automatic artifact correction algorithms (AAAs) are faced with deciding whether to reject these ambiguous components. Although artifact classification (whether with surface signals or ICs) by human raters is a very common approach, classification by human raters has liabilities. Researchers typically include or exclude ICs from subsequent analyses by visual inspection or quantitative analysis of a component’s time course, topography, and spectral features (i.e., pop_selectcomps and pop_prop in EEGlab), then classify the IC as artifactual or neural. Evidence suggests that certain features of the IC are especially useful for accurate and reliable classification of an IC as artifactual or neural, especially the power spectra (Delorme, Sejknowski, & Makeig, 2007), scalp topography (Mognon, Jovicich, Bruzzone, & Buiatti, 2011; Viola et al., 2009), and intracranial source complexity (Chaumon, , Bishop, & Busch, 2015; Delorme, Palmer, Onton, Oostenveld, & Makeig, 2012; Mcmenamin et al., 2010; Winkler et al., 2011).
Utility of AAAs for Identifying ICs with artifacts.
Despite these known features, human experts rely on unknown configurations of internal regression weights to identify these features (weights that vary within and between individuals), and classifications may not be reliable between experts or over time for a given expert (see Dawes, Faust, & Meehl, 1989 for a critical discussion of human vs actuarial judgment). Human interrater reliability is variable across studies; interrater reliability is high when raters: 1) classify artifacts on an interval-level scale (McMenamin, et al., 2009); 2) only classify ocular artifacts and discontinuities (Mognon et al., 2011); 3) are experienced with identifying particular artifacts (Viola et al., 2009); and 4) undergo training in IC classification (Hatz et al., 2015). Conversely, interrater reliability is lower when ICs are classified dichotomously (neural or artifact), or are a mixture of neural and artifactual data (i.e., ambiguous ICs). Researchers may also invest substantial time and energy classifying components (100 recordings multiplied by 62 components = 6200 components to classify), and training research assistants to classify components with a high degree of reliability (Hatz et al., 2015, McMenamin et al., 2010). In contrast, classification weights are known for AAAs, AAAs are perfectly reliable, and AAAs are less time-intensive to use for the researcher.
AAAs are often “trained” on one experimental setup or electrode montage, and performance can vary when an algorithm is used in a novel context (Chaumon et al., 2015). By comparison, a new automatic artifact correction tool—Multiple Artifact Rejection Algorithm (MARA)—uses an adapted classifier, which adjusts classification parameters as a function of electrode montage (Winkler, 2014). MARA (an EEGlab plugin) uses spectral, topographic, temporal, and source features of an IC for classification as neural or artifact, features previously observed to have good predictive validity for detecting artifact (Chaumon et al., 2015; Delorme et al., 2007; McMenamin et al., 2010; Mognon et al., 2011, Viola et al., 2009; Winkler et al., 2011). With more than 100 total participants, in continuous and event-related datasets, and with electrode montages varying from 16 to 128 electrodes, MARA has shown good performance for automatic classification of artifact (85 – 91% accuracy compared to experienced raters depending on experimental setup; Winkler et al., 2011, 2014). When research assistants have been trained to a high degree of reliability, or with fewer components to classify, MARA is also capable of semi-automatic correction: potential artifacts are highlighted, scores for classification features displayed, and a human rater can make the final classification. Given the superior reliability of AAAs over human raters, however, this method of human review may not enhance the reliability or artifact removal over the strictly automated approach.
Automatic EEG artifact Detection based on the Joint Use of Spatial and Temporal features (ADJUST; Mognon et al., 2011) is an EEGlab plugin that utilizes properties of time and space to classify ocular artifact and discontinuities (features with good predictive validity for detecting artifact; Chaumon et al., 2015; Mognon et al., 2011; Viola et al., 2009). ADJUST detects eye blink and discontinuity artifact, but, ADJUST does not select for myogenic or other artifact. ADJUST evaluates 5 features of an IC, then classifies an IC as artifactual when at least one temporal and one spatial feature are above threshold. Thresholds are created using an iterative Bayesian approach called Expectation-Maximization (Bruzzone & Prieto, 2000). Importantly, ADJUST identifies ocular artifact and discontinuities with exceptional accuracy (~95% accuracy compared to human raters).
Researchers are often especially concerned with AAAs committing false positives (i.e., incorrectly classifying neural data as artifact), but false positives are rare for many AAAs in general (Chaumon et al., 2015), and for MARA and ADJUST specifically (Mognon et al., 2011; Winkler et al., 2011, 2014). When automatic correction algorithms in fact make false positives, they are often ambiguous ICs that contribute very little to the actual data (<1–3% of variance; Chaumon et al, 2015; McMenamin, et al., 2010; Mognon et al., 2011) and that are also difficult for human raters to classify. One study reported that human raters produced the best data when removing all ambiguous ICs, even when they were mostly neural activity relative to myogenic activity (McMenamin et al., 2010); yet, even after this aggressive IC removal by human raters (70% of the IC variance), myogenic artifact was still apparent in the EEG. Similarly, Winkler et al. (2014) reported good brain-computer interface performance after removal of 60% of ICs, And Mognon and colleagues (2011) reported that ocular artifact alone accounted for 51% of the variance in their dataset, andanother report found that only 15 of 71 ICs were stable within-participant (Artoni et al., 2014). It seems to be the case that neural sources may account for as little as 25% of the variance in the data2, as ocular, myogenic, and line noise artifact are often much greater in magnitude than EEG signals and contribute the most variance (McMenamin et al., 2010).
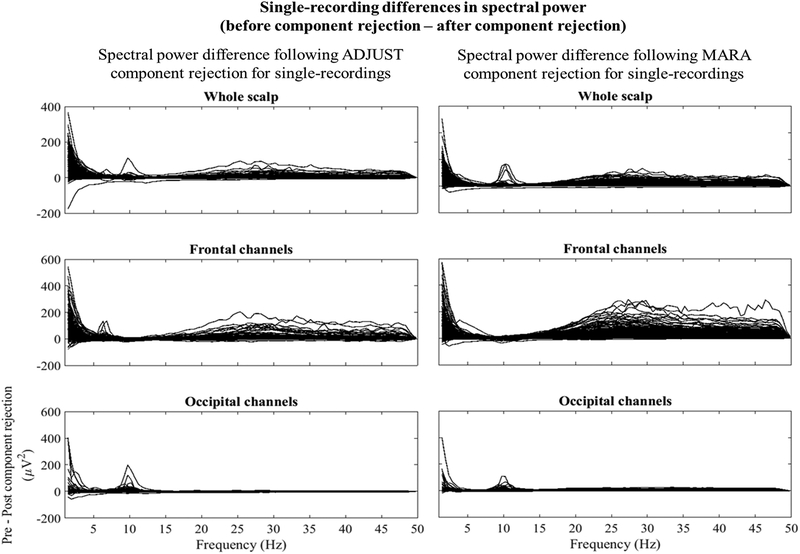
These observations suggest that researchers may want to consider the relative cost of false negatives and false positives when making artifact cleaning and analysis choices. Overall, human raters are likely imperfectly valid or reliable classifiers of artifact, whereas some AAAs (especially MARA and ADJUST) can use the most discriminative features of an IC to classify ICs as neural or artifact with high validity and perfect reliability. As detailed in Appendix A and shown in Figures 3 and 4, MARA aggressively rejected what appears to be high frequency frontalis electromyographic (EMG) activity as well as ocular activity; in contrast, ADJUST rejected ocular artifact only, and left the majority of the presumed frontalis activity in the data. In both cases, the distribution of alpha activity appears to be relatively unaltered, suggesting few false positive rejections of alpha band activity (see Figure A4).
