Abstract
Potential effects of shift work on health are probably related to the misalignment between the light-dark cycle and the human activity-rest cycle. Light exposure at night mediates these effects, including social misalignment and leads to an inversion of activity and rest, which, in turn, is linked to changes in behaviours. This article reviews the epidemiological evidence on the association between shift work and health, and possible mechanisms underlying this association. First, evidence from findings of the meta-analyses and systematic reviews published in the last 10 yr is presented. In addition, it reports the larger single-occupation studies and recent large population-based studies of the general workforce. Koch’s postulates were used to evaluate the evidence related to the development of disease as a result of exposure to shift work. Finally, we discussed limitations of the multiple pathways that link shift work with specific disorders and the methodological challenges facing shift work research. We concluded that the clearest indications of shift work being the cause of a disease are given when there is a substantial body of evidence from high quality field studies showing an association and there is good evidence from laboratory studies supporting a causal explanation of the link.
Keywords: Shift work, Nonstandard work hours, Health, Circadian system
Consensus Statements.
-
1)
There is strong evidence linking shift work and negative health outcomes such as cardiovascular diseases, gastrointestinal and metabolic disorders (type 2 diabetes; metabolic syndrome).
-
2)
There is less consistent evidence linking shift work to cancer, mental health problems and reproduction-related problems.
-
3)
It is not clear the extent to which the negative health effects of shift work are mediated—either directly and/or indirectly—by circadian misalignment, sleep restriction and social misalignment.
-
4)
We need to understand both the mechanisms behind the development of the negative health effects and how countermeasures can be applied under real life conditions.
-
5)
Men and women can respond differently to shift work and the risk of developing particular negative health effects may be gender-specific.
-
6)
Laboratory findings should be contextualised in the light of ‘real world’ knowledge obtained from field studies.
Consensus statements review expert panel: Thomas KANTERMANN1 (Chair), John AXELSSON2, João Silvestre SILVA3
1University of Applied Sciences for Economics and Management, Germany
2Karolinska Institute, Sweden
3National Social Security Institute, Brazil
Full consensus among panel members on all statements.
Introduction
This manuscript is part of a series of consensus papers developed by the Working Time Society, as commissioned by the International Commission on Occupational Health. The goal of this series is to provide guidance for a broad, international audience of researchers, industry representatives, workers, labor representatives, policy makers, and other stakeholders on managing health risks associated with non-standard working hours. Collectively, the papers provide overviews of the current state of research, identify health and safety risks, make recommendations for effective interventions, and suggest future research directions. Each paper presents a number of consensus statements, developed through the procedures outlined in Wong et al.1), and describes the background information on which the consensus statements are based. The present paper reviews the epidemiological evidence on the association between shift work and health, and possible mechanisms underlying this association.
While non-standard working times are clearly associated with an increased risk of negative health outcomes2,3,4,5), the physiological mechanisms underlying/mediating shiftwork-related disease risk are poorly understood. Several mechanisms have been proposed by which shift work increases disease risk including circadian misalignment, disturbed sleep, light induced suppression of melatonin levels at night, and reduction in circadian amplitude6, 7). These factors in turn are thought to disturb a number of physiological and behavioural processes that further contribute to and/or potentiate disease.
The circadian system synchronizes physiology and behaviour to the environment such that the body works as a finely tuned clock. A master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus coordinates 24-h rhythms in physiology and behaviours throughout the body including other brain regions and peripheral tissues. When aligned or entrained appropriately to the environment, the clock promotes sleep and related ‘anabolic’ functions at night (e.g., immune function and hormone release), and wakefulness and its related catabolic functions during the day (e.g., food intake and metabolism, physical activity)5, 8). The internal biological day and night are often demarcated by the endogenous melatonin rhythm, which is controlled by the SCN clock9, 10). In humans, high melatonin levels occur during the biological night and low melatonin levels during the biological day. Circadian alignment occurs when behavioural and physiological processes occur at appropriate biological times and optimise the circadian timing of physiology and behaviour. On the other hand, circadian misalignment results from a mismatch in internal circadian timing and the environment and/or behaviours/physiology, and is characterised by desynchrony among the timing of internal rhythms5, 11).
Due to psychosocial, physiological, and work schedule-related factors, some shift workers experience substantial misalignment between the circadian system and the imposed rest/work schedule, while others may show lesser degrees of misalignment. Whether shift workers with greater circadian misalignment are at increased risk for health problems has been hypothesised but is largely unknown. Behaviours associated with shift work may also contribute to internal desynchrony of circadian clocks throughout the body with possible implications for health and disease. For example, findings from non-human animal studies show that the biological clock located in the liver can be desynchronized from the master SCN clock by timed feeding at abnormal biological times—leading to metabolic dysfunction12,13,14). Specifically, the liver clock responds most strongly to the timing of food intake whereas the SCN clock responds most strongly to the light-dark cycle. Peripheral clocks can also be synchronized by endogenous temperature cycles15). Every day behaviours of shift workers that may contribute to internal desynchrony of circadian clocks include light exposure during the dark period, eating at night, being more physically active at night, and sleeping during the day—these factors feed back onto the circadian clock causing desynchronised rhythms and, therefore altered metabolic and body temperature cycles.
Shift work often requires wakefulness to occur at night and sleep to occur during the day. Shift workers obtain less sleep and less refreshing sleep when sleep occurs during the day because the circadian clock is promoting wakefulness, and sleep in shift workers is also often socially curtailed because of family, and work requirements16,17,18). Not surprisingly, most shift workers report reduced subjective sleep quality and total sleep time19). Sleep is most disturbed during rotational and night shift work schedules19). Early morning shift work can also lead to short sleep as it is difficult to go to bed early due to social requirements (e.g., child care) and to biological drives since the internal clock maximally promotes wake during the early evening20). This, and early wake times, can for many workers result in short and non-refreshing sleep4, 21). Findings from epidemiological studies suggest that short sleep duration, disturbed sleep, and insomnia symptoms are important factors that are associated with an increased risk of poor health and disease in shift workers22).
