Abstract
Objectives
To characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of lefamulin in the neutropenic murine thigh infection model to ascertain (i) which PK/PD index best correlates with efficacy and (ii) whether the magnitude of the index that drives efficacy varies for different pathogens.
Methods
We evaluated the in vivo PK/PD of lefamulin against five Streptococcus pneumoniae and five Staphylococcus aureus strains using a neutropenic murine thigh infection model. The relationships between bacterial burden in the thigh of normal and neutropenic mice after 24 h of lefamulin treatment and various PK/PD indices were determined.
Results
The kinetics of the three doses was linear by AUC. Rate of killing was maximal at concentrations near the MIC; suppression of regrowth was dose dependent, with a post-antibiotic effect of 3.0–3.5 and 1.0–1.5 h against S. pneumoniae and S. aureus, respectively. The efficacy of lefamulin correlated most strongly with the AUC0–24/MIC ratio; coefficient of determination was 79.9% for S. pneumoniae and 78.3% for S. aureus. The magnitude of the 24 h AUC/MIC of total drug required ranged from 9.92 to 32.1 for S. pneumoniae and 40.2 to 82.5 for S. aureus, corresponding to free drug values (∼20% free fraction) of 1.98–6.42 and 8.04–16.5, respectively.
Conclusions
Lefamulin, the first systemically available pleuromutilin in humans, exhibits time- and concentration-dependent killing. The presence of white blood cells had only a slight effect in enhancing the activity of the drug, indicating a leucocyte-independent effect. The identified driver of efficacy, the AUC0–24/MIC ratio and the ratios determined against various S. aureus and S. pneumoniae strains, will inform further non-clinical and clinical trials.
Introduction
To be effective, antimicrobial agents must penetrate the infected tissue at adequate concentrations that are maintained at a sufficient level and/or time course to achieve the desired antibacterial effect. The MIC, which is measured in vitro, does not provide information on the time course of antimicrobial activity in vivo, including effects that persist after drug tissue concentrations fall below the MIC [i.e. the in vivo post-antibiotic effect (PAE)]. Thus, optimal antimicrobial dosing regimens must be determined by evaluating the interrelationship between pharmacokinetics (PK) and pharmacodynamics (PD); i.e. the rate of antibacterial activity, its dependence on concentration or the time of exposure, and the presence or absence of a PAE. Studies of murine infection models have been used to identify the PK and PD measures that are associated with efficacy for many classes of agents.1–7 The results obtained from these studies in animal models have been similar to those observed in humans.8
Lefamulin is an antimicrobial agent from the pleuromutilin class that is in development for oral and intravenous administration in patients. In vitro studies have shown that lefamulin has potent activity against a variety of Gram-positive and Gram-negative bacteria that cause skin and respiratory tract infections.9–11 Previous in vitro and in vivo studies have shown that lefamulin achieves high intracellular concentrations, accumulates in the macrophages and provides good tissue and organ penetration.12,13 (See the article in this Supplement entitled ‘Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae’ for details on macrophage accumulation.14) Lefamulin was previously evaluated in a Phase 2 study in patients with skin and skin structure infections15,16 and was recently evaluated in two Phase 3 trials in patients with community-acquired bacterial pneumonia.17,18
The goal of the present studies was to characterize the PK and PD of lefamulin in the neutropenic murine thigh infection model to ascertain (i) which PK or PD index best correlates with the efficacy of lefamulin and (ii) whether the magnitude of the index that drives efficacy varied for different pathogens. These studies should provide guidance for dosing regimen selection for the successful administration of lefamulin for the treatment of patients with infections caused by drug-resistant, Gram-positive bacteria.
Materials and methods
Ethics
The murine infection model studies were approved by the Animal Research Committee of the University of Wisconsin and the William S. Middleton Memorial Veterans Hospital, and were carried out in adherence with the laws and animal care guidelines of the USA.
