Abstract
The circadian time structure (CTS) has long been the subject of research in occupational medicine, but not to industrial toxicology, including methods of setting threshold limit values (TLVs) and employee biological monitoring. Numerous animal and human investigations document vulnerability to chemical, contagion, and other xenobiotics varies according to the circadian time of encounter. Permanent and rotating nightshift personnel are exposed to industrial contaminants in the same or higher concentration as dayshift personnel, and because of incomplete CTS adjustment to night work, contact with contaminants occurs during a different biological time than day workers. Thus, the amount of protection afforded by certain TLVs, especially for employees of high-risk settings who work night and other nonstandard shift schedules, might be inadequate. The CTS seems additionally germane to procedures of employee biological monitoring in that high-amplitude 24 h rhythms in biomarkers indicative of xenobiotic exposure may result in misjudgment of health risks when data are not gathered in sufficient frequency over time and properly interpreted. Biological reference values time-qualified for their rhythmic variation, currently of interest to laboratory medicine practice, are seemingly important to industrial medicine as circadian time and work-shift specific biological exposure indices to improve surveillance of personnel, particularly those working nonstandard shift schedules.
Keywords: Circadian rhythms, Chronotoxicology, Threshold limit values, Biological monitoring, Biological exposure limits, Rotating shift work, Permanent night work, Nonstandard work schedules
Consensus Statements.
We propose three key consensus statements, founded on new applications of circadian rhythm research, that are of potentially high relevance to the health and wellbeing of permanent night and rotating shift workers. Recommendations that follow each key statement derive from numerous around-the-clock laboratory animal and human investigations that infer shift-dependent differences in vulnerability to workplace xenobiotics and also previously trialed innovative comprehensive methods of employee biological monitoring that entail serial waking-time selfassessments coupled with improved data interpretation. Since these are novel considerations in occupational medicine, some recommendations are research oriented.
-
1)
Permanent and rotating nightshift personnel are subjected to industrial contaminants in the same or higher concentrations as dayshift personnel. Numerous (more than 125) laboratory animal and human research investigations demonstrate that the adverse effects of chemical, contagion, physical, and other xenobiotic exposures can differ extensively according to the time of the encounter. Threshold limit values are often established on the basis of homeostatic theory, i.e., the unsubstantiated assumption of constancy of biological processes and functions, and databases of acute adverse events that lack qualification for biological time or work shift of occurrence. Adjustment of the circadian time structure of normally diurnally active humans when suddenly altered by night work is slow and often incomplete. Thus, exposure of permanent night and rotating shift workers to industrial contaminants is likely to occur during a different circadian time compared to dayshift workers. Therefore, the extent of protection afforded by some threshold limit values, particularly for employees of highrisk industrial and medical settings, might be inappropriate when working nights or other nonstandard schedules. Thus, we recommend:
Workplace xenobiotic and other biological stressors be researched for circadian differences in acute and chronic adverse effects, with findings utilized to create time-weighted average, short-term, and ceiling threshold limit values optimally protective for both day and night workers.
Databases inventory acute adverse health events according to hours of work on the given shift as well as clock hour, more preferably circadian time of the affected employee, to ensure protective threshold limit values, not only for day but permanent night and other nonstandard shift work personnel.
Novel approaches entailing transcriptomics and metabolomics, which are currently used in biological rhythm research to determine circadian time through single time-of-day sampling of blood, breath, and hair follicle or tissue, be researched for applications to industrial toxicology to identify the circadian time of acute adverse reaction to workplace xenobiotic and other biological stressors.
-
2)
Human beings by nature are a diurnal species. Under ordinary conditions of life, the circadian time structure is organized to support a routine of activity during the natural light of day and sleep during the natural darkness of night. Nonstandard permanent and rotating night work schedules require the circadian time structure be reorganized to the unnatural routine of nocturnal activity and diurnal sleep. Biological adjustment to such major alteration of the normal activity/sleep pattern is neither instantaneous nor harmonious and results in a transient state of circadian disruption, i.e., mismatch between the staging of 24 h rhythms of bodily processes and functions and cyclic environmental demands and challenges and/or alteration of the period of circadian rhythms from 24.0 h. Research involving non-human models infers circadian disruption, itself, can increase the vulnerability to chemical and other xenobiotic stressors. Additionally, animal and human experiments suggest circadian disruption can be a potential adverse effect of xenobiotics. Thus, we recommend:
Research be initiated to determine if the circadian disruption caused by working nonstandard permanent and rotating night schedules poses elevated risk for acute adverse reactions to workroom xenobiotic and hazards and, accordingly, whether it needs to be an additional criterion of consideration for establishing or adjusting short-term, time-weighted, and ceiling threshold limit values.
Research be initiated to determine whether disturbance of the circadian time structure—alteration from normal of the period, staging, and/or amplitude of individual circadian rhythms or the overall circadian time structure—can be an acute and/or chronic adverse effect of workplace exposures to xenobiotics and other biological stressors and thus an additional criterion of consideration in establishing threshold limit values.
-
3)
Current methods for the conduct of biological monitoring and interpretation of derived data against daytimederived reference biological exposure indices are founded on homeostatic theory, which incorrectly assumes biological constancy. Consequently, biological monitoring is typically restricted to single or before and after work shift assessments. Many biomarkers of exposure and biological exposure indices of reference are governed by circadian processes and exhibit predictable day/night variation, sometimes of considerable magnitude, suggesting the utility of so-called biological-time or shift-qualified reference values for greater precision of data interpretation. Thus, we recommend:
Biological monitoring be more extensively integrated into high-risk permanent and rotating night shift settings.
Biological monitoring, particularly in high-risk settings, entail employee serial self-assessments of biomarkers indicative of health status throughout the waking span, i.e., both at and off work. This is exemplified by surveillance programs consisting of: (i) serial self-measurement of peak flow rate (measure of airway patency and ease of respiration) by personnel of certain work environments using small, easy-to-use, inexpensive instrumentation to detect early signs of detrimental effects on the airways and lungs, and (ii) serial self-collections of body fluids, such as urine and/or saliva, by employees routinely exposed to dangerous chemical and other contaminates to better detect risk for acute and/or chronic adverse effects;
Research to assess the need for biological-time or shift-qualified reference values to improve interpretation of data derived from biological monitoring when high peak-to-trough 24 h variation is characteristic of reference biological exposure indices.
More extensive research be conducted to develop wearable small-scale, inexpensive, worker-acceptable biosensors/technology for convenient real-time biological monitoring, including for sampling blood or other bodily fluids, especially for personnel of high risk occupational settings engaged in nonstandard work schedules.
Companies responsibly manage personal information when collecting, storing, and processing biological monitored and other employee data. In Europe this policy now falls under the General Data Protection Regulation; https://www.eugdpr.org/. Data are increasingly gathered by internet, mobile phones, sensors, radiofrequency identification tags, and information systems, enabling management to build comprehensive personal biometric and psychometric profiles. The greater the amount of information collected and processed, the more complete the ‘fingerprint’ of the employee and the greater the difficulty of maintaining anonymity and security. Personal data should not be used or processed in ways incompatible with approved purposes. Workers need to be clearly informed about how their employer transmits, stores, processes, and protects their personal information, and they must know their rights, such as the right to withdraw consent for use of their biological monitored or other data without prejudice or penalty.
Consensus statements review expert panel: Arne LOWDEN1(Chair), Claire CARUSO2, Francesco PORTALUPPI3
1Stockholm University, Sweden
2National Institute for Occupational Safety and Health, USA
3American Association for Medical Chronobiology and Chronotherapeutics, USA
Full consensus among panel members on all statements.
Introduction
This consensus paper focuses on the pertinence of the human circadian time structure (CTS) to industrial toxicology, specifically threshold limit values and biological monitoring. It is one of several consensus papers developed by the Working Time Society, commissioned by the International Commission on Occupational Health1), published in this journal issue. Each describes the current state of knowledge, identifies health and safety risks, and offers recommendations for effective interventions and future research through a series key consensus statements developed utilizing procedures outlined in Wong et al1). Collectively, the reports provide guidance to a broad international audience of researchers, industry members, labor representatives, policy makers, workers, and other interested stakeholders on managing fatigue and ensuring health and safety of those routinely engaged in nonstandard work schedules.
Background
The impact of the CTS and its disruption by permanent night and rotating shift schedules on worker fatigue, performance, accidents, and health is well documented2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18). Its relevance to industrial toxicology, i.e., development of threshold limit values (TLVs) and conduct of biological monitoring (BM), for optimal protection of the health and wellbeing not only of day but night and other nonstandard shift workers, however, is far less appreciated. This is the situation even though more than 125 investigations substantiate the acute adverse effects of a wide array of chemical, biological, and other xenobiotic stressors often vary extensively according to the time of exposure with regard to the staging of responsive circadian rhythms, and the several published position papers that corroborate their relevance to industrial toxicology17, 19,20,21,22). Of additional relevance are recent findings reported by Jay et al.23) revealing permanent and rotating nightshift personnel are subjected to workroom contaminants in the same or higher concentration than dayshift personnel. Collective knowledge of circadian rhythms in vulnerability plus between-shift differences in exposure to harmful substances infers the nightshift may pose greater risk for adverse effects than the dayshift.
