Abstract
BACKGROUND/OBJECTIVES
The glycemic index (GI) is a measure of the postprandial glucose response (PPGR) to food items, and glycemic load (GL) is a measure of the PPGR to the diet. For those who need to maintain a healthy diet, it is beneficial to regulate appropriate levels of blood glucose. In reality, what influences the meal GI or GL depends on the macronutrient composition and the physical chemistry reactions in vivo. Thus, we investigated whether different macronutrients in a meal significantly affect the PPGR and the validity of calculated GI and GL values for mixed meals.
SUBJECTS/METHODS
12 healthy subjects (6 male, 6 female) were recruited at a campus setting, and subjects consumed a total of 6 test meals one by one, each morning between 8:00 and 8:30 am after 12 h of fasting. PPGR was measured after each consumed meal and serial finger pricks were performed at indicated times. Test meals included 1) 68 g oral glucose, 2) 210 g rice, 3) rice plus 170 g egg white (RE), 4) rice plus 200 g bean sprouts (RS), 5) rice plus 10 g oil (RO), and 6) rice plus, egg white, bean sprouts, and oil (RESO). The incremental area under the curve (iAUC) was calculated to assess the PPGR. Mixed meal GI and GL values were calculated based on the nutrients the subjects consumed in each of the test meals.
RESULTS
The iAUC for all meals containing two macronutrients (RS, RO, or RE) were not significantly different from the rice iAUC, whereas, the RESO iAUC (2,237.5 ± 264.9) was significantly lower (P < 0.05). The RESO meal's calculated GI and GL values were different from the actual GI and GL values measured from the study subjects (P < 0.05).
CONCLUSIONS
The mixed meal containing three macronutrients (RESO) decreased the PPGR in healthy individuals, leading to significantly lower actual GI and GL values than those derived by nutrient-based calculations. Thus, consuming various macronutrient containing meals is beneficial in regulating PPGR.
Keywords: Glycemic index, glycemic load, blood glucose, carbohydrate, meals
INTRODUCTION
The glycemic index (GI), developed by Wolster [1], is a measure used to characterize the blood glucose response to various carbohydrate-containing foods. A low-GI diet is beneficial in the management of diabetes and cardiovascular disease (CVD) [2,3,4,5]. For instance, a low-GI diet improved the blood glucose response for 24 hours along with a better fat oxidation profile in men with body mass indices (BMI) between 17 and 24 kg/m2 [6]. Moreover, three weeks of a low-GI diet compared to consuming a high GI diet improved insulin sensitivity among premenopausal women [7]. A high GI diet has been shown to raise blood triglycerides and reduce high-density lipoprotein cholesterol, factors that are linked to CVD and atherosclerosis [8]. Hence, a low-GI diet can be beneficial in not only maintaining low blood glucose levels but also in losing weight [9]. A low GI benefits the glucose metabolism by not having the stress related to a high spike in postprandial glucose, resulting in a greater control over food intake and contributing to weight loss [5]. Low GI diets have been found to have a small but useful effect on medium-term glycemic control in diabetic patients [2,3,4,5]. The GI has gained international recognition as a reliable source for predicting postprandial glycemia associated with carbohydrate consumption.
In spite of the well-recognized positive effects of a low GI, Dodd et al. [10] raised a controversial perspective regarding the utility and validity of using a calculated GI for mixed meals. Possibly, the controversy arose due to the diverse composition of nutrients present in mixed meals, which may affect the functioning of the digestive system. Physiologically, carbohydrates contribute significantly to the postprandial glucose response (PPGR) [11]. Moreover, consuming carbohydrates along with other components such as proteins, fats, and fiber is reported to be effective in reducing the glucose response [1]. Fiber postpones the absorption of glucose; therefore, the glycemic response differs when consuming the same amount of glucose with or without fiber, suggesting that a mixture of nutrients may not accurately predict the glycemic response [11,12,13]. Protein is also reported to reduce the blood glucose level by augmenting the secretion of insulin in response to an increase in blood sugars in diabetic patients [14,15]. Presence of fat slows the absorption rate; therefore, a glycemic response with fat present is slower than one after the consumption of high-carbohydrate foods alone [16]. A systematic review reported that a high-fat diet mixed with carbohydrate consumption induces late postprandial hyperglycemia [17]. A clinical study of healthy individuals has shown a dose-dependent reduction in the glycemic response when the meal was consumed with almonds compared to that after consuming the same meal without almonds [18]. To investigate the effects of consuming a mixed nutrient meal on PPGR, the study which choose to include protein, fat, and fiber (separately and together) in a carbohydrate-based meals needed. Previously, in fact, several studies have used this type of meal GI investigation method and produced disparate results [1,3,19].