Drug PK
Single-dose PK studies were conducted in thigh-infected ICR Swiss mice using a non-compartmental model. Neutropenia was induced chemically (see Murine infection model section below). Animals received single subcutaneous doses of 10, 40 and 160 mg/kg lefamulin; linear interpolation was used for PK of the in-between dose levels. For each dose, blood samples were obtained at 0.5, 1, 3, 6, 9 and 12 h after dosing by cardiac puncture (n = 3 per dose and timepoint). Samples were centrifuged, and serum was stored at –70°C. Lefamulin free base concentrations in mouse serum were measured by an unpublished fit-for-purpose method (data on file, Nabriva Therapeutics GmbH) based on protein precipitation followed by dilution with water and LC–MS/MS detection. The lower limit of quantification (LLOQ) was 5.00 ng/mL, and the upper limit of quantification was 5000ng/mL. The accuracy of quality control samples was between 75.9% and 113.8% (internal limit: ±30%), with a precision of ≤4.8% (internal limit: ≤30%). The R2 was between 0.995 and 1.000 (internal limit: ≥0.99). PK data were analysed using the sparse sampling non-compartmental method (Phoenix WinNonlin 6, Certera, Princeton, NJ, USA) based on nominal timepoints. AUC values were determined using the linear trapezoidal method. The level of plasma protein binding was determined by LC–MS/MS after equilibrium dialysis of pooled sera at 0.12, 0.53 and 3.25 mg/L.
Susceptibility studies
The study organisms and their lefamulin MICs are listed in Table 1. The susceptibilities to lefamulin of five S. pneumoniae strains and five S. aureus strains were evaluated using standard microdilution techniques from the CLSI.19
Table 1.
MICs of lefamulin for study organisms
| Organism | MIC (mg/L) | Resistance summary |
|---|---|---|
| S. aureus ATCC 25923 | 0.12 | MSSA |
| S. aureus ATCC Smith | 0.06 | MSSA |
| S. aureus ATCC 33591 | 0.06 | MRSA |
| S. aureus UW 307109 | 0.12 | HA-MRSA |
| S. aureus WIS-1 | 0.06 | HA-MRSA |
| S. pneumoniae ATCC 10813 | 0.12 | PSSP |
| S. pneumoniae CDC 145 | 0.06 | PRSP, MR |
| S. pneumoniae CDC 1020 | 0.12 | PRSP, MR |
| S. pneumoniae CDC 1293 | 0.12 | PRSP, MR |
| S. pneumoniae CDC 1329 | 0.06 | PRSP, MR |
HA, hospital-acquired; MR, macrolide resistant; PSSP, penicillin-susceptible S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
Murine infection model
The infection model studies used specific-pathogen-free, female ICR/Swiss mice weighing 23–27 g (Harlan Sprague Dawley, Madison, WI, USA). Neutropenia was induced chemically using two intraperitoneal injections of cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN, USA): one injection of 150 mg/kg 4 days before infection and one injection of 100 mg/kg on the day before infection, as described previously.3,4 The neutropenic murine thigh infection model was used for all experiments. S. aureus and S. pneumoniae strains with MICs ranging from 0.06 to 0.12 mg/L were used as infectious agents. Groups of two mice (four thighs per dosing regimen) were infected with 100 μL (containing ∼105–106 cfu of the infectious organism) by injection into one or both thighs 2 h before lefamulin was administered subcutaneously. The animals were sacrificed 24 h after the first administration, and thighs were removed aseptically and homogenized in a saline solution. To evaluate cfu, aliquots of 10-fold serial dilutions were plated (10 μL) on blood agar plates and incubated at 37°C for 20 h; LLOQ was 100 cfu. The common logarithms of cfu/thigh values were used for all calculations.