Threshold limit values
The American Conference of Governmental Industrial Hygienists defines a TLV as the level of a chemical, physical, or biological agent to which nearly all workers may be repeatedly exposed, day after day, without adverse health effects. Three types of TLVs regulate industrial exposures–time-weighted average ones applicable to typical 8 h/d, 40 h/wk exposures; short-term exposure limit ones applicable to brief (≤15 min) encounters of ≤4 times/d with ≥60 min between each; and ceiling limit, i.e., maximum allowed exposures ones—and industrial hygienists or other trained specialists are responsible for ensuring compliance24). A major theoretical construct underlying TLVs is homeostasis, i.e., the unsubstantiated assumption of constancy of biological functions and processes, implying the time or shift during the 24 h when workplace contaminants are experienced is inconsequential. The well corroborated concept of chronobiology—rhythmicity of biological functions and processes—in contrast, implies the opposite perspective, i.e., the time during the 24 h when exposure occurs can be a critical determinant of vulnerability and risk for adverse health effects24).
Circadian Time-Keeping
Human and other life forms are organized both spatially as cells, tissues, organs, and systems and also temporally as endogenous biological rhythms of different period [tau, τ], i.e., ultradian (τ<20 h), circadian (τ≈24 h), and infradian (τ>28 h)24). This consensus report focuses on the totality of the body’s circadian rhythms, which we term the circadian time structure (CTS), as the basis of the possible differential vulnerability of night versus day workers to contaminate xenobiotic chemical, contagion, and other potentially harmful stressors.
The CTS of mammals, including humans, is orchestrated by a central brain time-keeping axis composed of clock genes of the suprachiasmatic nuclei (SCN) located within the hypothalamus plus the pineal gland through the synthesis and circulation of the hormone melatonin only during the darkness of night. Collectively, the SCN and pineal gland coordinate the τ and staging, such as the peak and trough times, of the multitude of autonomous peripheral biological clocks that govern cellular and organ 24 h rhythms24, 25). Under usual circumstances, the CTS is organized by external time cues, i.e., natural 24 h cyclic phenomena, which in totality constitute the environmental time structure (ETS)25). The most important time cues for ancestral humanoids was natural sunrise and sunset, but for humans today, it is mainly the onset and offset times of artificial electric light sources18, 25, 26). These natural and artificial light cues are sensed by the photopigment melanopsin contained within intrinsically photoreceptive ganglion cells of the retinae and conveyed via the retinohypothalamic neural tract to the master brain clock, the SCN27, 28). Additional temporal cues, like time (s) of nutrient consumption, are also of importance, especially to peripheral cellular, tissue, and organ clocks24, 26). The τ and staging of the numerous endogenous circadian rhythms that compose the CTS under normal conditions of life are internally coordinated and synchronized relative to the τ and staging of cyclic 24 h phenomena that comprise the ETS. Thus, biological processes of human beings are organized in time to support optimal efficiency of metabolic, cognitive, and physical processes during the usual diurnal activity span and repair and restorative processes during the usual nocturnal sleep span24). Throughout evolution the well-integrated and highly organized CTS, precisely entrained to natural ambient light/dark and other cyclic environmental signals, conferred functional and survival advantage through differential biological and cognitive readiness during the 24 h to cope with usual predictable-in-time extraneous challenges24, 29).
Biomarkers of Circadian Time
Throughout the years a variety of biomarkers have been used to determine circadian time17, 30, 31); however, the peak time or other features of the body temperature and hormone melatonin circadian rhythms are perhaps the most popular ones32,33,34). Early research studies enlisted the cooperation of shift workers to self-record the clock time of sleep onset and offset plus oral temperature taken at frequent intervals during the wake span while working night and dayshifts and also during off days35). In many such studies, urine voids or saliva samples were also self-collected to determine melatonin concentration. Customarily, such data were analyzed by special time series, so-called Cosinor, analyses to objectively determine the peak or other characteristics of the respective biomarkers of circadian time17, 25, 31), and in the case of melatonin the dim light melatonin onset time34). These biomarkers and the methods utilized to obtain them, while appropriate for research investigation, are impractical for routine application in occupational medicine, because of likely noncompliance of workers to self-collect body fluids at designated times of the day and night plus high cost to industry to analyze such samples for melatonin or other constituents indicative of circadian time. New transcriptome and metabolome approaches coupled with interpretative algorithms enable determination of circadian time by once-a-day sampling of blood, breath, and hair follicle and other tissue36,37,38,39,40,41,42,43). Such methods are currently being explored in forensic medicine to estimate circadian time from analysis of single blood spots44), and seemingly it is worthy of research for future application to occupational medicine and industrial toxicology.
Impact of Nonstandard Work Schedules on Circadian Time-Keeping
The staging of individual rhythms of the CTS accommodate both to natural and unnatural alteration of the ETS, respectively, seasonal variation in daily photoperiod duration and rapid transmeridian displacement by aircraft or working permanent night and rotating shift schedules29, 31, 35). Indeed, nonstandard work schedules that entail artificial light at night exposure and abnormal meal timings disrupt the normal phase relationships between individual 24 h rhythms and/or perturb the circadian τ from 24.0 h26, 35, 45). Accommodation of the CTS to major alteration of the normal diurnal activity in light/nocturnal rest in darkness routine, for example, necessitated by nonstandard work schedules, is neither instantaneous nor harmonious30, 46, 47). For some persons, adjustment may be arduous or even impossible due to age, genetic, medical, or other factors, resulting in persistent circadian rhythm disruption with associated symptoms of disordered sleep, digestive system complaints, altered mood, and marked and persistent fatigue14, 29, 48).
Circadian Rhythms in Disposition of Xenobiotics
Uptake of chemical and other xenobiotic substances by the skin, lungs, and gastrointestinal tract and their distribution, metabolism, and elimination are governed by circadian processes24). Their absorption by skin is modulated by 24 h rhythms of tissue hydration, acid-base balance, porosity, blood flow, immunology, sebum formation, and keratinocyte, melanocyte, fibroblast, and basal cell processes24, 49, 50). Deposition of gasses, particulates, and other matter via the alveolar tissue of the lungs depends on endogenous 24 h periodicities of respiratory rate, tidal and minute volume, lung diffusion capacity, plus alveolar blood flow and cellular processes24, 51,52,53). Ingestion of chemical and solid matter by the gastrointestinal tract depends on nyctohemeral rhythms of gastric pH, enzyme concentration and activity, motility/transit time, blood flow, bacterial biome, active and passive transport phenomena, and local cellular processes24, 54). The metabolism, detoxification, and elimination of chemical substances also are governed by circadian rhythms of Phase I (e.g., P450 cytochromes, aldehyde dehydrogenases, and carboxylesterases), Phase II (e.g., glucuronosyltransferases, sulfotransferases, and glutathione S-transferases conjugations), and Phase III (uptake and efflux transporter) processes24, 54). These and other CTS-regulated activities are of proven relevance to clinical medicine24), and laboratory animal and human investigations clearly demonstrate they are the basis for the observed 24 h variation in vulnerability to a great variety of chemical, biological, and other xenobiotic stressors of relevance to industrial toxicology practice.
Chronotoxicology
Chronotoxicology is the study of biological time-dependent differences in the adverse effects of xenobiotic substances17, 24, 55, 56). Hundreds of animal and human research studies corroborate the CTS mediates the responses to and consequences of a wide variety of toxic challenges. Investigations involving other species, such as insects, fish, and plants, also document prominent disparity in vulnerability according to the circadian time of exposure to insecticides and pesticides—β-cyfluthrin, chlorfenapyr, dichlorodiphenyltrichloroethane, dursban, malathion, permethrin, and trichlorfon—and herbicides—glyphosate, glufosinate, fomesafen, and chlorimuron ethyl24, 57,58,59,60,61,62,63,64,65) —thus demonstrating the ubiquity across species of the phenomenon of chronotoxicity.
The concept of chronotoxicity also includes circadian disruption as an adverse effect of challenging exposures. Animal66,67,68,69) and human4, 18, 25, 26, 70,71,72,73,74) research demonstrates xenobiotics can induce alteration of molecular clock gene behavior giving rise to abnormalities of the amplitude, phase, and/or period (τ) of 24 h rhythms. Moreover, some investigations indicate circadian disruption, itself, can increase vulnerability to chemical and other challenges and thus pose enhanced risk for compromised health and wellbeing, even oncogenesis75, 76).
The current knowledge of the CTS-dependent reactions to and adverse outcomes of exposures to contagion, physical (noise, temperature, etc.), gaseous, chemical, metal, irritant, allergenic, carcinogenic, and teratogenic agents is summarized in the following sections. Because the literature is so vast, we often cite a recent review paper by some of the authors24) to respect journal guidelines concerning reference number.
Contagions
The immune system exhibits strong circadian organization77). Thus, it is not surprising that both laboratory animal experiments and human vaccination trials demonstrate prominent circadian time-dependent differences in the acute response to and consequence (morbidity and/or mortality in rodent investigations) of bacterial and viral contagions, such as Bacillus Calmette-Guérin, Brucella, E. coli endotoxin, coxsackie B3, Staphylococcus aureus pneumococcal, Salmonella Typhimurium, vesicular stomatitis, herpes, influenza, and malaria/plasmodium berghei challenges17, 24, 77,78,79,80,81). Moreover, case study reports link sudden nocturnal death of young workers from exposure to Staphylococcus aureus and other toxins of super-antigen properties that trigger strong inflammatory response82).
Physical agents
Loud noise-provoked seizure and death of laboratory rodents plus high ambient temperature-induced physiological responses and limits of tolerance of humans are CTS-dependent17, 24). Moreover, adverse effects and mortality of animals caused by ionizing and ultraviolet-B radiation are also governed by circadian time24, 83,84,85,86,87,88,89).
