Abstract
Background
Anaphylaxis is increasing in young children. The aim of the present study was to analyze the clinical characteristics of anaphylaxis in Korean infants, with a focus on food triggers.
Methods
The study analyzed the medical records of infants aged 0 to 2 years old who had been diagnosed with anaphylaxis in 23 secondary or tertiary hospitals in Korea.
Results
We identified 363 cases of infantile anaphylaxis (66.9% male). Cutaneous symptoms were most prevalent (98.6%), followed by respiratory (83.2%), gastrointestinal (29.8%), and neurologic (11.6%) symptoms. Cardiovascular symptoms were noted in 7.7% of the cases. Most of the cases of anaphylaxis (338; 93.1%) were induced by foods. The most common trigger food was cow's milk and cow's milk products (43.8%), followed by hen's eggs (21.9%), walnuts (8.3%), wheat (7.7%), peanuts (4.8%), other nuts (3.0%), and fish (2.1%). In cow's milk-induced anaphylaxis cases, more than half the cases had cow's milk specific immunoglobulin E (sIgE) levels that were lower than the diagnostic decision points (DDPs), which is 5 kUA/L for those under the age of 1 and 15 kUA/L for those over the age of 1. In anaphylaxis induced by hen's egg, most of the cases (91.8%) had hen's egg sIgE levels that were higher than the DDP, which is 2 kUA/L for those under the age of 2 and 7 kUA/L for those over the age of 2. Of the infantile anaphylaxis cases, 46.8% had been treated with epinephrine, and 25.1% had been prescribed an epinephrine auto-injector.
Conclusion
Cow's milk is the most frequent trigger food of anaphylaxis in Korean infants. However, we found no significant correlation between the sIgE level and clinical severity. Education is required regarding the importance of epinephrine as the first line therapy for anaphylaxis and on properly prescribing epinephrine for infants with a history of anaphylaxis.
Keywords: Anaphylaxis, Epinephrine, Food, Infant
Graphical Abstract
INTRODUCTION
Anaphylaxis is an acute, systemic allergic disease that is life-threatening. However, the prevalence and clinical characteristics of anaphylaxis, especially in infants, is not clear and showed diversity in many studies. The major epidemiology studies of anaphylaxis performed in 2006 reported the lifetime prevalence as 0.05%–2.0%.1 In 2014, a US nationwide cross-sectional telephone survey reported the estimated prevalence of anaphylaxis among adults as 1.6%–5.1%.2 There have been increasing concerns about food induced anaphylaxis and anaphylaxis in the young age group.3,4,5,6 National Health Interview Survey in America reported that food allergy increased 18% from 1997 to 2007 and that hospitalization related to food allergy has also increased.7 In a retrospective cohort study including children 6 months to 18 years of age with food-related anaphylaxis from 37 children's hospitals in US between 2007 and 2012, the number of cases of food-related anaphylaxis increased from 41 per 100,000 emergency department (ED) visits to 72 per 100,000 ED visits. They reported that the proportion of anaphylaxis cases in infants 6 months to 1 year of age among total anaphylaxis cases in children was 6% (428 episodes among total 7,303 episodes). And they also identified young age (< 1 year old) was an important factor for hospital admission (odds ratio, 1.8).8 As infants cannot express the symptoms of anaphylaxis well and often have difficulties in recognizing them, not only diagnosis is not easy, but also treatment is difficult, making them a unique age group. Although it is important to monitor this age group, reported data on the characteristics of infant anaphylaxis is rare.9 Among children, most anaphylaxis cases occurred by food allergen and frequent causative foods differ from country to country due to different food culture. We conducted a multicenter survey to analyze the clinical characteristics of anaphylaxis in Korean infants focused on triggers and clinical characteristics.
METHODS
Subjects
The anaphylaxis patients between the age of zero and two who were diagnosed by pediatric allergists were enrolled from the survey on the national scale of 23 secondary or tertiary hospitals to evaluate the status of pediatric anaphylaxis in Korea between 2009 to 2013 by the Food Allergy and Atopic Dermatitis (FAAD) Study Group in the Korean Academy of Pediatric Allergy and Respiratory Disease.10
Medical records were retrospectively analyzed to find the patients who have a diagnostic code that corresponds to anaphylaxis from the International Statistical Classification of Diseases, 10th revision diagnosis, that is, T78.2 (anaphylactic shock, unspecified), T78.0 (anaphylactic shock due to adverse food reaction), T80.5 (anaphylactic shock due to serum), T88.6 (anaphylactic shock due to adverse effect of correct drug or medicament properly administered), and T63.4 (toxic effect of venomous animals, venom of other arthropods, labeled insect sting anaphylaxis for this study). After a pediatric allergy specialist confirmed whether the patients' record meets the diagnosis criteria of anaphylaxis by the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network in 2006,11 the patients were enrolled in the 2009–2013 pediatric anaphylaxis survey.