The GI calculation for a mixed meal reflects the quantity and possibly quality of the glucose response [20]. However, some studies have reported that when the subjects ate a mixed meal (not only carbohydrate but also protein, fat, and fiber in the meal), the GI meal calculation was inaccurate [21,22]. Perhaps, that inaccuracy is related to interactions among nutrients, which may override the carbohydrate contribution to the meal's GI. One study fed a mashed potato meal with chicken breast, salad, and rapeseed oil and reported that the calculated GI and the actual GI were different [21], with the measured meal GI lower than that obtained using the GI meal calculation method. Furthermore, another study reported a similar result suggesting that incorporation of all types of macronutrients elicits an improvement in glucose metabolism [22]. Thus, we examined the potential for conflicting results by investigating the differences between actual and estimated GI results produced by consuming a typical Asian dish.
GI values have been published for individual food items, but food is commonly consumed as a mixture of several food items. Therefore, glycemic load (GL) is used to measure the quantity of carbohydrates in a mixed meal and can be used in addition to the GI, which only reflects the quantity of the carbohydrates [23]. Moreover, most studies related to the GI have been based on Western diets rather than Asian diets in which carbohydrates, notably rice, are a staple food [24,25]. Therefore, examining both the GI and the GL within mixed meals from Asian countries, where carbohydrate content consumption is as high as 70% of the whole diet, is highly recommended [26]. Thus, these traditional components of Asian dishes provided a scientific rationale for our investigation of both GI and GL calculations within a carbohydrate-rich meal.
We hypothesized that the different components included in the mixed meal significantly influence the PPGR. Our goals were to examine whether carbohydrates alone should be the main consideration when analyzing a meal's GI and whether the meal's GI calculation can be used to estimate the actual GI of a mixed meal. We also examined the effects on blood glucose levels of nutrient quality/quantity in a mixed meal by measuring GL.
SUBJECTS AND METHODS
Subjects
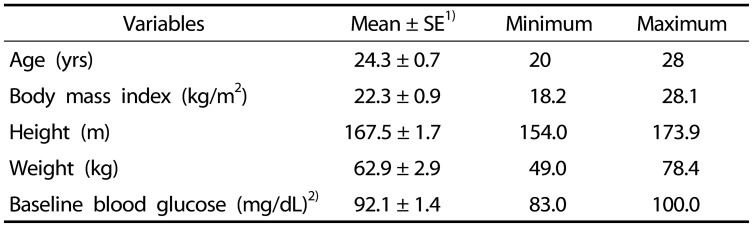
Twelve healthy subjects were recruited (6 men, 6 women). Table 1 summarizes the characteristics of the subjects. Their mean ± SD (min-max) age was 24.3 ± 0.7 years (20–28 years), BMI was 22.3 ± 0.9 kg/m2 (18.2–28.1 kg/m2), height was 167.5 ± 1.7 cm (154.0–173.9 cm), body weight was 62.9 ± 2.9 kg (49.0–78.4 kg), and baseline fasting blood glucose was 92.1 ± 1.4 mg/dL (83.0–100.0 mg/dL). Subjects' weight and height were measured via bio-impedance (Inbody770, South Korea), and blood glucose was measured by a finger prick method using Accu-chek Performa Blood Glucose Meter (Accu-chek, USA). The BMI was calculated by weight (kg)/height (m)2. The subjects answered questionnaires about medication intakes and the existence of metabolic disorders in their family members. We included participants with BMI > 25 (overweight but not controlled); however, they were required to meet our health condition criteria as stated on the enrollment questionnaire. Thus, we did not exclude overweight subjects. Subjects were recruited from on campus via flyers describing the research. The flyers were distributed over the March to April period. No participants reported allergies to rice, bean sprouts, egg white, or oil, and there were no symptoms related to glucose intolerance detected during pre-examination. All subjects consented to and were informed about the test protocol. The test procedure was approved by Kookmin University's Institutional Review Board (IRB) (KMU-201401-BR-007-01).
Table 1. Subject characteristics.
n = 12 (6 men; 6 women)
1)SE: Standard Error
2)Fasting glucose level at initial screening (fasted for 12 h)
Test meals
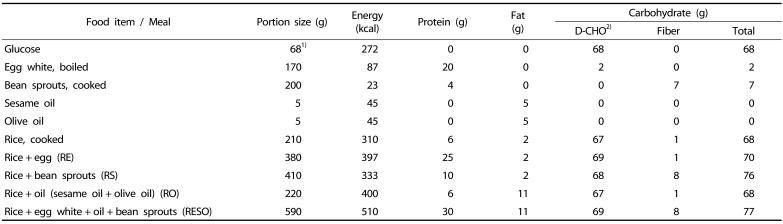
The six types of test meals were: 1) oral glucose (weight: 68 g) dissolved in warm water (200 mL), 2) cooked white rice (210 g) with soy sauce (3 g) served alone or served together with 3) 170 g egg white (RE), 4) 200 g black bean sprouts (RS), 5) 10 g oil (RO) (5 g sesame oil and 5 g olive oil), or 6) egg white, bean sprouts, and oil (RESO). For the preparation of the meals, white rice (CJ Corporation, South Korea) was microwaved for 1.5 min. The egg white was removed from cooked eggs (12–15 eggs boiled in 500 mL of cold water for 13 mins), and the black bean sprouts (Pulmuone, South Korea) were microwaved for 2 min per serving size (200 g). The sesame oil was from Ottugi (South Korea), and the olive oil brand was Stella (USA). Soy sauce was served to increase the palatability of the test meals.