Gases
Human research documents day/night difference in carboxyhemoglobin elimination, and animal experiments validate circadian-time disparity in vulnerability to oxygen-induced seizures, ozone-provoked injury of alveolar and terminal bronchiole tissue of the airways, and carbon monoxide lethality24, 90). CTS-related variation in animal and/or human responses to and adverse effects of low oxygen partial pressure (hypoxia of elevated altitude), rapid hyperbaric decompression, and central nervous system toxicity of hyperbaric oxygen is also reported24, 67, 91).
Chemicals
Investigations reveal differences according to circadian time in methyl isocyanate tolerance of humans and alcohol-provoked hypothermia of humans and rodents and also alcohol-induced death of laboratory animals24). Additionally, the adverse effects of a wide array of chemical xenobiotics, including death of animals from a so-called 50% lethality dose—dose expected to cause 50% mortality of the test population—show circadian dependency. This is illustrated by studies entailing nicotine, nikethamide, strychnine, potassium cyanide, urethane [ethyl carbamate], and cholinesterase inhibitors—the pesticides of soman [O-Pinacolyl methylphosphono-fluoridate], chlorpyrifos, dichlorvos, malathion, parathion, phenitrothion, and trichlorometaphos-3—plus herbicides paraquat and glufosinate, plant insecticide dinitro-ortho-cresol, and bacteriostat florfenicol17, 24, 92,93,94,95). Additionally, abnormalities of sperm structure, total sperm count, and serum testosterone concentration of male rodents caused by the pyrethroid insecticide fenvalerate vary in extent relative to the biological time of exposure24). CTS dependency is also displayed for neurobehavioral deficits induced by trichloroethylene; hyperthermia caused by aconitum napellus; hepatotoxicity provoked by bromobenzene, carbon tetrachloride, chloroform, dichloroethylene, trichloroethylene, and styrene [ethenylbenzene]; and nephrotoxicity produced by carbon tetrachloride24, 93, 96,97,98,99).
Metals
Laboratory animal studies substantiate substantial circadian differences in risk for renal injury and death from several metals, including cadmium, mercury, lithium, lead, nickel, chromium, copper, and zinc24, 93, 100,101,102,103,104,105,106,107).
Irritants/allergens
Circadian rhythms modulate the intensity of irritant and immune reactions to various substances, e.g., delayed and recall immune system responses of rodent skin to oxazolone and of human skin to sodium lauryl sulfate and lapyrium chloride24). Furthermore, they influence the magnitude of the erythema and wheal (induration) responses of human skin to the capillary dilator histamine and antigens of house dust, grass, ragweed, and penicillin24). The scale of the airways inflammatory and constricting reactions of both laboratory animals and humans to inhaled ozone, house dust, histamine, methacholine, and acetylcholine is additionally CTS-dependent24).
Carcinogens/teratogens
Laboratory animal research validates the critical importance of circadian time in determining the vulnerability of the skin, brain, and gastrointestinal tissues to tumor induction by the chemical carcinogens methylnitrosourea, beta-propiolactone, and 7,12-dimethylbenz[a]anthracene, and also Ehrlich ascites tumor cells17, 24, 78, 108,109,110,111). Furthermore, prominent circadian-stage discrepancy is demonstrated in laboratory animals for the cytotoxicity and genotoxicity of the immunosuppressant mycophenolate mofetil and alkylating agent nitrosourea24, 112, 113). Finally, circadian time disparity in risk for fetal teratogenesis during gestation is demonstrated through laboratory animal investigations entailing alcohol, dexamethasone, hydroxyurea, 5-fluorouracil, cyclophosphamide, and Th-R [N-mustard]24, 114).
Magnitude of circadian time-dependent toxicity
The extent of variation during the 24 h in vulnerability to the diverse xenobiotic stressors is not trivial. For instance, in laboratory animals the lethality of a dose of potassium cyanide expected to produce 50% mortality is actually 100% when administered at the commencement of the nocturnal activity span, while 12 h later at the commencement of the rest span it is only 20%24). Fatality of laboratory animals to a single whole body x-irradiation dose of 550r when timed during the middle of the nocturnal activity span is 100%, while when timed near the end of the diurnal rest span it is 0%24). Finally, exposure of gravid animals to the classical teratogen hydroxyurea at the commencement of the nocturnal activity span is safe and does not cause any malformations, while exposure of animals of the same gestational stage but at a different circadian time 12 h later that corresponds to the end of the activity span causes numerous abnormalities24, 114).
Industrial Toxicology and Threshold Limit Values
Modern concepts of industrial toxicology, especially concerning threshold limit values, are credited to Theodore Hatch, who in the 1960s envisioned adverse effects of chemical exposures in terms of two—impairment and disability—risk scales, with impairment perceived as a precursor of disability, i.e., reversible and irreversible pathology, and death in the extreme115). Hatch proposed occupational exposures be limited in intensity to the capability of inherited regulatory homeostatic and adaptive compensatory coping mechanisms, the latter exemplified by induction of liver enzymes with repeated moderate chemical exposures, improved efficiency of heat loss with repeated exposures to hot environments, and increased oxygen carrying capacity plus enhanced pulmonary, cardiac, and metabolic efficiency with repeated exposures to low oxygen partial pressure at high altitude116,117,118,119,120). Hatch’s perspective, which is consistent with the concept and goals of preventive medicine, constitutes the theoretical basis for the promulgation of workplace TLVs.
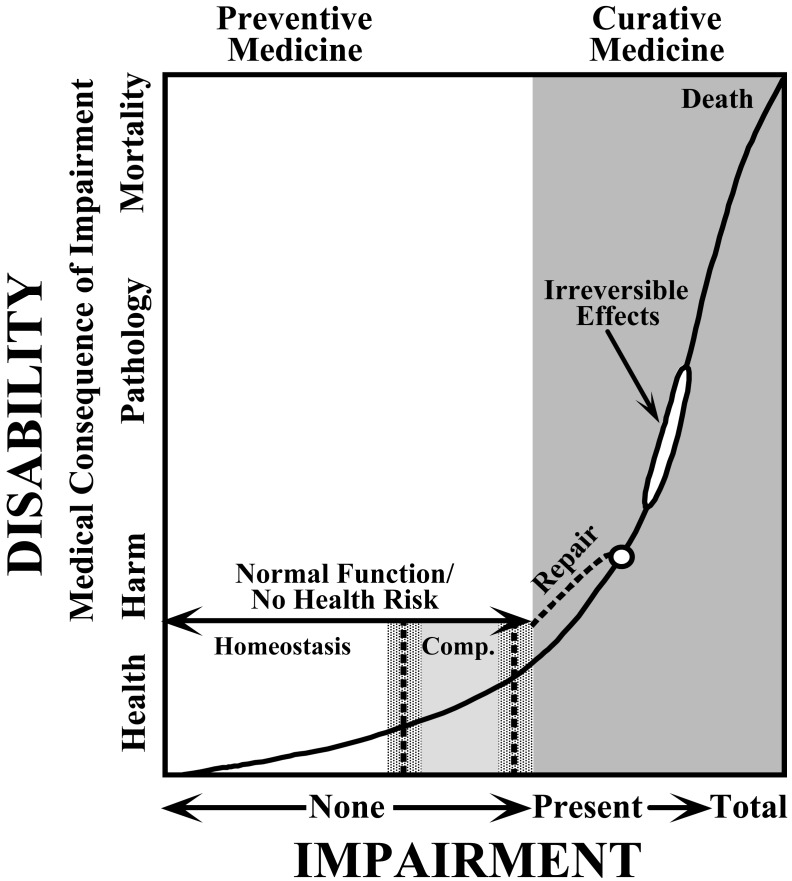
Figure 1 is a modification of Hatch’s Fig. 2 of his 1968 Archives of Environmental Health publication115). It displays risk for worker disability, i.e., pathology (left vertical axis), over a hypothetical range of xenobiotic exposures of different intensities relative to risk for physiologic impairment (horizontal axis). The upper limits of tolerance to such stressors, based on the functional capabilities of inherited homeostatic and compensatory (labeled ‘Comp’) mechanisms, are depicted at the mid-center and right center portions of the lower horizontal axis by two emboldened short vertical hatched lines. The curve extending from near the left lower corner to upper right corner symbolizes in a kind of dose-effect manner the potential magnitude of disability according to the extent of biological impairment that might be induced by chemical or other workroom contaminants. Exposures that do not exceed biological tolerance, i.e., capabilities of inherited homeostatic and compensatory mechanisms, do not cause impairment or disability, i.e., are no threat to health, and are compatible with the tenets of preventative medicine. Exposures that exceed the upper limit of biological tolerance, depicted by the gray tone shading at the far right of the figure, have pathological consequences that necessitate curative medical intervention, and which in the extreme might culminate in death.
Fig. 1.
Theodore Hatch’s scales of biological impairment and functional disability as the conceptual basis for the promulgation of TLVs115). Impairment due to excessive industrial exposures is viewed as a precursor of disability—reversible and irreversible pathology, and death in the extreme. Curve extending from bottom left corner to upper right corner depicts the potential level of disability relative to the severity of biological impairment stemming from xenobiotic exposures. Exposures not exceeding the capability of inherited regulatory homeostatic and compensatory mechanisms (labeled ‘Comp’), whose respective upper limits are denoted by two short vertical hatched lines at the mid-center and right-center portions of the lower horizontal axis, are unlikely to provoke impairment or disability for the vast majority of workers. This perspective is consistent with the tenets of preventive medicine. Exposures beyond the biological limits of tolerance that increase the risk for biological impairment and disability–pathology (gray shading of the far right-hand portion of figure)–entail curative medical interventions. Accordingly, TLVs that respect normal homeostatic and compensatory processes avert impairment and disability. New to Hatch’s original 1968 figure is the depiction by stippled shading of arbitrary width of the predictable-in-time 24 h variation of the limits of involved inherited homeostatic and compensatory coping mechanisms; the shading thus symbolizes the potential magnitude of the day/night difference in the vulnerability of shift-workers to xenobiotic and other workspace stressors. Figure is a modification of both Hatch115) and Smolensky et al24).