In vivo PAEs
At 2 h after infection with either S. pneumoniae (ATCC 10813) or S. aureus (ATCC 25923), mice were treated with single subcutaneous doses of lefamulin (10, 20 or 40 mg/kg). Groups of two treated mice (four thighs) and saline-treated control mice were sacrificed at the timepoints of 0, 2, 4, 6, 9, 12 and 24 h. The thighs were removed at each timepoint and were processed immediately for cfu determination. PAE was calculated using dose-specified parameters by the following equation: PAE = T – C, where C is the time for the growth of 1 log10 cfu/thigh in control animals, and T is the time for the growth of 1 log10 cfu/thigh in treated animals after total and free-drug levels in plasma had fallen below the MIC.20
PD parameter determination
For each dosage regimen studied, the PK/PD index that correlates best with efficacy was evaluated by relating the number of bacteria in the thigh at the end of 24 h of therapy with (i) Cmax/MIC ratio, (ii) 24 h AUC/MIC ratio, and (iii) T>MIC; %T>MIC was calculated using dose-specified parameters. Neutropenic mice were infected, as described above, with penicillin-susceptible S. pneumoniae ATCC 10813 or methicillin-susceptible S. aureus ATCC 25923. Total daily doses of lefamulin between 5 and 160 mg/kg were fractionated into one, two, four or eight doses to vary the PK/PD indices and limit the interdependence among parameters with dose escalation. Drug doses were administered subcutaneously in 0.2 mL volumes. The mice were sacrificed after 24 h of therapy, and the thighs were removed and processed for cfu determination. Saline-treated control mice were sacrificed just before treatment as early control and after 24 h.
PK/PD target determination
To determine the 24 h fAUC/MIC required for a static effect, we studied the activity of twice-daily (q12h) dosing regimens of lefamulin against five S. aureus or S. pneumoniae strains with MICs ranging from 0.06 to 0.12 mg/L. The total daily dose of lefamulin varied from 5 to 320 mg/kg subcutaneously q12h. Thus, in infected mice, 10 mg/kg was the lowest dose tested; linearity was assumed below 10 mg/kg. The mice (two mice/four thighs per dosing regimen) were sacrificed after 24 h of therapy, and the thighs were removed and processed for cfu determination. Saline-treated control mice were sacrificed just before treatment as early control and after 24 h.
Data analysis
The correlations between efficacy and the PK/PD indices (Cmax/MIC ratio, AUC0–24/MIC ratio and T>MIC) were determined by non-linear least-squares multivariate regression for each dosage regimen and PK/PD index studied. The coefficient of determination (R2) was used to estimate the variance that could be due to regression for each PK/PD index. Free-drug concentrations were used to calculate the magnitude of the various indices. The dose–response results of these studies were analysed using the sigmoid dose–effect model. The model is derived from the Hill equation, E = (Emax × DN)/( + DN), where E is the effect or, in this case, the log change in the number of cfu per thigh between treated mice and untreated control mice after the 24 h period of study; Emax is the maximum effect; D is the total dose administered over 24 h; ED50 is the dose required to achieve 50% of Emax; and N is the slope of the dose–effect curve. The indices Emax, ED50 and N were calculated by non-linear least-squares regression. SigmaStat was used for data analysis, and both upper and lower bounds were included.
Results
Drug PK
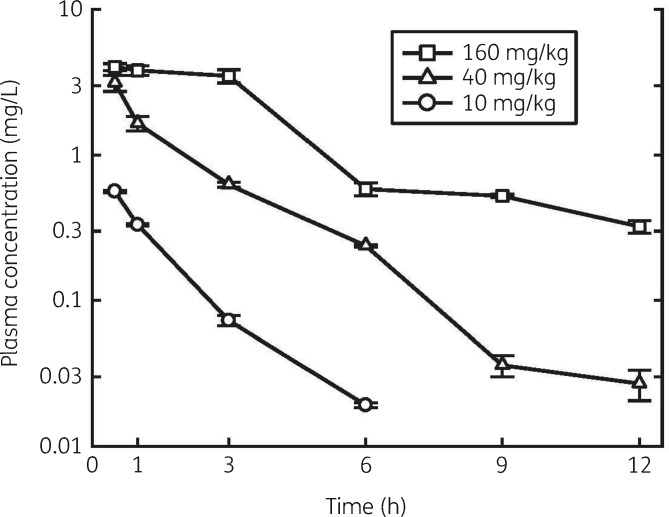
The serum concentration–time curves after single subcutaneous doses of 10, 40 and 160 mg/kg lefamulin are shown in Figure 1. In general, the 160 mg/kg dose of lefamulin was well tolerated by the mice. The 10 and 40 mg/kg doses of lefamulin administered subcutaneously were likewise well tolerated by all the animals. Linear regression showed that the kinetics of the three doses was linear by AUC. PK parameters calculated using a non-compartmental approach are listed in Table 2. The plasma concentration of lefamulin ranged from 0.02 to 4.2 mg/L: 80% of the drug was bound to protein in the plasma, staying constant over concentrations of 1–10 mg/L.