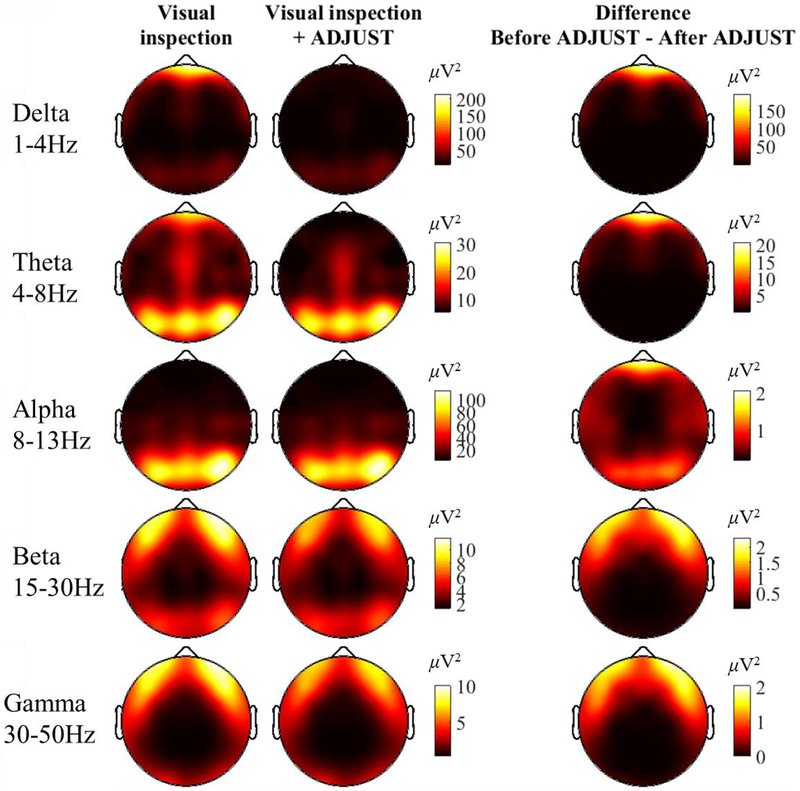
Figure 3.
Spectral power across the scalp for five frequency bands of interest before and after automatic IC-based correction using the ADJUST algorithm. The depicted scale (inμV2) varies across frequency bins, and is the same for the first two columns (Visual inspection only, Visual inspection only + ADJUST; the difference score has its own scale)). Overlapping-epochs were hamming-windowed prior to FFT to mitigate edge-artifacts. The FFT results for each epoch were averaged for each subject, then across all subjects (i.e., a grand-average). Spectral points were averaged within canonical frequency bands.
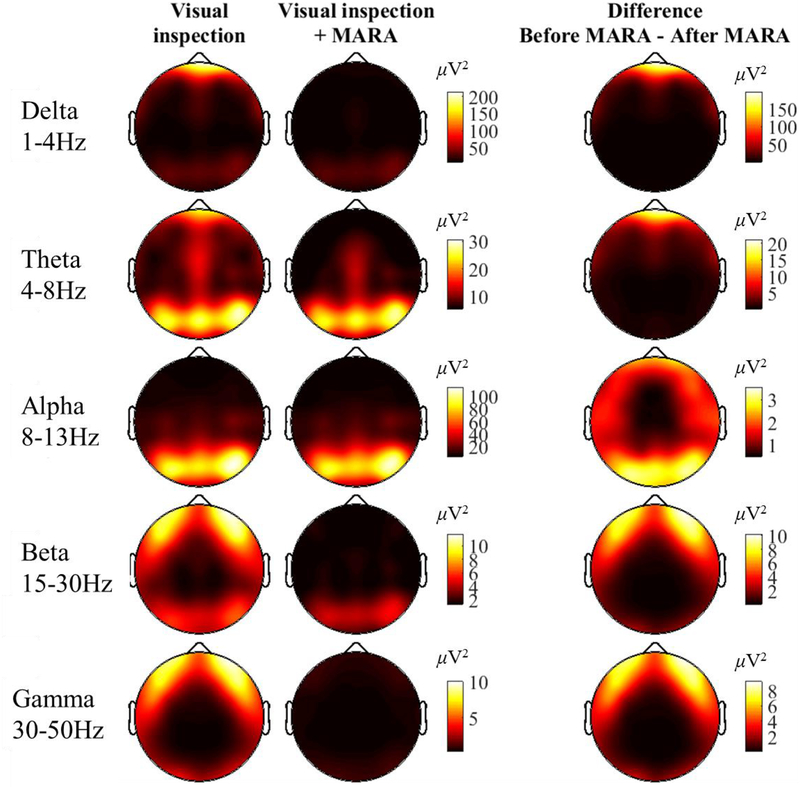
Figure 4.
Spectral power across the scalp for five frequency bands of interest before and after automatic IC-based correction using the MARA algorithm. The depicted scale (in μV2) varies across frequency bins, and is the same for the first two columns (Visual inspection only, Visual inspection only + MARA; the difference score has its own scale)). Overlapping-epochs were hamming-windowed prior to FFT to mitigate edge-artifacts. The FFT results for each epoch were averaged for each subject, then across all subjects (i.e., a grand-average). Spectral points were averaged within canonical frequency bands.
Conceptualizing Frontal Asymmetry: Statistical Approaches and Theoretical Inferences
In addition to methodological issues of recording, reference, artifact removal, and power extraction, frontal asymmetry research may benefit from careful consideration of statistical approaches. Theory should drive the development of conceptual models, with theory-driven research extending and challenging such models. Frontal asymmetry is widely relevant in models ranging from emotional processing to psychopathology and often reported as a predictor, outcome, mediator, or moderator. As such, it is important that researchers consider the role of frontal asymmetry in any given model and what theoretical clarity this role will provide. Understanding and consideration of the statistical approach at the outset of study design will help researchers advance theory by designing studies aimed at improving models of emotion, motivation, and psychopathology.
As such, statistical approaches that are most commonly used in frontal asymmetry research are outlined below, with consideration of how statistical approaches can ensure the utility of frontal asymmetry within the context of a particular model. A decade ago, significant advances were made in statistical approaches to frontal asymmetry by a review distinguishing between predictor and outcome (Cacioppo, 2004) and another distinguishing between mediator and moderator (Coan & Allen, 2004). We will suggest the continued relevance of such important distinctions and provide a brief introduction to frontal asymmetry as a predictor, outcome, mediator, and moderator.
Frontal Asymmetry as a Psychological and Neural Index
As discussed above and in a number of reviews (Allen & Reznik, 2015; Coan & Allen, 2004; Nusslock et al., 2015), frontal asymmetry research can generally be summarized by two major research approaches. The first approach examines frontal asymmetry during rest as a trait variable related to various psychological constructs (Davidson, 1994; Stewart et al., 2010; Sutton & Davidson, 1997) and predictive of future emotional behavior or psychopathology (Blackhart et al., 2006; Nusslock et al., 2011; Papousek, Reiser, Weber, Freudenthaler, & Schulter, 2012; Wheeler, Davidson, & Tomarken, 1993). The second approach studies state-related changes in frontal asymmetry as a function of current emotional state or behavior (Coan et al., 2001; Harmon-Jones, Vaughn-Scott, Mohr, Sigelman, & Harmon-Jones, 2004; Harmon-Jones & Sigelman, 2001; Killeen & Teti, 2012).
It is important to note that the vast majority of research from both approaches focuses on frontal asymmetry as an index of psychological phenomena (e.g., approach motivation, well-being, risk for psychopathology). As a neurophysiological measure, however, frontal asymmetry may index both: 1) psychological phenomena; and 2) neural activity reflecting potential mechanisms. A handful of studies have examined the generators of scalp-recorded asymmetry to date, and have implicated different regions of both the left and right frontal regions (Gable, Mechin, Hicks, & Adams, 2015; Koslov, Mendes, Pajtas, & Pizzagalli, 2011; Pizzagalli, Sherwood, Henriques, & Davidson, 2005; Saletu, Anderer, & Saletu-Zyhlarz, 2006; Shackman, McMenamin, Maxwell, Greischar, & Davidson 2009). It is important to remember that different regions within the frontal lobes with varying functions can result in similar downstream processes like self-reported affect; for example, increased reward sensitivity has been linked with more leftward intracranial activity (Pizzagalli et al., 2005), whereas increased behavioral inhibition has been linked with more rightward intracranial activity (Shackman et al., 2009), and aberration in either system could potentially result in a depression-like presentation. Further studies are needed to isolate the neural structures and mechanisms that contribute to asymmetrical frontal alpha power at the scalp and increase risk for depression (Allen & Reznik, 2015; Cacioppo, 2004; Davidson, 2004). Simultaneous EEG-functional magnetic resonance imaging (fMRI) recordings (Allen, Hewig, Hecht, Miltner, & Schnyer, 2013), magnetoencephalography (Domschke et al., 2015; Onoda et al., 2007), source estimation (Pizzagalli et al., 2005; Smith, Cavanagh, & Allen, 2013), time-frequency analyses (Allen & Cohen, 2010), and scalp-level functional connectivity metrics may all be useful for revealing neural circuitry that contributes to frontal alpha asymmetry. Ultimately, a comprehensive model should examine frontal asymmetry as an indicator of both psychological and neural phenomena.
