Abstract
Objectives
Lefamulin is a semi-synthetic intravenous and oral pleuromutilin antibiotic with activity against pathogens commonly associated with community-acquired bacterial pneumonia. Using data from two Phase 1 studies, a population pharmacokinetics (PPK) model for lefamulin in plasma and epithelial lining fluid (ELF) was constructed.
Methods
Plasma pharmacokinetic (PK) data from a crossover, bioavailability, food-effect study and plasma and ELF PK data from a tissue penetration study in normal healthy volunteers were used to construct a PPK model for lefamulin. Model development involved refinement of a previous PPK model for intravenous and oral administration, followed by application of the model to plasma and ELF data from the tissue penetration study. The ELF penetration ratio of lefamulin was determined using model-based simulations.
Results
The PPK analysis data set contained 1103 plasma and 12 ELF lefamulin concentrations from 32 subjects. A three-compartment model with non-linear protein binding and two parallel absorption processes provided precise and unbiased estimated plasma concentration–time profiles. The absorption rate was slower and bioavailability was decreased after a high-fat/high-calorie meal. ELF data were well described using first-order rate constants into and out of the ELF compartment. The median predicted lefamulin total-drug ELF AUC0–24/free-drug plasma AUC0–24 ratio was ∼5:1 after intravenous or oral administration.
Conclusions
The final PPK model allowed precise characterization of plasma and ELF exposures after intravenous and oral administration. The high ELF penetration ratio suggests that the penetration of lefamulin into the effect site is rapid and extensive, irrespective of route of administration.
Introduction
Lefamulin (also known as BC-3781) is a semi-synthetic pleuromutilin antibiotic whose mechanism of action is ribosomal protein synthesis inhibition.1 Lefamulin has activity against pathogens commonly associated with acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia (CABP), including MRSA and MDR Streptococcus pneumoniae.1 Lefamulin is currently in Phase 3 development by Nabriva Therapeutics (Vienna, Austria and King of Prussia, PA, USA) for treatment of patients with CABP.
Data from multiple Phase 1 studies and a Phase 2 study had previously been utilized to develop a population pharmacokinetics (PK) model for lefamulin.2 The previous model, which had added data from 1167 PK samples from 129 patients to the 1677 PK samples from 66 subjects in an earlier population PK model, determined that the most appropriate model was a three-compartment model with zero-order infusion and first-order (linear) elimination. Saturable (non-linear) protein binding was also incorporated into that model. Studies used to construct and refine previous models included single intravenous (iv) doses of lefamulin ranging from 25 to 400 mg, twice-daily doses of 75, 100 or 150 mg iv, and single oral doses of 600 mg.
The objectives of this analysis were to refine the previously developed population PK model for lefamulin using data from a Phase 1, bioavailability and food-effect study. The refined model was then used to describe the PK of lefamulin in pooled plasma and epithelial lining fluid (ELF) data obtained from healthy subjects. The final objective was to use the population PK model to predict the ELF penetration ratio of lefamulin after iv or oral administration in the fed and fasted state.
Methods
Study design
Data for the analyses described here were obtained from two Phase 1 studies: (i) Study NAB-BC-3781-1107 (Study 1107), a four-period crossover study to assess the bioavailability of lefamulin (data on file, Nabriva Therapeutics GmbH; ClinicalTrials.gov identifier: NCT02557789) and (ii) Study NAB-BC-3781-1005 (Study 1005), a single-dose study to assess the penetration of lefamulin into ELF.3 All subjects provided written informed consent before study initiation. All studies were approved by independent ethics committees or institutional review boards and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Study 1107 was a Phase 1, single-centre, single-cohort, randomized, crossover study with healthy subjects receiving a single dose of lefamulin in four study sessions:
Treatment A: lefamulin as a 600 mg immediate-release (IR) tablet in the fasted state.
Treatment B: lefamulin as a 600 mg active pharmaceutical ingredient in a capsule (three 200 mg capsules) in the fasted state.
Treatment C: lefamulin as 150 mg iv in 250 mL of citrate-buffered saline infused over 1 h.