Figure 1.
Serum concentrations of lefamulin in thigh-infected neutropenic mice following subcutaneous dosing. Error bars represent SD.
Table 2.
PK parameters of lefamulin after subcutaneous administration
| Dose (mg/kg) | Tmax (h) | Cmax (mg/L) | AUC0–24 | Half-life (h) |
|---|---|---|---|---|
| 10 | 0.5 | 0.563 ± 0.010 | 0.823 | 1.55 |
| 40 | 0.5 | 3.136 ± 0.400 | 5.55 | 1.84 |
| 160 | 0.5 | 4.025 ± 0.225 | 18.8 | 2.87 |
In vivo time–kill and PAEs
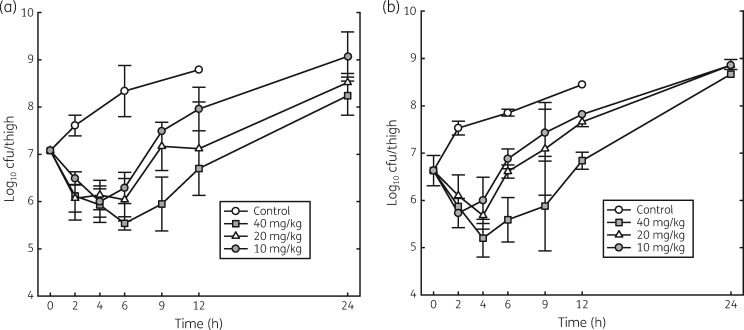
The effects of single doses of 10, 20 and 40 mg/kg of lefamulin on the in vivo killing and regrowth of S. pneumoniae ATCC 10813 and S. aureus ATCC 25923 are shown in Figure 2. For both organisms, the rate of killing was maximal at the lowest dose studied; however, the duration of regrowth suppression was dose dependent. Regrowth of S. pneumoniae began 4 h after administration of the 10 mg/kg dose and 6 h after administration of the 40 mg/kg dose. Regrowth of S. aureus began 2 h after administration of the 10 mg/kg dose and 4 h after administration of the 40 mg/kg dose. For both organisms, regrowth after the 20 mg/kg dose was intermediate between that of the other two doses. These results demonstrate that the in vivo PAE of lefamulin against S. pneumoniae was ∼3.0–3.5 h and against S. aureus was ∼1.0–1.5 h.
Figure 2.
Effect of single doses of lefamulin (0, 10, 20 and 40 mg/kg) on the time course of antimicrobial activity with S. pneumoniae ATCC 10813 (a) and S. aureus ATCC 25923 (b) in the thighs of neutropenic mice. Each point represents the mean value from three mice. Error bars represent SD.