Frontal Asymmetry as a Simple Correlate
Empirical work and reviews of the frontal asymmetry literature (Coan & Allen, 2004) often combine studies examining frontal asymmetry as a predictor or outcome variable, which may reduce theoretical clarity. Although direction cannot be assumed in the large portion of the literature that focuses on identifying the psychological correlates of frontal asymmetry (for review see Coan & Allen, 2004; Nusslock et al., 2015), an important distinction between the metric as a predictor and outcome variable can be made in directional models (Cacioppo, 2004). When frontal asymmetry is treated as a predictor variable, results indicate the probability of the outcome variable conditional on the level of frontal asymmetry—P(outcome measure | EEG asymmetry). On the other hand, when frontal asymmetry is treated as the outcome variable, the assumption is that the probability of a particular frontal asymmetry score depends on values in the predictor variable—P(EEG asymmetry | predictor variable). For example, virtually all studies relating frontal alpha asymmetry to risk for psychopathology examine frontal asymmetry as an outcome variable (asymmetry scores are measured as a function of presence or history of psychopathology; e.g., Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1990, 1991; Stewart et al., 2010), when in fact the conceptual model guiding the investigation would be one where frontal asymmetry is a predictor. Findings of frontal EEG asymmetry as a simple correlate (outcome) are not inconsistent with its role as a predictor, but such findings are not sufficient to support a theoretical model of frontal EEG asymmetry as a predictor variable. Of course to establish it as a predictor of psychopathology, resource-intensive longitudinal designs are needed such as that of Nusslock, Shackman, Harmon-Jones, Alloy, Coan, & Abramson (2011), wherein frontal EEG asymmetry predicted the development of a first episode of depression over the ensuing three years. Although findings of frontal EEG asymmetry as a simple correlate (outcome) are not inconsistent with its role as a predictor, such findings are not sufficient to support a theoretical model of frontal EEG asymmetry as a predictor variable. Thus it remains to be determined conclusively in large samples whether EEG asymmetry is a risk indicator for future depression or a residual “scar” (Lewinsohn, Steinmetz, Larson, & Franklin, 1981) of previous psychopathology. Increased consideration of directionality of models in frontal asymmetry research may provide further theoretical clarity.
Moderation analysis
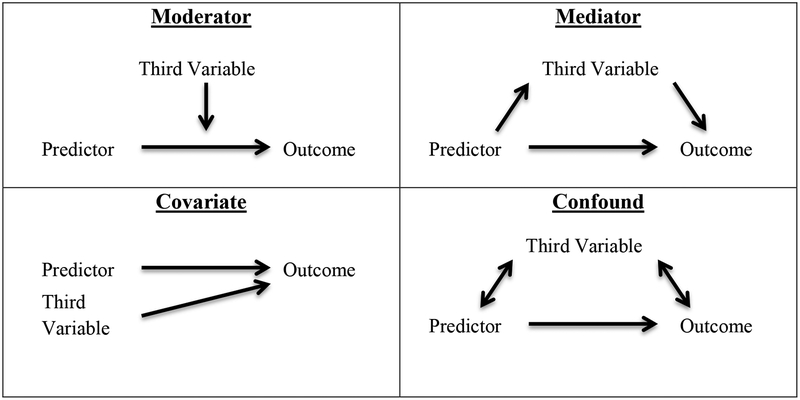
Moderators are third variables that may advance theory by explication of for whom or under what circumstances a given relationship exists. More generally, a moderator variable alters the relationship between the predictor and outcome (i.e., an interaction effect); as depicted in Figure 4, moderation suggests that the relationship between an outcome and predictor variable differs as a function of the third variable (Baron & Kenny, 1986). For example, frontal asymmetry would serve as a moderator of treatment response in the hypothetical case in which a given psychotherapeutic intervention decreases depressive symptoms only for individuals with greater relative left frontal activity, but not individuals with greater relative right frontal activity (e.g., Bruder et al., 2001, 2008). A moderator may also exist in a case where all participants respond to treatment, but those with greater relative left frontal activity respond with significantly more improvement than their counterparts with greater relative right frontal activity. As such, frontal asymmetry moderation analyses typically examine whether relationships change as a function of trait-like resting “activity” but may also examine change in frontal asymmetry. While a large amount of research has examined frontal asymmetry as a moderator of emotional processing (see Coan & Allen, 2004), only a small number of studies have examined frontal asymmetry as potential moderator of treatment response; frontal asymmetry has been implicated as a potential moderator of treatment response in cognitive-behavioral therapy (Moscovitch et al., 2011) and pharmacological treatment for depression (Arns et al., 2015; Bruder et al., 2001, 2008) but was found not to moderate treatment response for behavioral activation (Gollan et al., 2014). Although findings on EEG asymmetry as a moderator of treatment response are in the preliminary phases, there is some convergent evidence that more left than right frontal activity may interact with pharmacological and cognitive interventions in terms of predicting positive clinical outcomes.
Statistically, moderators are represented as interactions between third variables and predictor variables (Figure 4). While there must be a statistically significant interaction between the moderator and the predictor on the outcome variable, there may or may not be independent main effects of the moderator and predictor variables on the outcome variable. Moderators can be distinguished from covariates, which are third variables that reduce the variance in the relationship between the predictor and the outcome by adjusting for the effect of a third variable statistically related to the outcome (MacKinnon, Lockhart, Baraldi, & Gelfand, 2013). True covariates explain variance in the outcome but not predictor variables (Figure 4). If a third variable explains variance in both the outcome and predictor, including such a variable as a covariate may lead to ambiguous results (Miller & Chapman, 2001). For example, because time of day has been found to influence frontal asymmetry (Velo et al., 2012), it may be beneficial to adjust for time of day when examining frontal asymmetry as an outcome. However, if time of day also explains variance in one’s predictor variable (e.g. all alternative treatment groups occurred in the morning), removing variance associated with time of day affects not only frontal asymmetry but also the group variable.
Mediation Analysis
Further insight into how a process occurs, and, as such, the model of a process, may be revealed through mediators. A mediator accounts for the relationship between a predictor and outcome variable (Figure 4). Mediation indicates that the extent to which a predictor variable changes an outcome variable depends (or partially depends) on another variable through which a process of change may occur (Baron & Kenny, 1986). Importantly, a mediator may not itself be the mechanism of change but may be a larger representation of a number of variables that change (Kazdin, 2007). This distinction may be particularly important when operating under the assumption that frontal EEG asymmetry is an indicator of some neural mechanism. As such, frontal asymmetry serves as a mediator that indexes neural change. For example, Allen et al. (2001) used biofeedback training to alter frontal asymmetry scores and found that increased relative right frontal activity led to decreased positive affect; the effect of biofeedback training on affective responding was mediated via frontal asymmetry. Additionally, a number of studies have examined frontal asymmetry as an outcome of treatment that may be linked to symptom change in future research (Barnhofer et al., 2007; Fachner, Gold, & Erkkilä, 2013; Woo, Kim, Kim, Petruzzello, & Hatfield, 2009).
