Abstract
Objectives
Lefamulin is a semi-synthetic intravenous (iv) and oral pleuromutilin antibiotic active against community-acquired bacterial pneumonia (CABP) pathogens. Pharmacokinetic/pharmacodynamic (PK/PD) target attainment analyses were carried out to evaluate lefamulin 150 mg iv q12h and 600 mg orally q12h under fed and fasted conditions for the treatment of patients with CABP.
Methods
The analyses undertaken used a population PK model based on Phase 1 PK data, non-clinical PK/PD targets for efficacy and in vitro surveillance data for Streptococcus pneumoniae (SP) and Staphylococcus aureus (SA), and Monte Carlo simulation. Percentage probabilities of PK/PD target attainment by MIC on day 1 were determined using median total-drug epithelial lining fluid (ELF) and free-drug plasma AUC:MIC ratio targets associated with 1 and 2 log10 cfu reductions from baseline.
Results
Percentage probabilities of attaining the total-drug ELF AUC:MIC ratio target for a 1 log10 cfu reduction from baseline for SP were ≥99.2% at the MIC90 of 0.12 mg/L and 96.7%, 82.1% and 96.3% for iv and oral dosing regimens under fed and fasted conditions, respectively, at the MIC99 of 0.25 mg/L. Percentage probabilities of attaining the free-drug plasma AUC:MIC target for the same endpoint at the SP MIC99 were 100% for each regimen. For the SA MIC90 of 0.12 mg/L and AUC:MIC ratio targets for the same endpoint, percentage probabilities were 92.7%–100% for iv and oral dosing regimens.
Conclusions
These data provide support for lefamulin 150 mg iv q12h and 600 mg orally q12h for the treatment of patients with CABP and suggest that doses may not need to be taken under fasted conditions.
Introduction
Community-acquired bacterial pneumonia (CABP) is a leading cause of infection-related hospitalization and mortality, and is associated with increased burden in certain populations, including the elderly.1–4 Among those with CABP, Streptococcus pneumoniae is the most common causative pathogen.1,2 Given the current prevalence of S. pneumoniae resistance to β-lactams, macrolides and older-generation tetracyclines,5 the treatment of CABP can be challenging. In the USA, the estimated numbers of hospitalizations and deaths per year that have been attributed to antibiotic-resistant S. pneumoniae are 19000 and 7000, respectively.6 Given these data, the availability of additional treatment choices for patients with CABP, especially oral therapy, is needed.
Lefamulin (BC-3781) is an antimicrobial agent from the pleuromutilin class7 that demonstrates in vitro microbiological activity against a wide range of bacterial pathogens, including common pathogens causing CABP and acute bacterial skin and skin structure infections. These pathogens include S. pneumoniae and Staphylococcus aureus, including MRSA.8–12 Two Phase 3 clinical studies, LEAP1 and LEAP2, one evaluating intravenous (iv) to oral lefamulin and the other evaluating oral lefamulin for the treatment of CABP, were recently completed. For each study, lefamulin met the FDA primary endpoint of non-inferiority compared with moxifloxacin for early clinical response assessed 72–120 h following initiation of therapy in the intent-to-treat (ITT) patient population. Lefamulin also met the EMA primary endpoint for non-inferiority (10.0% margin) compared with moxifloxacin based on an investigator assessment of clinical response 5–10 days following the completion of study drug dosing in the modified ITT and clinically evaluable at test of cure patient populations.13,14
The current paradigm for the development of antimicrobial agents involves the use of non-clinical infection models that characterize pharmacokinetic/pharmacodynamic (PK/PD) relationships for efficacy. Studies using such models enable the identification of the PK/PD index associated with efficacy and calculation of the magnitudes of this index required to achieve various levels of reduction in bacterial burden (i.e. PK/PD targets).15 The application of these PK/PD targets for efficacy, together with a population PK model developed using Phase 1 and/or 2 data, in vitro surveillance data and Monte Carlo simulation, enables the evaluation of dosing regimens for future Phase 3 studies.16–18 The evaluation of non-clinical PK/PD targets for efficacy with early clinical PK data is now advocated in contemporary regulatory guidance documents, such as the infectious disease-specific FDA guidance documents for developing drugs and the EMA guidance for developing antibacterial agents.19–21
In the analyses described herein, non-clinical PK/PD targets for efficacy for S. pneumoniae and S. aureus (see article in this Supplement entitled ‘Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae’22), a population PK model developed based on Phase 1 PK data (see article in this Supplement entitled, ‘Prediction of lefamulin epithelial lining fluid penetration after intravenous and oral administration using Phase 1 data and population pharmacokinetics methods’23), in vitro surveillance data12 and Monte Carlo simulation were used to assess the PK/PD target attainment of lefamulin 150 mg iv q12h and 600 mg orally q12h for the treatment of patients with CABP.