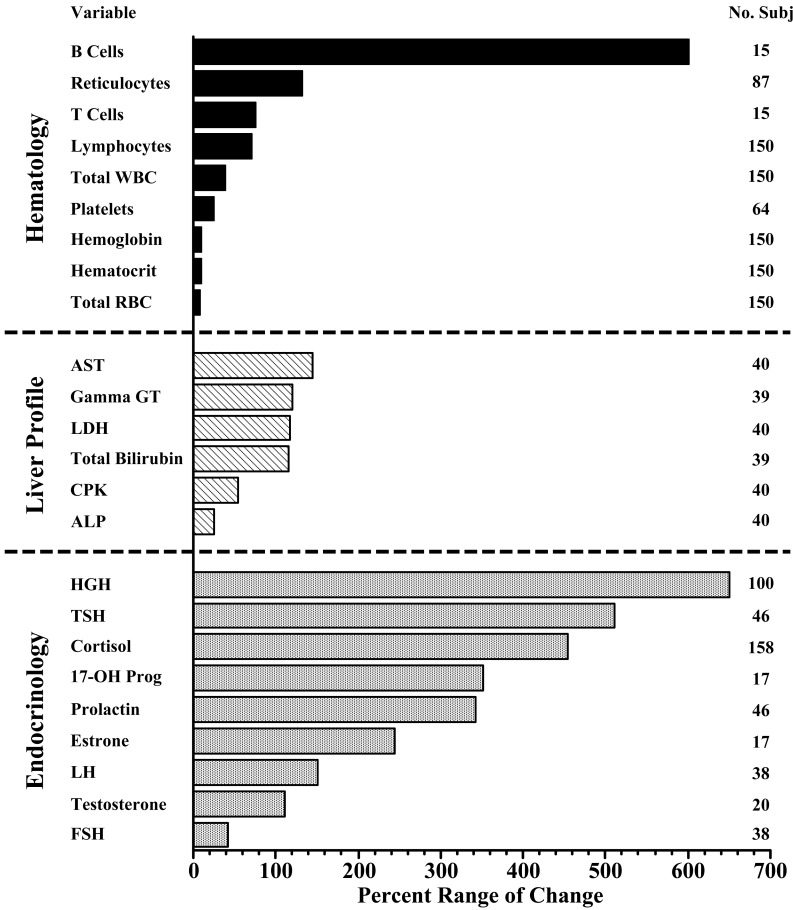
Fig. 2.
Illustrated rationale of the necessity of BTQRVs as BEIs. Figure created using databases of several different studies in which blood samples were collected at 1 to 4 h intervals throughout a single 24 h span from working-age healthy men and women adhering to a routine of diurnal activity and nocturnal sleep and consuming breakfast, lunch, and dinner meals at usual times122,123,124,125,126). Length of the individual horizontal vectors represents the total amount of the 24 h variation displayed in blood samples by selected hematology, liver, and hormone variables. Average magnitude of the day/night variation per variable across subjects is calculated as the difference between the highest and lowest value divided by the 24 h mean of all values multiplied by 100 and expressed in %. Temporal variation in hematological variables is depicted in the top panel; for hemoglobulin, hematocrit, and total RBC (total red blood cells) it is ≤10% and negligible, while for total WBC (total white blood cells) and platelet blood cells it is moderate, and for total lymphocytes, T and B cell lymphocytes, and reticulocytes it is high, ≥100%. Middle panel displays mainly liver function variables; AST (aspartate aminotransferase, also known as serum glutamate oxaloacetate transaminase [SGOT]), Gamma GT (gamma glutamyl transferase), LDH (lactate dehydogenase), CPK (creatine phosphokinase isoenzymes–more indicative of heart, muscle, and brain than liver tissue status), total bilirubin, and ALP (alkaline phosphatase) exhibit extensive, ≥100%, 24 h variation. Bottom endocrinology panel depicts the range of 24 h disparity in the serum concentration of reproductive and other hormones. Most display great, ≥100%, day/night variation; this is particularly evident for HGH (human growth hormone), TST (thyroid stimulating hormone), cortisol, 17-OH Prog (17-OH progesterone), prolactin, estrone (an estrogen), and testosterone, although not for LH (luteinizing hormone).
Half a century ago there was little knowledge of circadian rhythms and their relevance to industrial toxicology, particularly potential between-shift differences in susceptibility to xenobiotic stresses. We added shadings of arbitrary width to the above-described boundaries of involved homeostatic and compensatory processes to denote, in accordance with the findings of the above-reviewed animal and human literature, CTS-dependent differences in biological state that can give rise to work-shift discrepancy in risk for impairment and disability. This updated figure thus incorporates circadian differences in risk/vulnerability of employees relative to the protection afforded by TLVs, not only for those working standard day but also nonstandard permanent night and rotating shift schedules for whom CTS adjustment is likely to be incomplete. It is noteworthy Hatch also advocated TLVs take into consideration age-dependent and inter-individual disparity in vulnerability, including that due to diminished health status arising from previously experienced work-induced disability115).
Circadian Rhythms and Methods of Biological Monitoring
Biological monitoring (BM) is a common procedure in modern medicine exemplified by around-the-clock electrocardiology and ambulatory blood pressure patient studies for heart disease and hypertension, wrist actigraphy for appraisal of activity and sleep disorders, daytime serial peak expiratory flow rate [PEFR] self-assessments for determination of airways health of asthma sufferers, and collection of urine voids and blood samples for routine diagnostics and dose-titration of medications. Since the 1960s, BM in the form of autorhythmometry—serial self-measurement of biological and behavioral variables and/or self-collection of sequential urine, saliva, or other body fluids at frequent intervals during the wake or entire 24 h span for several consecutive days—has been extensively utilized to explore the chronobiology of human health and disease24, 121). It has also been the principle means of investigating the speed of adjustment of the CTS of workers to rotating and permanent nightshift schedules and travelers to rapid time-zone displacement by jet aircraft30, 31, 35, 45).
BM has also long been incorporated into occupational medicine to assess worker exposures and health status, although without consideration of the implications posed by the CTS in its conduct. Formal endorsement in the United States of BM of workers dates to the early 1970s; although, it was not until the early 1980s that the American Conference of Governmental Industrial Hygienists created a committee to recommend normative reference BEIs to aid interpretation of values24). Typically, bodily fluids and/or biological measures are collected before and after work shifts, with data evaluated relative to biological exposure indices (BEIs), generally informed by no-observed-adverse-effect, lowest-observed-adverse-effect, and/or benchmark dose values that lack work-shift and clock-time specification24).
BEIs, like TLVs, are promulgated without consideration of possible CTS influences. Some blood, saliva, and urine constituents show such small 24 h variation they do not necessitate special referencing for circadian time of sampling. However, judgment of the normality or abnormality of BM-derived variables that exhibit large predictable-in-time difference during the 24 h is likely to benefit from reference values qualified for biological time. Such biological time-qualified reference values (BTQRVs) have been proven valuable in clinical medicine to assess normality of determined blood constituents known to display high-amplitude circadian variation122,123,124,125,126), and they seem relevant to occupational medicine and industrial toxicology to improve interpretation of BM data, particularly those obtained at atypical times from night workers24).
Rationale for the need of BTQRVs as BEIs for certain biomarkers is illustrated in Fig. 2. It presents by the length of individual horizontal vectors the total extent of the day/night variation of several hematology, liver, and hormone variables of blood samples. The figure is created using databases of several studies in which blood samples were collected at 1 to 4 h intervals throughout a single 24 h span from working-age healthy men and women adhering to a routine of diurnal activity and nocturnal sleep and consuming breakfast, lunch, and dinner meals at usual times122,123,124,125,126). The length of the horizontal vector represents the average magnitude of the 24 h variation of the given variable across all studied subjects, calculated as the highest value minus the lowest value divided by the 24 h mean of all values multiplied by 100 and expressed in %. The number of subjects represented per variable is listed in the right-hand column of the figure. The amount of temporal variation in the hematological variables is shown in the top panel; that of hemoglobulin, hematocrit, and total RBC (total red blood cells) is negligible, ≤10%, that of total WBC (total white blood cells) and platelet blood cells is moderate, and that of total lymphocytes, T and B cell lymphocytes, and reticulocytes is high, ≥100%. The middle panel depicts primarily liver function variables; AST (aspartate aminotransferase, also known as serum glutamate oxaloacetate transaminase [SGOT]), Gamma GT (gamma glutamyl transferase), LDH (lactate dehydogenase), CPK (creatine phosphokinase isoenzymes that are more indicative of heart, muscle, and brain than liver tissue status), total bilirubin, and ALP (alkaline phosphatase) exhibit extensive, ≥100%, 24 h variation. The bottom endocrinology panel depicts the extent of the 24 h disparity in the serum concentration of reproductive and other hormones. Most display great, ≥100%, temporal variation; this is particularly evident for HGH (human growth hormone), TST (thyroid stimulating hormone), cortisol, 17-OH Prog (17-OH progesterone), prolactin, estrone (an estrogen), and testosterone, although not LH (luteinizing hormone). It is obvious that many biomarkers of health and wellbeing are characterized by substantial 24 h variation, thereby implicating the need of BTQRVs to properly interpret whether values obtained through employee BM are normal or abnormal according to the circadian time of biomarker sampling. In many occupational settings, 8 h or 12 h shift-specific BTQRVs may be more easily applicable than clock-hour or circadian-time ones. Biological variables additionally exhibit weekly and annual rhythms, and in young women menstrual rhythms24, 122,123,124,125,126,127,128); however, the relevance of BTQRVs for these time domains to better interpret BM-derived values awaits appraisal.