Although the multiple independent regression models approach to mediation outlined by Baron and Kenny (1986) is still widely used, the Sobel test may provide a more direct test of mediator effects. The Sobel test is a particular t-test that compares the effect of the mediator and the null hypothesis that the mediator has no effect. The effect of the mediator can be considered the product of: 1) the predictor to mediator path; and 2) the mediator to outcome path. Further conceptual discussion of the Sobel test and detailed procedures can be found in Preacher & Hayes (2004). Importantly, mediators share the same statistical relationships with another third variable, confounders. Yet, there is a conceptual distinction between mediators and confounders in that confounders cannot reasonably be the cause by which the predictor variable affects the outcome (MacKinnon et al., 2013). In nearly all cases, a researcher’s good judgment and theoretical knowledge should be used when identifying a variable as a mediator; in other words, it is misguided to rely on p-values alone, which can result in the identification of a confound as a mediator. This distinction highlights the importance of theory-driven research; only theory may distinguish frontal asymmetry as a confounder from frontal asymmetry as a mediator. Examining frontal asymmetry as a mediator may significantly advance theory by allowing insight into a potential mechanism by which a process occurs.
Conclusions and Best Practices
The availability of turn-key EEG systems, cheap and powerful computers for analysis, and increasing pressure from granting agencies to include biological measures in proposals has led to an increase in the number of researchers using or considering EEG as a solution for addressing questions related to motivation and psychopathology. Despite increasing availability and nearly 40 years’ worth of work, one could wish for greater consensus regarding its recording and processing parameters and theoretical conceptualization. In that spirit, we offer a few suggestions for researchers in an attempt to standardize the recording and interpretation of frontal alpha asymmetry studies:
Assessing state mood before and after EEG preparation can provide an index of whether any unintended emotional manipulations transpired.
Transforming the online reference to the surface Laplacian (i.e., CSD) can mitigate contamination from nonfrontal alpha power that may be unrelated to motivational/emotional states and traits, and can improve localization of EEG activity. This may be a good first step towards isolating intracranial sources of frontal alpha asymmetry.
Moving beyond simple resting state recordings, either by selecting specific segments within the resting state (e.g. Allen & Cohen, 2010) or by using emotional challenges to increase relevant signal related to emotion and motivational processes, may result in more reliable frontal asymmetry metrics and ideally larger effect sizes.
Stringently cleaning ocular and myogenic artifact as well as signal discontinuities with ICA-based approaches may improve signal-to-noise ratios and mitigate the effect of confounding variables like muscle tension on frontal alpha asymmetry. The use of automated artifact correction algorithms can enhance reliability of artifact correction over human raters’ decisions.
Attempting to link frontal EEG asymmetry not just to psychological constructs, but to other measures of neural function can identify potential mechanisms underlying frontal asymmetry and reveal more about the pathway from lateralized alpha power to psychological functioning (i.e., motivational states and traits).
Giving careful consideration of frontal alpha asymmetry as an outcome or predictor, and using experimental designs that are amenable to the specific conceptualization. More prospective studies need to be performed in this regard, testing frontal asymmetry as a predictor of psychopathology.
Giving careful consideration of frontal alpha asymmetry as a mediator or moderator when designing experiments and testing hypotheses.
Even after nearly 40 years of research, at least two fundamental questions remain regarding frontal alpha asymmetry: where does it come from and what does it indicate? Improvements in recording and processing techniques as well as theoretical models have accelerated developments in the field. It is in that spirit that the present review is offered, with the hopes that these recommendations will continue this progress.
Figure 5.
This figure illustrates conceptual differences between the third variables of moderator, mediator, covariate, and confound, adapting diagrams from Baron and Kenny (1986). As described in further detail in the text, a moderator is a third variable that changes the relationship between a predictor and outcome variable. Covariates do not alter but can clarify the relationship between predictor and outcome by adjusting for variance between a third variable and the outcome. On the other hand, mediators are third variables through which a predictor variable changes an outcome variable. Mediators may be distinguished from confounds, which are third variables that share the same statistical relationships but cannot reasonably be the cause by which the predictor affects change.
Acknowledgements
This project was supported in part by grants from the National Institutes of Health (R01 MH066902 and R21 MH101398). The authors wish to thank Michael Goldstein for discussions concerning IC-based artifact correction approaches.
Appendix A: A Comparison of Mara and Adjust for IC-Based Artifact Removal
Comparison of Features Weighed by Each Algorithm
MARA was created by a machine learning algorithm trained on human raters’ classification of 1290 components. An initial feature set including 13 features of the IC time course, 9 spectral features, and 16 topographic features (scalp and source topography) were evaluated as potential classifiers. These 38 features were narrowed down to 6 features that were non-redundant and the best classifiers relative to expert ratings: 1) The complexity of a minimum l2-norm solution to the inverse problem. More complex solutions are indicative of artifact; 2) The logarithm of the difference between minimal and maximal scalp activations. A larger difference is more indicative of artifact; 3) The mean absolute local skewness for a 15s window, where more skewness is indicative of artifact; 4) A steep frequency spectra, especially in terms of elevated 20–50Hz power (noted as λ by Winkler et al., 2011). Higher values are more indicative of artifact; 5) Power in the 8–13Hz band, where less power is indicative of artifact; 6) Deviation from 1/f, where lower values are indicative of artifact (i.e., the IC approximates 1/f).
MARA is the first algorithm to combine source estimation with scalp-based features for IC artifact classification. Notably, MARA was compared against datasets that were bandpass filtered (i.e., 0.1–40Hz) or had noisy channels removed prior to evaluation by MARA, and the ability of MARA to classify ICs that were predominantly line noise is not addressed by the authors. Also, MARA was evaluated on data that was reduced in dimensionality (reduced to 30 components with principal components analysis (PCA)) prior to ICA, which the authors suggest may improve performance (although PCA prior to ICA may introduce non-linearities into the data; see http://tinyurl.com/nox9fc6 for a tutorial on PCA-ICA decomposition).
ADJUST considers 5 features, classifying as artifact an IC that has at least one temporal and one spatial feature suggestive of artifact: 1) The difference between anterior and posterior electrodes, and more relative anterior activity is indicative of artifact (e.g., blinks or vertical eye movements); 2) The presence of rare and high-amplitude events (i.e., temporal kurtosis) is also suggestive of rapid and high-amplitude artifact (i.e., blinks); 3) Excessive variance within an epoch, where greater variance suggests artifact; 4) Large differences in activity between left and right frontal channels, especially activity that is in anti-phase, is also an artifact indicator (e.g., horizontal eye movements); and, 5) Large differences between activity at one channel and activity at surrounding channels indexes discontinuity.
Empirical Comparison
To compare the performance of MARA and ADJUST, resting-state recordings (2502 recordings from 323 participants; see Stewart et al., 2010 for details on sample) were used. The sample size varied slightly across analyses because some participants had missing data, and/or ADJUST’s Estimation-Maximization algorithm failed to discriminate neural versus artifact ICs. All analyses included at least 2480 recordings. Human raters visually inspected and rejected non-biological signals (e.g., amplifier clipping, discontinuities). Ocular and myogenic artifact was not rejected by human raters. Most data processing steps were implemented with native EEGlab functions or EEGlab plugins. Data were downsampled from 1000Hz to 250Hz (using pop_resample), and then bandpass filtered 1–50Hz using a custom zero-phase shift optimal FIR filter generated following the recommendations of Cook and Miller (1992) via the fir2 function in Matlab, although pop_eegfiltnew will also work. Channels marked as bad by human raters were also removed (pop_select). Segments of 2.048 sec were cut from the continuous data (pop_epoch). Segments overlapped by 75%. The mean of each epoch was subtracted from each epoch to remove DC offset (pop_rmbase). Segmented data were demixed using the pop_runica function in EEGlab using the FastICA toolbox using the symmetrical approach (available as an EEGlab plugin at: http://tinyurl.com/zxvtscl). Following ICA decomposition, the processing streams diverged. The data that was cleaned with visual inspection had bad channels interpolated, and then was transformed to the CSD montage, and processing was finished. A duplicate set of data underwent automated MARA or ADJUST IC classification implemented in SASICA (a plugin for EEGlab that contains many AAAs from different researchers; eeg_SASICA; Chaumon et al., 2015). After fully automatic IC classification by MARA or ADJUST, components classified as artifactual were subtracted from the data and converted back into channel-level data (pop_subcomp), bad channels were then interpolated (eeg_interp), and the cleaned data were CSD transformed using the approach summarized by Perrin, Pernier, Bertrand, & Echallier (1989, 1990) and implemented with the function laplacian_perrinX from Cohen (2014).