Treatment D: lefamulin as a 600 mg IR tablet administered 1 h after a standard FDA high-fat/high-calorie breakfast.
The population PK model was refined using data from healthy subjects under treatments A, C and D only. Blood samples for determination of lefamulin plasma concentrations were collected pre-dose and 10, 20, 30 and 45 min and 1, 1.25, 1.5, 2, 2.5, 3, 4, 5, 8, 12, 24 and 36 h post-dose at each session.
Study 1005 was a Phase 1, single-dose study conducted in 12 healthy adult male volunteers. All subjects received lefamulin 150 mg iv over 1 h. Plasma PK samples were to be drawn pre-dose and at 0.5, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h after the start of infusion. One ELF sample per patient was also taken via bronchoalveolar lavage (BAL). ELF could be collected at 1, 2, 4 or 8 h after the start of infusion.
For both studies, the actual dates and times of dose administration and PK sample collection were used in the construction of the population PK analysis data set. An outlier was defined as an aberrant observation that substantially deviated from the rest of the observations within an individual. Outliers were excluded from this analysis because of the potential to negatively impact the convergence and/or parameter estimates.4 Suspected outlier observations were tested by fitting candidate models to the data with and without the suspected outlier(s) and evaluating the resulting goodness of fit. If exclusion of the point(s) significantly improved the fit to the other observations, the point was declared an outlier and excluded from the analysis. Concentrations in the data set that were below the limit of quantification were flagged.
To characterize the analysis populations and to evaluate their ability to explain a portion of the inter-individual variability (IIV) of selected PK parameters, subject demographics were collected before study drug administration. Demographic information included sex, age, height, weight and BMI. Race information was also collected. BMI was calculated as height in metres divided by weight in kilograms squared. The only laboratory information included in the analysis was serum creatinine (to calculate CLCR) and serum albumin. CLCR was calculated from the baseline serum creatinine level, age and body weight by using the Cockcroft–Gault equation and then normalizing to a body surface area of 1.73 m2.5
Population PK model development: iv and oral administration
All population PK analyses were conducted using NONMEM® (ICON Development Solutions, Ellicott City, MD, USA), implementing the first-order conditional estimation method with η–ε interaction.6–8 Based on the results of previous analyses, model development focused on a three-compartment model with linear clearance and non-linear protein binding. Model refinement was initially conducted using the data from Study 1107, in order to estimate oral bioavailability of lefamulin and quantify the impact of food using the population PK model. The refined model was then applied to the data from Study 1005 in order to estimate the penetration of lefamulin into ELF under various dosing conditions.
The first step of the PK model development involved fitting the previous population PK model to the iv data, followed by modifications to the model to enable simultaneous fitting of data collected after the iv and oral administration from Study 1107. If the previous model was found inadequate in describing the data, a series of two- or three-compartment models with more complex atypical protein binding and absorption were evaluated as necessary. Although previous analyses suggested that lefamulin exhibited dose-dependent absorption, linear absorption models were also evaluated to assess the potential for concentration-independent absorption.
Base structural model development began with IIV estimated for free-drug CL and volume of the central compartment (Vc) using the exponential error model. This model for IIV assumes that the variance is constant with respect to the log of the typical value of the PK parameter, and the estimates are presented as percentage coefficients of variation (%CV). IIV in other PK parameters was to be evaluated as necessary. Refinements in the model, such as inter-occasion variability, were made based on the need to adequately fit the data.
Residual variability (RV), a composite of model mis-specification, assay variability, intra-individual variability, errors in the data and other unexplained errors, was initially estimated with a constant CV error model. This model for RV assumes that the variance increases in proportion to the squared predicted concentrations, and the estimate was presented as %CV. If warranted, additive or additive plus constant CV models were evaluated for RV. An additive model for RV assumed that the variance was constant, and the estimate was presented as a standard deviation. Refinements in the final model, such as inter-occasion variability, were made based on the need to adequately fit the data.