Correlation of PK and PD indices
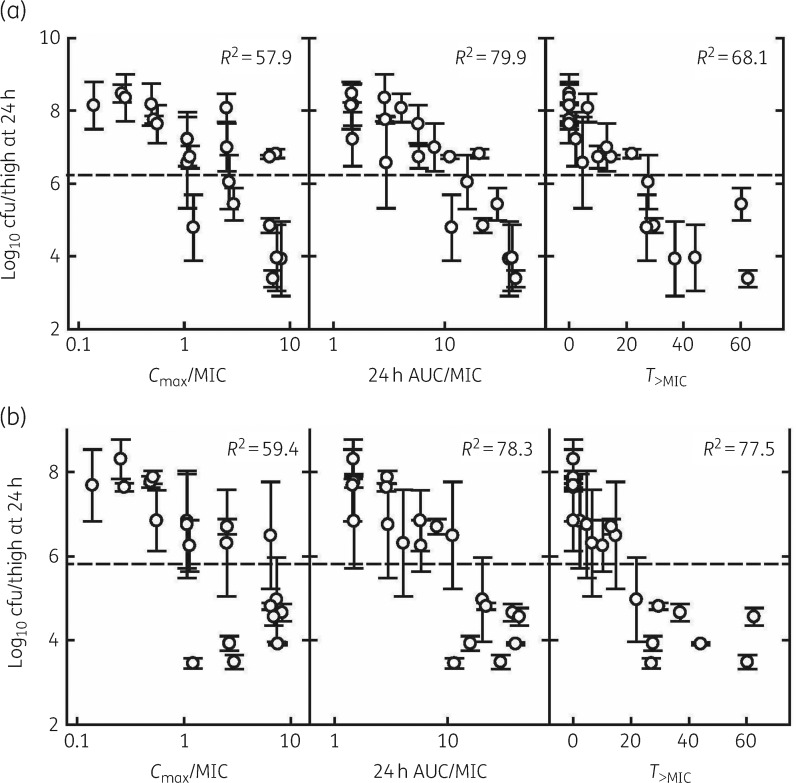
Correlation was assessed by visual inspection and then determination of R2 for the observed relationship between the number of bacteria in the thigh at the end of 24 h of therapy and PK/PD indices for S. pneumoniae (Figure 3a) and S. aureus (Figure 3b). The efficacy of lefamulin correlated best with the AUC0–24/MIC ratio, followed by the T>MIC. The R2 value for the AUC0–24/MIC ratio was 79.9% for S. pneumoniae and 78.3% for S. aureus. The R2 value for the T>MIC was 68.1% for S. pneumoniae and 77.5% for S. aureus.
Figure 3.
Relationship of different pharmacokinetic and pharmacodynamic indices to the antimicrobial activity of lefamulin against S. pneumoniae ATCC 10813 (a) and S. aureus ATCC 25923 (b) in the thighs of neutropenic mice. Error bars represent SD. The horizontal dashed line represents the early control cfu per thigh before the start of treatment.
PK/PD target determination
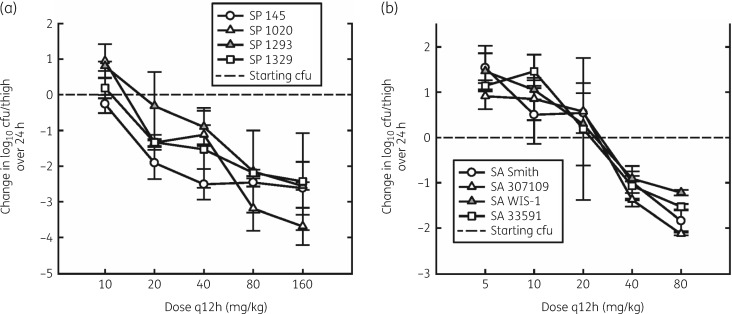
The dose–response curves for lefamulin against the various strains of S. pneumoniae and S. aureus are shown in Figure 4(a and b), respectively. In general, the shape of the dose–response curves was similar for all strains. However, the location of the dose–response curve was related to the MIC of the organism, showing a good in vitro/in vivo correlation. The static dose, maximum bactericidal effect and the 24 h AUC/MIC required for the static effect are shown in Table 3. The mean static and 1 log kill doses were 8.97 and 16.8 mg/kg q12h, respectively, for S. pneumoniae and 25.1 and 52.0 mg/kg q12h for S. aureus. The magnitude of the AUC0–24/MIC ratio required for static dose ranged from 9.92 to 82.5, corresponding to free-drug fAUC0–24/MIC static effect ratios ranging from 1.98 to 16.5 (Table 3).