Methods
Simulated patient population
Monte Carlo simulation was performed using a previously described population PK model for lefamulin developed using Phase 1 data (see article in this Supplement entitled ‘Prediction of lefamulin epithelial lining fluid penetration after intravenous and oral administration using Phase 1 data and population pharmacokinetics methods’23) and NONMEM® version 7.1.224 (ICON Development Solutions, Ellicott City, MD, USA). The population PK model describing the disposition of lefamulin was a three-compartment model with non-linear protein binding and two parallel first-order absorption processes. The only significant covariate relationships identified for the plasma PK were the effect of food on the rate and extent of absorption after oral administration. First-order rate constants were used to describe lefamulin transfer into and out of the epithelial lining fluid (ELF) compartment from plasma. As described below, given the food effect findings, data from simulated patients who received oral lefamulin under fed and fasted conditions were evaluated.
Using the mean parameter vector and the variance–covariance matrix from the above-described population PK model, PK parameter estimates were simulated for 2000 patients. These population PK parameter estimates were used to generate total-drug ELF and free-drug plasma concentration–time profiles from 0–24 h on day 1 for each simulated patient following lefamulin 150 mg iv q12h and 600 mg orally q12h under fed and fasted conditions. Day 1 total-drug ELF and free-drug plasma AUC0–24 values were calculated using the linear trapezoidal rule. These exposures were then divided by MIC values ranging from 0.015 to 16 mg/L to calculate the ratio of the AUC0–24 to the MIC (AUC:MIC ratio), the PK/PD index of primary interest for lefamulin (see article in this Supplement entitled ‘In vivo pharmacodynamics of lefamulin, the first systemic pleuromutilin for human use, in a neutropenic murine thigh infection model’25).
Non-clinical PK/PD targets for efficacy
Median total-drug ELF and free-drug plasma AUC:MIC ratio targets for S. pneumoniae and S. aureus efficacy based on data from a neutropenic murine lung infection model, as described earlier in this Supplement,22 are summarized in Table 1. The bacterial reduction endpoints of interest for the AUC:MIC ratio targets for S. pneumoniae and S. aureus were 1 and 2 log10 cfu reductions from baseline. Greater emphasis was given to PK/PD target attainment results based on the former, the basis for which was guided by the results of a recent assessment that evaluated the relationship between the probability of PK/PD target attainment using this endpoint and the probability of obtaining regulatory approval for an antibacterial dosing regimen for patients with pneumonia. Results of this analysis, which were based on data for 19 development programmes for antibacterial agents for pneumonia, demonstrated that, as the probability of PK/PD target attainment for a given dosing regimen in the context of that agent’s MIC distribution for the relevant pathogen increased, so too did the probability of regulatory approval for the same dosing regimen.26
Table 1.
Summary of AUC:MIC ratio targets for S. pneumoniae and S. aureus efficacy
| Pathogen | Bacterial reduction endpoint (log10 cfu reduction from baseline) | Median AUC:MIC ratio targeta |
|
|---|---|---|---|
| total-drug ELF | free-drug plasma | ||
| S. pneumoniae | 1 | 14.0 | 1.37 |
| 2 | 22.0 | 2.15 | |
| S. aureus | 1 | 21.7 | 2.13 |
| 2 | 63.9 | 6.24 | |
Median AUC:MIC ratio targets were determined based on the evaluation of data for five S. pneumoniae and five S. aureus isolates using a neutropenic murine lung infection model described in this Supplement (see ‘Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae’22).
In vitro surveillance data
The MIC distributions for lefamulin against S. pneumoniae and S. aureus were determined for isolates collected between 2015 and 2016.12 A total of 3923 S. pneumoniae isolates were collected from 122 medical centres worldwide (60 in the USA, 38 in Europe and the Mediterranean region, 15 in the Asia-Pacific region, 9 in Latin America). For S. aureus, a total of 2919 isolates were collected from 124 medical centres worldwide (62 in the USA, 38 in Europe and the Mediterranean region, 14 in the Asia-Pacific region, 10 in Latin America). MIC values for S. pneumoniae isolates ranged from ≤0.008 to 1 mg/L; MIC50 and MIC90 values were 0.06 and 0.12 mg/L, respectively. MIC values for S. aureus isolates ranged from 0.03 to >16 mg/L; MIC50 and MIC90 values for all isolates and the MRSA subset (n = 1981) were 0.06 and 0.12 mg/L.
Evaluation of PK/PD target attainment
Percentage probabilities of PK/PD target attainment by MIC value and weighted over the above-described MIC distributions based on total-drug ELF and free-drug plasma exposures for lefamulin were determined for each of the AUC:MIC ratio targets described in Table 1.