Exposure to light at night (including room light levels that many night shift workers are subject to during their work shifts) can reduce circulating melatonin levels23). If the light is bright enough, endogenous nocturnal melatonin levels can be completely suppressed. Evidence from studies of cancer in mouse models has suggested, for example, an association between reduced melatonin levels by light at night and increased risk of cancer24).
It is largely unknown how alterations in circadian amplitude may contribute to health and disease in shift workers and therefore additional research is needed to assess the potential physiological role of circadian amplitude in the real world as well as under controlled laboratory conditions. Higher circadian amplitude can provides robustness of the circadian system to perturbation25). Lower circadian amplitude has been hypothesized to contribute to disease (e.g., irregular sleep-wake rhythm disorder, depression)26,27,28,29).
It is possible that the any or all of the mechanisms described above interact to influence health and disease in shift workers. Circadian misalignment, short sleep, reduced melatonin levels, and reduced circadian amplitude may also disturb the health of shift workers by alteration of a number of physiological and behavioural processes that contribute to the development of disease or exacerbate existing disease. These include inflammation, oxidative stress, alterations in the temporal patterns or levels of hormones, reductions in physical activity, and poor dietary habits.
In addition to physiological disruption, changes in shift workers’ lifestyle, behaviours and habits play an important role in the impact that work schedules have upon health outcomes. There is some evidence that shift workers are more likely to engage in ‘unhealthy’ behaviours based on reports of higher incidence of smoking and alcohol intake, together with higher BMI and lower physical activity levels5, 30, 31). Such behaviours and habits are inexorably linked with work schedules as work schedules inevitably dictate free time (both amount of timing) and thus drive behaviour. For example, working at night dictates sleeping during the day and therefore shifts eating opportunities to the night hours.
It has been suggested that the health consequences of shiftwork appear realtively non-specific and more consistent with what has been labelled as ‘lifestyle illness’. That is, shift work may predispose individuals to lifestyle illnesses and that some of the increased health risk for shift workers is due in part to the effects of (mal) adaptive non-circadian lifestyle factors. However, disentangling the relative contribution of circadian and non-circadian influences in ‘real-world’ settings has proved complex, as many field study designs do not permit the statistical separation of maladaptive lifestyle behaviours from the inherent circadian disruption associated with shift work.
In short, the potential effects of shift work on health are probably related to the misalignment between the light-dark cycle and the human activity-rest cycle. Light exposure at night mediates these effects, including social misalignment and leads to an inversion of activity and rest, which, in turn, is linked to changes in behaviours and habits. This complex interaction of biopsychosocial factors leads to an increase in ‘at risk’ lifestyle behaviours. What is not yet clear is the relative contribution of physiological and behavioural factors. While this may seem a relatively minor point it is critical with respect to risk mitigation. If the physiological factors are the primary determinants of disease mechanisms, there is probably little we can do to alter these effects short of eliminating shift work. On the other hand, if the effects are mediated primarily through (mal) adaptive changes in behaviour that increase the risk of lifestyle illnesses, these are potentially modifiable through appropriate training and education.
Evidence from Observational Studies
Given the very large numbers of observational studies that have examined the links between shift work and health, the first part of this section will focus primarily on the findings of the meta-analyses and systematic reviews published in the last 10 yr (summarized in Table 1). In addition, it will report the larger single-occupation studies (in terms of participants) published since those reviews, within each disease category (summarized in Table 2). The second part of this section will examine recent large population-based studies of the general workforce.
Table 1. Meta-analyses and reviews of the relationship between shift work and health.
| Authors | Type of article | Number of studies/ participants |
Summary findings | Designs |
|---|---|---|---|---|
| Cardiovascular Heart Disease | ||||
| Vyas et al., 2012 | Meta-analysis | 34 studies | Shift work associated with myocardial infarction (RR=1.23), ischemic stroke (RR=1.05) and coronary event (RR=1.24); highest risks associated with night work. | Prospective cohort; retrospective cohort; case-control |
| Frost et al., 2009 | Systematic review | 14 studies | Limited epidemiologic evidence of a causal association between shift work and ischemic heart disease. | Retrospective cohort; case-control; nested |
| Metabolic syndrome | ||||
| Wang et al., 2014 | Meta-analysis | 13 studies | Dose-response relationship with night work (RR=1.77 with longer exposure); with women at higher risk than men. | Cross-sectional; case-control; prospective cohort; nested |
| Obesity | ||||
| van Drongelen et al., 2011 | Systematic review | 8 studies | Crude association between night work and body weight increase; insufficient evidence of confound-adjusted relationship. | Prospective cohort; retrospective cohort |
| Proper et al., 2016 | Systematic review | 11 studies | Shift work associated with body weight gain, risk for overweight and impaired glucose tolerance. | Prospective cohort; retrospective cohort |
| Type-2 diabetes | ||||
| Gan et al., 2014 | Meta-analysis | 12 studies | Shift work associated with increased risk (OR=1.09); greater for males (OR=1.37) and rotating shifts. | Prospective cohort; retrospective cohort; cross-sectional |