Figure 4.
Dose–response relationships for q12h subcutaneous dosing of lefamulin against various strains of S. pneumoniae (SP) (a) and S. aureus (SA) (b) in the thighs of neutropenic mice. Error bars represent SD. SA, S. aureus; SP, S. pneumoniae.
Table 3.
Static dose, maximum killing and 24 h AUC/MIC ratio required for a static effect of lefamulin (q12h) against 10 organisms
| Organism/ strain | MICa (mg/L) | Comments | Static dose (mg/kg/12 h) | Total 24 h AUC/MIC derived from static dose | 24 h fAUC/MICb derived from static dose | 1 log kill dose (mg/kg/12 h) | 24 h fAUC/MICb derived from 1 log kill dose | Maximal killing (log10 cfu/thigh) |
|---|---|---|---|---|---|---|---|---|
| S. pneumoniae | ||||||||
| ATCC 10813 | 0.12 | PSSP | 18.6 | 32.1 | 6.42 | 34.7 | 15.12 | –2.86 |
| CDC 145 | 0.06 | PRSP, MR | 5.45 | 15.0 | 3 | 7.98 | 4.38 | –2.62 |
| CDC 1020 | 0.12 | PRSP, MR | 7.23 | 9.92 | 1.984 | 11.9 | 3.48 | –3.69 |
| CDC 1293 | 0.12 | PRSP, MR | 8.34 | 11.4 | 2.28 | 19.8 | 7.00 | –2.56 |
| CDC 1329 | 0.06 | PRSP, MR | 5.25 | 14.4 | 2.88 | 9.59 | 5.26 | –2.43 |
| mean (range) | 8.97 (5.25–18.6) | 16.6 (9.92–32.11) | 3.31 (1.98–6.42) | 16.8 (7.98–34.7) | 7.04 (3.48–15.12) | –2.83 | ||
| S. aureus | ||||||||
| ATCC 25923 | 0.12 | MSSA | 31.9 | 67.4 | 13.48 | 56.0 | 21.8 | –1.98 |
| ATCC 33591 | 0.06 | MRSA | 22.2 | 81.9 | 16.38 | 42.9 | 38.0 | –1.53 |
| ATCC Smith | 0.06 | MSSA | 13.2 | 40.2 | 8.04 | 44.6 | 38.8 | –1.84 |
| WIS-1 | 0.06 | HA-MRSA | 22.3 | 82.5 | 16.5 | 52.1 | 42.2 | –1.22 |
| UW 307109 | 0.12 | HA-MRSA | 36.1 | 79.8 | 15.96 | 64.3 | 23.4 | –2.13 |
| mean (range) | 25.1 (13.2–36.1) | 70.4 (40.2–82.5) | 14.07 (8.04–16.50) | 52.0 (42.9–64.3) | 32.8 (21.8–42.2) | –1.74 |
HA, hospital-acquired; MR, macrolide resistant; PSSP, penicillin-susceptible S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
MICs were determined in Mueller–Hinton broth (MHB) by standard CLSI microdilution techniques. MHB was supplemented with 3% lysed horse blood for MIC determinations with S. pneumoniae. MICs were determined twice in triplicate; mean values are shown.
A value of 20% unbound lefamulin was used for calculations of 24 h fAUC/MIC, corresponding to the value of in vitro plasma protein binding assay at 3 mg/L.