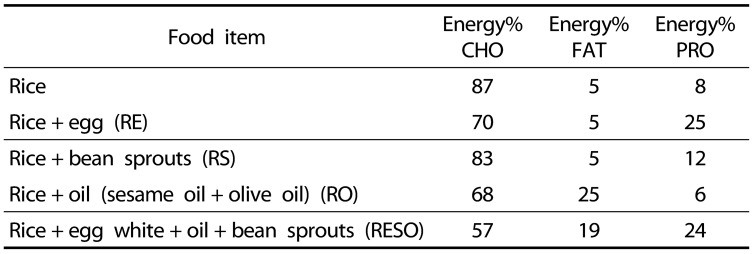
The food items were chosen based on presenting a range of macronutrients within a meal; egg presents protein, oil presents fat, and bean sprouts present fiber. Moreover, these items are frequently incorporated within Asian meals. The nutritional composition of each food item and its energy content (Table 2) were obtained from the data provided by each item's commercial product label. Each test meals' percentage of energy, in kilocalories, from each of the major macronutrients (carbohydrate, protein, fat) are shown in Table 3. This table demonstrates the different ratios of the three macronutrients in each of the test meals.
Table 2. Composition of the test meals consumed by the subjects.
1)Dissolved in 200 mL water
2)Digestible carbohydrate
Table 3. Percentages of total energy from macronutrients within the test meals*.
*Data obtained from the commercially available nutrition information on each item
Study procedure
Subjects were provided a verbal explanation of the study and directed to follow the instructions on the study description document. All subjects consented to participate in the study.
Additional participant screening included an oral glucose tolerance test (OGTT) to ascertain their fasting blood glucose level [22]. Following an overnight fasting period (12 h), OGTTs occurred at a public health and dietetic laboratory between approximately 08:00 and 08:30 h. A subject's blood glucose level was tested prior to consuming a glucose meal (68 g glucose dissolved in 200 mL of warm water) within approximately 15 min. Blood glucose levels were tested at 15, 30, 45, 60, 90, and 120 min after the consumption of oral glucose. Subjects that did not exhibit a fasting glucose level indicative of pre-diabetic or diabetic status (fasting glucose level below 110 mg/dL) were able to participate in the study [27].
Each meal testing session included one of six meals. In the morning, the subjects arrived after overnight fasting and consumed a randomly selected test meal. The subject's blood glucose level was measured via the finger pricking method as described above for the OGTT. The total energy values of the test meals ranged from 272–510 kcal and the carbohydrate content ranged from 68–77 g. Each test meal's resultant blood glucose level was used to calculate the incremental area under the curve (iAUC) [1]. The calculated meal GI (GI-C) for each meal type was obtained by multiplying the food item GI by the amount of food carbohydrates consumed and dividing by meal's total carbohydrate content of the mixed meal [28]:
The calculated meal GL (GL-C) was calculated by multiplying the GI-C by the amount of carbohydrates consumed, then dividing by 100 [29].
The meal GI-C and GL-C values were then compared with the respective measured GI and GL values obtained from the subjects [30]. The measured meal GI (GI-S) and measured meal GL (GL-S) values were calculated as [30]:
Statistical analysis
All data are expressed as mean±standard error (SE) values. The iAUC, GI, and GL, as well as the blood glucose responses at each test time, were analyzed by using the Wilcoxon signed-rank test. The Wilcoxon signed-rank test was chosen for comparisons between two measures within subjects due to the small sample sizes in this study. Every statistical analysis comparison test used the response to rice as the reference to which all the other mixed meals' blood glucose responses were compared. All statistical analysis was performed using the IBM SPSS 21 statistical program (IBM, USA). The presence of statistical significance was set at P < 0.05.