The structural population PK model identified as appropriate was then used to assess the ability of the subject covariates to explain a portion of IIV. Population PK covariate model development was undertaken by using forward selection followed by a backward elimination procedure. Forward selection was first performed using NONMEM® as a univariable analysis of each patient covariate with an observable trend. In forward selection, one parameter–covariate pair is added at a time to the model; the one that results in the largest significant improvement in the objective function (α = 0.05) is kept in the model. This process was repeated until there were no further covariates that produced significant changes in the objective function. The resulting full multivariable model was examined for any remaining biases with respect to the IIV and RV models. A backward elimination procedure was then performed in which each covariate was removed from the parameter equation separately. The most non-significant covariate was then removed from the model to produce the new base multivariable model. This process was repeated until all remaining covariates were significant.
Once the final model was identified, a visual predictive check (VPC, a graphical comparison of observations and simulated predictions) was used to evaluate the ability of the final model to adequately describe the observed lefamulin concentration–time profiles in healthy subjects and patients.7,8 VPC plots were generated through Monte Carlo simulations of 500 data sets using the final model estimated parameters, and the 5th, 50th and 95th percentiles of simulated data were compared with observed data to validate the final model.
Population PK model development: ELF pharmacokinetics
To characterize the time course of lefamulin concentrations in ELF, the final model from the process described above was applied to the plasma data from Study 1005 to obtain Bayesian, post hoc estimates of the plasma PK parameters for each subject. Plasma PK parameters were then fixed, and the model was applied to the ELF data to estimate parameters describing the transit of lefamulin from plasma to ELF. Different functional forms for the ELF PK were used as necessary to provide an adequate fit to the observed data. Sensitivity analyses were performed to ensure appropriate simulation of ELF concentrations with varying conditions. VPC plots were used to assess the robustness of the plasma/ELF model.
Monte Carlo simulations: ELF penetration
After completion of the population PK analysis, Monte Carlo simulations were conducted to calculate predicted lefamulin ELF penetration after iv or oral administration. Covariates were randomly selected from distributions similar to those in Study 1005. The cumulative total-drug AUC for both plasma and ELF was calculated by integrating the PK profile over time for each subject. Using the cumulative AUC, total-drug plasma and ELF AUC0–24 values were computed for each simulated patient. The plasma:ELF penetration ratio was determined by dividing the plasma AUC0–24 by the ELF AUC0–24.
Results
Analysis data
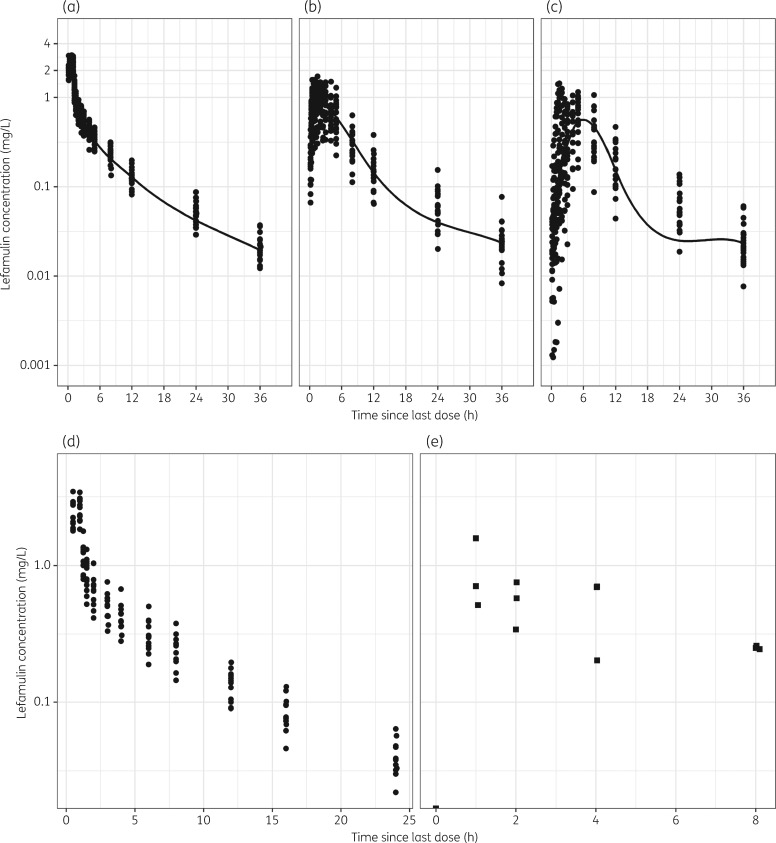
The demographic characteristics of the subjects from Studies 1107 and 1005 are provided in Table 1. Twenty subjects and 959 plasma concentration records were available from Study 1107 and used as the data set for population PK model refinement. All subjects completed all three treatment sessions. No plasma samples were assayed as below the limit of quantification, and no significant outliers were identified. The 12 subjects from Study 1005 contributed a total of 144 plasma samples and 12 ELF samples to the analysis. Similar to Study 1107, no samples were assayed as below the limit of quantification, and no significant outliers were observed. Semi-log scatterplots of both plasma and ELF lefamulin concentrations are provided in Figure 1.