Using total-drug ELF and free-drug plasma AUC:MIC ratio targets for S. pneumoniae and S. aureus and MIC distributions for each pathogen, overall percentage probabilities of PK/PD target attainment were determined by multiplying the percentage probability of PK/PD target attainment for the AUC:MIC ratio target at a given MIC value by the probability of occurrence of that MIC value. The sum of these percentages (i.e. the overall PK/PD target attainment) was then determined.
Evaluation of non-clinical PK/PD relationships for efficacy relative to simulated total-drug ELF AUC:MIC ratios
Using parameter estimates from Hill models constructed using data from murine lung-infection models for S. aureus and S. pneumoniae (see article in this Supplement entitled, ‘Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae’22), fitted functions for the relationship between change in log10 cfu from baseline at 24 h and lefamulin total-drug ELF AUC:MIC ratio were generated for each pathogen. Total-drug ELF AUC:MIC ratios for simulated patients were generated by taking the day 1 total-drug ELF AUC values for each simulated patient after administration of iv and oral lefamulin dosing regimens and dividing this AUC value by an MIC value that was randomly assigned from the observed distribution for the global collection of isolates for each pathogen. To interpret the non-clinical PK/PD relationships for efficacy relative to total-drug ELF AUC:MIC ratios for simulated patients, box-and-whisker plots of total-drug ELF AUC:MIC ratios were then overlaid on the above-described fitted functions.
Results
Summary of simulated exposures
Table 2 provides the summary statistics for day 1 AUC0–24 values among simulated patients after administration of lefamulin iv and oral dosing regimens.
Table 2.
Summary statistics for day 1 AUC0–24 values among simulated patients after administration of lefamulin iv and oral dosing regimens
| Lefamulin dosing regimen (mg) | Route of administration | Food status | Exposure matrix | Mean (%CV) | Median (range) |
|---|---|---|---|---|---|
| 150 | iv | fasted | total-drug plasma | 13.2 (13.5) | 13.1 (8.76–19.5) |
| free-drug plasma | 1.67 (13.9) | 1.65 (1.06–2.45) | |||
| total-drug ELF | 9.46 (47.6) | 8.58 (2.06–33.4) | |||
| 600 | oral | fasted | total-drug plasma | 13.4 (19.6) | 13.3 (6.49–23.6) |
| free-drug plasma | 1.59 (22.2) | 1.56 (0.69–3.04) | |||
| total-drug ELF | 8.92 (49.6) | 8.00 (1.20–40.9) | |||
| fed | total-drug plasma | 10.1 (23.1) | 9.94 (4.13–18.3) | ||
| free-drug plasma | 1.12 (25.5) | 1.10 (0.42–2.17) | |||
| total-drug ELF | 6.07 (51.4) | 5.38 (0.788–28.0) |
Evaluation of PK/PD target attainment
Figure 1 shows the percentage probabilities of PK/PD target attainment by MIC on day 1 for lefamulin dosing regimens based on the evaluation of the total-drug ELF and free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline, overlaid on the MIC distribution for S. pneumoniae. A tabular summary of percentage probabilities of PK/PD target attainment by MIC for total-drug ELF and free-drug plasma AUC:MIC ratio targets associated with 1 and 2 log10 cfu reductions from baseline for S. pneumoniae is provided in Table S1 (available as Supplementary data at JAC Online).
Figure 1.
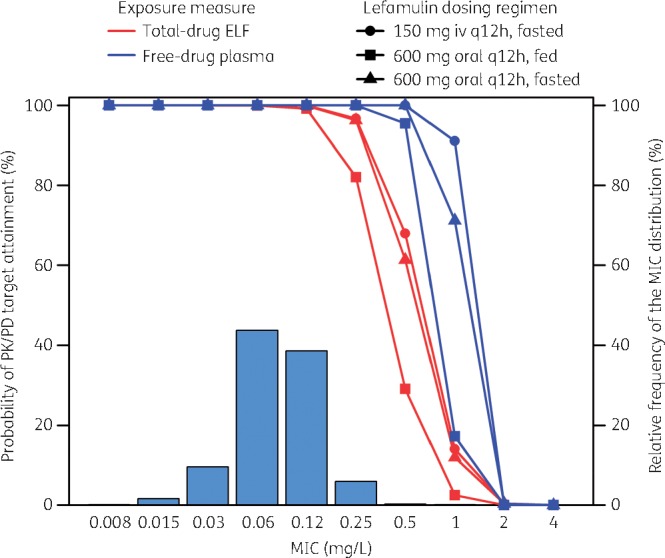
Percentage probabilities of PK/PD target attainment by MIC for lefamulin iv and oral dosing regimens based on the evaluation of the total-drug ELF and free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. pneumoniae, overlaid on the MIC distribution for S. pneumoniae.