Case report form
We used the case report form (CRF) which was developed by FAAD Study Group in the Korean Academy of Pediatric Allergy and Respiratory Disease and used in our previous study published in 2016.10 It is composed of demographics, personal allergy disease history and family history, main triggers, clinical manifestation, diagnostic test on suspicious allergen and treatment contents. The clinical manifestations of anaphylaxis are categorized into 5 organ symptoms such as cutaneous, respiratory, gastrointestinal, neuromuscular and cardiovascular symptoms. The time interval between the exposure to trigger and symptom development, biphasic reaction and blood pressure were also included in the CRF.
Laboratory data analysis
We collected laboratory data confirming the triggers in anaphylaxis patients between the age of zero and two. In skin prick test results, the cases that had a wheal diameter over 3 mm or equal to or greater than histamine control was defined as positive. Among the patients who received ImmunoCAP (Thermo Fisher Scientific, Uppsala, Sweden) as a diagnosis test, those with cow's milk and hen's egg anaphylaxis were further analyzed. It was checked whether their specific immunoglobulin E (sIgE) level exceeds the cutoff values of sIgE announced in 2001 by Sampson, which enable food allergy diagnosis even if no challenge test is conducted.12 How well it conforms to the cutoff value of sIgE titer that has a diagnostic decision point (DDP) level with a positive predictive value at 95% (98% for hen's egg white) or over was examined, which is 5 kUA/L (under the age of one) or 15 kUA/L (over the age of one) in cases of cow's milk and 2 kUA/L (under the age of two) or 7 kUA/L (over the age of two) in cases of hen's egg white.
Statistical analysis
SPSS for Windows, version 21.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Continuous variable that does not have normal distribution was expressed in median values.
Ethics statement
This study was approved by the independent ethics committee at each hospital including Institutional Review Board (IRB) of Hallym University Dongtan Sacred Heart Hospital (approval No. 2014-045) and the need for informed consent was waived by the board.
RESULTS
Demographics
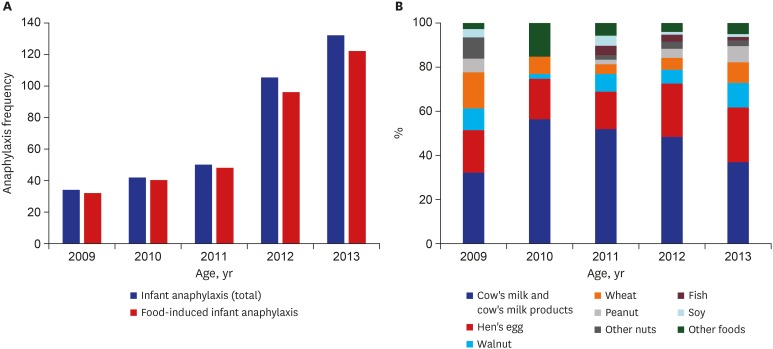
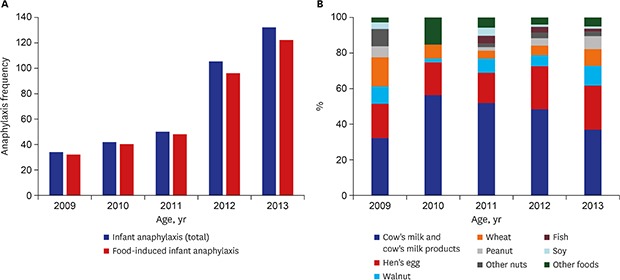
A total of 363 anaphylaxis patients between the age of zero and two years old were enrolled and their median age was 15 months (range, 0–35). There were more boys than girls with its proportion 66.9%. Among the patients, 74% were already aware of the food allergy and 56.7% had accompanying atopic dermatitis. Forty-seven patients among 363 infants (12.9%) had allergic rhinitis, 41 (11.3%) had asthma, 7 patients (1.9%) had chronic urticaria and patients who had drug allergy was 7 (1.9%). Family history of allergic disease was observed in 45.2% of the patients. Annual variation of the number of anaphylaxis in infants showed an increasing trend, with the number of cases nearly quadrupling between 2009 to 2013 (Fig. 1A).
Fig. 1. Annual variation of anaphylaxis in infants. (A) The number and (B) common trigger food.