Table 1.
Summary statistics or counts of subject demographic characteristics of the analysis population
| Variable | Study 1107a |
Study 1005b |
||
|---|---|---|---|---|
| n | median (min–max) | n | median (min–max) | |
| Age (years) | 20 | 31.5 (20–55) | 12 | 24 (20–48) |
| Weight (kg) | 20 | 76.8 (54–100.5) | 12 | 76.3 (59.7–99.1) |
| Height (cm) | 20 | 170.5 (154–188) | 12 | 181 (175–195) |
| Body surface area (m2) | 20 | 1.9 (1.5–2.2) | 12 | 1.94 (1.73–2.3) |
| Serum albumin (g/dL) | 20 | 4.7 (4.1–5.6) | 12 | 4.4 (3.8–4.9) |
| CLCR (mL/min/1.73 m2) | 20 | 114.6 (77.8–156.7) | 12 | 97.6 (80.9–127.1) |
Study 1107 included 12 (60%) males and 8 (40%) females.
Study 1005 included 12 (100%) males.
Figure 1.
Lefamulin concentration–time data from Studies 1107 and 1005. (a) Study 1107, iv 150 mg. (b) Study 1107, 600 mg; oral, fasted. (c) Study 1107 600 mg; oral, fed. (d) Study 1005, plasma. (e) Study 1005, ELF.
Population PK model: iv and oral administration
Ultimately, a three-compartment disposition model with linear elimination, plus the incorporation of a saturable protein binding sub-model, provided an adequate fit to the iv data from healthy subjects. As shown in Figure 1, lefamulin exhibited biphasic absorption characteristics within subjects after oral administration. A model containing parallel immediate and delayed absorption, with the delayed absorption described using transit compartments, well characterized the double-peak absorption kinetics and provided adequate fits to iv and oral data simultaneously. Following oral administration, lefamulin was partially absorbed into plasma via the immediate absorption rate (Ka), resulting in a rapid absorption peak reached at ∼2 h. Additionally, a large proportion (FS, the fraction absorbed through the slow process) of lefamulin was gradually absorbed into plasma via a delayed absorption rate (Ka2) subsequent to a delayed onset of absorption, leading to the observed biphasic absorption profiles.
Additionally, Figure 1 indicates that fed status can delay and decrease lefamulin absorption after oral administration. Incorporation of fed status as a descriptor of absorption rates and bioavailability provided an improved fit to the pooled fasted and fed PK data; these relationships were retained in the base structure model, which served as the comparator for subsequent covariate analysis. Note that all PK parameters (e.g. clearances and volumes) were conditioned on lefamulin unbound concentrations.