A common method of BM in some occupational settings comprises serial measurements conducted at regular intervals during work and off hours. For instance, in the United Kingdom, BM of workers routinely exposed to chemicals, irritants, and allergens that can adversely affect the airways may entail repeated self-assessments throughout the waking span of peak expiratory flow rate (PEFR), a measure of the patency of the airways and ease of breathing, using an inexpensive, simple-to-use, personal light-weight, hand-held peak flow meter. Published normative reference values take into account ethnicity, age, sex, height, and weight24); however, like all other BEIs they are regarded as if static, i.e., without circadian variation. The peak-to-trough circadian variation (rhythm amplitude) in PEFR values of diurnally active healthy young adults is small, equal only to 3–5% of the overall 24 h mean. Typically PEFR is highest during the middle and late afternoon—9–12 h after commencement of the daytime activity period—and lowest between the middle and latter span of nighttime sleep24). The PEFR 24 h mean of individuals with obstructive or reactive airways disease is significantly lower than normative age and sex reference values, and the circadian amplitude is larger, generally 20–25%, but sometimes as much as 40–50%, of the 24 h mean. The more severe the airways insult and pathology, the lower the PEFR 24 h mean and greater the PEFR circadian amplitude. Additionally, the time of day of best airway function may be significantly advanced or delayed from the normal reference time24).
The PEFR circadian rhythm is well known to most pulmonary medicine and some occupational health physicians. Nonetheless, BM of employees exposed to respiratory irritants and allergens typically is restricted to one or two PEFR measurements—either only after or both before and after the work shift—to assess airways heath. Frequent self-assessment of PEFR during work and remaining wake span has been utilized as a more comprehensive method of BM for employees exposed to polyvinylchloride and toluene diisocyanate aerosols as well as grain and cork dusts. Adverse effects of such exposures are manifested both by a lower than predicted PEFR 24 h mean and higher than normal between-measurement variation24). Even more comprehensive BM has involved serial PEFR self-measurements done throughout the waking span of consecutive work and off days. Using this approach, Randem et al.129) found solder workers routinely exposed to fumes of pine resin sap, i.e., colophony, display relative to normative reference values a lower PEFR 24 h mean, larger circadian amplitude of variation, and somewhat altered clock time of best airways flow rates on work compared to non-work weekend and vacation days.
The findings of Randem et al.129) are in line with those reported almost six decades ago by McKerrow et al.130), who noted employees toiling in dusty cotton mills are prone to chest tightness and breathing difficulty on Mondays following off-work weekends and vacations. These symptoms, which are indicative of the lung disease byssinosis, progressively worsen during the work shift. In the early stages of this occupational illness, symptoms are limited to the first workday, Monday, of each work week, but in advanced stages they additionally manifest Tuesdays or even every work and off day. The BM by McKerrow et al.130) of cotton workers involved serial PEFR self-assessments done throughout the waking span of the daytime work shift, finding the PEFR 24 h mean to be markedly depressed by 35–40% and the PEFR circadian amplitude enhanced by 2–3-fold relative to the respective normative reference values. Furthermore, the peak time of the PEFR circadian rhythm (time of day of greatest airflow rate that denotes best airway function) was greatly advanced—by 9 to 12 h to the early morning at 06:30 h—from afternoon/early evening as expected for diurnally active workers. This later alteration is indicative of the progressive increase during the daytime work shift of breathing distress produced by cotton dust exposure. Thus, a proven BM procedure for early detection of occupationally induced adverse effects and pathology of the airways is frequent serial self-assessment during work and off time of PEFR using an inexpensive personal peak flow meter. Early indicators of acute adverse effects of workplace contaminants on the airways include alteration of not just one but as many as three parameters of the PEFR circadian rhythm—the 24 h mean, amplitude, and peak time.
Serial self-collection of urine voids is an additional means of biologically monitoring chemical and other xenobiotic exposures, e.g., to chemical sanitizing agents. Chlorine-based disinfectants are routinely utilized by cleaning personnel to control microbial contamination and minimize risk for infection. Such chemicals enter the body by inhalation or other means during work and are metabolized by liver enzymes into harmful, i.e., cancer-inducing trihalomethane, byproducts. Gängler et al.131) collected consecutive urine voids from human volunteers working with chlorine disinfectants at different times of the day and night, finding the time of day of their use determined the urinary concentration of the surrogate biomarker 4-hydroxynonenal and detrimental trihalomethane metabolites. Urinary biomarker concentrations were low following morning-time exposures but considerably higher following afternoon and nighttimes ones, substantiating significant circadian rhythm-dependent differences in the body burden of the harmful byproducts of the chlorine disinfectants. The time-of-day differences were not due to corresponding disparities in environmental exposures; rather, they resulted from circadian rhythm-dependent differences in the activity of the liver enzyme CYP2E1 that metabolizes chlorine chemicals. Circadian rhythms in benzene metabolism and biomarkers also have been identified by Watts132) that additionally demonstrate the need for TQFVs as BEIs according to the time of urine sampling.
Conclusions
Personnel of nonstandard work schedules, especially those engaged in permanent and rotating nightshift work, may be exposed to harmful xenobiotic contaminants in the same or even greater concentrations than dayshift workers. Moreover, night workers are likely to be exposed to workroom contaminants during a different circadian time that may be deterministic of higher vulnerability than day workers. Establishment of threshold limit values and methods of biological monitoring, including interpretation of derived values against reference biological exposure indices, are based solely on homeostatic theory, the unsubstantiated assumption of biological constancy, and databases of adverse events that lack qualification for biological time or work shift. Rarely, if ever, has the vulnerability of personnel to industrial contaminates and other stressors been explored according to work shift or circadian time. The goal of this consensus paper is to call attention to the extensive literature pertaining to laboratory animal and human investigations that document the importance of the circadian time structure to industrial toxicology. The collective findings of the many investigations reviewed herein entailing contagion, physical, gaseous, chemical, metal, irritant, allergenic, carcinogenic, and teratogenic xenobiotics suggest circadian factors ought to be an additional important criterion used to decide threshold limit values, particularly to protect personnel working nonstandard work schedules. Furthermore, realization that biomarkers of adverse workplace xenobiotic exposures may be governed by high-amplitude circadian rhythms should serve to better inform the methods of biological monitoring and data interpretation, not only for night but also day workers.
Acknowledgments
We authors acknowledge the suggestions of Dr. Marc Riedel (Université François Rabelais de Tours, France) to improve the content of the manuscript and also Ms. Linda Sackett-Lundeen (University of Minnesota, Minneapolis, MN) for her invaluable assistance in constructing the figures for this consensus article.