The percentage probability of attaining the total-drug ELF AUC:MIC ratio target associated with a 1 log10 cfu reduction from baseline for S. pneumoniae at the MIC99 value of 0.25 mg/L was 96.7% for the iv dosing regimen. Percentage probabilities of PK/PD target attainment at the same MIC value were 82.1% and 96.3% for the fed and fasted oral dosing regimens, respectively. At the MIC90 value of 0.12 mg/L for S. pneumoniae, percentage probabilities of PK/PD target attainment ranged from 99.2% to 100% for the iv and oral dosing regimens. For the free-drug plasma AUC:MIC ratio target associated with the same endpoint and an MIC value of 0.25 mg/L, percentage probabilities were 100% for each dosing regimen.
The percentage probability of attaining the total-drug ELF AUC:MIC ratio target associated with a 2 log10 cfu reduction from baseline for S. pneumoniae was 99.2% at an MIC90 value of 0.12 mg/L for the iv dosing regimen. Percentage probabilities were 92.3% and 99.2% for the fed and fasted oral dosing regimens, respectively, at the same MIC value. For the free-drug plasma AUC:MIC ratio target associated with the same endpoint and an MIC value of 0.12 mg/L, percentage probabilities were 100% for each dosing regimen.
As shown in Table 3, overall percentage probabilities of PK/PD target attainment based on total-drug ELF or free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. pneumoniae were ≥98.3% for the iv and oral (fed and fasted) dosing regimens.
Table 3.
Overall percentage probabilities of PK/PD target attainment for lefamulin dosing regimens based on the evaluation of total-drug ELF or free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. pneumoniae and S. aureus
| Pathogen | Exposure matrix | Overall percentage probabilities of PK/PD target attainmenta |
||
|---|---|---|---|---|
| iv | oral |
|||
| fed | fasted | |||
| S. pneumoniae | total-drug ELF | 99.6 | 98.3 | 99.5 |
| free-drug plasma | 100 | 99.9 | 100 | |
| S. aureus | total-drug ELF | 99.5 | 98.5 | 99.4 |
| free-drug plasma | 99.7 | 99.7 | 99.7 | |
Based on the MIC distributions for S. pneumoniae or S. aureus isolates collected as part of the 2015–16 SENTRY Antimicrobial Surveillance Program from global regions.
Figure 2 shows the percentage probabilities of PK/PD target attainment by MIC for lefamulin dosing regimens based on the evaluation of the total-drug ELF and free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. aureus, overlaid on the MIC distribution for S. aureus. A tabular summary of the percentage probabilities of PK/PD target attainment by MIC for total-drug ELF and free-drug plasma AUC:MIC targets associated with 1 and 2 log10 cfu reductions from baseline for S. aureus is provided in Table S2.
Figure 2.
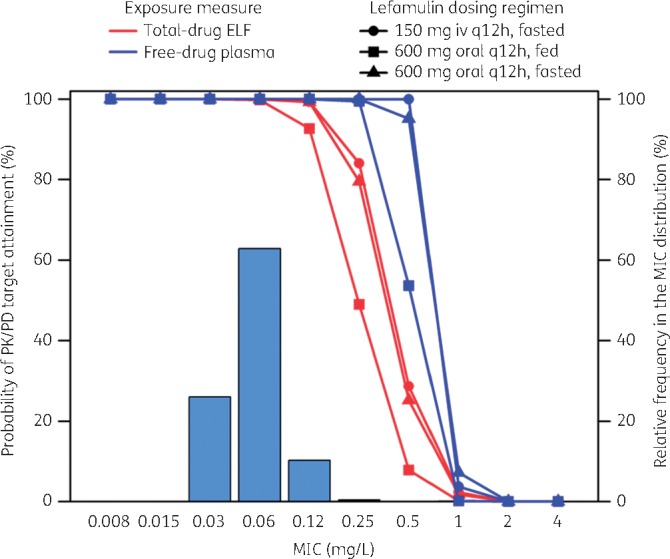
Percentage probabilities of PK/PD target attainment by MIC for lefamulin iv and oral dosing regimens based on the evaluation of the total-drug ELF and free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. aureus, overlaid on the MIC distribution for S. aureus.
The percentage probability of attaining the total-drug ELF AUC:MIC ratio target associated with a 1 log10 cfu reduction from baseline for S. aureus was 99.3% at the MIC90 value of 0.12 mg/L for the iv dosing regimen. Percentage probabilities of PK/PD target attainment at the same MIC value were 92.7% and 99.3% for the fed and fasted oral dosing regimens, respectively. For the free-drug plasma AUC:MIC ratio target associated with the same endpoint and an MIC99 value of 0.25 mg/L, percentage probabilities were ≥99.5% for each dosing regimen.