| Anothaisintawee et al., 2016 | Meta-analysis | 10 studies | Shift work associated with increased risk (RR=1.40). | Prospective cohort; retrospective cohort |
| Breast Cancer | ||||
| He et al., 2015 | Meta-analysis | 15 studies | Shift work associated with increased risk (RR=1.12). | Cohort; case-control |
| Wang et al., 2013 | Meta-analysis | 10 studies | Night work associated with increased risk (RR=1.19); dose-response relationship with night work (RR=1.03 for every 5 yr increase of exposure to night work). | Prospective cohort; nested; case-control |
| Jia et al., 2013 | Meta-analysis | 13 studies | Night work associated with increased risk (RR=1.20). | Cohort; case-control |
| Ijaz et al., 2013 | Meta-analysis | 16 studies | Night work associated with increased risk in case- control studies (RR=1.09 for every 5 yr of exposure) but not in cohort studies (RR=1.01). | Prospective cohort; retrospective cohort; nested |
| Viswanathan et al., 2009 | Meta-analysis | 8 studies | Night work associated with increased risk (RR=1.40). | Prospective cohort; case-control |
| Erren et al., 2008 | Meta-analysis | 7 studies | Shift work associated with increased risk (RR=1.40). | Cohort; case-control |
| Megdal et al., 2005 | Meta-analysis | 6 studies | Night work associated with increased risk (RR=1.51). | Prospective cohort; retrospective cohort; nested; case-control |
| Lin et al., 2015 | Meta-analysis | 16 studies | Night work associated with increased risk (RR=1.029 for 5 yr, 1.025 for 5–10 yr, 1.074 for 10–20 yr, and 1.088 for >20-yr). | Prospective cohort |
| Kamdar et al., 2013 | Meta-analysis | 15 studies | Borderline significant association with ever exposed to night work (RR=1.21). | Cohort; case-control |
| Travis et al., 2016 | Meta-analysis | 10 studies | Night work not associated with increased risk. | Prospective cohort |
| Other cancer | ||||
| Rao et al., 2015 | Meta-analysis | 8 studies | Night work associated with increased risk of prostate cancer (RR=1.24). | Cohort; case-control |
| Wang et al., 2015 | Meta-analysis | 6 studies | Night work associated with increased risk of colorectal cancer (OR=1.32). | Cohort; case-control |
| Reproduction | ||||
| Stocker et al., 2014 | Meta-analysis | 15 studies | Shift work associated with increased menstrual disruption (OR=1.22). Night work associated with increased risk of early miscarriage (OR=1.29). | Cross-sectional; case-control; prospective cohort; retrospective cohort |
| Bonde et al., 2013 | Meta-analysis | 13 studies | Permanent night work associated with increased risk of miscarriage (RR=1.51). | Cross-sectional; case-control; prospective cohort; retrospective cohort |
| Palmer et al., 2013 | Meta-analysis | 21 studies | Shift work predicted small increase in risk of pre-term delivery (RR=1.04–1.18). | Cross-sectional; case-control; prospective cohort; retrospective cohort |
| Palmer et al., 2013 | Meta-analysis | 11 studies | Shift work not significantly associated with small for gestational age. | Cross-sectional; case-control; prospective cohort; retrospective cohort |
| van Melick et al., 2014 | Meta-analysis | 11 studies | Shift work not associated with pre-term birth. | Cross-sectional; case-control; prospective cohort; retrospective cohort |
| Gastrointestinal disorders | ||||
| Knutsson & Bøggild, 2010 | Systematic review | 20 studies | Shift work associated with gastrointestinal symptoms and peptic ulcer. | Cross-sectional; cohort. |
| Depression | ||||
| Lee et al., 2017 | Meta-analysis | 11 studies | Night shift work associated with increased risk of depression (RR=1.43). | Cross-sectional; longitudinal; prospective cohort. |
| Angerer et al., 2017 | Systematic review & Meta-analysis | 11 studies (5 included in meta-analysis) | Night shift work associated with non-significant increase in risk of depression (RR=1.42). | Prospective cohort. |
‘Shift work’ defined as any pattern of irregular work hours that may or may not involve night work. ‘Night work’ defined as any pattern of shift work that includes night working. OR: odds ration; RR: relative risk; HR: hazard ratio. ‘Cohort’ can be defined as a longitudinal study in which a group of people who share some characteristic is followed through a selected period. It can be retrospective when goes back in time or prospective when requires new data collected ahead.
Table 2. Single observational studies of the relationship between shift work and health.
| Authors | Type of article | Number of studies/ participants |
Summary findings |
|---|---|---|---|
| Cardiovascular Heart Disease | |||
| Vetter et al., 2016 | Prospective cohort | N=189,158 | Small but significant increase in risk among nurses with prolonged exposure (≥10 yr) to rotating night work (HR=1.15). |
| Breast cancer | |||
| Wegrzyn et al., 2017 | Prospective cohort | N=78,516 & N=114,559 | Increased risk associated with ≥ 20 yr exposure to night work, particularly among women exposed during young adulthood (Not significant in first study, women with ≥30 yr of shift work, follow-up primarily after retirement. HR=2.15 in second study, younger women with ≥20 yr of shift work; HR=1.4 in second study with updated exposure information). |
| Metabolic syndrome | |||
| Guo et al., 2015 | Cross-sectional | N=25,382 | Dose-response relationship with night work among women but not men (OR=1.10 for every 10 yr increase in shift work). |
| Type-2 diabetes | |||
| Silva-Costa et al., 2015 | Cross-sectional | N=14,427 | Increased risk associated with >20 yr exposure to night work in both sexes (RR=1.42 for women; 1.06 for men). |
| Hansen et al., 2016 | Prospective cohort | N=19,873 | Increased risk associated with night (OR=1.58) and evening shifts (OR=1.29), but not rotating shifts. |
| Reproduction | |||
| Gaskins et al., 2015 | Prospective cohort | N=1,739 | No association between shift work and fecundity. |
‘Shift work’ defined as any pattern of irregular work hours that may or may not involve night work. ‘Night work’ defined as any pattern of shift work that includes night working. OR: odds ration; RR: relative risk; HR: hazard ratio.