Signal-to-noise ratio was calculated as the ratio of power in a frequency bin to the average power of the surrounding ±5 Hz, but excluding ±1 Hz around the frequency bin of interest (Cohen, 2014). For example, discontinuities and noise in EEG data produce a ‘flat’ spectrum, and thus the ratio between neighboring spectral points will be low. By comparison, a robust alpha peak between 9–11Hz that is much greater than activity at 8 or 12 Hz will have a large ratio, and SNR will be large between 9–11Hz.
A mixed-linear model (MLM) was calculated in SPSS 23 to evaluate the relationship between alpha asymmetry scores and depression status (see Stewart et al., 2010 for information about sample and participant selection) using the alpha asymmetry scores derived from the different processing approaches. In brief, 143 participants met criteria for a Major Depressive Episode (MDE) at some point in their life, and 163 participants had no history of depression in their lifetime. A previous report showed that the participants with at least one MDE had less left-than-right frontal alpha activity (Stewart et al., 2010). MLM main effects were evaluated for MDE history, Electrode site, Day, and Session; additionally a MDE × Electrode interaction was also calculated. Only the main effect of MDE history is reported here.
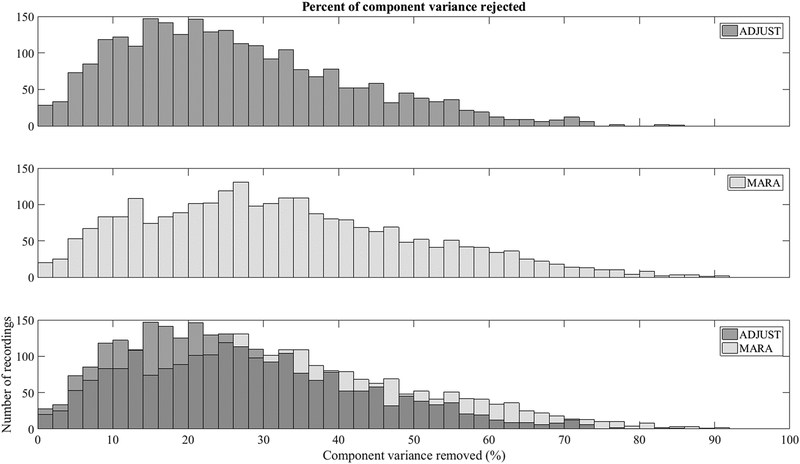
MARA rejected 32% of component variance on average (range 0–92%). ADJUST rejected 26% of component variance (range 0 – 86%). The distribution of rejected component variance is displayed in Figure A1. On one hand, the values of 32% and 26% are somewhat lower than has been reported previously in the literature, and may be due to the fact that MARA and ADJUST were computed on data that had some artifact removed prior to IC classification. On the other hand, the range of rejected data, as well as the histograms in Figures A1 and A4, suggests that in a small number of cases, either the AAAs over-corrected, or a handful of recordings were dominated by artifact and potentially unusable. Given the range of data rejected, it would probably be wise for investigators to closely inspect files that have had a substantial portion of component variance marked for removal.
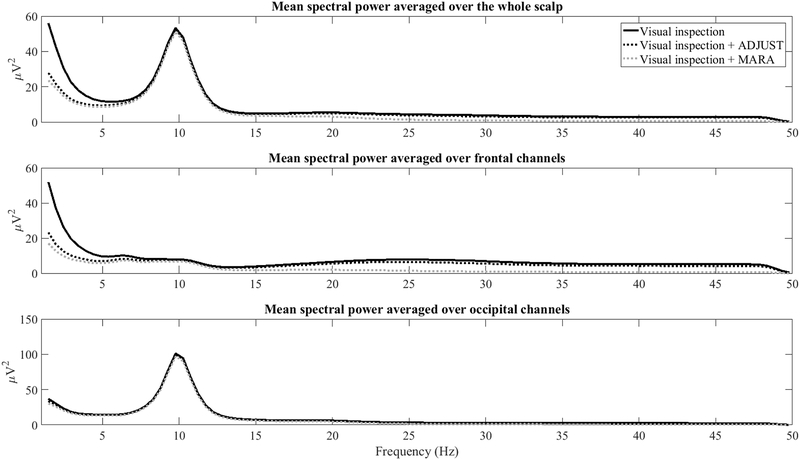
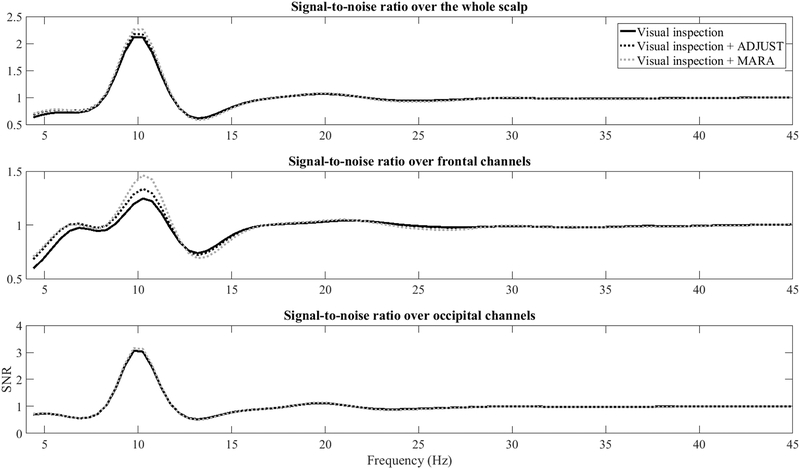
As detailed in this review and shown in Figures 3, 4, and A4, both ADJUST and MARA preserve alpha activity and its topography in most cases, but they differ in terms of how aggressively they rejected what appears to be high frequency frontalis EMG activity as well as ocular activity. The preservation of alpha activity is apparent in the frequency spectrum across the scalp (Figure A2, top panel) as well as frontal leads specifically (Figure A2, middle panel) following both ADJUST and MARA. It is also apparent that MARA attenuated high frequency power and low frequency power more aggressively than ADJUST. Signal-to-noise ratios in the alpha band over frontal channels also improved following MARA component removal, and to a lesser extent ADJUST component removal (Figure A3).
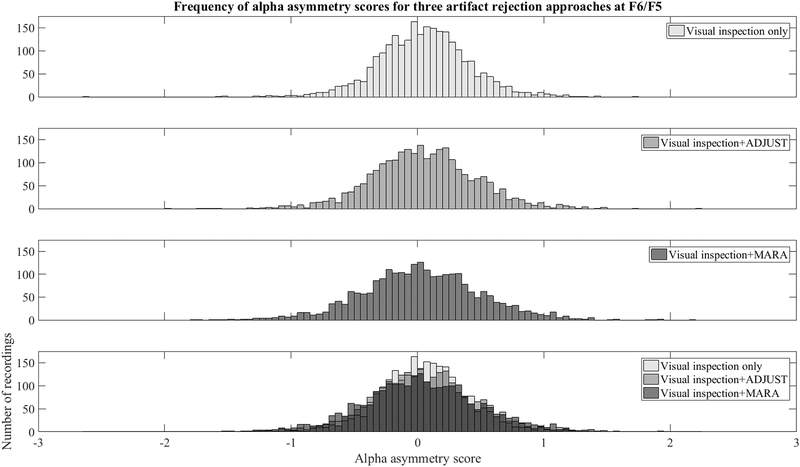
To examine the impact on asymmetry scores, the 8–13 Hz power at each left hemisphere site was log transformed and subtracted from the 8–13 Hz power at homologous right hemisphere sites (i.e., alpha asymmetry score, see Coan & Allen, 2004). The variability across participants in asymmetry scores is displayed for data before and after AAA cleaning in Figure A5. It appears to be the case that cleaning slightly improves the distribution in the asymmetry scores (the distribution is slightly less kurtotic). This may be a result of a reduction in the bilateral low- and high-frequency noise: bilateral high frequency power that overlapped the alpha band would create a pointed distribution centered on zero following subtraction of right-sided from left-sided electrodes. This is more so the case for F6/F5, which appears to be a hot spot for high-frequency activity in the beta and gamma bands in the topoplots of Figures 3 and 4..