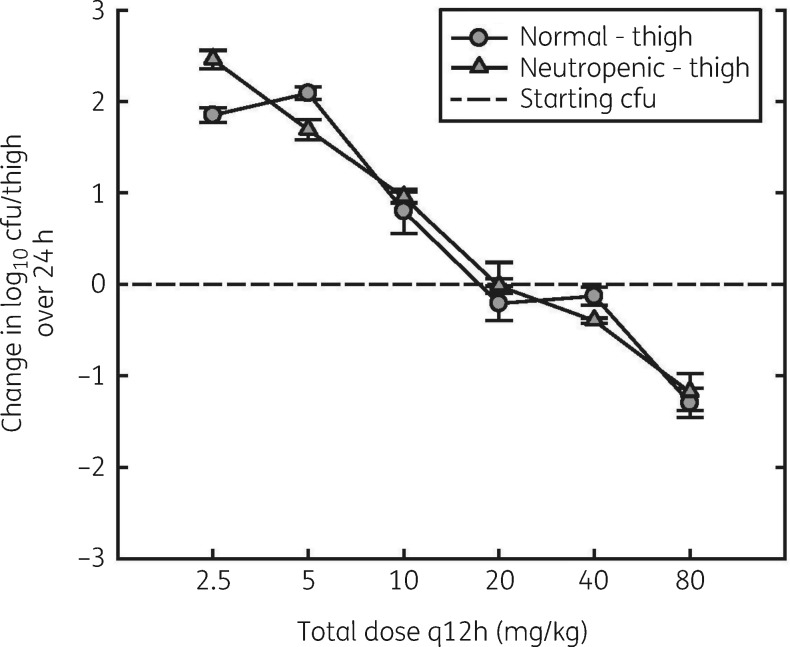
Impact of neutrophils
No appreciable difference in activity of lefamulin against S. pneumoniae was observed between neutropenic and normal mice (Figure 5). The static dose was 22.5 mg/kg q12h for normal mice and 21.0 mg/kg q12h for neutropenic mice. The corresponding AUC0–24/MIC ratios were 41.2 and 37.5.
Figure 5.
Dose–response relationships for q12h dosing of lefamulin against S. pneumoniae ATCC 10813 in the thighs of normal and neutropenic mice. Error bars represent SD.
Discussion
In our studies, we evaluated the in vivo PK and PD of lefamulin, the first systemically available pleuromutilin for human use. Lefamulin had potent activity against various S. pneumoniae and S. aureus strains tested. For antimicrobials of a new class, the evaluation of the relationship between PK and PD is crucial for the development of efficacious dose regimens.1 We evaluated the PK and PD of lefamulin in a murine model that has been used for dose evaluations of other agents.1–7 The results showed that lefamulin exhibited time-dependent killing with modest in vivo PAEs. The rate of killing was not enhanced by higher lefamulin concentrations; however, dose-dependent regrowth was observed, particularly at the highest dose studied (40 mg/kg).
The free-drug AUC0–24/MIC ratio was the most important index driving efficacy, followed by the percentage of time that free-drug concentrations exceeded the MIC. This result is consistent with other neutropenic thigh S. aureus infection models, where AUC/MIC was the best predictor of efficacy.6 The magnitude of the 24 h AUC/MIC of total drug required for bacteriostasis ranged from 9.92 to 32.1 for S. pneumoniae strains and 40.2 to 82.5 for S. aureus. This would correspond to free-drug values (∼20% free fraction) ranging from 1.98 to 6.42 and 8.04 to 16.5 for S. pneumoniae and S. aureus, respectively, which is somewhat lower than the 24 h AUC/MIC values obtained with fluoroquinolones, tetracyclines, clindamycin and macrolides for similar organisms.1,8 The presence of white blood cells had no appreciable effect in enhancing the activity of the drug in the thighs. Based on five different isolates of each S. pneumoniae and S. aureus strain in the thigh infection model, a bacteriostatic total 24 h AUC/MIC ratio of ∼70 should be targeted for efficacy. Consequently, the target 24 h fAUC/MIC ratio is 14 (8–16.5), taking into account a 20% free fraction of lefamulin in mouse plasma. These non-clinical fAUC/MIC targets should be bridged with PK data from humans to predict doses and regimens resulting in a free-drug AUC/MIC ratio of 14 (8–16.5) that would be adequate to treat most patients when population variations are considered.
Funding
This work was supported by Nabriva Therapeutics. Editorial support for this article was funded by Nabriva Therapeutics.