Triggers
The most frequent trigger was food, accounting for 93.1% (338/363), followed by drugs (3%, 11/363). The rest included three cases of food-dependent exercise-induced anaphylaxis, two cases of insect bite-induced anaphylaxis, and nine cases with unknown cause. In the food trigger group, cow's milk and cow's milk products occupied the largest proportion at 43.8% (148/338) and hen's egg occupied the second largest proportion at 21.9% (74/338), followed by walnut, wheat, peanut, other nuts (pine nut, almond, pecan), fish and soy (Table 1). Time trend of common trigger foods of infant anaphylaxis is showed in Fig. 1B.
Table 1. Triggers in 363 infants with anaphylaxis.
| Variables | No. (%) | |
|---|---|---|
| Food | 338 (93.1) | |
| Cow's milk & cow's milk products | 148 (43.8) | |
| Hen's egg | 74 (21.9) | |
| Walnut | 28 (8.3) | |
| Wheat | 26 (7.7) | |
| Peanut | 16 (4.7) | |
| Other nutsa | 10 (3.0) | |
| Fish | 7 (2.1) | |
| Soybean | 6 (1.8) | |
| Others | 20 (5.9) | |
| No data | 3 (0.9) | |
| Drugb | 11 (3.0) | |
| Food-dependent exercise-induced anaphylaxisc | 3 (0.9) | |
| Insect bite | 2 (0.6) | |
| Unknown | 9 (2.5) | |
aPine nut (5 cases), almond (4 cases), pecan (1 case); bAntibiotics (5 cases), anesthetics (2 cases), NSAIDs (1 case), vaccine (1 case), vitamin (1 case), steroid (1 case); cCow's milk and cow's milk product (2 cases), buckwheat (1 case).
Clinical manifestation
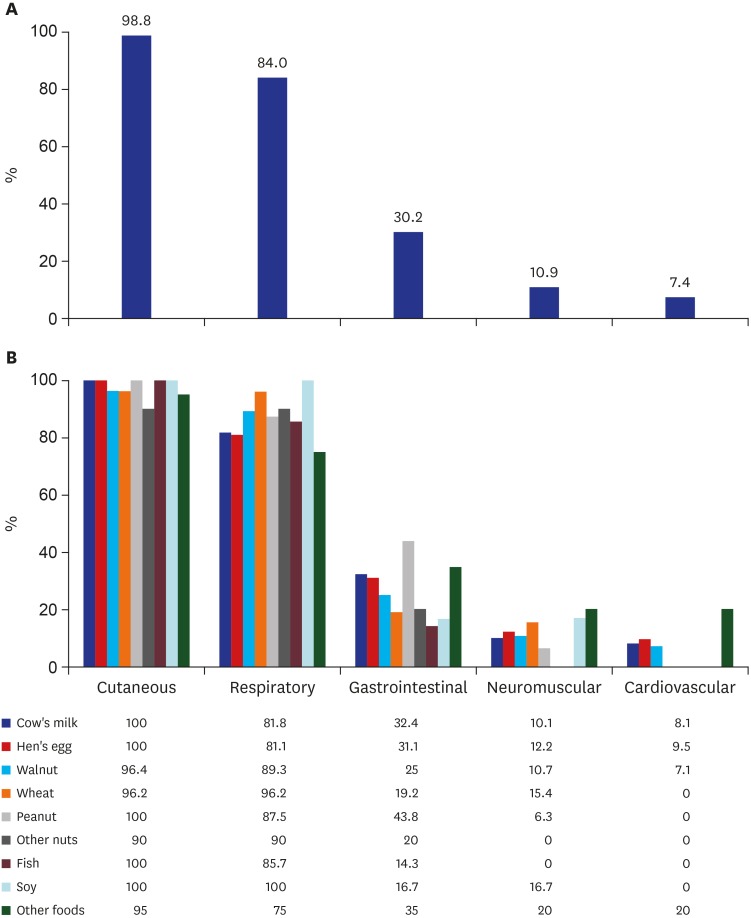
As for the symptoms of anaphylaxis, cutaneous symptoms were most frequently observed, with a proportion of 98.6%, and respiratory symptoms were 83.2%, followed by gastrointestinal symptoms and neuromuscular symptoms. There were only 7.7% of cardiovascular symptoms (Fig. 2). Only in 9.1% of the cases, blood pressure was measured.
Fig. 2. Clinical manifestation of 338 food-induced infant anaphylaxis. Percentage of (A) each clinical symptom and (B) clinical manifestation by trigger food.