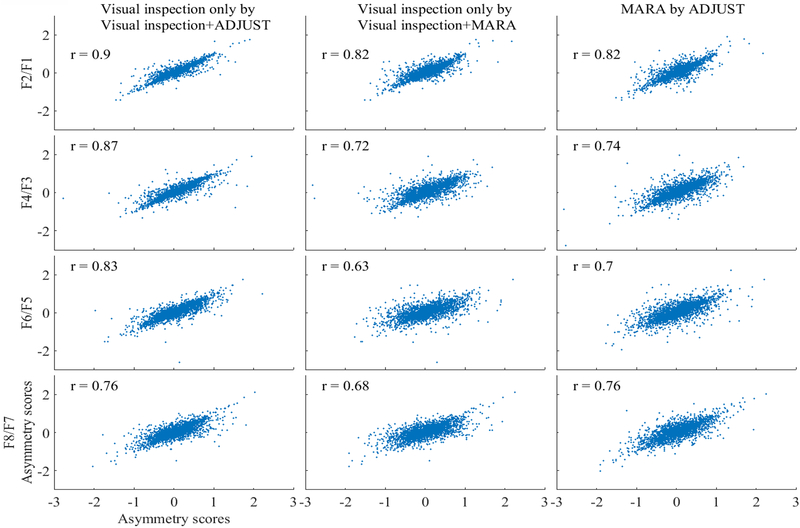
Pearson correlation coefficients between asymmetry scores for data cleaned using MARA and ADJUST as well as for asymmetry scores calculated from visually inspected data are depicted in Figure A6. The correlations are uniformly high, although descriptively smallest at F6/F5. Altogether, the results suggest that automatic correction algorithms may adequately clean data recordings in many cases. In individual cases, data may be over-or-under cleaned (i.e., the range of data removed by correction algorithms and Figure A1 and A4). Automatic cleaning resulted in distributions of asymmetry scores that more closely approximated normality, reduced high frequency activity unrelated to 8–13Hz activity, and preserved frontal and scalp-wide alpha power. In fact, the effect sizes of the relationship between alpha asymmetry and lifetime depression history were similar or improved following AAA: for visually inspected data the relationship between lifetime depression status and alpha asymmetry was (F(1,9356.29)=54.07, p<.001), for visual inspection + ADJUST component removal data the relationship was (F(1,9355.85)=53.46, p<.001), and for visual inspection + MARA component removal data the relationship was (F(1,9050.30)=79.29, p<.001). Altogether, the results suggest that MARA in fact reduced EMG noise unrelated to the relationship between frontal alpha asymmetry and depression status. In.
Figure A1.
Histograms of percent of variance rejected for ADJUST (top panel), MARA (middle panel), and the overlap between ADJUST and MARA (bottom panel).
Figure A2.
Power across all scalp sites (top panel), frontal channels only (F1, F2, F3, F4, F5, F6, F7, F8, and Fz, middle panel), occipital channels only (POz, Oz, O1, and O2, bottom panel) at each spectral point for data that was visually inspected data, visually inspected + ADJUST, and visually inspected + MARA.. All 3 conditions show a robust alpha peak, MARA and ADJUST similarly attenuate delta and theta power, and MARA attenuates high frequency power at frontal channels.
Figure A3.
Signal-to-noise ratio across all scalp sites (top panel), frontal channels only (F1, F2, F3, F4, F5, F6, F7, F8, and Fz, middle panel), occipital channels only (POz, Oz, O1, and O2, bottom panel) at each spectral point for data that was visually inspected data, visually inspected + ADJUST, and visually inspected + MARA. MARA and ADJUST increase SNR over frontal channels.
Figure A4.
Spectral power differences for single recordings (N≥2480) across all scalp sites (top panel), frontal channels only (F1, F2, F3, F4, F5, F6, F7, F8, and Fz, middle panel), occipital channels only (POz, Oz, O1, and O2, bottom panel) at each spectral point for data that was visually inspected data, visually inspected + ADJUST, and visually inspected + MARA. MARA and ADJUST produce generally consistent results across recordings. Both ADJUST and MARA adequately preserved alpha power in most, but not all cases. MARA more frequently reduced high frequency power over frontal channels than ADJUST.
Figure A5.
Histograms of asymmetry scores for different artifact mitigation approaches at a frequently used asymmetry site (F6/F5). Histograms of asymmetry scores following visual inspection (top panel), visual inspection + ADJUST (top-middle panel), and visual inspection + MARA (bottom-middle panel), and the overlap between three artifact approaches (bottom panel) show that MARA reduces the pointedness of the distribution in asymmetry scores around zero compared to visual inspection only, and visual inspection + ADJUST.
Figure A6.
Correlations for asymmetry scores between different methods of artifact mitigation at four frontal electrodes. Pearson correlations between 3 datasets are presented: visually inspected data, visually inspected + ADJUST, and visually inspected + MARA cleaned data.. Correlations between visually inspected data and visually inspected + component cleaned data were high in every case. Interestingly, the lowest correlations were between visual inspected data and MARA, and between ADJUST and MARA, at those electrodes that also tended to have the greatest high frequency power, presumably from frontalis activity (i.e., F4/F3 and F6/F5).
Footnotes
Matlab computes a symmetrical FFT. To get power from the Matlab fft function, first load an epoch into the fft function, then discard the second half of the fft output (the fft results are symmetrical), and take the absolute value of what remains (i.e., magnitude). Scale the magnitude by the number of points (magnitude / sample points). Square the scaled magnitude to get power. Multiple non-DC and non-Nyquist spectral points by 2 (because half of fft results were discarded previously; DC and Nyquist spectral points are unique and not multiplied by 2). The final result will be spectral power for the number of spectral points determined by epoch length (i.e., frequency precision).
The proportion of neural activity to artifact (~25%) may not generalize to experimental designs with fewer artifacts or greater signal-to-noise ratios; for example, sleep studies or EEG recordings in paralyzed or anesthesized patients may have a larger proportion of neural activity.