An evaluation was made of each of the meta-analyses and reviews presented here, focussing on their criteria for selecting studies. First, it was assessed whether all of the selected studies in a review found significant associations between shift work and the outcome. Significant findings are more likely to be published than non-significant findings32) and therefore if a review only includes studies with significant findings, this might suggest that the selection process for the review has not been sufficiently thorough (e.g. has not included searching for unpublished reports). There were no such indications of selection bias in the reviews and meta-analyses described below and thus all were evaluated positively in this regard. Second, each review was assessed in terms of whether it included studies conducted in more than one occupational sector. This was evaluated as a positive characteristic, as findings based on data from multiple occupations are less likely to be occupation-specific and may therefore be applicable to occupational groups beyond those studied. All of the reviews and meta-analyses described below were evaluated positively in this regard.
Meta-analyses, systematic reviews and selected single-occupation studies
A meta-analysis examining the links between shift work and cardiovascular heart disease (CHD) identified that shift workers have higher risk of myocardial infarction, with the highest risks associated with night work33). However, an earlier systematic review was equivocal about a causal link, concluding that it was not possible to rule out biases such as inadequate confounder control, selection bias, and misclassification due to very crude measures of night work exposure34). Since these reviews, a large prospective cohort study of female nurses based on the Nurses Health Study (NHS) observed a small but significant increase in risk among nurses with prolonged exposure (≥5 yr) to rotating shift work that includes night work3). Uniquely, they also found that cardiovascular risk waned after the cessation of shift work (Table 2).
Turning to the links between shift work and metabolic disorders, a meta-analysis reported a dose-response relationship between night work (number of years exposed) and metabolic syndrome (i.e., the association of visceral obesity, dyslipidemia, abnormal blood pressure, and serum glucose levels in the same individual)35). Female night workers were found to be at higher risk than their male counterparts, which it was suggested might have been due to women being more prone to vitamin D insufficiency. Subsequent to that review, a large study of retired workers found a dose-response relationship between duration of exposure and risk of metabolic syndrome among female but not male night workers (Table 2)36). Two recent systematic reviews considered obesity in shift workers37, 38). Both identified associations between shift/night work and body weight or BMI, but the earlier review38) concluded that there was insufficient evidence of a significant relationship after adjusting for confounders such as age, gender, bodyweight at baseline, and physical activity. Significant confounder-adjusted associations between shift work and type 2 diabetes were demonstrated in two recent meta-analyses39, 40). The earlier review39) found that risk was greater for male shift workers than for females shift workers. Since these two reviews, a large cross-sectional study identified associations between exposure to night work for >20 yr and type 2 diabetes, but in this case with stronger effects in women than in men41). Another recent large prospective cohort study, which compared risk on different shift patterns worked by nurses, found increased risk of type 2 diabetes associated with night and evening shifts, but not rotating shifts42).
The link between shift/night work and breast cancer has been examined by at least 10 meta-analyses to date. Eight of the 10 analyses reported a significant overall association43,44,45,46,47,48,49,50), while another reported an association bordering on significance51). However, the reviews’ authors expressed varying levels of caution regarding the size and reliability of the association, and whether it represented a causal link. The most recent meta-analysis found no significant association between shift work and breast cancer52). Since that most recent review, a paper reporting results from two large prospective studies based on the NHS reported that increased risk of breast cancer was primarily associated with long-term exposure to rotating night work during early adulthood53); a finding which may go some way to explaining the lack of association reported by Travis et al52), as discussed by Schernhammer54). Meta-analyses have also identified dose-response relationships between night work and prostate cancer55), and between night work and colorectal cancer56).
A meta-analysis of reproductive outcomes reported that shift work was associated with increased menstrual disruption but not infertility, after adjustment for confounds57). In addition, night work was associated with increased risk of early miscarriage after adjustment. A meta-analysis of outcomes later in pregnancy observed a marked increase in the risk of miscarriage associated with permanent night work, while the risk for rotating night work was smaller and not significant58). Another meta-analysis found that shift work predicted only small increases in risk of pre-term delivery and small-for-gestational-age at birth59). A fourth meta-analysis found no association between shift work and risk of pre-term birth60). Among the more recent investigations in this area, a prospective cohort study of women trying to get pregnant found no association between shift work (various patterns) and fecundity61) (Table 2).
Shift workers are more likely than their day working counterparts to report gastrointestinal symptoms and peptic ulcer, according to a systematic review of field studies62). However, it is important to note that many of the reviewed studies were cross-sectional and lacked good experimental control of potentially confounding effects.
Two systematic reviews and meta-analyses have recently been published evaluating the links between shift work and depression. The first was a meta-analysis which incorporated the results of 11 studies, 9 of which were cross-sectional, including two conference poster presentations63). They reported that night shift work was associated with an increased risk of depression. They also reported sub-group analyses in which they found that the association was significant among women but not men; and that the association was significant in both studies of nurses (3 studies) and studies of other occupations. They found no link between duration of shift work exposure and risk of depression (based on two studies). Another systematic review that was based exclusively on prospective studies (9 studies identified) was more equivocal in its findings64). They found no association among three studies of nurses which examined exposure to night work of up to 2 yr. However, in five studies that drew their samples from the general population, they found evidence of increased risk due to night in at least some sub groups over a long observation period (>4 yr), although with no uniform pattern. Thus one study found an association in women only, while another an association among men working night shifts and women working rotating shifts. Another study found an association in white collar workers but not blue collar workers. A supplementary meta-analysis of five of the studies found a non-significant trend for increased risk.