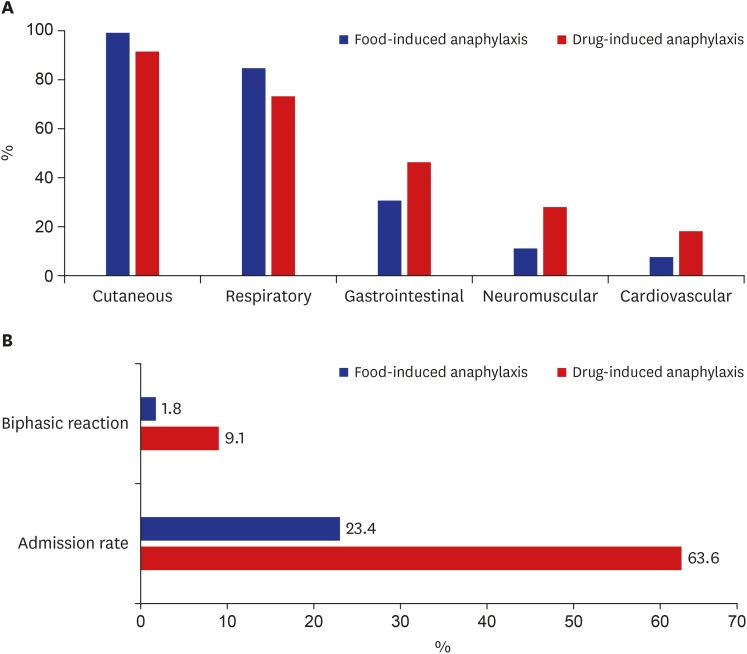
Comparing the symptoms between food-induced anaphylaxis and drug-induced anaphylaxis, there were some differences in involved organs and pattern of clinical symptoms. In the case of drug induced anaphylaxis, the proportion of cardiovascular symptoms was 2.5 times higher than that in the case of food-induced anaphylaxis (18.2% vs. 7.4%). Drug anaphylaxis also had 2.5 times (27.3% vs. 10.9%) the proportion of neuromuscular symptoms and 1.5 times (45.5% vs. 30.2%) the proportion of gastrointestinal symptoms than the case of food-induced anaphylaxis. While 4.7% of the patients with infant anaphylaxis experienced biphasic reaction, the proportion was as high as 9.1% in the case of drug anaphylaxis. This is approximately five times higher than that of patients with food-induced anaphylaxis at 1.8%. The proportion of those who needed hospitalization was 2.7 times higher in the case of drug cause than the case of food cause (63.6% vs. 23.4%) (Fig. 3).
Fig. 3. Differences between food and drug anaphylaxis. (A) Involved organs and (B) clinical course.
Among the total patients, 51% (185/363) showed symptoms within 30 minutes after the exposure to trigger. Excluding the cases that the time until the occurrence of symptoms after the exposure is unknown (91 cases), 68% (185/272) of the patients showed symptoms within 30 minutes. There were some cases (31 cases, 8.6%) which showed symptoms after two hours (Table 2).
Table 2. Time until the occurrence of symptom after the exposure in infant anaphylaxis.
| Variables, hr | No. (%) |
|---|---|
| Immediate | 69 (19.0) |
| < 0.5 | 116 (32.0) |
| 0.5–2 | 56 (15.4) |
| 2–4 | 18 (5.0) |
| > 4 | 13 (3.6) |
| Unknown | 91 (25.0) |
| Total | 363 (100) |
Laboratory tests to confirm the triggers
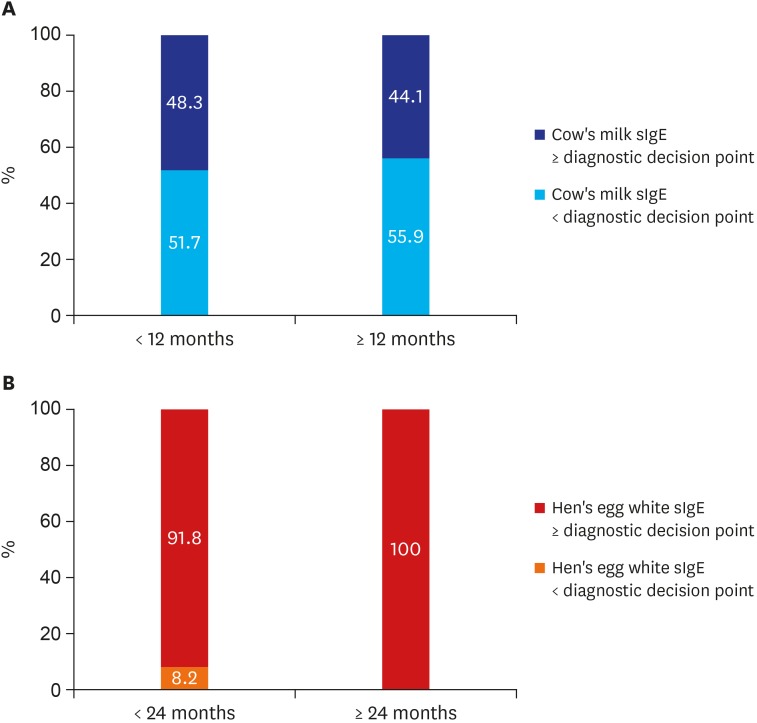
There were 307 out of 363 patients (84.6%) who received a diagnostic test to confirm anaphylaxis triggers. ImmunoCAP was the most frequently performed test with its proportion at 81.8%, followed by multiple allergen simultaneous test (28.4%), skin prick test (2.5%), intradermal test (0.6%), and 9.4% received an oral challenge test. Out of 148 cow's milk anaphylaxis patients, 79.9% received cow's milk sIgE by ImmunoCAP and 9.3% received casein sIgE. Median value of cow's milk sIgE was 6.8 kUA/L and there was a case where anaphylaxis occurred at a very low level of 0.37 kUA/L. Median value of casein sIgE was 7.76 kUA/L and minimum value was 0.59 kUA/L. We categorized 119 cow's milk anaphylaxis cases into two groups according to the value of DDP of cow's milk sIgE12 measured by immunoCAP. The patients with a value less than DDP of 5 kUA/L was 31 out of 60 (51.7%) in cases of those under 12-months-old. And the patients with a value less than DDP of 15 kUA/L was 33 out of 59 (55.9%) in cases of those over 12-months-old. More than half of the cow's milk anaphylaxis cases developed the anaphylactic symptoms in values less than the value of DDP (Table 3 and Fig. 4A).