References
- Ahern GL, & Schwartz GE (1985). Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia, 23, 745–755. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67, 183–218. [DOI] [PubMed] [Google Scholar]
- Allen JJB, & Cohen MX (2010). Deconstructing the “resting” state: Exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Frontiers in Human Neuroscience, 4, 232 doi: 10.3389/fnhum.2010.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJB, Hewig JS, Hecht H, Miltner WHR, & Schnyer DM (2013). Linking resting state EEG asymmetry to resting state fMRI with simultaneous recordings. Psychophysiology, 50, S35. [Google Scholar]
- Allen JJB, McKnight KM, Moreno FA, Demaree HA, & Delgado PL (2009). Alteration of frontal EEG asymmetry during tryptophan depletion predicts future depression. Journal of Affective Disorders,115,189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJB, & Reznik SJ (2015). Frontal EEG asymmetry as a promising marker of depression vulnerability: summary and methodological considerations. Current Opinion in Psychology, 4, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Urry HL, Hitt SK, & Coan JA (2004). The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology, 41, 269–280. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, & Vallesi A (2016). Asymmetry in prefrontal resting-state EEG spectral power underlies individual differences in phasic and sustained cognitive control. Neuroimage, 124, 843–857. [DOI] [PubMed] [Google Scholar]
- Arns M, Bruder G, Hegerl U, Spooner C, Palmer D, Etkin A, … Gordon E (2015). EEG alpha asymmetry as a gender-specific predictor of outcome to acute treatment with different antidepressant medications in the randomized iSPOT-D study. PLoS One, 127, 509–519. doi: 10.1016/j.clinph.2015.05.032 [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Stewart JG, Stanton CH, Mueller EM, & Pizzagalli DA (2015). Emotion-processing biases and resting EEG activity in depressed adolescents. Depression and Anxiety, 32(9), 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, & Kenny D. a. (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Barnhofer T, Duggan D, Crane C, Hepburn S, Fennell MJV, & Williams JMG (2007). Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport, 18(7), 709–712. doi: 10.1097/WNR.0b013e3280d943cd [DOI] [PubMed] [Google Scholar]
- Bigdely-Shamlo N, Mullen T, Kothe C, Su KM, & Robbins KA (2015). The PREP pipeline: standardized preprocessing for large-scale EEG analysis. Frontiers in Neuroinformatics, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismark AW, Moreno FA, Stewart JL, Towers DN, Coan JA, Oas J, Erickson RP, & Allen JJB (2010). Polymorphisms of the 5HT1A allele are linked to frontal brain electrical asymmetry. Biological Psychology, 83, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart GC, Minnix JA, & Kline JP (2006). Can EEG asymmetry patterns predict future development of anxiety and depression? Biological Psychology, 72, 46–50. doi: 10.1016/j.biopsycho.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Boksem MA, Kostermans E, Tops M, & De Cremer D (2012). Individual differences in asymmetric resting-state frontal cortical activity modulate ERPs and performance in a global-local attention task. Journal of Psychophysiology, 26(2), 51. [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, & Quitkin FM (2001). Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biological psychiatry, 49(5), 416–425. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, & Tenke CE (2008). Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biological Psychiatry, 63, 1171–1177. doi: 10.1016/j.biopsych.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone L, & Prieto DF (2000). Automatic analysis of the difference image for unsupervised change detection. Geoscience and Remote Sensing, IEEE Transactions on, 38, 1171–1182. [Google Scholar]
- Cacioppo JT (2004). Feelings and emotions: Roles for electrophysiological markers. Biological Psychology, 67, 235–243. doi: 10.1016/j.biopsycho.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Chaumon M, Bishop DV, & Busch NA (2015). A practical guide to the selection of independent components of the electroencephalogram for artifact correction. Journal of neuroscience methods,, 250, 47–63. [DOI] [PubMed] [Google Scholar]
- Çiçek M, & Nalçaci E (2001). Interhemispheric asymmetry of EEG alpha activity at rest and during the Wisconsin Card Sorting Test: relations with performance. Biological psychology, 58(1), 75–88. [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2003). The state and trait nature of frontal EEG asymmetry in emotion In Hugdahl K, Davidson RJ (Eds.), The Asymmetrical Brain, second ed. MIT Press, Cambridge, MA, pp. 565–615. [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–49. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ, & Harmon-Jones E (2001). Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology, 38, 912–925. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, & McKnight PE (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EW, & Miller GA (1992). Digital filtering: Background and tutorial for psychophysiologists. Psychophysiology, 29, 350–362. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1998). Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology, 35, 607–614. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2004). What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology, 6, 219–234. doi: 10.1016/j.biopsycho.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1994). Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology, 6(04), 741–758. [Google Scholar]
- Davidson RJ, Schaffer CE, & Saron C (1985). Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed Subjects. Psychophysiology, 22, 353–364. [DOI] [PubMed] [Google Scholar]
- Dawes RM, Faust D, & Meehl PE (1989). Clinical versus actuarial judgment. Science, 243, 1668–1674. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Cozzuto G, Caprara GV, & Alessandri G (2013). Relations among EEG-alpha asymmetry, BIS/BAS, and dispositional optimism. Biological Psychology, 94, 198–209. [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, & Makeig S (2007). Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage, 34, 1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Palmer J, Onton J, Oostenveld R, & Makeig S (2012). Independent EEG sources are dipolar. PloS one, 7, e30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Khoe W, & Mangun GR (2007). Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Human Brain Mapping, 28, 742–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J (2010). Evaluating two-step PCA of ERP data with geomin, infomax, oblimin, promax, and varimax rotations. Psychophysiology, 47, 170–183. [DOI] [PubMed] [Google Scholar]
- Domschke K, Zwanzger P, Rehbein MA, Steinberg C, Knoke K, Dobel C, … & Pantev C (2015). Magnetoencephalographic correlates of emotional processing in major depression before and after pharmacological treatment. International Journal of Neuropsychopharmacology, 19(2), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachner J, Gold C, & Erkkilä J (2013). Music therapy modulates fronto-temporal activity in rest-EEG in depressed clients. Brain Topography, 26(2), 338–54. doi: 10.1007/s10548-012-0254-x [DOI] [PubMed] [Google Scholar]
- Fitzgibbon SP, Powers DM, Pope KJ, & Clark CR (2007). Removal of EEG noise and artifact using blind source separation. Journal of Clinical Neurophysiology, 24, 232–243. [DOI] [PubMed] [Google Scholar]
- Fox NA, & Davidson RJ (1986). Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia, 24, 417–422. [DOI] [PubMed] [Google Scholar]
- Gable PA, Mechin N, Hicks J, & Adams DL (2015). Supervisory control system and frontal asymmetry: neurophysiological traits of emotion-based impulsivity. Social cognitive and affective neuroscience, 10(10), 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan JK, Hoxha D, Chihade D, Pflieger ME, Rosebrock L, & Cacioppo J (2014). Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biological Psychology, 99, 198–208. doi: 10.1016/j.biopsycho.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw GM, Foster JJ, & Corballis PM (2014). Frontal and parietal EEG asymmetries interact to predict attentional bias to threat. Brain and cognition, 90, 76–86. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Makeig S, & Kutas M (2009). Identifying reliable independent components via split-half comparisons. NeuroImage, 45, 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D (2004). Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biological Psychology, 67, 157–182. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, & Thayer JF (2001). The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology, 38, 847–857. [PubMed] [Google Scholar]
- Hagemann D Naumann E, Thayer JF, & Bartussek D (2002). Does resting electroencephalograph asymmetry reflect a trait?: An application of latent state-trait theory. Journal of Personality and Social Psychology, 82, 619–641. [PubMed] [Google Scholar]
- Harmon-Jones E (2003). Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology, 40, 838–848. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJ (1998). Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology, 74, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, & Peterson CK (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology, 84, 451–462. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Peterson CK, & Harris CR (2009). Jealousy: novel methods and neural correlates. Emotion, 9, 113–117. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Sigelman J (2001). State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology, 80, 797–803. [PubMed] [Google Scholar]
- Harmon-Jones E, Vaughn-Scott K, Mohr S, Sigelman J, & Harmon-Jones C (2004). The effect of manipulated sympathy and anger on left and right frontal cortical activity. Emotion, 4, 1–7. [DOI] [PubMed] [Google Scholar]
- Hatz F, Hardmeier M, Bousleiman H, Rueegg S, Schindler C, & Fuhr P (2015). Reliability of fully automated versus visually controlled pre-and post-processing of resting-state EEG. Clinical Neurophysiology, 126, 268–274. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, & Miller GA (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106(3), 376. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, & Davidson RJ (1998). Baseline EEG asymmetries and performance on neuropsychological tasks. Neuropsychologia, 36(12), 1343–1353. [DOI] [PubMed] [Google Scholar]
- Jung TP, Humphries C, Lee TW, Makeig S, McKeown MJ, Iragui V, & Sejnowski TJ (1998). Extended ICA Removes Artifacts from Electroencephalographic Recordings. Advances in Neural Information Processing Systems, 10, 894–900. [Google Scholar]