Population-based studies
One of the advantages of single-occupation cohort studies over population-based studies of the general workforce is that the former type of study design limits confounding due to differences between occupations (e.g., gender, work environment, shift pattern). However, one of the major limitations of single-occupation cohort studies is that the reported associations between shift work and health may not be applicable to the general population. This section contains a review of recent large population-based studies of the general workforce that have examined associations between shift work and health after adjusting for multiple lifestyle and work-related confounding factors.
Wyse et al.65) reported a cross-sectional study investigating whether shift work was associated with obesity, diabetes, and well-being in 277,168 working adults who were recruited as part of UK Biobank. Shift work was defined as a work schedule outside usual working hours (i.e., 9am–5pm weekdays). A number of variables were assessed, including body mass index (BMI), body composition, self-reported diabetes and/or cardiovascular disease, immune activation (calculated as the ratio of neutrophils to lymphocytes), neuroticism, and depressed mood. Associations between shift work and body composition, prevalent disease, and mood parameters were investigated using regression models, which were adjusted for a range of lifestyle and work-related confounding factors. Shift workers reported higher BMI, waist circumference, and a higher body fat percentage than non-shift workers. Shift workers were more likely to report diabetes, hypertension and angina, as well as higher immune activation (thought to contribute to the pathophysiology of diabetes) than non-shift workers. Neuroticism scores were higher in shift workers, and shift workers were also more likely to report mood instability, feeling depressed, and lack of enthusiasm, compared with non-shift workers. These findings were independent of multiple confounding factors and suggest that shift work not only compromises metabolic health, but reduces overall well-being.
As noted in the Introduction, one of the proposed pathways linking shift work to chronic disease is behavioural change, or more specifically, a reduction in physical activity (see also ‘Pathways for health effects among shift workers’, below). To investigate this issue, Neil-Sztramko et al.66) used data collected from the Canadian Health Measures Survey, a national cross-sectional survey conducted by Statistics Canada, to compare levels of physical activity between day workers (self-reported “regular daytime schedule or day shift”) and shift workers (self-reported “regular night shift” or “rotating night shift”). Physical activity data were collected with 4,323 participants using waist-worn accelerometers for seven d. Main outcome variables included the number of min/d where activity occurred at intensities higher than 3.0 metabolic equivalents in bouts of 10 min or more (i.e., moderate-vigorous physical activity) and the number of min/d at intensities less than 1.0 metabolic equivalents (i.e., sedentary time). Mean daily levels of moderate-vigorous physical activity and sedentary time were not different between shift workers and day workers. It is important to note that in this study, overall low levels of physical activity were observed in both day workers and shift workers—with only 15.4% of participants meeting the recommended physical activity guidelines66, 67).
Poor diet is another potential behavioural change that may contribute to the negative health outcomes associated with shift work. Wirth et al.68) examined the inflammatory potential of diet in a nationally representative sample of 7,643 shift workers and day workers as part of the National Health and Examination Survey, in the United States. Participants were characterised as either shift workers (evening, night, rotating shifts; n=1,445) or day workers (regular daytime; n=6,198). Diet was assessed using a population-based dietary inflammatory index (DII)69). The DII, which was developed to characterise an individual’s diet on a continuum from maximally anti-inflammatory to pro-inflammatory, is based on two different sources of dietary intake information (24-h dietary recall and a 7-d dietary recall). Higher DII scores (i.e., more pro-inflammatory) have been associated with impairments in glucose tolerance and circulating inflammatory markers consistent with metabolic syndrome68). Participation in any shift work was associated with higher adjusted mean DII values (i.e., more pro-inflammatory) compared to day workers.
Pathways for Health Effects among Shift Workers
Our understanding of the multiple pathways that link shift work with specific disorders is limited for two reasons. First, there are many potential factors that could lead to a disease besides the circadian disruption that is a consequence of inverting day- and night-time activity. Social aspects, inappropriate coping mechanisms, genetic characteristics, type of job, and stress at work are just some of the factors that may increase shift workers’ susceptibility to certain diseases70). Second, the term “shift work” has a broad meaning and it is difficult to conceptualise a dose-response relationship. Permanent night work, early morning shift work, alternating shifts, and irregular work hours are among the many types of work schedule that may be deemed as shift work. This makes it difficult to draw reliable conclusions regarding the association between shift work and disease because we do not have an agreed measure of the ‘dose’ experience by a shiftworker. Thus, for the purposes of this discussion we present some of the most well-known mechanisms for explaining the effects of shift work workers’ lives on health and disease risk, regardless of the type of work schedule.
The effects of shift work on circadian misalignment have, for the most part, been examined in simulated shift- and night-work studies focusing on health-related outcomes, using markers of metabolic function, inflammatory responses, and/or cardiovascular measures. Consistently, albeit with some variation, it has been found that these outcomes are altered during ‘night shifts’ as compared to ‘day shifts’, during periods of circadian misalignment. With regard to the impact of shift work on sleep, a large body of research, which will not be reviewed here, reports changes in physiological measures associated with sleep restriction in the absence of circadian misalignment (for review see4, 71,72,73). The effects of shift work on family and community are discussed elsewhere in this special issue (see Arlinghaus et al. “Working Time Society Consensus: Evidence-based effects of shift work and non-standard working hours on workers, family and community”). The sleep disturbances reported by shift workers21) may be due to circadian misalignment, sleep restriction, or a combination of both. Disturbed sleep may, in turn, be an underlying cause of other symptoms and/or diseases that are prevalent among shift workers, such as inflammatory responses and increased sensitivity to pain, as well as altered metabolism.