Table 3. Laboratory data of infant anaphylaxis.
| Variables | No. (%) | Mean ± SD | Median (Min.–Max.) | ||
|---|---|---|---|---|---|
| ImmunoCAP data of cow's milk anaphylaxis infants (n = 148) | |||||
| Total IgE, kUA/L | 125 (84.5) | 353.45 ± 669.71 | 128.00 (4.30–5,001.00) | ||
| Specific IgE, kUA/L | |||||
| Cow's milk | 119 (80.4) | 22.16 ± 46.18 | 6.80 (0.37–427.00) | ||
| Casein | 14 (9.5) | 14.75 ± 16.66 | 7.76 (0.59–54.60) | ||
| ImmunoCAP data of hen's egg anaphylaxis infants (n = 74) | |||||
| Total IgE, kUA/L | 66 (89.2) | 389.18 ± 600.68 | 155.00 (13.50–3,282.00) | ||
| Hen's egg white sIgE, kUA/L | 59 (79.7) | 24.45 ± 30.17 | 10.40 (1.03–100.00) | ||
SD = standard deviation, IgE = immunoglobulin E.
Fig. 4. The DDP for food allergy by Sampson12 and sIgE of anaphylaxis infants. (A) More than half of the cow's milk anaphylaxis cases developed the anaphylactic symptoms in value less than the value of DDP. (B) In hen's egg anaphylaxis, most of the cases had hen's egg white sIgE levels above the DDP.
DDP = diagnostic decision point, sIgE = specific immunoglobulin E.
Among the 74 hen's egg anaphylaxis patients, 59 cases (79.7%) were confirmed as the hen's egg white sIgE using immunoCAP. Median value was 10.4 kUA/L and minimum value was 1.03 kUA/L. We categorized 59 hen's egg anaphylaxis cases into two groups according to the value of DDP of hen's egg sIgE.12 The patients with the values higher than DDP of 2 kUA/L was 91.8% in cases of those under 24-months-old and all of the patients over 24-months-old showed hen's egg sIgE higher than DDP of 7 kUA/L (Table 3 and Fig. 4B).
Initial management and prescription of epinephrine
For 175 patients, their initial treatment of anaphylaxis occurred in the emergency room (ER). Type of medications used for initial management of anaphylaxis were analyzed in cases of anaphylaxis patients who visited the ER. Antihistamine was used in 82.3% of the cases, intravenous fluid injection was used in 65.7%, systemic steroid in 57.4% and epinephrine was used in 46.8% of the cases. Bronchodilator was used in 28% and oxygen was used in 24.6% (Table 4).
Table 4. Initial treatment of 175 infant anaphylaxis in emergency room.
| Treatment | No. (%) |
|---|---|
| Antihistamine | 144 (82.3) |
| Intravenous fluid | 115 (65.7) |
| Systemic steroid | 101 (57.4) |
| Intramuscular epinephrinea | 82 (46.8) |
| Bronchodilator | 49 (28) |
| Oxygen supply | 43 (24.6) |
aIncluding the 3 patients (1.7%) who had epinephrine injection before the arrival at hospital.