- Jung TP, Humphries C, Lee TW, Makeig S, McKeown MJ, Iragui V, & Sejnowski TJ (1998, August). Removing electroencephalographic artifacts: comparison between ICA and PCA In Neural Networks for Signal Processing VIII, 1998. Proceedings of the 1998 IEEE Signal Processing Society Workshop (pp. 63–72). IEEE. [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, Mckeown MJ, Iragui V, & Sejnowski TJ (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37, 163–178. [PubMed] [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3, 1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432 [DOI] [PubMed] [Google Scholar]
- Killeen LA, & Teti DM (2012). Mothers’ frontal EEG asymmetry in response to infant emotion states and mother-infant emotional availability, emotional experience, and internalizing symptoms. Development and Psychopathology, 24, 9–21. doi: 10.1017/S0954579411000629 [DOI] [PubMed] [Google Scholar]
- Koslov K, Mendes WB, Pajtas PE, & Pizzagalli DA (2011). Asymmetry in resting intracortical activity as a buffer to social threat. Psychological Science, 22(5), 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockhart G, Baraldi AN, & Gelfand LA (2013). Evaluating Treatment Mediators and Moderators. The Oxford Handbook of Research Strategies for Clinical Psychology, (April), 1–27. doi: 10.1093/oxfordhb/9780199793549.013.0015 [DOI] [Google Scholar]
- Makeig S, Bell AJ, Jung TP, & Sejnowski TJ (1996). Independent component analysis of electroencephalographic data. Advances in Neural Information Processing Systems, 145–151. [Google Scholar]
- Makeig S, Debener S, Onton J, & Delorme A (2004). Mining event-related brain dynamics. Trends in Cognitive Sciences, 8, 204–210. [DOI] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, & Kemp AH (2008). Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion, 8, 560–572. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, & Gratton G (2011). Pulsed Out of Awareness: EEG Alpha Oscillations Represent a Pulsed-Inhibition of Ongoing Cortical Processing. Frontiers in Psychology, 2, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Greischar LL, & Davidson RJ (2009). Validation of regression-based myogenic correction techniques for scalp and source-localized EEG. Psychophysiology, 46, 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Bachhuber DR, Koppenhaver AM, Greischar LL, & Davidson RJ (2010). Validation of ICA-based myogenic artifact correction for scalp and source-localized EEG. Neuroimage, 49, 2416–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Smeets T, Giesbrecht T, Quaedflieg CW, Smulders FT, Meijer EH, & Merckelbach HL (2015). The role of frontal EEG asymmetry in post-traumatic stress disorder. Biological Psychology, 108, 62–77. [DOI] [PubMed] [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, & Kovacs M (2002). Regional patterns of brain activity in adults with a history of childhood-onset depression: gender differences and clinical variability. American Journal of Psychiatry, 159, 934–940. [DOI] [PubMed] [Google Scholar]
- Miller GM, & Chapman JP (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. doi: 10.1037//0021-843X.110.1.40 [DOI] [PubMed] [Google Scholar]
- Miskovic V, & Schmidt LA (2010). Frontal brain electrical asymmetry and cardiac vagal tone predict biased attention to social threat. International Journal of Psychophysiology, 75(3), 332–338. [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, & Buiatti M (2011). ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology, 48, 229–240. [DOI] [PubMed] [Google Scholar]
- Moscovitch D. a, Santesso DL, Miskovic V, McCabe RE, Antony MM, & Schmidt L. a. (2011). Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biological Psychology, 87, 379–85. doi: 10.1016/j.biopsycho.2011.04.009 [DOI] [PubMed] [Google Scholar]
- Mullen T (2012). NITRC: CleanLine: Tool/Resource Info. Available online at: http://www.nitrc.org/projects/cleanline (Accessed March 22, 2015).
- Nusslock R, Shackman AJ, Harmon-jones E, Alloy LB, Coan JA, & Abramson LY (2011). Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology, 120, 497–503. doi: 10.1037/a0022940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Walden K, & Harmon-Jones E (2015). Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: An RDoC perspective. International Journal of Psychophysiology, 98, 249–261. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Shishida K, Hashizume A, Ueda K, Yamashita H, & Yamawaki S (2007). Anticipation of affective images and event-related desynchronization (ERD) of alpha activity: an MEG study. Brain research, 1151, 134–141. [DOI] [PubMed] [Google Scholar]
- Onton J, Westerfield M, Townsend J, & Makeig S (2006). Imaging human EEG dynamics using independent component analysis. Neuroscience & Biobehavioral Reviews, 30, 808–822. [DOI] [PubMed] [Google Scholar]
- Ota T, Toyoshima R, & Yamauchi T (1996). Measurements by biphasic changes of the alpha band amplitude as indicators of arousal level. International journal of psychophysiology, 24(1), 25–37. [DOI] [PubMed] [Google Scholar]
- Papousek I, Reiser EM, Weber B, Freudenthaler HH, & Schulter G (2012). Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology, 49, 489–98. doi: 10.1111/j.1469-8986.2011.01324.x [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Kujawa A, Nelson SK, Cole C, & Zapp DJ (2013). The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain and cognition, 82(3), 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, & Echallier JF (1989). Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical neurophysiology, 72, 184–187. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, & Echallier JF (1990). Corrigenda. Electroencephalography and Clinical Neurophysiology, 76, 565–566. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, & Davidson RJ (2005). Frontal brain asymmetry and reward responsiveness a source-localization study. Psychological Science, 16(10), 805–813. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36, 717–731. doi: 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Quinn CR, Rennie CJ, Harris AW, & Kemp AH (2014). The impact of melancholia versus non-melancholia on resting-state, EEG alpha asymmetry: Electrophysiological evidence for depression heterogeneity. Psychiatry Research, 215, 614–617. [DOI] [PubMed] [Google Scholar]
- Quraan M. a, Protzner AB, Daskalakis ZJ, Giacobbe P, Tang CW, Kennedy SH, … McAndrews MP (2014). EEG power asymmetry and functional connectivity as a marker of treatment effectiveness in DBS surgery for depression. Neuropsychopharmacology, 39, 1270–81. doi: 10.1038/npp.2013.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SA, Duke LM, & Allen JJB (1998). Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology, 35, 389–404. [PubMed] [Google Scholar]
- Saletu B, Anderer P, & Saletu-Zyhlarz GM (2006). EEG topography and tomography (LORETA) in the classification and evaluation of the pharmacodynamics of psychotropic drugs. Clinical EEG and neuroscience,37(2), 66–80. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, & Davidson RJ (2009). Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science, 20(12), 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer CE, Davidson RJ, & Saron C (1983). Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biological Psychiatry, 18, 753–762. [PubMed] [Google Scholar]
- Schmeichel BJ, Crowell A, & Harmon-Jones E (in press). Exercising self-control increases relative left frontal cortical activation. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Cavanagh JF, & Allen JJB (2013). Where it’s at: Estimating sources of resting-state frontal EEG alpha asymmetry. Psychophysiology, 50, S36. [Google Scholar]
- Smith EE, Zambrano-Vazquez L, & Allen JJ (2016). Patterns of alpha asymmetry in those with elevated worry, trait anxiety, and obsessive-compulsive symptoms: A test of the worry and avoidance models of alpha asymmetry. Neuropsychologia, 85, 118–126. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, & Allen JJ (2010). Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology, 119, 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, & Allen JJ (2011). Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. Journal of Affective Disorders, 129, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, & Allen JJ (2014). Resting and task-elicited prefrontal EEG alpha asymmetry in depression: Support for the capability model. Psychophysiology, 51, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, & Davidson RJ (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8, 204–210. [Google Scholar]
- Thibodeau R, Jorgensen RS, & Kim S (2006). Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology, 115, 715–729. [DOI] [PubMed] [Google Scholar]
- Towers DN, & Allen JJ (2009). A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology, 46, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM (1981). Lateral brain function, emotion, and conceptualization. Psychological Bulletin, 89, 19–46. [PubMed] [Google Scholar]
- Tullett AM, Harmon-Jones E, & Inzlicht M (2012). Right frontal cortical asymmetry predicts empathic reactions: Support for a link between withdrawal motivation and empathy. Psychophysiology, 49, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Velo JR, Stewart JL, Hasler BP, Towers DN, & Allen JJ (2012). Should it matter when we record? Time of year and time of day as factors influencing frontal EEG asymmetry. Biological Psychology, 91, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola FC, Thorne J, Edmonds B, Schneider T, Eichele T, & Debener S (2009). Semi-automatic identification of independent components representing EEG artifact. Clinical Neurophysiology, 120, 868–877. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ (1993). Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology, 82–89. [DOI] [PubMed] [Google Scholar]
- Winkler I, Haufe S, & Tangermann M (2011). Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behavioral and Brain Functions, 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Brandl S, Horn F, Waldburger E, Allefeld C, & Tangermann M (2014). Robust artifactual independent component classification for BCI practitioners. Journal of Neural Engineering, 11,35013–35022. [DOI] [PubMed] [Google Scholar]
- Woo M, Kim S, Kim J, Petruzzello SJ, & Hatfield BD (2009). Examining the exercise-affect dose-response relationship: Does duration influence frontal EEG asymmetry? International Journal of Psychophysiology, 72(2), 166–172. doi: 10.1016/j.ijpsycho.2008.12.003 [DOI] [PubMed] [Google Scholar]