RESULTS
Glycemic response to test meals
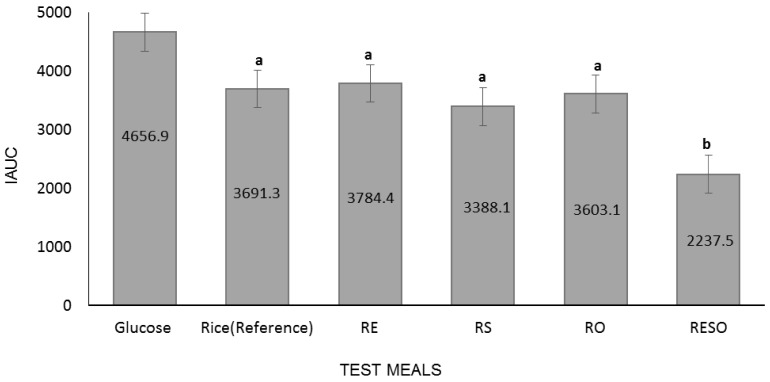
Fig. 1 shows the average iAUC values for the participant-consumed test meals. Analysis of the glycemic responses revealed that the iAUC (2,237.5 ± 264.9) of RESO was significantly lower than that of rice (3,691.3 ± 419.0). Although RS (3,388.1 ± 268.6) and RO (3,603.1 ± 473.4) iAUC values were lower than that of rice, and the RE (3,784.4 ± 247.3) iAUC was greater than that of rice, none of the differences were statistically significant.
Fig. 1. Incremental area under the curve (iAUC) for participant-consumed test meals (different letters indicate significant differences between meal iAUC values; Wilcoxon signed-rank test, P < 0.05; n = 12, 6 men and 6 women).
RE, Rice+ egg; RS, Rice+ bean sprouts; RO, Rice+ oil; RESO, Rice+ egg+ bean sprouts+ oil.
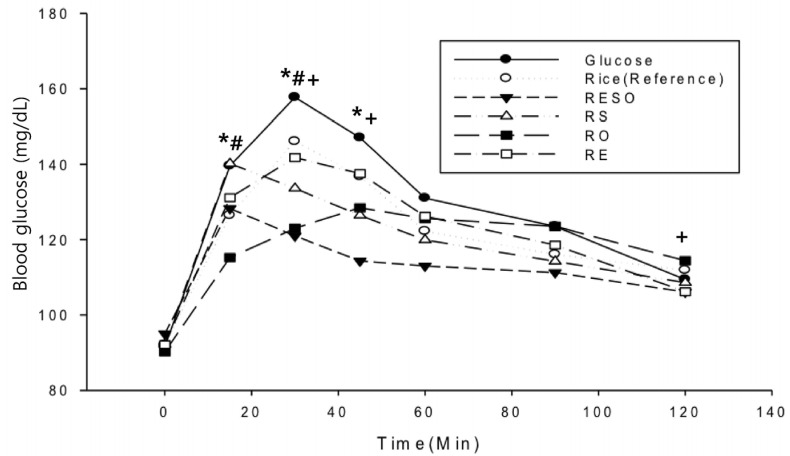
Fig. 2 presents the meal-specific PPGRs within 120 min after meal consumption. At 15 min after the meal, the blood glucose response for the RS meal (140.1 ± 4.1 mg/dL) was higher than the rice meal's PPGR (126.4 ± 5.5 mg/dL). At 15 min after the meal, the RO meal produced the lowest PPGR level (115.3 ± 4.2 mg/dL; P < 0.05). At 30 min after meal consumption, the PPGR levels for the RO (123.0 ± 5.6 mg/dL), RESO (121.0 ± 4.4 mg/dL), and RS (133.6 ± 5.1 mg/dL) meals were significantly lower than that to the rice meal (146 ± 8.0 mg/dL). Moreover, at 45 mins, the RESO (114.3 ± 2.9 mg/dL) and RS (126.4 ± 6.3 mg/dL) meals PPGR values were significantly lower than that of the rice meal (136.8 ± 7.0 mg/dL). However, at 120 min after meal consumption, only the RESO meal's PPGR level (106 ± 2.4 mg/dL) was significantly lower than that observed for rice (112 ± 2.4 mg/dL).
Fig. 2. Temporal changes in subjects' postprandial blood glucose responses to the test meals (n = 12, 6 men and 6 women; Signifiant differences from the reference value (rice) indicated by * for RS, # for RO, and + for RESO; Wilcoxon signed-rank test, P < 0.05).
RE, Rice+ egg; RS, Rice+ bean sprouts; RO, Rice+ oil; RESO, Rice+ egg+ bean sprouts+ oil.