Among the 193 patients' data that had record, 4.7% of the cases showed biphasic reaction and 14.5% experienced repeated anaphylaxis episodes. Only 25.1% of the cases had a prescription of epinephrine auto-injector.
DISCUSSION
This study found a steep increase in infantile anaphylaxis in Korea, with the number of cases nearly quadrupling between 2009 to 2013. Australia reported a steady increase in hospital admissions due to anaphylaxis (8.8 percent per year) between 1993 and 2005. In particular, the number of cases of food-induced anaphylaxis among children under the age of 5 has increased significantly.4 The prevalence of food allergy in Korean elementary school children was 4.2% in a 1995 national survey and 5.2% in a 2006 survey.13,14 The increase in anaphylaxis may be due to an increase in food allergies that has in turn increased the number of cases of anaphylaxis caused by food. However, there is the possibility that the rate of diagnosis of anaphylaxis has increased as patients and guardians have become more aware of it.
Food was the most frequently observed cause of anaphylaxis, and cow's milk and cow's milk products were the most common foods followed by hen's egg, walnut, wheat, peanut, other nuts, fish, and soy in infants between the age of zero and two years old. In a multicenter study which was performed in 14 tertiary hospitals in Korea between September 2014 and August 2015 by Korean Academy of Pediatric Allergy and Respiratory Diseases (KAPARD), FAAD Study Group investigated frequent food allergen of 2,056 children with immediate-type food allergy aged 0 to 18 years old. The study reported that hen's egg (27.4%), cow's milk (26.6%), walnut (7.2%), wheat (6.2%), peanut (5.5%), soy (2.4%), shrimps (2.2%), buckwheat (1.7%) were the frequent food allergens in order.15 Another multicenter study performed by KAPARD in 2009–2013 reported the frequent food allergens sensitized to 740 children with food induced anaphylaxis aged 0 to 18 years old, were cow's milk (28.4%), hen's egg (13.6%), walnut (8.0%), and wheat (7.2%), buckwheat (6.5%), and peanut (6.2%), in order.10
These two previous Korean studies show that the common causes of food allergy and those of anaphylaxis are somewhat different. Hen's egg was the most common food allergen sensitized in children with immediate-type food allergy, however, cow's milk was the most common trigger food in children with anaphylaxis in Korea. Buckwheat is lower in the common food ranking that causes immediate-type food allergy, but is ranked higher among the causes of anaphylaxis in Korean children. This suggests that cow's milk and buckwheat can cause relatively severe allergic reactions. In the study, cow's milk was the most common food that caused anaphylaxis in infants aged 0–2 years, accounting for 43.8%, and even at very low levels of cow's milk sIgE. It was remarkable that walnut (8.3%) was more frequently observed than peanut (4.7%) in cases of anaphylaxis in Korean infants. It is probably because Koreans consume walnut more frequently than peanut.16
A study by a single institute research study in America that examined triggers of anaphylaxis among children by age reported that the most frequent causes are cow's milk, peanut, tree nut and hen's egg in cases of infant, tree nut, peanut and cow's milk in cases of preschool age and shellfish, tree nut and fruit in cases of adolescent.17 A nationwide multi-institute survey in America revealed that hen's eggs, fruit, peanuts, and tree nuts are frequently observed in cases of children under five and shellfish is common among children over six.18 A European study indicated tree nut (cashew, in particular) and peanut as the most frequent cause of pediatric anaphylaxis and it reported that hen's egg and cow's milk are the most frequent among children under three.19 In Japan, hen's egg, cow's milk and wheat were proven to be the most frequent cause of food allergy, occupying 90% in cases of children under the age of one in particular, followed by crustacean, fruit, buckwheat, fish and peanut.20 As such, the causal food of food-induced anaphylaxis and food allergy show different distribution due to the difference of food culture by the nations.
The most frequently observed symptoms of infant anaphylaxis were cutaneous symptoms and respiratory symptoms, regardless of the cause. However, compared to food-induced anaphylaxis, drug anaphylaxis had high proportion of cardiovascular symptoms, gastrointestinal symptoms, and nervous system symptoms. In domestic survey on children and adolescent anaphylaxis conducted by our research group, cardiovascular symptoms were observed in 14.3% of the total children under the age of 18.10 Comparing to this, cardiovascular symptoms were as low as 7.7% in the case of young age group between zero and two years in this study. Difficulty of recognizing cardiovascular symptoms in young age can be one of the reasons. Considering the small number of records on blood pressure, it can be inferred that blood pressure measurements were often omitted when a young infant came with anaphylaxis symptoms. Since reduced blood pressure is one of the diagnostic criteria of anaphylaxis, blood pressure measurement must be performed even in infants when anaphylaxis is suspected.