Inflammatory responses and pain
Vallieres et al.74) showed that insomnia contributes to chronic pain, mainly among rotating shift workers. Although the relationship between insomnia and pain is not exclusively seen in shift workers75), the sleep restriction and circadian misalignment observed in this population may lead to additional immune/inflammatory changes76, 77). This was supported by evidence from a laboratory study involving chronic sleep disruption and circadian misalignment, in which circadian misalignment was found to reduce cortisol levels and increase levels of pro- and anti-inflammatory proteins78). Given that inflammation plays a role in the development of several chronic diseases associated with shift work, including metabolic syndrome, cardiovascular disease, cancer and diabetes, diets that promote inflammatory responses may represent a target for behavioural interventions to reduce the health impacts of shift work68).
Metabolic dysfunction
Metabolic dysfunction (i.e. the failure of bodily process such as the conversion of consumed food into energy) is more prevalent among shift workers1, 79,80,81), as is one of its main symptoms, namely obesity2, 79,80,81). Higher levels of obesity may in turn underlie the increased prevalence of obstructive sleep apnea (OSA) that is found among shift workers82). OSA, which is characterised by bouts of interrupted breathing during sleep83), has a well-established association with obesity84).
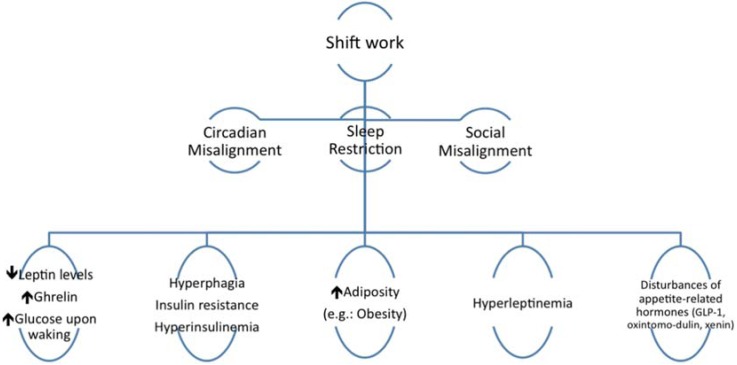
Experimental and observational studies have indicated that circadian disruption and insufficient/poor quality sleep maybe part of the mechanism by which shift work leads to impaired metabolism85,86,87,88,89,90,91), as summarized in Fig. 1.
Fig. 1.
Mechanisms underlying metabolic dysfunction in shift workers.
Seminal work by Van Cauter and colleagues demonstrated the effect of circadian disruption in glucose and insulin response to meals92). A series of studies followed which examined the impacts of a phase shift on glucose and insulin responses in the laboratory. A 9-h phase advance resulted in significant changes to glucose and insulin responses to a test meal. Using the same protocol, the team also demonstrated changes to lipid metabolism93, 94). These earlier studies were some of the first to point to possible mechanisms by which shift work may impact long-term health, as inappropriate glucose and insulin responses can result, over time, in type 2 diabetes.
In addition to circadian disruption, sleep loss has also been shown to influence metabolic function. For example, a recent study used an 11-d protocol involving three control nights of sleep followed by eight periods of sleep restriction to up to 5 h either during the night hours, or during the day hours. Insulin sensitivity was decreased and inflammation was increased when participants were sleep restricted and these changes were greater when the sleep was scheduled during the day (i.e., sleep loss plus circadian misalignment)91). Similarly, a circadian misalignment protocol was used to separate the effects of the behavioural rhythm, circadian timing, and circadian disruption on glucose and insulin response to meals95). In addition to investigating circadian misalignment by shifting sleep to the biological day, meal alignment effects were tested by providing meals at the same point in the behavioural rhythm (breakfast upon waking) but at different points in the circadian rhythm (night sleep breakfast at 0800 h, day sleep breakfast at 2,000 h). Glucose tolerance was reduced following evening meals compared to morning meals (i.e., the circadian effect was independent from the behavioural rhythm). Similarly, glucose response to meals was lower in the misaligned condition, independent of circadian time95). Together the findings add to our understanding of the mechanisms by which shift work may increase risk of metabolic disorder and diabetes.
While not ‘simulated shift-work’ per se, forced desynchrony studies also provide vital insights into the potential mechanisms underlying increased disease risk in shift work. The forced desynchrony protocol is an experimental research design that allows the researcher to separate the relative effects of the sleep homeostatic and circadian systems. Using this protocol, Scheer and colleagues reported that circadian misaligned states were associated with decreased leptin (the body’s appetite suppressant) and increased glucose levels, compared to the aligned states. In addition, 3/8 participants in the study reached pre-diabetic states87). Moreover, increased glucose response following meals was reported when participants were in a misaligned state in the forced desynchrony protocol96) and this study also reported decreased leptin levels with circadian misalignment.
Preferences for certain food types are also reported to change with insufficient sleep73, 97), with participants displaying preferences for high-fat foods following one night shift98). The combination of circadian misalignment and insufficient sleep promote overeating as well as reduced capacity of the body to cope with the food. Thus both the timing of eating and the content of the meals are key factors99, 100). Energy expenditure may also be altered by shift work, with one laboratory study finding that three consecutive simulated night shifts were associated with reduced total daily energy expenditure101). Such effects, in combination with low physical activity levels in shift workers noted previously, could further contribute to weight gain and obesity.