The percentage probability of attaining the total-drug ELF AUC:MIC ratio target associated with a 2 log10 cfu reduction from baseline for S. aureus was 100% at the MIC value of 0.03 mg/L for the iv dosing regimen. Percentage probabilities of PK/PD target attainment were 98.4% and 99.9% for the fed and fasted oral dosing regimens, respectively, at the same MIC value. At the MIC value of 0.06 mg/L, percentage probabilities exceeded 90% for the iv and oral fasted dosing regimens but not for the oral fed dosing regimen (76.0%). For the free-drug plasma AUC:MIC ratio target associated with the same endpoint and MIC value of 0.12 mg/L, percentage probabilities were ≥91.9% for each dosing regimen.
As shown in Table 3, overall percentage probabilities of PK/PD target attainment based on total-drug ELF or free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. aureus were ≥98.5% for the iv and oral (fed and fasted) dosing regimens.
Figures 3 and 4 show the fitted functions for the relationship between change in log10 cfu from baseline at 24 h and lefamulin total-drug ELF AUC:MIC ratio, based on Hill-type models fit to data from the neutropenic murine lung infection models for S. pneumoniae and S. aureus, respectively. As reported previously, the coefficient of determination for each relationship was 0.65 and 0.69, respectively. Horizontal box-and-whisker plots of total-drug ELF AUC:MIC ratios based on day 1 total-drug ELF AUC values for simulated patients after the iv and oral lefamulin dosing regimens and MIC values from the global collection of isolates for each pathogen are shown overlaid on the fitted functions.
Figure 3.
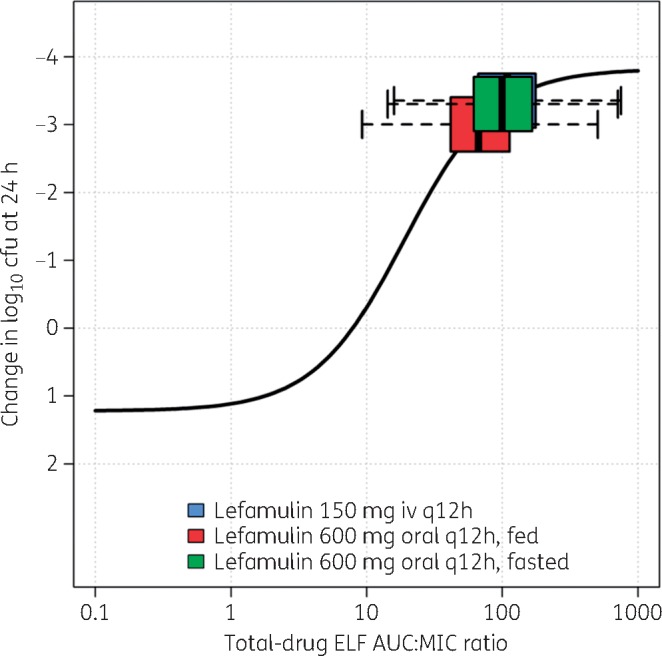
Non-clinical PK/PD relationship for efficacy for S. pneumoniae, overlaid with box-and-whisker plots of total-drug ELF AUC:MIC ratios for simulated patients after administration of lefamulin iv and oral dosing regimens. Horizontal box-and-whisker plots of total-drug ELF AUC:MIC ratios for simulated patients after iv and oral (under fed and fasted conditions) dosing regimens are shown overlaid on the PK/PD relationship based on data from a neutropenic murine-lung infection model for S. pneumoniae. For each boxplot, the edge of the box represents the 25th and 75th percentiles of the distribution for total-drug ELF AUC:MIC ratio. The line within the box represents the median total-drug ELF AUC:MIC ratio. The whiskers extend to the nearest value among those represented by 1.5 × IQR of the box edges, where IQR is the distribution of total-drug ELF AUC:MIC ratio from the 25th to the 75th percentiles. Note: the box-and-whisker plot for the distribution of total-drug ELF AUC:MIC ratios for lefamulin 150 mg iv q12h shown in blue is obscured by that for lefamulin 600 mg orally q12h administered under fasted conditions, shown in green.
Figure 4.