References
- 1.Wong IS, Dawson D, Van Dongen HPA. (2019) International consensus statements on non-standard working time arrangements and occupational health and safety. Ind Health 57, 135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colquhoun WP, Rutenfranz J. (Eds.) (1980) Studies of shiftwork. Taylor & Francis, London. [Google Scholar]
- 3.Guo Y, Liu Y, Huang X, Rong Y, He M, Wang Y, Yuan J, Wu T, Chen W. (2013) The effects of shift work on sleeping quality, hypertension and diabetes in retired workers. PLoS One 8, e71107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haus EL, Smolensky MH. (2013) Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17, 273–84. [DOI] [PubMed] [Google Scholar]
- 5.Haus E, Reinberg A, Mauvieux B, Le Floc’h N, Sackett-Lundeen L, Touitou Y. (2016) Risk of obesity in male shift workers: a chronophysiological approach. Chronobiol Int 33, 1018–36. [DOI] [PubMed] [Google Scholar]
- 6.Johnson LC, Tepas DL, Colquhoun WP, Colligan MJ. (Eds.) (1981) Biological rhythms, sleep and shift work. SP Medical & Scientific Books, New York. [Google Scholar]
- 7.Kecklund G, Axelsson J. (2016) Health consequences of shift work and insufficient sleep. BMJ 355, i5210. [DOI] [PubMed] [Google Scholar]
- 8.Kogi K, Miura T, Saito H .(Eds.) (1982) Shiftwork: Its practice and improvements. Center for Academic Publications, Tokyo. [Google Scholar]
- 9.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, Mori M, Washio M, Sakauchi F, Ito Y, Yoshimura T, Tamakoshi A. (2006) Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol 164, 549–55. [DOI] [PubMed] [Google Scholar]
- 10.Mosendane T, Mosendane T, Raal FJ. (2008) Shift work and its effects on the cardiovascular system. Cardiovasc J Afr 19, 210–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, Burgos J, Gómez-Acebo I, Llorca J, Peiró R, Jimenez-Moleón JJ, Arredondo F, Tardón A, Pollan M, Kogevinas M. (2015) Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer 137, 1147–57. [DOI] [PubMed] [Google Scholar]
- 12.Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RCH, van der Beek AJ, van Steeg H, van Kerkhof LWM. (2016) The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am J Prev Med 50, e147–57. [DOI] [PubMed] [Google Scholar]
- 13.Reinberg A, Smolensky MH, Riedel M, Touitou Y, Le Floc’h N, Clarisse R, Marlot M, Berrez S, Pelisse D, Mauvieux B. (2015) Chronobiologic perspectives of black time—accident risk is greatest at night: an opinion paper. Chronobiol Int 32, 1005–18. [DOI] [PubMed] [Google Scholar]
- 14.Rutenfranz J, Knauth P, Angersbach D .(1981) Shift work research issues. In: Biologcal rhythms, sleep, and shift work, Johnson LC, Tepas DI, Colquhoun WP, Colligan MJ (Eds.), 165–96, SP Medical & Scientific Books, New York. [Google Scholar]
- 15.Silva-Costa A, Rotenberg L, Coeli CM, Nobre AA, Griep RH. (2016) Night work is associated with glycemic levels and anthropometric alterations preceding diabetes: baseline results from ELSA-Brasil. Chronobiol Int 33, 64–72. [DOI] [PubMed] [Google Scholar]
- 16.Siqueria K, Griep R, Rotenberg L, Silva-Costa A, Mendes da Fonseca MJ. (2016) Weight gain and body mass index following change from daytime to night shift—a panel study with nursing professionals. Chronobiol Int 33, 776–9. [DOI] [PubMed] [Google Scholar]
- 17.Smolensky MH, Scheving LE, Paustenbach D .(1985) In: Biological rhythms in shift work and occupational health, Cralley LJ, Cralley L (Eds.), Patty’s theory and practice of industrial hygiene, Edition II, Volume 3B Biological responses, 175–311, John Wiley and Sons, New York. [Google Scholar]
- 18.Stevens RG. (2016) Circadian disruption and health: Shift work as a harbinger of the toll taken by electric lighting. Chronobiol Int 33, 589–94. [DOI] [PubMed] [Google Scholar]
- 19.Franco G, Lorena M. (1987) [Chronobiology and industrial medicine: approach to the evaluation of the toxicological risk in occupational exposure to xenobiotics]. G Ital Med Lav 9, 125–40 (in Italian). [PubMed] [Google Scholar]
- 20.Gaffuri E, Costa G. (1986) Applied aspects of chronoergohygiene. Chronobiologia 13, 39–51. [PubMed] [Google Scholar]
- 21.Miura N, Ohtani K. (2015) [Application of “chronotoxicology” to occupational health]. Sangyo Eiseigaku Zasshi 57, 21–5 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 22.Smolensky MH, Reinberg A. (1990) Clinical chronobiology: relevance and applications to the practice of occupational medicine. Occup Med 5, 239–72. [PubMed] [Google Scholar]
- 23.Jay SM, Gander PH, Eng A, Cheng S, Douwes J, Ellison-Loschmann L, McLean D, Pearce N, ‘tMannetje A. (2017) New Zealanders working non-standard hours also have greater exposure to other workplace hazards. Chronobiol Int 34, 519–26. [DOI] [PubMed] [Google Scholar]
- 24.Smolensky MH, Reinberg AE, Sackett-Lundeen L. (2017) Perspectives on the relevance of the circadian time structure to workplace threshold limit values and employee biological monitoring. Chronobiol Int 34, 1439–64. [DOI] [PubMed] [Google Scholar]
- 25.Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L, Portaluppi F. (2016) Circadian disruption: new clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int 33, 1101–19. [DOI] [PubMed] [Google Scholar]
- 26.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, Vermeulen R, Carreón T, Caruso CC, Lawson CC, Thayer KA, Twery MJ, Ewens AD, Garner SC, Schwingl PJ, Boyd WA. (2017) Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 607-608, 1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kofuji P, Mure LS, Massman LJ, Purrier N, Panda S, Engeland WC. (2016) Intrinsically photosensitive retinal ganglion cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS One 11, e0168651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smolensky MH, Sackett-Lundeen LL, Portaluppi F. (2015) Nocturnal light pollution and underexposure to daytime sunlight: complementary mechanisms of circadian disruption and related diseases. Chronobiol Int 32, 1029–48. [DOI] [PubMed] [Google Scholar]
- 30.Vieux N, Ghata J, LaPorte A, Migraine C, Nicolai A, Reinberg A. (1979) Adjustment of shift workers adhering to a three- to four-day rotation (Study 2). Chronobiologia 6 Suppl 1, 37–42. [Google Scholar]
- 31.Reinberg AE, Smolensky MH .(1992) Night and shift work and transmeridian space flights. In: Biologic rhythms in clinical and laboratory medicine, Touitou Y, Haus E (Eds.), 243–55, Springer-Verlag, Berlin. [Google Scholar]
- 32.Mirick DK, Davis S. (2008) Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomarkers Prev 17, 3306–13. [DOI] [PubMed] [Google Scholar]
- 33.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’hermite-Balériaux M, Zee PC. (2005) Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms 20, 178–88. [DOI] [PubMed] [Google Scholar]
- 34.Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, Kayumov L. (2007) Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry 31, 1–11. [DOI] [PubMed] [Google Scholar]
- 35.Reinberg A. (Ed.) (1979) Chronobiological field studies of oil refinery workers. Chronobiologia 4 Suppl. 1, 3–119. [Google Scholar]
- 36.Akashi M, Soma H, Yamamoto T, Tsugitomi A, Yamashita S, Yamamoto T, Nishida E, Yasuda A, Liao JK, Node K. (2010) Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc Natl Acad Sci USA 107, 15643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anafi RC, Francey LJ, Hogenesch JB, Kim J. (2017) CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci USA 114, 5312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun R, Kath WL, Iwanaszko M, Kula-Eversole E, Abbott SM, Reid KJ, Zee PC, Allada R. (2018) Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci USA 115, E9247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughey JJ. (2017) Machine learning identifies a compact gene set for monitoring the circadian clock in human blood. Genome Med 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Lozano Sinues P, Tarokh L, Li X, Kohler M, Brown SA, Zenobi R, Dallmann R. (2014) Circadian variation of the human metabolome captured by real-time breath analysis. PLoS One 9, e114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinues PM, Kohler M, Zenobi R. (2013) Monitoring diurnal changes in exhaled human breath. Anal Chem 85, 369–73. [DOI] [PubMed] [Google Scholar]
- 42.Van Dycke KC, Pennings JL, van Oostrom CT, van Kerkhof LW, van Steeg H, van der Horst GT, Rodenburg W. (2015) Biomarkers for circadian rhythm disruption independent of time of day. PLoS One 10, e0127075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A, Wisniewski S, Zaleska M, Bartok O, Ashwal-Fluss R, Lammert H, Herzel H, Hummel M, Kadener S, Kunz D, Kramer A. (2018) High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 128, 3826–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lech K, Liu F, Ackermann K, Revell VL, Lao O, Skene DJ, Kayser M. (2016) Evaluation of mRNA markers for estimating blood deposition time: towards alibi testing from human forensic stains with rhythmic biomarkers. Forensic Sci Int Genet 21, 119–25. [DOI] [PubMed] [Google Scholar]
- 45.Reinberg A, Riedel M, Brousse E, Floc’h NL, Clarisse R, Mauvieux B, Touitou Y, Smolensky MH, Marlot M, Berrez S, Mechkouri M. (2013) Circadian time organization of professional firemen: desynchronization-tau differing from 24.0 hours-documented by longitudinal self-assessment of 16 variables. Chronobiol Int 30, 1050–65. [DOI] [PubMed] [Google Scholar]