In this study, 4.7% of the cases with record data showed biphasic reaction. A study in Thailand reported that 6.3% of the anaphylaxis patients who visited ER showed biphasic reaction and that the time interval from onset to administration of epinephrine was a predicator.21 A multi-institution research study on pediatric anaphylaxis in Turkey reported 3.1% of biphasic reaction.22
Drug anaphylaxis had high portion of biphasic symptoms at 9.1%, which is approximately five times higher than that in case of food-induced anaphylaxis. Proportion of severe cases that required hospitalization was also 2.7 times higher in drug anaphylaxis. A previous study reported that history of drug anaphylaxis is a contributing factor to induce biphasic reaction. A meta-analysis of patients with biphasic anaphylaxis reported that unknown trigger and hypotension are correlated with biphasic anaphylaxis, which seldom develops in food-induced cases.23 There is a study reported that biphasic reactions were associated with a history of prior anaphylaxis, unknown precipitant, symptoms of diarrhea, and wheezing.24
Although hen's egg anaphylaxis developed in most cases over the DDP of hen's egg white sIgE by Sampson,12 more than half of cases with cow's milk anaphylaxis developed anaphylaxis when cow's milk sIgE value was less than the DDP, implying that the correlation between cow's milk sIgE and anaphylaxis cannot be as high as we thought. In Japan, a paper was published in 2007 that reported the cutoff value of sIgE of cow's milk allergy at 5.8 in cases of infants under one, 38.6 in cases of one-year-olds, and 58.3 kUA/L in cases of those over two.25 There was one paper published in Korea that examined DDP of cow's milk sIgE in Korean children. However, the subject was limited to pediatric atopic dermatitis patients.26 For clearer conclusion, detailed correlation analysis between the severity of symptoms and cow's milk sIgE using a whole sample of cow's milk allergy patients will be required.
In our study, 46.8% of anaphylactic infants received epinephrine treatment as initial management. A research study conducted in ten European countries reported that the percentage of epinephrine treatment when an adolescent visited ER due to anaphylaxis increased from 12% in 2011 to 25% in 2014.27 In a study in Australia, the epinephrine prescription rate of pediatric severe anaphylaxis cases who visited ER was 39.3%.28 According to a study in America, the percentage of anaphylaxis patients receiving epinephrine increased over time (40% in 2002 to 59% in 2006).7
In this study, prescription rate of epinephrine auto-injector was as low as 25%. Among infant anaphylaxis patients, a large portion had weight less than 15 kg. The fact that there is only 0.15 mg dose of epinephrine auto-injector for children can be one of the reasons of low prescription rate. The position paper by European Academy of Allergy and Clinical Immunology Taskforce on Anaphylaxis in Children argued that an ample of epinephrine and syringe can be given to small children to account for weight but it is more practical to use 0.15 mg epinephrine auto-injector in case of healthy infants over 7.5 kg.29 According to a recent study in Japan, no other side effects were reported except pain in the injection site in anaphylaxis children under the age of 3 using 0.15 mg of epinephrine auto-injector.30 We believe that using this kind of method can increase the prescription rate of epinephrine among infants.
As for the conclusion of this study, although cow's milk is the most frequent trigger food of infant anaphylaxis in Korea, correlation was not high between cow's milk sIgE level and clinical anaphylaxis. Hence, it is necessary to carefully interpret the results of low cow's milk sIgE in case of patients who show suspicious symptoms of cow's milk anaphylaxis and oral challenge test will be required for confirmation. Moreover, education is required regarding the importance of epinephrine as the first line therapy for anaphylaxis and on properly prescribing epinephrine for infants with a history of anaphylaxis.
ACKNOWLEDGMENTS
We thank the members of the Korean Academy of Pediatric Allergy and Respiratory Diseases for collecting valuable patient data.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee S, Pyun BY.
- Data curation: Min TK, Jeon YH.
- Formal analysis: Lee SY, Kim KW.
- Investigation: Kim HH, Yum HY.
- Methodology: Kim WK, Park YM, Kim JH, Ahn K.
- Software: Lee YJ, Jang GC, Song TW, Kim J.
- Validation: Jeong KU, Kim YH.
- Writing - original draft: Jeon YH.
- Writing - review & editing: Lee SY, Pyun BY.