Transparency declarations
W. W. W. is an employee of and holds stock in Nabriva Therapeutics plc. D. A. has none to declare.
Scott Newcomer, MS, of Davenport Scientific Services, LLC, assisted in the preparation of this article but did not meet the criteria for authorship. Editorial support for development of this article was provided by Lycely del C. Sepulveda-Torres, PhD, and Michael S. McNamara, at C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and funded by Nabriva Therapeutics.
This article forms part of a Supplement sponsored by Nabriva Therapeutics.
References
- 1. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–12. [DOI] [PubMed] [Google Scholar]
- 2. Craig WA. Pharmacodynamics of antimicrobials: general concepts and applications In: Nightingale CH, Ambrose PG, Drusano GL. et al. , eds. Antimicrobial Pharmacodynamics in Theory and Clinical Practice. New York: Marcel Dekker, 2007; 1–19. [Google Scholar]
- 3. Andes D, Craig WA.. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother 1998; 42: 2375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andes D, van Ogtrop ML, Peng J. et al. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 2002; 46: 3484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DG, Murakami Y, Andes DR. et al. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 105 and 107 CFU injected into opposite thighs of neutropenic mice. Antimicrob Agents Chemother 2013; 57: 1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepak AJ, Marchillo K, Craig WA. et al. In vivo pharmacokinetics and pharmacodynamics of the lantibiotic NAI-107 in a neutropenic murine thigh infection model. Antimicrob Agents Chemother 2015; 59: 1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lepak AJ, Marchillo K, Pichereau S. et al. Comparative pharmacodynamics of the new oxazolidinone tedizolid phosphate and linezolid in a neutropenic murine Staphylococcus aureus pneumonia model. Antimicrob Agents Chemother 2012; 56: 5916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ambrose PG, Bhavnani SM, Rubino CM. et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 2007; 44: 79–86. [DOI] [PubMed] [Google Scholar]
- 9. Paukner S, Sader HS, Ivezic-Schoenfeld Z. et al. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother 2013; 57: 4489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sader HS, Biedenbach DJ, Paukner S. et al. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2012; 56: 1619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sader HS, Paukner S, Ivezic-Schoenfeld Z. et al. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J Antimicrob Chemother 2012; 67: 1170–5. [DOI] [PubMed] [Google Scholar]
- 12. Paukner S, Krause K, Gruss A. et al. Accumulation of the pleuromutilin antibiotic BC-3781 in murine macrophages and effect of lung surfactant on the BC-3781 in vitro activity. In: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, USA, 2013. Abstract A-011.
- 13. Wicha WW, Ivezic-Schoenfeld Z, Novak R. Pharmacokinetic, mass balance and tissue distribution of [14C]-BC-3781 in non-pigmented rats. In: 20th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 2010. Abstract P909.
- 14. Wicha WW, Strickmann DB, Paukner S.. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother 2019; 74 Suppl 3: iii11--iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prince WT, Ivezic-Schoenfeld Z, Lell C. et al. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2013; 57: 2087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubino CM, Xue B, Bhavnani SM. et al. Population pharmacokinetic analyses for BC-3781 using phase 2 data from patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59: 282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.File TM Jr, Goldberg L. Das A et al. Efficacy and safety of iv-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 trial. Clin Infect Dis 2019; doi:10.1093/cid/ciz090. [DOI] [PMC free article] [PubMed]
- 18.Alexander E, Goldberg L, Das A et al. Oral lefamulin is safe and effective in the treatment of adults with community-acquired bacterial pneumonia (CABP): results of the Lefamulin Evaluation Against Pneumonia (LEAP 2) study. In: IDWeek, San Francisco, CA, 2018. Abstract 74297.
- 19.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard :11th Edition. CLSI, Wayne, PA, 2012. [Google Scholar]
- 20. Craig WA, Gudmundsson W.. Postantibiotic effect In: Lorian V, ed. Antibiotics in Laboratory Medicine. Baltimore, MD: Williams & Wilkins, 1996: 296–329. [Google Scholar]