Comparison of GI calculation and actual glycemic response for test meals
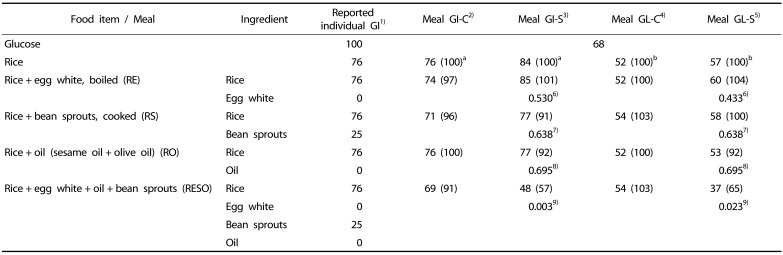
Table 4 presents each meals' GI and GL values based on either actual measurements (GI-S and GL-S) or on component calculation (GI-C and GI-C) methods. In the rice meal, the GI-S was 84 (Table 4), which is more than the GI-C value (76), previously reported GI of rice. For the RE, RS and RO meals, the GI-S values were similar to the rice GI-S value, whereas for the RESO meal, the GI-S (48) was significantly lower than the rice GI-S value (P < 0.05). This trend was similar in the meal GL-S comparison (RE, RS, RO and RESO vs rice). The RESO meal's GI-C (69) was not much different from the previously reported rice GI-C (76), whereas the RESO meal's GI-S (48) was 43% lower value of rice's GI-S (84). The measured GL-S values showed similar outcomes with the only significant difference between the rice's and RESO's GL-S values (57 vs 37, respectively) whereas GL-C values of those were not much different (52 vs 54) (Table 4).
Table 4. Comparison of calculated GI and GL and actual GI and GL of the individual test meals.
aNumbers in parentheses are percentages of the rice GI
bNumbers in parentheses are percentages of the rice GL
1)GI obtained from the University of Sydney (http://www.glycemicindex.com) and nutrition data (http://nutritiondata.self.com/topics/glycemic-index#ixzz35Q0hDq93) databases
2)Calculated meal glycemic index (Meal GI-C) =
3)Measured meal glycemic index (Meal GI-S) = Food iAUC × 100/glucose iAUC
4)Calculated Meal glycemic load (Meal GL-C) = (Food's carbohydrate (g) × Calculated meal GI)/100
5)Measured meal glycemic load (Meal GL-S) = (Food's carbohydrate (g) × Meal GI from the study)/100
6)P-value for comparison between rice and RE (Wilcoxon signed-rank test)
7)P-value for comparison between rice and RS (Wilcoxon signed-rank test)
8)P-value for comparison between rice and RO (Wilcoxon signed-rank test)
9)P-value for comparison between rice and RESO (Wilcoxon signed-rank test)
n = 12 (6 men and 6 women)
GI, glycemic index; GL, glycemic load; iAUC, incremental area under the curve.
DISCUSSION
The results show that a mixed meal, that is, one that includes additional fat, protein, and fiber, produces a lower PPGR than that produced following an unmixed meal with the same amount of available carbohydrate. Moreover, the results demonstrate that the calculated GI and GL values for a mixed meal overestimate the actual GI and GL values, a trend that was also observed in another study [10]. Our meal containing rice, egg, oil, and bean sprouts had a significantly lower glucose iAUC than that of the rice-only meal, suggesting that the presence of different macronutrients affects metabolism and the postprandial glucose level. This may be due to synergistic effects among the metabolisms of the fat, protein, and carbohydrate macronutrients, effects that are manifested in the postprandial glucose levels. Thus, the GI calculation method suggested by Wolever and Jenkins [1], which reflects only carbohydrates, does not accurately account for the other nutrients included in the meal since the other macronutrients, such as fat and protein, may significantly influence the glycemic response to a mixed meal. Our data support those in other studies that observed differences between the calculated and actual GI measurements [10,31,32,33].
In our study, the addition of protein, fat, and fiber to carbohydrates, such as in the RESO meal, produced a marked decrease in both the iAUC and the glycemic response, while the presence of only one additional macronutrient in the other mixed meals had a minimal effect on PPGR. A study examining the effects of tuna and cheese consumed with potatoes and pasta reported that the tuna protein added to potato or pasta altered the GI [33,34,35], whereas the egg protein in our RE meal did not change the GI from that in the reference, rice meal. In those studies, compared to a potato or pasta alone meal, there was a reduction in the GI when subjects consumed a potato or pasta meal with either tuna or cheese. Meals containing high levels of protein and fat may influence the body's carbohydrate metabolism by either inducing enhancement of insulin activity or slowing the rate of gastric emptying. Protein is known to enhance the secretion of insulin via the release of glucagon-like peptide-1 or neuropeptide YY (incretin); thus, increasing the efficiency of glucose disposal. Similarly, a study investigating a mixture of carbohydrates and 0–30 g of proteins in liquid showed reductions in PPGR [36]. However, the rate of absorption of a liquid passing through the gastrointestinal tract is notably different from the rate of digestion and absorption of a meal. Moreover, Our RE meal was composed of nearly 70% carbohydrate, and the lack of a significant change in GI or GL may indicate that level of added protein may have been inadequate to detect such a change. Fat digestion and absorption are comparatively slower than those of other macronutrients. Fat can delay the absorption of carbohydrates due to the deceleration of gastric emptying, in turn delaying the absorption of glucose into the bloodstream. A study investigating mixtures of mashed potato with olive oil or water reported that gastric emptying was slower and the PPGR was slower with oil than with water, as well there was an increase in GLP-1 level in the blood of type 2 diabetes patients mashed potato-olive oil mixture [37]. Our RO meal data showed the addition of oil prolonged glucose disposal, which delayed gastric emptying and possibly increasing satiation. The PPGR to the RO meal peaked at 45 min whereas in the other groups (RESO, RS, RE, rice, and glucose) the PPGR peaked at either 15 or 30 min. However, there was no significant difference in the iAUC values of the RO and rice meals, indicating that we may need to incorporate a higher level of fat in the diet in actual measure. Moreover, in the study that reported an effect from a diet that included fat [37], almost 50% of the kilocalories were from fat, whereas the level in our RO meal was only 25%. This difference in composition may alter the effect of fat on gastric emptying.