The covariate screening plots revealed multiple potential relationships between subject descriptors and primary PK parameters. However, the only covariate:parameter relationship that was statistically significant using forward selection was between distributional clearance to the first peripheral compartment (CLD1) and serum albumin [drop in minimum value of the objective function (MVOF) of 4 units]. This full multivariable model did not require modification and was thus subjected to backward elimination, at which point the CLD1:albumin relationship was determined to be insignificant based on the more strict criteria employed for backward elimination (P > 0.001). However, removal of fed status on Ka and Ka2 resulted in a significant increase in the MVOF [>10.83 units (α = 0.001, 1 degree of freedom)]. Removal of fed status on absolute absorption bioavailability (Ftot) failed to meet the statistical threshold but was retained based on clinical relevance and consistency with previous analyses (data on file, Nabriva Therapeutics GmbH). Thus, the relationships of fed status on Ka, Ka2 and Ftot were retained in the final population PK model describing the PK of lefamulin after iv and oral administration.
The population PK parameter estimates and associated standard errors for the model are provided in Table 2. The precision of the PK parameter estimates was high throughout. In general, the magnitude of the IIV was relatively modest for the majority of the parameters (≤37.1%) and higher for Ka, Ka2 and FS (108.2%, 54.8% and 55.7%, respectively) because of the variable absorption profiles across subjects. The intra-individual (residual) variability was estimated at <0.1 SD of lefamulin concentrations, which indicates a low extent of unexplained RV in the model fit. The effect of fed status on the parameters defining drug absorption are provided in Equations (1–3) below:
| (1) |
| (2) |
| (3) |
where Fed = 0 represents the fasted condition and Fed = 1 represents the fed condition.
Table 2.
Population PK model parameter estimates
| Parametera | Population mean |
Magnitude of IIV |
||
|---|---|---|---|---|
| estimate | SEM (%) | estimate | SEM (%) | |
| Plasma | ||||
| CL (L/h) | 159 | 5.61 | 16.1 | 57.7 |
| Vc (L) | 53.1 | 9.66 | 13.4 | fixed |
| CLD1 (L/h) | 86.6 | 42.1 | 37.1 | 77.5 |
| Vp1 (L) | 656 | 27.4 | 24.7 | fixed |
| CLD2 (L/h) | 199 | 16.1 | 23.5 | 69.1 |
| Vp2 (L) | 259 | 8.17 | NE | N/A |
| Ka (1/h) | 1.20 | 11.6 | 108.2 | 38.5 |
| Ka2 (1/h) | 2.12 | 26.0 | 54.8 | 63.3 |
| Ftot | 0.24 | 7.99 | 22.8 | 84.6 |
| FS | 0.80 | 8.35 | 55.7 | 74.2 |
| proportion of Ka when fed | 0.04 | 14.7 | NE | NA |
| proportion of Ka2 when fed | 0.44 | 3.55 | NE | NA |
| proportion of Ftot when fed | 0.81 | 9.13 | NE | NA |
| ELF | ||||
| Kin (1/h) | 2.71 | 17.0 | 31.6 | fixed |
| Kout (1/h) | 0.51 | 27.5 | 31.6 | fixed |
| Protein binding | ||||
| Fumin | 0.0997 | |||
| Fumax | 0.259 | |||
| Cup50 (mg/L) | 1.35 | |||
| RV | ||||
| plasma proportional error | 0.007 | 9.48 | NE | NA |
| ELF proportional error | 0.05 | fixed | NE | NA |
CL, total clearance of free drug; CLD1, distributional clearance to first peripheral compartment; CLD2, distributional clearance to second peripheral compartment; Cup50, concentration at which protein binding is half-maximal; Fumax, maximum extent of non-linear protein binding; Fumin, minimum extent of non-linear protein binding; NA, not applicable; NE, not evaluated; Vp1, volume of first peripheral compartment; Vp2, volume of second peripheral compartment.
Volumes and clearances are scaled to free-drug concentrations.