- 46.Archer SN, Laing EE, Möller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, Smith CP, Dijk DJ. (2014) Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci USA 111, E682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kervezee L, Cuesta M, Cermakian N, Boivin DB. (2018) Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proc Natl Acad Sci USA 115, 5540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinberg A, Ashkenazi I. (2008) Internal desynchronization of circadian rhythms and tolerance to shift work. Chronobiol Int 25, 625–43. [DOI] [PubMed] [Google Scholar]
- 49.Bruguerolle B, Giaufre E, Prat M. (1991) Temporal variations in transcutaneous passage of drugs: the example of lidocaine in children and in rats. Chronobiol Int 8, 277–82. [DOI] [PubMed] [Google Scholar]
- 50.Matsui MS, Pelle E, Dong K, Pernodet N. (2016) Biological rhythms in the skin. Int J Mol Sci 17, E801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hesseltine GR, Wolff RK, Hanson RL, McClellan RO, Mauderly JL. (1985) Comparison of lung burdens of inhaled particles of rats exposed during the day or night. J Toxicol Environ Health 16, 323–9. [DOI] [PubMed] [Google Scholar]
- 52.Mahajan KK, Mahajan SK, Mishra N. (1990) Diurnal variations in lung transfer factor and its components. Indian J Physiol Pharmacol 34, 209–11. [PubMed] [Google Scholar]
- 53.Marek W. (1997) [Chronobiology of the bronchial system]. Pneumologie 51 Suppl 2, 430–9 (in German). [PubMed] [Google Scholar]
- 54.Bélanger PM, Bruguerolle B, Labreque G .(1997) Rhythms in pharmacokinetics; absorption, distribution, metabolism, and excretion. In: Physiology and pharmacology of biological rhythms, Redfern PH, Lemmer B (Eds.), 177–204, Springer, Berlin. [Google Scholar]
- 55.Pons M, Cambar J. (1996) [Chronotoxicology, a relatively unknown approach in toxicology]. Pathol Biol (Paris) 44, 555–63 (in French). [PubMed] [Google Scholar]
- 56.Cambar J, Pons M .(1997) New trends in chronotoxicology. Rhythms in pharmacokinetics; absorption, distribution, metabolism, and excretion. In: Physiology and pharmacology of biological rhythms, Redfern PH, Lemmer B (Eds.), 537–88, Springer, Berlin. [Google Scholar]
- 57.Campiranon S, Koukkari WL. (1976) Circadian periodic response of Phaseolus vulgaris l. to 2,4-dichlorophenoxyacetic acid. Chronobiologia 3, 137–48. [PubMed] [Google Scholar]
- 58.Fernandez AT, Randolph NM. (1967) A photoperiodic effect on the daily susceptibility of the house fly to trichlorfon. J Econ Entomol 60, 1633–6. [DOI] [PubMed] [Google Scholar]
- 59.Frudden L, Wellso SG. (1968) Daily susceptibility of house flies to malathion. J Econ Entomol 61, 1692–4. [DOI] [PubMed] [Google Scholar]
- 60.Roberts D, Smolensky MH, Hsi B, Scanlon JE .(1974) Circadian pattern in susceptibility of Aedes Aegypti (L.) larvae to Dursban. In: Chronobiology, Scheving LE, Halberg F, Pauly JE (Eds.), 612–6, Igaku Shoin, Tokyo, Japan. [Google Scholar]
- 61.Gosselink JG, Standifer LC. (1967) Diurnal rhythm of sensitivity of cotton seedlings to herbicides. Science 158, 120–1. [DOI] [PubMed] [Google Scholar]
- 62.Maliszewska J, Piechowicz B, Maciąga G, Zaręba L, Marcinkowska S. (2018) Pyrethroid residue dynamics in insects depends on the circadian clock. J Environ Sci Health B 53, 441–6. [DOI] [PubMed] [Google Scholar]
- 63.McLeay DJ, Munro JR. (1979) Photoperiodic acclimation and circadian variations in tolerance of juvenile rainbow trout (Salmo gairdneri) to zinc. Bull Environ Contam Toxicol 23, 552–7. [DOI] [PubMed] [Google Scholar]
- 64.Miller RP, Martinson KB, Sothern RB, Durgan BR, Gunsolus JL. (2003) Circadian response of annual weeds in a natural setting to high and low application rates of four herbicides with different modes of actions. Chronobiol Int 20, 299–324. [DOI] [PubMed] [Google Scholar]
- 65.Rikin A, John JB, Wergin WP, Anderson JD. (1984) Rhythmical changes in the sensitivity of cotton seedlings to herbicides. Plant Physiol 76, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crouzier D, Le Crom VB, Four E, Lallement G, Testylier G. (2004) Disruption of mice sleep stages induced by low doses of organophosphorus compound soman. Toxicology 199, 59–71. [DOI] [PubMed] [Google Scholar]
- 67.Eynan M, Biram A, Mullokandov M, Kronfeld-Schor N, Paz-Cohen R, Menajem D, Arieli Y. (2017) The transition from day-to-night activity is a risk factor for the development of CNS oxygen toxicity in the diurnal fat sand rat (Psammomys obesus). Chronobiol Int 34, 578–86. [DOI] [PubMed] [Google Scholar]
- 68.Fang MZ, Zhang X, Zarbl H. (2010) Methylselenocysteine resets the rhythmic expression of circadian and growth-regulatory genes disrupted by nitrosomethylurea in vivo. Cancer Prev Res (Phila) 3, 640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao B, Chen TM, Zhong Y. (2016) Possible molecular mechanism underlying cadmium-induced circadian rhythms disruption in zebrafish. Biochem Biophys Res Commun 481, 201–5. [DOI] [PubMed] [Google Scholar]
- 70.Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. (2013) Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J Natl Cancer Inst 105, 1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haus E, Smolensky M. (2006) Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 17, 489–500. [DOI] [PubMed] [Google Scholar]
- 72.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2010) IARC monographs on the evaluation of carcinogenic risks to humans; Painting, firefighting, and shiftwork [1. Circadian Rhythm 2. Fires 3. Occupational Exposure 4. Neoplasms-etiology 5. Paint − adverse effects 6. Work Schedule Tolerance], (Meeting 2007: Lyon, France). II. Series, 98, International Agency for Research on Cancer, Lyon. [PMC free article] [PubMed] [Google Scholar]
- 73.Song P, Li Z, Li X, Yang L, Zhang L, Li N, Guo C, Lu S, Wei Y. (2017) Transcriptome profiling of the lungs reveals molecular clock genes expression changes after chronic exposure to ambient air particles. Int J Environ Res Public Health 14, E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V, WHO International Agency For Research on Cancer Monograph Working Group (2007) Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8, 1065–6. [DOI] [PubMed] [Google Scholar]
- 75.Filipski E, Lévi F. (2009) Circadian disruption in experimental cancer processes. Integr Cancer Ther 8, 298–302. [DOI] [PubMed] [Google Scholar]
- 76.Filipski E, Subramanian P, Carrière J, Guettier C, Barbason H, Lévi F. (2009) Circadian disruption accelerates liver carcinogenesis in mice. Mutat Res 680, 95–105. [DOI] [PubMed] [Google Scholar]
- 77.Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P. (2013) Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci USA 110, 9897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsai TH, Burns RE, Scheving LE. (1979) Circadian influence on the immunization of mice with live Bacillus Calmette-Guérin (BCG) and subsequent challenge with Ehrlich ascites carcinoma. Chronobiologia 6, 187–201. [PubMed] [Google Scholar]
- 79.Gagnidze K, Hajdarovic KH, Moskalenko M, Karatsoreos IN, McEwen BS, Bulloch K. (2016) Nuclear receptor REV-ERBα mediates circadian sensitivity to mortality in murine vesicular stomatitis virus-induced encephalitis. Proc Natl Acad Sci USA 113, 5730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB. (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci USA 113, 10085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langlois PH, Smolensky MH, Glezen WP, Keitel WA. (1995) Diurnal variation in responses to influenza vaccine. Chronobiol Int 12, 28–36. [DOI] [PubMed] [Google Scholar]
- 82.Al Madani O, Gordon AE, Weir DM, Raza MW, Busuttil A, Blackwell C. (1999) Pyrogenic toxins of Staphylococcus aureus in sudden unexpected nocturnal deaths in adults and older children: factors influencing the control of inflammatory responses to toxic shock syndrome toxins. FEMS Immunol Med Microbiol 25, 207–19. [DOI] [PubMed] [Google Scholar]
- 83.Arbogast BJ, Gerbes Al (1984) Chronobioassay of radiation injury in mice with and without time shift. Ann Rev. Chronopharmacol 1, 369–72. [Google Scholar]
- 84.Gaddameedhi S, Selby CP, Kemp MG, Ye R, Sancar A. (2015) The circadian clock controls sunburn apoptosis and erythema in mouse skin. J Invest Dermatol 135, 1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lappenbusch WL. (1970) Effect of circadian rhythm on the radiosensitivity of the rough-skinned newt (Taricha granulosa). J Radiat Res (Tokyo) 11, 134–7. [DOI] [PubMed] [Google Scholar]
- 86.Lappenbusch WL. (1972) Effect of circadian rhythm on the radiation response of the Chinese hamster (Cricetulus griseus). Radiat Res 50, 600–10. [PubMed] [Google Scholar]
- 87.Pizzarello DJ, Isaak D, Chua KE, Rhyne AL. (1964) Circadian rhythmicity in the sensitivity of two strains of mice to whole body radiation. Science 145, 286–91. [DOI] [PubMed] [Google Scholar]
- 88.Sarkar S, Gaddameedhi S. (2018) UV-B-induced erythema in human skin: the circadian clock is ticking. J Invest Dermatol 138, 248–51. [DOI] [PubMed] [Google Scholar]