The evidence reviewed above suggests that the increased risk of metabolic dysfunction in shift workers compared with day workers may be linked to dietary pattern, which is often altered in shift workers by the inversion of the sleep-wake cycle. Since shiftworkers can be relatively ‘time poor’, they are more likely to eat easily prepared foods that are of low nutritional quality and that are rich in saturated fats and sugars100, 102). Shift workers also tend to consume lower quantities of healthy foods such as vegetables and fruits103, 104). As well as the potential negative impact on metabolic processes, disrupted eating habits may also underlie the higher prevalence of gastric disturbances among shift workers (e.g. heartburn, abdominal pains, constipation, borborygmus, flatulence); as well as more serious diseases such as chronic gastritis, gastroduodenitis, peptic ulcer, and colitis105). These problems have been linked to poor eating habits such as binge eating to remain awake at night100), as well as to the limited availability of healthy foods in the workplace at night. Considering that the circadian temporal system regulates the appetite106) as well as the gastric activity (intestinal motility, for example), the nocturnal eating behaviour observed in shift workers can contribute to gastrointestinal disturbances62).
Behavioural countermeasures to the negative effects of shift work and night work on health have been tested in simulated settings. A small study demonstrated beneficial effects on blood pressure during a night shift when participants engaged in moderate exercise immediately prior to the start of the shift. Blood pressure was lower throughout the shift compared to a no exercise condition107). It remains an open question as to how much additional physical activity shift workers should undertake in order to maintain good health. Preliminary work investigating the effects of fasting during a simulated night shift has shown a reduced glucose response to breakfast as compared to eating during a night shift108). While the implementation of such countermeasures may have broader ramifications, these findings point towards potential strategies to minimise the negative impacts of shift and night-work on health.
Research showing that shift workers are more likely to engage in unhealthy behaviours is sometimes interpreted in terms of shift workers’ adopting inappropriate coping strategies. However, while eating at night may be viewed as an unhealthy behaviour due to circadian misalignment of digestive rhythms, the work pattern requires it. Rather than advocating a simplistic argument that shift workers have poor coping strategies (i.e. blaming the victim), it might be more useful to suggest that there are significant additional risk factors for shift workers (sleep and circadian disruption) which mean that healthy behaviours are even more important for long-term health and well-being. A recent study showed that despite shift workers engaging in levels of physical activity below those recommended for good health, the levels were not substantially different from non-shift workers66). Despite the health benefits of physical activity being well known, and central to most public health campaigns, only 15% of people in the study met the levels in the guidelines. All workers engage in ‘unhealthy’ behaviours and thus the messaging might usefully focus on benefits of health choices rather than the negative consequences. It may be that healthy habits and behaviours are even more important for shift workers but the studies have yet to be designed that can provide such guidance.
Cancer
In 1987, Stevens first proposed a connection between light exposure at night and breast cancer109, 110). Such a link may have significant implications for night workers who are regularly exposed to artificial light (Fig. 1). As noted above, investigations of the connection between shift work and cancer have produced mixed findings, possibly due to differences between studies with respect to the types of shift being worked, the waveform and intensity of the light, the duration of exposure, and the potentially confounding effects related to concurrent exposure to other possible carcinogens. As also noted above, exposure to artificial light can potentially suppress melatonin, production. Suppressed melatonin production has been linked to reproductive functions of the gonadal pituitary axis and prolactin secretion111). This suggests that disturbance of melatonin levels may lead to modulation of the individual’s reproductive physiology and subsequent susceptibility to neoplastic growth.
Mental health
Studies of the impact of shift work on mental health have highlighted the potential impact of disturbed social and biological rhythms112) and decreased exposure to daylight113). However, there is no clear evidence regarding the mechanisms involved, and there are inconsistencies between the studies114). McClung115) and Richter and colleagues116) pointed out that both circadian misalignment and sleep problems caused by shift work can lead to the development of mood disorders117). These mood effects could, in turn, trigger or exacerbate depressive episodes.
Gender
Gender differences in the effects of shift work have mixed effects, as was seen in some of the systematic reviews and meta-analyses described above. Female shift workers tend to be at greater risk of mortality, disability pension, obesity, fatigue and sleepiness. On the other hand, female shift workers have been found to be at lower risk of certain health complaints118). Several factors may contribute to such gender differences. Female shift workers are said to face the ‘double burden’ of having to cope with demanding work schedules in combination with a higher domestic workload than their male counterparts. The irregular work hours of a rotating shift schedule may be especially disadvantageous for those with childcare responsibilities (i.e. most often women), given the invariant nature of childcare from day to day and week to week. Females may be more likely than men to work highly demanding shift patterns, such as split shifts (i.e. two or more periods of work within a single day) and ‘quick returns’ (i.e. short intervals between the end of one shift and the start of the next)119). Psychobiological factors may also play a role, with women having been shown to experience more sleep disruption and cognitive impairment in connection with night shifts possibly due to greater sleep disruption120).
Methodological issues are likely to underlie the mixed picture that emerges from gender comparisons in the effects of shift work. Most such studies are based on samples from multiple occupational groups. As female and male shift workers tend to work in different occupations, there is a risk that such gender comparisons are confounded by occupational factors.
Overall Evaluation
To evaluate the evidence related to the development of disease as a result of exposure to shift work we have considered Koch’s postulates. Koch’s postulates are a series of basic criteria, first formulated by Robert Koch, for identifying the causative agent of a particular disease. The postulates were originally developed as a method of establishing a causative relationship between a microorganism and a disease, but they have since been adapted and are considered an accepted method for determining cause-effect relationships in the study of human disease121). The criteria include: strength of association, biologic credibility, consistency with other investigations, time sequence, and dose-response relationship. As a convention, more than one criterion must be met to support a hypothesis that a specific pathogen—in this case shift work—is the cause of the disease; the more criteria that are met, the stronger the evidence for a cause-effect relationship122).