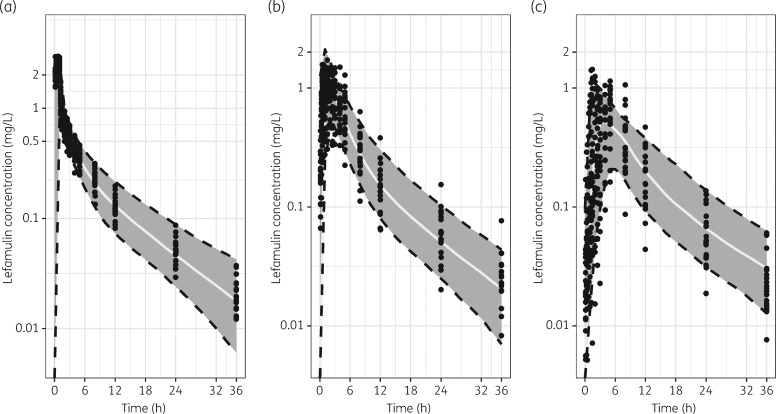
The VPC plots for the iv/oral model are provided in Figure 2. Generally, the bulk of the observed PK data are contained within the prediction intervals, suggesting that the final population PK model provided an accurate and unbiased fit of the lefamulin PK data in these healthy subjects. Additionally, model simulations (based on the final model) reasonably replicated external data from healthy subjects and patients after multiple dosing (data not shown), qualifying the applicability of the current model to future studies in patients. Collectively, the final model is expected to provide robust and reliable estimates of lefamulin exposure after iv or oral dosing.
Figure 2.
VPC plots for the model applied to lefamulin concentration–time data from Study 1107. (a) iv 150 mg. (b) 600 mg; oral, fasted. (c) 600 mg; oral, fed. The solid line and grey shaded area represent median and 90% CI of model simulations for 500 subjects, respectively. Solid dots represent observed PK data.
Population PK model: ELF pharmacokinetics
Application of the population PK model described above to the plasma data from the 12 subjects from Study 1005 provided a robust fit (r2 = 0.983 for the regression of observed to individual fitted plasma concentrations) with a relatively low degree of IIV (<40% for all parameters). The lefamulin ELF concentration–time data from the 12 subjects from Study 1005 were well described using simple first-order rate constants into (Kin) and out of (Kout) the ELF compartment (r2 = 0.966). The population PK parameter estimates and associated standard errors for the final model are provided in Table 2.
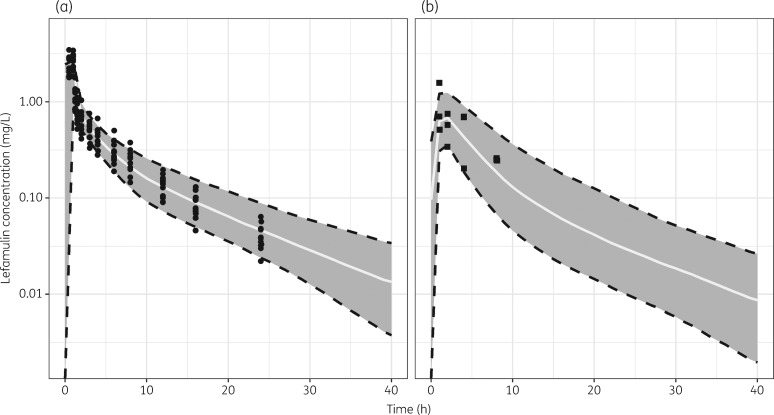
The VPC plots created as a model qualification step for the plasma and ELF data are shown in Figure 3. The VPC plots indicated that the model was appropriately representing the observed data. In both the plasma and ELF, there appeared to be reasonable agreement between the 5th, 50th and 95th percentiles of the observed and the individual simulated lefamulin concentrations across time intervals.
Figure 3.
VPC plots for the model applied to lefamulin concentration–time data from Study 1005. (a) Plasma. (b) ELF. The solid line and grey shaded area represent median and 90% CI of model simulations for 500 subjects, respectively. Solid dots represent observed PK data.