- 89.Spalding JF, McWilliams P. (1965) Radiation lethality and circadian rhythmicity in mice. Health Phys 11, 647–51. [DOI] [PubMed] [Google Scholar]
- 90.Witschi H, Espiritu I, Pinkerton KE. (1997) Pulmonary cell kinetics and morphometry after ozone exposure: day versus night and dose response in rats. Am J Physiol 272, L1152–60. [DOI] [PubMed] [Google Scholar]
- 91.Heckmann C, Hariri A. (1984) Circadian variation in the response to hypoxia studies in animals and man. Ann Rev Chronopharmacol 1, 393–6. [Google Scholar]
- 92.Krüger S, Herzog M, Kuhlmey H, Werner E. (1991) [Circadian rhythm of the toxicokinetics and toxicodynamics of the plant protection substance dinitro-ortho-cresol in rabbits]. Dtsch Tierarztl Wochenschr 98, 376–81 (in German). [PubMed] [Google Scholar]
- 93.Tsai TH, Scheving LE, Pauly JE .(1982) Circadian variation in host susceptibility in mercuric chloride and paraquat in Balb/Cann female mice. In: Toward chronopharmacology, 249–55, Takahashi R, Halberg F, Walker CA (Eds.), Pergamon Press, New York. [Google Scholar]
- 94.Yoshioka H, Nonogaki T, Fukuishi N, Shinohara Y, Hwang GW, Ohtani K, Miura N. (2017) Chronotoxicity of bromobenzene-induced hepatic injury in mice. J Toxicol Sci 42, 251–8. [DOI] [PubMed] [Google Scholar]
- 95.Yoshiyama Y, Kobayashi T, Kondo R, Tomonaga F, Ohwada T. (1995) Chronotoxicity of glufosinate ammonium in mice. Vet Hum Toxicol 37, 22–3. [PubMed] [Google Scholar]
- 96.Bruckner JV, Luthra GM, Kyle GM, Muralidhara S, Ramanathan R, Acosta D. (1984) Influence of time of exposure to carbon tetrachloride on toxic liver injury. Ann Rev Chronopharmacol 1, 373–6. [Google Scholar]
- 97.Desgagne M, Belanger PM. (1986) Chronotoxicity of styrene in rat. Ann Rev Chronopharmacol 3, 102–6. [Google Scholar]
- 98.Desgagne M, Boutet M, Belanger PM. (1988) The mechanism of the chronohepatotoxicity of chloroform in rat: correlation between binding to hepatic subcellular fractions and histologlic changes. Ann Rev Chronopharmacol 5, 235–8. [Google Scholar]
- 99.Jaeger RJ, Conolly RB, Murphy SD. (1973) Diurnal variation of hepatic glutathione concentration and its correlation with 1,1-dichloroethylene inhalation toxicity in rats. Res Commun Chem Pathol Pharmacol 6, 465–71. [PubMed] [Google Scholar]
- 100.Cal JC, Cambar J. (1984) Time dependent mercuric chlorine induced acute renal failure in rats and mice. Ann Rev Chronopharmacol 1, 377–80. [Google Scholar]
- 101.Cambar J, Pons M. (1982) Etude des variations circadiennes de la dose léthale 50 du chlorure mercurique chez la souris. CR Acad Sci 294, 149–52. [PubMed] [Google Scholar]
- 102.Tamashiro H, Arakaki M, Akagi H, Hirayama K, Smolensky MH. (1986) Methylmercury toxicity in spontaneously hypertensive rats (SHR). Bull Environ Contam Toxicol 36, 668–73. [DOI] [PubMed] [Google Scholar]
- 103.Tamashiro H, Arakaki M, Akagi H, Hirayama K, Murao K, Smolensky MH. (1986) Sex differential of methylmercury toxicity in spontaneously hypertensive rats (SHR). Bull Environ Contam Toxicol 37, 916–24. [DOI] [PubMed] [Google Scholar]
- 104.Miura N, Yoshioka H, Ashimori A, Ohtani K, Hasegawa T, Hwang GW, Ikeda M, Nonogaki T. (2017) Multidirectional analyses of hepatic chronotoxicity induced by cadmium in mice. J Toxicol Sci 42, 597–604. [DOI] [PubMed] [Google Scholar]
- 105.Hawkins R, Kripke DF, Janowsky DS. (1978) Circadian rhythm of lithium toxicity in mice. Psychopharmacology (Berl) 56, 113–4. [DOI] [PubMed] [Google Scholar]
- 106.Shito T, Ando T, Okamoto T, Nakano S. (1992) Chronopharmacokinetics and chronotoxicity of lithium in mice eating normal and low-sodium diets. Chronobiol Int 9, 114–23. [DOI] [PubMed] [Google Scholar]
- 107.Yoshioka H, Nonogaki T, Shinohara Y, Suzui M, Mori Y, Hwang GW, Ohtani K, Miura N. (2018) Lethal chronotoxicity induced by seven metal compounds in mice. J Toxicol Sci 43, 129–34. [DOI] [PubMed] [Google Scholar]
- 108.Beland FA, Dooley KL, Sheldon WG, Delongchamp RR. (1988) Circadian variation in the induction of intestinal tumors by N-methyl-N-nitrosourea in male C57BL/6N mice. J Natl Cancer Inst 80, 325–30. [DOI] [PubMed] [Google Scholar]
- 109.Iversen OH, Iversen UM. (1976) Is there a diurnal variation in the susceptibility of mouse skin to the tumorigenic action of methylcholanthrene? A study of tumour yield with special reference to the variation between cages. Acta Pathol Microbiol Scand [A] 84, 406–14. [DOI] [PubMed] [Google Scholar]
- 110.Iversen OH, Iversen UM. (1995) A diurnal variation in the tumorigenic response of mouse epidermis to a single application of the strong short-acting chemical carcinogen methylnitrosourea. A dose-response study of 1, 2 and 10 mg. In Vivo 9, 117–32. [PubMed] [Google Scholar]
- 111.Schreiber D, Wessel H, Musil A. (1977) Brain tumour induction by methylnitrosourea. Influence of the circadian rhythm on tumour induction by nitrosourea. Neuropatol Pol 15, 137–44. [PubMed] [Google Scholar]
- 112.Dridi I, Grissa I, Ezzi L, Chakroun S, Ben-Cherif W, Haouas Z, Aouam K, Ben-Attia M, Reinberg A, Boughattas NA. (2016) Circadian variation of cytotoxicity and genotoxicity induced by an immunosuppressive agent “Mycophenolate Mofetil” in rats. Chronobiol Int 33, 1208–21. [DOI] [PubMed] [Google Scholar]
- 113.Itoh K, Masumori S, Nakajima M, Hayashi M, Sakakibara H, Shimoi K. (2012) Differences in micronucleus induction in peripheral blood reticulocytes of mice exposed to N-ethyl-N-nitrosourea at light and dark dosing times. J Toxicol Sci 37, 427–30. [DOI] [PubMed] [Google Scholar]
- 114.Sauerbier I .(1992) Rhythms in drug-induced teratogensis. In: Biologic rhythms in clinical and laboratory medicine, Touitou Y, Haus E (Eds.), 151–7, Springer-Verlag, Berlin. [Google Scholar]
- 115.Hatch TF. (1968) Significant dimensions of the dose-response relationship. Arch Environ Health 16, 571–8. [DOI] [PubMed] [Google Scholar]
- 116.Henane R, Valatx JL. (1973) Thermoregulatory changes induced during heat acclimatization by controlled hypothermia in man. J Physiol 230, 255–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Houston CS, Riley RL. (1947) Respiratory and circulatory changes during acclimatization to high altitude. Am J Physiol 149, 565–88. [DOI] [PubMed] [Google Scholar]
- 118.Sakuma T, Ohtake M, Katsurayama Y, Jarukamjorn K, Nemoto N. (1999) Induction of CYP1A2 by phenobarbital in the livers of aryl hydrocarbon-responsive and -nonresponsive mice. Drug Metab Dispos 27, 379–84. [PubMed] [Google Scholar]
- 119.Williams GM, Iatropoulos MJ. (2002) Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol 30, 41–53. [DOI] [PubMed] [Google Scholar]
- 120.Zheng JL, Parfett C, Williams A, Yagminas A, Zhou G, Douglas GR, Yauk CL. (2011) Assessment of subclinical, toxicant-induced hepatic gene expression profiles after low-dose, short-term exposures in mice. Regul Toxicol Pharmacol 60, 54–72. [DOI] [PubMed] [Google Scholar]
- 121.Koukkari WL, Sothern RB .(2006) Introducing biological rhythms, Springer Science + Business Media, USA. [Google Scholar]
- 122.Nicolau GY, Haus E. (1989) Chronobiologic reference values in clinical chemistry. Endocrinologie 27, 197–230. [PubMed] [Google Scholar]
- 123.Haus E, Lakatua DJ, Swoyer J, Sackett-Lundeen L. (1983) Chronobiology in hematology and immunology. Am J Anat 168, 467–517. [DOI] [PubMed] [Google Scholar]
- 124.Haus E, Nicolau GY, Lakatua D, Sackett-Lundeen L. (1988) Reference values for chronopharamcology. Ann Rev Chronopharmacol 4, 333–424. [Google Scholar]
- 125.Haus E, Nicolau GY, Lakatua DJ, Sackett-Lundeen L, Petrescu E, Swoyer J. (1993) Chronobiology in laboratory medicine. Ann Ist Super Sanita 29, 581–606. [PubMed] [Google Scholar]
- 126.Haus E, Nicolau GY, Lakatua DJ, Sackett-Lundeen L, Swoyer J .(1990) Circadian rhythms in laboratory medicine. In: Referencev values and chronobiology. Fanfani M and Tarquini B (Eds.), 21–33, XV World Cong of Anatomic and Clinical Pathology. Arand and Brent, Florence.
- 127.Reinberg AE, Dejardin L, Smolensky MH, Touitou Y. (2017) Seven-day human biological rhythms: an expedition in search of their origin, synchronization, functional advantage, adaptive value and clinical relevance. Chronobiol Int 34, 162–91. [DOI] [PubMed] [Google Scholar]
- 128.Reinberg A, Smolensky MH, Touitou Y. (2016) The full moon as a synchronizer of circa-monthly biological rhythms: chronobiologic perspectives based on multidisciplinary naturalistic research. Chronobiol Int 33, 465–79. [DOI] [PubMed] [Google Scholar]
- 129.Randem B, Smolensky MH, Hsi B, Albright D, Burge S. (1987) Field survey of circadian rhythm in PEF of electronics workers suffering from colophony-induced asthma. Chronobiol Int 4, 263–71. [DOI] [PubMed] [Google Scholar]
- 130.McKerrow CB, McDermott M, Gilson JC, Schilling RSF. (1958) Respiratory function during the day in cotton workers: a study in byssinosis. Br J Ind Med 15, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gängler S, Charisiadis P, Seth R, Chatterjee S, Makris KC. (2018) Time of the day dictates the variability of biomarkers of exposure to disinfection byproducts. Environ Int 112, 33–40. [DOI] [PubMed] [Google Scholar]
- 132.Watts JD .(1974) Urinary phenol excretion in a non-occupational exposed population [Master’s Dissertation]. The University of Texas-Houston School of Public Health, Houston.