References
- 1.Lieberman P, Camargo CA, Jr, Bohlke K, Jick H, Miller RL, Sheikh A, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 2.Wood RA, Camargo CA, Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32(1):35–50. doi: 10.1016/j.iac.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993–1994 to 2004–2005. J Allergy Clin Immunol. 2007;120(4):878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol. 2015;135(4):956–963.e1. doi: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudders SA, Banerji A, Vassallo MF, Clark S, Camargo CA., Jr Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol. 2010;126(2):385–388. doi: 10.1016/j.jaci.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124(6):1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 8.Parlaman JP, Oron AP, Uspal NG, DeJong KN, Tieder JS. Emergency and hospital care for food-related anaphylaxis in children. Hosp Pediatr. 2016;6(5):269–274. doi: 10.1542/hpeds.2015-0153. [DOI] [PubMed] [Google Scholar]
- 9.Simons FE, Sampson HA. Anaphylaxis: unique aspects of clinical diagnosis and management in infants (birth to age 2 years) J Allergy Clin Immunol. 2015;135(5):1125–1131. doi: 10.1016/j.jaci.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Ahn K, Kim J, Jang GC, Min TK, Yang HJ, et al. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res. 2016;8(6):535–540. doi: 10.4168/aair.2016.8.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 13.Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, et al. Prevalences of symptoms of asthma and other allergic diseases in Korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001;16(2):155–164. doi: 10.3346/jkms.2001.16.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suh M, Kim HH, Sohn MH, Kim KE, Kim C, Shin DC. Prevalence of allergic diseases among Korean school-age children: a nationwide cross-sectional questionnaire study. J Korean Med Sci. 2011;26(3):332–338. doi: 10.3346/jkms.2011.26.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S. A Study for Prevention and Control of Food Allergy. Research Report of Grant from Korea Ministry of Food and Drug Safety (MFDS) in 2015. Cheongju: Ministry of Food and Drug Safety; 2015. [Google Scholar]
- 16.Hwnag YJ. The Table of Food Supply and Demand in 2007. Seoul: Korea Rural Economic Institute; 2008. [Google Scholar]
- 17.Rudders SA, Banerji A, Clark S, Camargo CA., Jr Age-related differences in the clinical presentation of food-induced anaphylaxis. J Pediatr. 2011;158(2):326–328. doi: 10.1016/j.jpeds.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121(1):166–171. doi: 10.1016/j.jaci.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Vetander M, Helander D, Flodström C, Ostblom E, Alfvén T, Ly DH, et al. Anaphylaxis and reactions to foods in children--a population-based case study of emergency department visits. Clin Exp Allergy. 2012;42(4):568–577. doi: 10.1111/j.1365-2222.2011.03954.x. [DOI] [PubMed] [Google Scholar]
- 20.Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, et al. Japanese guideline for food allergy 2014. Allergol Int. 2014;63(3):399–419. doi: 10.2332/allergolint.14-RAI-0770. [DOI] [PubMed] [Google Scholar]
- 21.Lertnawapan R, Maek-a-nantawat W. Anaphylaxis and biphasic phase in Thailand: 4-year observation. Allergol Int. 2011;60(3):283–289. doi: 10.2332/allergolint.10-OA-0256. [DOI] [PubMed] [Google Scholar]
- 22.Orhan F, Canitez Y, Bakirtas A, Yilmaz O, Boz AB, Can D, et al. Anaphylaxis in Turkish children: a multi-centre, retrospective, case study. Clin Exp Allergy. 2011;41(12):1767–1776. doi: 10.1111/j.1365-2222.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408–416.e1-2. doi: 10.1016/j.jaip.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Bellolio MF, Hess EP, Campbell RL. Predictors of biphasic reactions in the emergency department for patients with anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(3):281–287. doi: 10.1016/j.jaip.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007;119(5):1272–1274. doi: 10.1016/j.jaci.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Kim HY, Park MR, Choi J, Shim JY, Kim MJ, et al. Diagnostic decision points of specific IgE concentrations in Korean children with egg and cow's milk allergies. Allergy Asthma Immunol Res. 2015;7(4):332–338. doi: 10.4168/aair.2015.7.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137(4):1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Braganza SC, Acworth JP, Mckinnon DR, Peake JE, Brown AF. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006;91(2):159–163. doi: 10.1136/adc.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, et al. The management of anaphylaxis in childhood: position paper of the European Academy of Allergology and Clinical Immunology. Allergy. 2007;62(8):857–871. doi: 10.1111/j.1398-9995.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 30.Ito K, Ono M, Kando N, Matsui T, Nakagawa T, Sugiura S, et al. Surveillance of the use of adrenaline auto-injectors in Japanese children. Allergol Int. 2018;67(2):195–200. doi: 10.1016/j.alit.2017.07.002. [DOI] [PubMed] [Google Scholar]