Monte Carlo simulations: ELF penetration
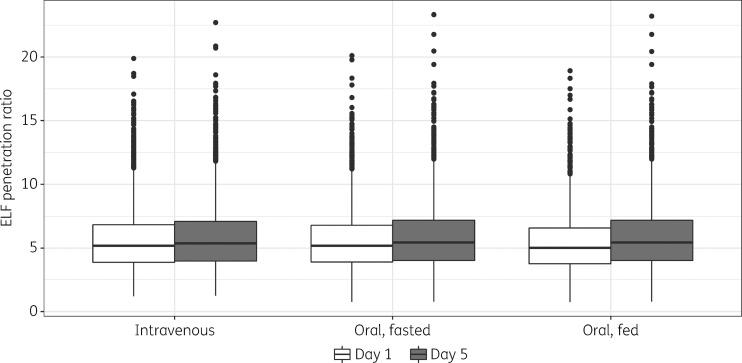
Using model-predicted exposures, the median lefamulin total-drug ELF AUC0–24:free-drug plasma AUC0–24 ratio was ∼5:1 after iv or oral administration among simulated patients (Figure 4). This ratio indicates that drug penetration is extensive irrespective of the route of administration and irrespective of a fed or fasted state when administered orally.
Figure 4.
Comparison of simulated lefamulin penetration ratio in the ELF on days 1 and 5 of treatment, by route of administration and fed status. Solid line in the middle of the box represents the median, box limits are the IQR, vertical lines (whiskers) extend to ±1.5 × IQR, and solid circles represent values outside the whiskers.
Discussion
The objectives of this analysis were three-fold. The first objective was to refine a prior population PK model using data from a crossover study in which subjects received lefamulin via iv infusion and via an oral tablet given in the fed and fasted states. For the second objective, this refined model was then applied to pooled plasma and ELF data from a second Phase 1 study in order to describe the time course of lefamulin concentrations in ELF after administration of lefamulin via iv infusion. The third objective was to predict the ELF penetration ratio of lefamulin after iv or oral administration using Monte Carlo simulations.
The previous population PK model, which had been developed using data from multiple Phase 1 studies and a Phase 2 study, had determined that a three-compartment model with linear clearance and non-linear protein binding best fit the data.2 The results of the current analysis confirm this model and emphasize its robust description of lefamulin PK in plasma after iv administration. Of note, it was necessary to fix the parameters that defined the non-linear protein binding process to those values obtained from the in vitro protein binding study (data on file, Nabriva Therapeutics GmbH). Subsequent analyses of lefamulin plasma concentration data collected after oral administration were complicated by the variable absorption profiles both within and across subjects. Lefamulin exhibited multi-phase absorption kinetics, and a complex absorption model with parallel immediate and delayed first-order absorption processes was necessary to capture the double-peak absorption profiles. Following oral administration, lefamulin was partially and immediately absorbed into plasma via a fast absorption rate constant (Ka), resulting in a rapid absorption peak reached at ∼2 h. Additionally, the majority of the lefamulin dose (∼80%) was gradually absorbed into plasma via a slower process subsequent to a delayed onset of absorption, leading to the observed biphasic absorption profile. Ultimately, when fit to the iv and oral data simultaneously, inclusion of the complex oral absorption model allowed precise and unbiased fits to lefamulin PK data after iv and oral administration. Model-based simulation diagnostics (i.e. VPC plots) confirmed the robustness of this model for not just the model development population (Study 1107) but also the external studies assessing the PK of lefamulin in both healthy subjects and patients administered lefamulin for up to 7 days. This provides important support for the robustness of the model, given that only single-dose studies were included in the present analysis.
Consistent with the observed data, fed status was deemed a significant predictor of the rate of lefamulin absorption, resulting in significantly delayed absorption in healthy subjects. Covariate analysis indicated that fed status was not a statistically significant covariate on the extent of absorption (bioavailability); however, this relationship was retained based on clinical relevance as well as for consistency with previous analyses. The CL was estimated to be 159 L/h, and bioavailability under fasted and fed status was estimated to be 0.24 and 0.19, respectively. These key population mean PK parameter estimates for this refined analysis were consistent with previous analyses.2 No other subject demographic factors were significantly associated with the IIV in lefamulin PK parameters.