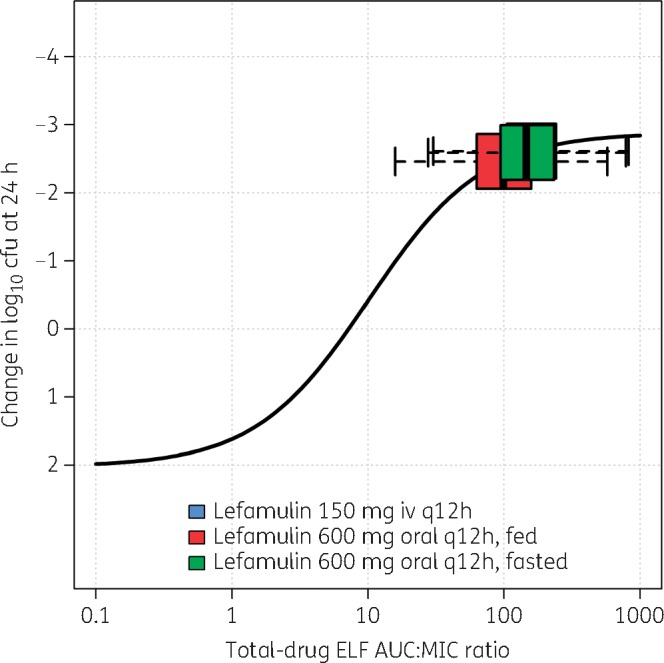
Non-clinical PK/PD relationship for efficacy for S. aureus, overlaid with box-and-whisker plots of total-drug ELF AUC:MIC ratios for simulated patients after administration of lefamulin iv and oral dosing regimens. Horizontal box-and-whisker plots of total-drug ELF AUC:MIC ratios for simulated patients after iv and oral (under fed and fasted conditions) dosing regimens are shown overlaid on PK/PD relationship based on data from a neutropenic murine-lung infection model for S. aureus. For each boxplot, the edges of the box represent the 25th and 75th percentiles of the distribution for total-drug ELF AUC:MIC ratio. The line within the box represents the median total-drug ELF AUC:MIC ratio. The whiskers extend to the nearest value among those represented by 1.5×IQR of the box edges, where IQR is defined by the distribution of total-drug ELF AUC:MIC ratio from the 25th to the 75th percentiles. Note: the box-and-whisker plot for the distribution of total-drug ELF AUC:MIC ratios for lefamulin 150 mg iv q12h shown in blue is obscured by that for lefamulin 600 mg orally q12h administered under fasted conditions, shown in green.
Discussion
The objectives of these analyses were to use non-clinical PK/PD targets for efficacy for S. pneumoniae and S. aureus (see article in this Supplement entitled ‘Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae’22) and a previously developed population PK model (see article in this Supplement entitled ‘Prediction of lefamulin epithelial lining fluid penetration after intravenous and oral administration using Phase 1 data and population pharmacokinetics methods’23), in vitro surveillance data12 and Monte Carlo simulation to assess the PK/PD target attainment of lefamulin 150 mg iv q12h and 600 mg orally q12h for the treatment of patients with CABP.
The population PK model used for these analyses was developed using a data set that contained 959 plasma concentrations from 20 subjects enrolled in a Phase 1 food-effect study27 and 144 plasma concentrations and 12 ELF concentrations from 12 subjects enrolled in the Phase 1 tissue penetration study.28 A three-compartment model with non-linear protein binding and two parallel first-order absorption processes, which was consistent with the results of previous population PK analyses of Phase 1 and 2 data,29,30 provided precise and unbiased estimates of lefamulin plasma concentration–time profiles. Covariate analyses demonstrated that the absorption rate was slower and bioavailability was decreased after a high-fat/high-calorie meal compared with the fasted condition. When applied to the data from the tissue penetration study, the model provided an unbiased fit to the plasma data after iv administration. The ELF data from these 12 subjects were well described using first-order rate constants into and out of the ELF compartment.
AUC:MIC ratio targets for both S. pneumoniae and S. aureus were identified using PK/PD relationships for efficacy based on data from a neutropenic murine lung infection model, a model that is appropriate for translations to patients with CABP. AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. pneumoniae and S. aureus represented the model endpoint of focus. This choice is supported by the results of a recent assessment of the relationship between the probability of PK/PD target attainment for antibacterial dosing regimens in patients with pneumonia, including those with CABP, and the probability of obtaining regulatory approval for that same dosing regimen. As described earlier, these data demonstrated that, as the probability of attaining the non-clinical PK/PD target associated with a 1 log10 cfu reduction from baseline increased for a given dosing regimen, so too did the probability of gaining regulatory approval for that same dosing regimen.26
The use of a neutropenic murine lung infection model and the collection of ELF PK from mice (see article in this Supplement entitled ‘Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae’22) allowed the estimation of AUC:MIC ratio targets based on drug exposures at the effect site. Given the above-described availability of ELF data from healthy subjects and the ability to estimate ELF exposures in simulated patients, the PK/PD target attainment of lefamulin iv and oral dosing regimens was evaluated based on total-drug ELF exposures and total-drug ELF AUC:MIC ratio targets for efficacy. Although the results of PK/PD target attainment analyses based on both total-drug ELF and free-drug plasma AUC:MIC ratio targets were examined, emphasis was placed on the results for the former. As described previously,18,31,32 the lack of consideration of effect site exposures can lead to poorly determined dosing regimens and even failure of the drug development programme. As described herein, the results of the analyses for lefamulin based on both exposure measures were similar.