Bean sprouts contain a higher amount of insoluble fiber relative to their kilocalorie level. Based on continuous glucose monitoring for 72 h, a clinical trial in Japan reported a decrease in the PPGR after a meal in which patients consumed vegetables (salad) 15 min prior to the actual meal [38]. Moreover, patients with type 2 diabetes who ate vegetables prior to their meal demonstrated that their daily PPGR was lower than those who did not eat vegetables, suggesting that fiber regulates glucose metabolism in type 2 diabetes patients, and that effect may also occur in healthy individuals. In our study, the RS meal produced the second lowest iAUC; thus, the lowest overall spike in glucose level occurred in a meal with a slightly higher carbohydrate level than that in the reference meal, rice. However, the RS meal did not produce a significant decrease in iAUC with the glucose response higher at 15 mins than it was in the rice meal. Also, the blood glucose level of RS was significantly lower than that in rice after 30 and 45 min, suggesting that it spiked early via a lower level of glucose available in the bloodstream and better glucose disposal to tissue. This indicates the benefits of fiber consumption. The magnitude of the effects observed may also be related to the ratio of fiber ingested to the level of digestible carbohydrates. Further inquiry into the effect of fiber on PPGR is needed.
The PPGR of the RESO in our study leads to questions about how a mixture of individual macronutrient metabolisms affects PPGR. Mixing all types of macronutrients in the RESO meal elicited the greatest effects on regulating PPGR after 30, 45, and 120 mins. Clinical studies have demonstrated that when you consume a meal that contains different macronutrients such as protein and fat, individually or together, the effect is beneficial [13,28]. Our results support this notion and suggest that all of the macronutrients commonly present in a typical Asian dish setting are beneficial. Overall, our results indicate the benefit of consuming a variety of nutrients in one mixed meal. Therefore, consuming a combination of several nutrients at once is useful for managing the PPGR in healthy young adults.
A recent study indicated that only counting carbohydrates cannot be definitive in predicting the blood glucose level after mixed meals [35]. The GI calculation concept assumes that other macronutrients have a minimal influence on the PPGR despite the different effects of each nutrient in vivo. Based on our results, the GI may be most useful for single food items, such as meals that are predominantly carbohydrate. In our meals, we utilized rice as the main carbohydrate source because it is regarded as a food staple in Asia. In our mixed meals, we included boiled eggs, cooked sprouts, and sesame oil, which are also commonly used as ingredients in Asian dishes. Thus, our study fed a typical Asian mixed meal to healthy individuals to investigate the PPGR from different macronutrients.
When we compared our measured GI-S and GL-S values with the calculated GI-C and GL-C values, the GI-S and GL-S of the RESO were substantially lower than the respective GI-C and GL-C values. The GI-S values for RS, RE, and RO were similar to the GI-Cs, which were based on the reported GI of rice (76). However, the reported GI of rice was lower than the GI-S of the rice (84). This result indicates the rice used in this study might have a GI value that is similar to that reported for japonica short white rice GI [20]. Regardless, the RESO GI-S (48) and GL-S (37) values were markedly different from the GI-C (69), and GL-C (54) values. Thus, calculating a mixed meal GI from individual food GI values appears to be inappropriate when assessing the PPGR in vivo. A previous study has also pointed out the inaccuracy of GI calculations for a mixed meal [34]. Thus, the calculated GI of a typical Asian mixed meal may need adjustment when predicting the actual PPGR.
In this study, we examined mixed meals with different nutrient components to examine their influence on the glycemic response in healthy individuals. Despite the small number of participants, eating a mixed meal diet that includes all three macronutrients (protein, fat, carbohydrate) is beneficial for reducing the PPGR spike in healthy individuals. However, when eating only protein with carbohydrates, only fat with carbohydrates, or only fiber with carbohydrates, there were no significant differences in the PPGR; thus, there may be a need to have all three macronutrients present in a meal to induce a beneficial post-prandial glucose effect. Overall, the results should encourage a person to change their diet in order to improve their glucose metabolism.
Additionally, both the American Dietetic Association and Korean Dietetic Association recommend eating “a rainbow” (a diversity) of nutrients to meet their daily nutritional recommendation [39]. The data from the present study can also be utilized during nutritional education to help explain the need to eat various kinds of food and nutrients. The results show that consumption of multiple nutrient types elicits a lower glycemic response than consuming meals with only one or two macronutrients. Thus, the types of nutrients in a meal should be considered as potential factors influencing the PPGR.