When applied to the Phase 1 study in which subjects provided BAL samples for determination of lefamulin concentrations in ELF, a relatively simple model provided a precise and unbiased characterization of lefamulin plasma and ELF concentration–time data pooled from healthy volunteers after iv administration. Given the issues with parameter identifiability in the setting of sparse BAL sampling employed in this study, a sensitivity analysis was performed to ensure that the PK parameters describing the movement of lefamulin from plasma to ELF were not overly sensitive to the plasma parameters. Of note, a previous model of lefamulin had suggested that ELF exposure was sensitive to the changes in Vc such that ELF concentrations were predicted to increase with increasing Vc (data on file, Nabriva Therapeutics GmbH). Because the model described here was found to be insensitive to differences in Vc and thus insensitive to the route of administration, simulation of ELF penetration after oral administration is expected to be reliable regardless of whether subjects were administered lefamulin in a fed or fasted state. Furthermore, the current model is conservative in that it does not predict that ELF concentrations increase with increasing Vc. Considerable drug penetration into the effect site, regardless of route of administration, was demonstrated by the model’s total-drug ELF AUC0–24:free-drug plasma AUC0–24 ratio of ∼5:1 after iv or oral administration. This is consistent with previous reports for this study.9,10
Although this model provides for robust estimates of ELF concentrations in normal, healthy volunteers enrolled in the Phase 1 study, one potential limitation is in the extrapolation of these results to infected patients. In theory, data obtained from healthy volunteers may not be representative of the ELF penetration in infected patients. However, given the limitations of conducting BAL sampling in infected patients, these studies in healthy volunteers are generally accepted as a reasonable alternative.10,11
Ultimately, any future PK–pharmacodynamic target attainment analyses using the population PK model described here will enable predictions that are based on robust estimations of ELF and plasma exposures in simulated subjects.
Funding
This work was supported by Nabriva Therapeutics. Copyediting support for this article was funded by Nabriva Therapeutics.
Transparency declarations
W. W. W. is an employee of and holds stock in Nabriva Therapeutics plc. C. M. R. and S. M. B. are employees of Institute for Clinical Pharmacodynamics, which was contracted by Nabriva Therapeutics to perform the analyses described here. L. Z. was employed by the Institute for Clinical Pharmacodynamics when the study was conducted. Copyediting support for this article was provided by Lycely del C. Sepulveda-Torres, PhD, and Michael S. McNamara, MS from C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and funded by Nabriva Therapeutics.
This article forms part of a Supplement sponsored by Nabriva Therapeutics.
References
- 1. Paukner S, Sader HS, Ivezic-Schoenfeld Z. et al. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother 2013; 57: 4489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubino CM, Xue B, Bhavnani SM. et al. Population pharmacokinetic analyses for BC-3781 using phase 2 data from patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59: 282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeitlinger M, Schwameis R, Burian A. et al. Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid. J Antimicrob Chemother 2016; 71: 1022–6. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Guidance for Industry: Population Pharmacokinetics.https://www.fda.gov/downloads/drugs/guidances/UCM072137.pdf.
- 5. Cockcroft DW, Gault MH.. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 6. Bauer RJ. NONMEM 7, Version 7.1.2. Ellicott City, MD, USA: ICON Development Solutions, 2010. [Google Scholar]
- 7. Mould DR, Upton RN.. Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2013; 2: e38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen TH, Mouksassi MS, Holford N. et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol 2017; 6: 87–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubino CM, Forrest A, Bhavnani SM. et al. Population pharmacokinetics of BC-3781 using phase 1 data. In: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, USA,2010. Abstract A1-018.
- 10. Rodvold KA, George JM, Yoo L.. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 2011; 50: 637–64. [DOI] [PubMed] [Google Scholar]
- 11. Rodvold KA, Hope WW, Boyd SE.. Considerations for effect site pharmacokinetics to estimate drug exposure: concentrations of antibiotics in the lung. Curr Opin Pharmacol 2017; 36: 114–23. [DOI] [PubMed] [Google Scholar]