Percentage probabilities of attaining the total-drug ELF AUC:MIC ratio target associated with a 1 log10 cfu reduction from baseline for S. pneumoniae were ≥99.2% at the MIC90 value of 0.12 mg/L and 96.7%, 82.1% and 96.3% for the iv dosing regimen and oral dosing regimens under fed and fasted conditions, respectively, at the MIC99 of 0.25 mg/L. For the free-drug plasma AUC:MIC ratio target associated with the same endpoint and an MIC value of 0.5 mg/L, percentage probabilities were ≥95.5% for each dosing regimen. Percentage probabilities of PK/PD target attainment for total-drug ELF and free-drug plasma AUC:MIC ratio targets associated with a 1 log10 cfu reduction from baseline for S. aureus were also high at the MIC90 value of 0.12 mg/L, ranging from 92.7% to 100% for the iv and oral dosing regimens. Overall percentage probabilities of PK/PD target attainment based on total-drug ELF or free-drug plasma AUC:MIC ratio targets associated with 1 and 2 log10 cfu reductions from baseline and MIC distributions for each pathogen collected from regions worldwide were ≥98.3%.
Finally, evaluation of fitted functions for the non-clinical PK/PD relationships based on Hill-type models fit to data from the neutropenic murine lung infection models for S. pneumoniae and S. aureus relative to distributions of total-drug ELF AUC:MIC ratios for simulated patients after administration of lefamulin iv and oral dosing regimens also provided dose selection support. The distribution of simulated total-drug ELF AUC:MIC ratios relative to where maximal bacterial kill is observed on the Hill function is consistent with that observed for other antibacterial agents for which clinical trials demonstrated high rates of efficacy.33
In conclusion, results of these analyses provide support for the selection of lefamulin 150 mg iv q12h and 600 mg orally q12h for the treatment of patients with CABP and suggest that doses may not need to be taken under fasted conditions. Future efforts to collect PK data for lefamulin from patients with CABP will enable refinement of the population PK model and confirmation that the lefamulin exposures generated for the simulated patient population receiving the iv and oral dosing regimens, as described herein, reflect those of the target patient population with CABP.
Funding
This work was supported by Nabriva Therapeutics. Copyediting support for this article was funded by Nabriva Therapeutics.
Transparency declarations
S. P. G. and W. W. W. are employees of and hold stock in Nabriva Therapeutics plc. S. M. B., J. P. H., C. M. R., J. C. B. and P. G. A. are employees of the Institute for Clinical Pharmacodynamics. L. Z. was an employee of the Institute for Clinical Pharmacodynamics at the time the analyses described herein were performed. H. S. S. is an employee of JMI Laboratories. The Institute for Clinical Pharmacodynamics was contracted by Nabriva Therapeutics to perform the analyses described herein. Copyediting support for this article was provided by Lycely del C. Sepulveda-Torres, PhD, and Michael S. McNamara, MS, at C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and funded by Nabriva Therapeutics.
This article forms part of a Supplement sponsored by Nabriva Therapeutics.
Supplementary Material
References
- 1. File TM, Marrie TJ.. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010; 122: 130–41. [DOI] [PubMed] [Google Scholar]
- 2. Welte T, Torres A, Nathwani D.. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67: 71–9. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
- 4. Violi F, Cangemi R, Falcone M. et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin Infect Dis 2017; 64: 1486–93. [DOI] [PubMed] [Google Scholar]
- 5. Pfaller MA, Farrell DJ, Sader HS. et al. AWARE Ceftaroline Surveillance Program (2008-2010): trends in resistance patterns among Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States. Clin Infect Dis 2012; 55: S187–93. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013 https://www.cdc.gov/drugresistance/threat-report-2013/index.html.