However, there is a need to examine further the accuracy of the GI calculation method on different target populations and with different mixed meals. We served 68 g of carbohydrates to match the standard OGTT per person. Moreover, the rice portion size was established to portray a typical one meal size for the Korean population. However, this study did not consider the typical one serving portions of sprouts, eggs, and oils. Also, the servings were not proportioned to the various body sizes of the subjects. Future studies need to incorporate these factors, and such limitations should be considered when interpreting the results presented herein. Despite observing a reduction in the glucose response after consumption of a mixed meal, we only included one mixed meal in our study, hence, more meal types and larger sample sizes are needed to verify our results.
Our results indicate a need to incorporate an increased variety of food items within a mixed meal, however, the study's sample size was small. To increase the statistical power of the study further testing with an increased sample size is needed. However, a study design involving experiments within subjects and utilizing individuals as controls would reduce the possible differences that can occur between subjects. In addition, we could expand this type of study to different demographic groups, such as diabetes patients, to accurately measure how overestimations of the GI calculation can occur within different groups of individuals.
In conclusion, despite the similar amounts of carbohydrates present with or without fat, protein, and fiber in a meal, the GI and GL measurements were lowest within subjects consuming a mixed meal, and the consumption of the mixed meal resulted in the lowest PPGR. This outcome indicates the presence of synergistic effects among the three macronutrients on the PPGR in vivo. The meal GI calculation mainly reflects the carbohydrate content, and, based on our results, this appears to be insufficient for accurately predicting the PPGR to a mixed meal. However, further studies with different diet types to test whether the calculation of the GI of a mixed meal is sufficient when carbohydrates, fat, protein, and fiber are present are warranted. Overall, we conclude that a diet that includes a tolerable amount of carbohydrates, protein, fat, and fiber in a mixed meal is beneficial for maintaining an appropriate PPGR.
ACKNOWLEGMENTS
The authors thank Pulmuone's cooperation and financial support, as well as the kind cooperation of the participants of this study.
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–172. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 2.McMillan-Price J, Petocz P, Atkinson F, O'neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166:1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- 3.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 5.Wolever TM, Jenkins DJ, Vuksan V, Jenkins AL, Wong GS, Josse RG. Beneficial effect of low-glycemic index diet in overweight NIDDM subjects. Diabetes Care. 1992;15:562–564. doi: 10.2337/diacare.15.4.562. [DOI] [PubMed] [Google Scholar]
- 6.Camps SG, Kaur B, Quek RY, Henry CJ. Does the ingestion of a 24 hour low glycaemic index Asian mixed meal diet improve glycaemic response and promote fat oxidation? A controlled, randomized cross-over study. Nutr J. 2017;16:43. doi: 10.1186/s12937-017-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost G, Leeds A, Trew G, Margara R, Dornhorst A. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic diet. Metabolism. 1998;47:1245–1251. doi: 10.1016/s0026-0495(98)90331-6. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Carey VJ, Anderson CA, Miller ER, 3rd, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, White K, Yee K, Appel LJ. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA. 2014;312:2531–2541. doi: 10.1001/jama.2014.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs JD, Bell D, Hall R, Parry-Strong A, Docherty PD, Clarke K, Chase JG. Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with type 2 diabetes. J Am Coll Nutr. 2013;32:11–17. doi: 10.1080/07315724.2013.767630. [DOI] [PubMed] [Google Scholar]
- 10.Dodd H, Williams S, Brown R, Venn B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am J Clin Nutr. 2011;94:992–996. doi: 10.3945/ajcn.111.012138. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Wolever TM, Jenkins AL, Lee R, Wong GS, Josse R. Glycemic response to wheat products: reduced response to pasta but no effect of fiber. Diabetes Care. 1983;6:155–159. doi: 10.2337/diacare.6.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Hamberg O, Rumessen JJ, Gudmand-Høyer E. Blood glucose response to pea fiber: comparisons with sugar beet fiber and wheat bran. Am J Clin Nutr. 1989;50:324–328. doi: 10.1093/ajcn/50.2.324. [DOI] [PubMed] [Google Scholar]
- 14.Fajans SS, Floyd JC, Jr, Knopf RF, Conn FW. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- 15.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham KM, Read NW. The effect of incorporating fat into different components of a meal on gastric emptying and postprandial blood glucose and insulin responses. Br J Nutr. 1989;61:285–290. doi: 10.1079/bjn19890116. [DOI] [PubMed] [Google Scholar]
- 17.Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38:1008–1015. doi: 10.2337/dc15-0100. [DOI] [PubMed] [Google Scholar]
- 18.Josse AR, Kendall CW, Augustin LS, Ellis PR, Jenkins DJ. Almonds and postprandial glycemia--a dose-response study. Metabolism. 2007;56:400–404. doi: 10.1016/j.metabol.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Miller JB, Pang E, Bramall L. Rice: a high or low glycemic index food? Am J Clin Nutr. 1992;56:1034–1036. doi: 10.1093/ajcn/56.6.1034. [DOI] [PubMed] [Google Scholar]
- 20.Wolever TM. Is glycaemic index (GI) a valid measure of carbohydrate quality? Eur J Clin Nutr. 2013;67:522–531. doi: 10.1038/ejcn.2013.27. [DOI] [PubMed] [Google Scholar]
- 21.Hätönen KA, Virtamo J, Eriksson JG, Sinkko HK, Sundvall JE, Valsta LM. Protein and fat modify the glycaemic and insulinaemic responses to a mashed potato-based meal. Br J Nutr. 2011;106:248–253. doi: 10.1017/S0007114511000080. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Ranawana DV, Leow MK, Henry CJ. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur J Nutr. 2014;53:1719–1726. doi: 10.1007/s00394-014-0678-z. [DOI] [PubMed] [Google Scholar]
- 23.Monro JA, Shaw M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr. 2008;87:237S–243S. doi: 10.1093/ajcn/87.1.237S. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Mizukuchi A, Kise M, Aoto H, Yamamoto S, Yoshihara R, Yokoyama J. Postprandial blood glucose and insulin responses to pre-germinated brown rice in healthy subjects. J Med Invest. 2005;52:159–164. doi: 10.2152/jmi.52.159. [DOI] [PubMed] [Google Scholar]
- 25.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54:846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 26.Kim EK, Lee JS, Hong H, Yu CH. Association between glycemic index, glycemic load, dietary carbohydrates and diabetes from Korean National Health and Nutrition Examination Survey 2005. Korean J Nutr. 2009;42:622–630. [Google Scholar]
- 27.World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva: World Health Organization; 2006. pp. 1–50. [Google Scholar]
- 28.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 29.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama M, Tang AC, Wakaki Y, Koyama W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr. 2003;57:743–752. doi: 10.1038/sj.ejcn.1601606. [DOI] [PubMed] [Google Scholar]
- 31.Collier GR, Wolever TM, Wong GS, Josse RG. Prediction of glycemic response to mixed meals in noninsulin-dependent diabetic subjects. Am J Clin Nutr. 1986;44:349–352. doi: 10.1093/ajcn/44.3.349. [DOI] [PubMed] [Google Scholar]
- 32.Bornet FR, Costagliola D, Rizkalla SW, Blayo A, Fontvieille AM, Haardt MJ, Letanoux M, Tchobroutsky G, Slama G. Insulinemic and glycemic indexes of six starch-rich foods taken alone and in a mixed meal by type 2 diabetics. Am J Clin Nutr. 1987;45:588–595. doi: 10.1093/ajcn/45.3.588. [DOI] [PubMed] [Google Scholar]
- 33.Gulliford MC, Bicknell EJ, Scarpello JH. Differential effect of protein and fat ingestion on blood glucose responses to high- and low-glycemic-index carbohydrates in noninsulin-dependent diabetic subjects. Am J Clin Nutr. 1989;50:773–777. doi: 10.1093/ajcn/50.4.773. [DOI] [PubMed] [Google Scholar]
- 34.Henry CJ, Lightowler HJ, Kendall FL, Storey M. The impact of the addition of toppings/fillings on the glycaemic response to commonly consumed carbohydrate foods. Eur J Clin Nutr. 2006;60:763–769. doi: 10.1038/sj.ejcn.1602380. [DOI] [PubMed] [Google Scholar]
- 35.Wolever TM, Bhaskaran K. Use of glycemic index to estimate mixed-meal glycemic response. Am J Clin Nutr. 2012;95:256–257. doi: 10.3945/ajcn.111.026880. [DOI] [PubMed] [Google Scholar]
- 36.Moghaddam E, Vogt JA, Wolever TM. The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr. 2006;136:2506–2511. doi: 10.1093/jn/136.10.2506. [DOI] [PubMed] [Google Scholar]
- 37.Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, Rayner CK, Horowitz M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab. 2006;91:2062–2067. doi: 10.1210/jc.2005-2644. [DOI] [PubMed] [Google Scholar]
- 38.Imai S, Fukui M, Kajiyama S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J Clin Biochem Nutr. 2014;54:7–11. doi: 10.3164/jcbn.13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Academy of Nutrition and Dietetic. How to Explain Basic Nutrition Concepts [Internet] Chicago, IL: Academy of Nutrition and Dietetic; 2018. [cited 2018 May 11]. Available from: https://www.eatrightpro.org/practice/practice-resources/international-nutrition-pilot-project/how-to-explain-basic-nutrition-concepts. [Google Scholar]