- 7. Paukner S, Riedl R.. Pleuromutilins: potent drugs for resistant bugs-mode of action and resistance. Cold Spring Harb Perspect Med 2017; 7: a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sader HS, Paukner S, Ivezic-Schoenfeld Z. et al. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J Antimicrob Chemother 2012; 67: 1170–5. [DOI] [PubMed] [Google Scholar]
- 9. Sader HS, Biedenbach DJ, Paukner S. et al. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2012; 56: 1619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paukner S, Sader HS, Ivezic-Schoenfeld Z. et al. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY Antimicrobial Surveillance Program in 2010. Antimicrob Agents Chemother 2013; 57: 4489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paukner S, Streit JM, Flamm RK. et al. In vitro activity of lefamulin against bacterial pathogens commonly causing acute bacterial skin and skin structure infections (ABSSSI) and bloodstream infections (BSI): global SENTRY surveillance 2016. In: ASM Microbe, Atlanta, GA, USA,2018. Abstract 6852.
- 12. Paukner S, Gelone SP, Arends SJR. et al. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015–2016). Antimicrob Agents Chemother 2019; doi:10.1128/AAC.02161-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexander E, Goldberg L, Das A. et al. Oral lefamulin is safe and effective in the treatment of adults with community-acquired bacterial pneumonia (CABP): results of Lefamulin Evaluation Against Pneumonia (LEAP 2) study. In: IDWeek, San Francisco, CA, USA,2018. Abstract 74297.
- 14. File TM Jr, Goldberg L, Das A. et al. Efficacy and safety of iv-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 trial. Clin Infect Dis 2019; doi:10.1093/cid/ciz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craig WA. Pharmacodynamics of antimicrobials: general concepts and applications In: Nightingale CH, Ambrose AG, Drusano GL, eds. Antimicrobial Pharmacodynamics in Theory and Clinical Practice. New York, NY, USA: Marcel Dekker, 2007; 1–19. [Google Scholar]
- 16. Drusano GL, Preston SL, Hardalo C. et al. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother 2001; 45: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhavnani SM, Hammel JP, Cirincione BB. et al. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob Agents Chemother 2005; 49: 3944–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trang M, Dudley MN, Bhavnani SM.. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr Opin Pharmacol 2017; 36: 107–13. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Guideline on the Use of Pharmacokinetics and Pharmacodynamics in the Development of Antimicrobial Medicinal Products http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500210982.pdf.
- 20.US Food and Drug Administration. Guidance for Industry. Community-Acquired Bacterial Pneumonia: Developing Drugs for Treatment US Department of Health and Human Services. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM123686.pdf.
- 21.US Food and Drug Administration. Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment US Department of Health and Human Services. https://www.fda.gov/downloads/Drugs/…/Guidances/ucm071185.pdf.
- 22. Wicha WW, Strickmann DB, Paulkner S.. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother 2019; 74 Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Wicha WW, Bhavnani SM. et al. Prediction of lefamulin epithelial lining fluid penetration after intravenous and oral administration using Phase 1 data and population pharmacokinetics methods. J Antimicrob Chemother 2019; 74 Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bauer RJ. NONMEM 7, Version 7.1.2. Ellicott City, MD, USA: ICON Development Solutions, 2010. [Google Scholar]
- 25. Wicha WW, Craig WA, Andes D.. In vivo pharmacodynamics of lefamulin, the first systemic pleuromutilin for human use, in a neutropenic murine thigh infection model. J Antimicrob Chemother 2019; 74 Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bulik C, Bhavnani S, Hammel J. et al. Relationship between regulatory approval and pharmacokinetic-pharmacodynamic target attainment: focus on community- and hospital-acquired pneumonia. In: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, USA,2013. Abstract A-295.
- 27. Wicha WW, Lell C, Seltzer E. et al. Pharmacokinetics and safety of an oral, immediate-release (IR) tablet formulation of lefamulin in fed and fasted healthy subjects. In: ECCMID, Vienna, Austria,2017. Abstract P1336.
- 28. Zeitlinger M, Schwameis R, Burian A. et al. Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid. J Antimicrob Chemother 2016; 71: 1022–6. [DOI] [PubMed] [Google Scholar]
- 29. Rubino CM, Xue B, Bhavnani SM. et al. Population pharmacokinetic analyses for BC-3781 using phase 2 data from patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59: 282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubino CM, Forrest A, Bhavnani SM. et al. Population pharmacokinetics of BC-3781 using phase 1 data. In: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, USA,2010. Abstract A1-018.
- 31. Rodvold KA, Hope WW, Boyd SE.. Considerations for effect site pharmacokinetics to estimate drug exposure: concentrations of antibiotics in the lung. Curr Opin Pharmacol 2017; 36: 114–23. [DOI] [PubMed] [Google Scholar]
- 32. Ambrose PG, Bhavnani SM, Ellis-Grosse EJ. et al. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin Infect Dis 2010; 51: S103–10. [DOI] [PubMed] [Google Scholar]
- 33. Ambrose PG. Antibacterial drug development program successes and failures: a pharmacometric explanation. Curr Opin Pharmacol 2017; 36: 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