Strength of association
The strength of association describes the strength of the relationship between exposure to the proposed cause and symptoms of the disease. Studies examining the relationships between shift work and health typically use odds ratios or risk ratios to provide an indication of the strength of association. Odds ratios are used to compare the relative odds of the occurrence of the outcome of interest (e.g., disease or disorder), given exposure to the variable of interest (e.g., shift work), whereas risk ratios describe the ratio of the probability of an event occurring (e.g., developing a disease) in an exposed group (e.g., shift workers) to the probability of the event occurring in a comparison, non-exposed group (e.g., day workers). Tables 1 and 2 summarise the relative odds and risk ratios for particular diseases as a result of exposure to shift work. In most cases, the odds ratios or risk ratios are greater than one, indicating a strong association between exposure to shift work and the development of various diseases related to health.
Biologic credibility
Several mechanisms have been proposed by which shift work increases disease including circadian misalignment, disturbed sleep, light induced suppression of melatonin levels at night, and reduction in circadian amplitude. Much of our understanding of these proposed mechanisms comes from tightly controlled laboratory studies in which variables are experimentally manipulated (i.e., sleep dose, rest/activity cycle, etc.) and changes in the outcome variables of interest are measured. Laboratory studies involving sleep restriction and/or simulated night/shift work show altered glucose and insulin responses to meals87, 93, 96), alterations in appetite hormones87), and changes in preference for particular types of food, as well as reductions in total daily energy expenditure101). Over time, such changes could lead to overweight and obesity, type 2 diabetes, and metabolic syndrome. More recently, changes in gut microbiota as a result of sleep loss and/or circadian misalignment have been proposed as a potential pathway linking shift work to gastro-intestinal dysfunction (for review see122)). Field studies are generally supportive of an association between cardiovascular heart disease and shift work; however questions remain regarding the causal nature of that relationship. Laboratory studies indicate alterations in cortisol levels, inflammatory markers, blood pressure and other measures following a period of simulated shift work, all of which may provide potential pathways for the development of cardiovascular disease following exposure to shift work but further replication is required.
The observational evidence linking shift work to reproduction problems and to impaired mental health is somewhat inconsistent, and there is a considerable lack of laboratory studies examining the causal relationship between shift work and the development of these conditions. Perhaps the most hotly debated issue in the arena of shift work and health is the link between shift work exposure and breast cancer. The evidence from field studies is largely supportive of a link, with nine out of ten meta-analyses finding an association, but laboratory studies have yet to provide a conclusive account of an underlying mechanism.
Consistency with other investigations
This criterion refers to a pattern of similar results found by different investigators at different times and in different populations. In Tables 1 and 2, we report the findings from over 300 studies conducted in the last 10 yr that have examined the relationship between health and exposure to shiftwork. These studies have examined shift workers from countries around the world (e.g., Brasil, Denmark, England, Finland, France, Italy, Japan, Sweden, USA, Australia) who are engaged in occupations across a range of industries (e.g., health care, transportation, manufacturing, hospitality). The studies linking shift work and negative health outcomes such as cardiovascular diseases, gastrointestinal, metabolic disorders (e.g., type 2 diabetes, metabolic syndrome) and cancer are consistent, reporting similar patterns of results. There is less consistent evidence linking shift work to mental health problems and reproduction-related problems.
Time sequence
In order for an hypothesized cause-effect relationship between shift work and the development of a particular disease to be supported, exposure to shift work must occur before the development of disease itself. Prospective or longitudinal investigations provide good evidence regarding either the incidence of an outcome or the relative risk of an outcome based on exposure. Tables 1 and 2 identify a number of prospective/longitudinal studies in which individuals are recruited at baseline prior to commencing shift work, and then followed for several years later for incidence of a particular disease. A majority of these studies show clear evidence of temporal sequence; that is, individuals who are disease free prior to shift work exposure are more likely to develop type 2 diabetes, metabolic syndrome, cardiovascular heart disease, mental ill health, and breast cancer following exposure to shift work when compared with individuals who have never been exposed to shift work.
Dose-response relationship
This criterion describes a relationship in which greater exposure to the etiologic agent results in greater risk of contracting the disease. A number of studies reported in Tables 1 and 2 have examined the impact of exposure to shift work (i.e., number of years worked) and the risk of developing disease. The risk of developing metabolic syndrome, type 2 diabetes, and breast cancer increases with increasing exposure to shift to work; with some evidence to suggest prolonged exposure to shift work results in a small but significant increase in the risk of developing cardiovascular disease. There also appears to be a gender difference in the dose-response relationship between exposure to shift work and development of some diseases, suggesting that some of the health effects of shift work and the proposed mechanisms may be different in men and women.
Methodological Challenges Facing Shift Work Research
A common theme that occurs in several of the reviews cited above is that firm conclusions regarding the link between shift work and impaired health cannot be drawn due to the paucity of reliable evidence from high quality studies. Cross-sectional studies, studies with small and/or unrepresentative samples, and studies that rely on un-validated self-reported indices can—at best—only be suggestive of a causal link (or an absence of a link) between work schedule and the studied health outcome. Most of the systematic reviews draw upon one or other of the various published sets of criteria for assessing the quality of such studies (e.g. Downs et al.123), Wells et al.124)). These highlight the need for longitudinal studies that are based on large representative samples, using reliable and accurate assessments of exposure and outcome, with adjustment for relevant confounders and an appropriate length of follow-up.
Within the reviews of shift work research, one of the most frequently cited methodological weaknesses is the inadequate specification of exposure and the related issue of misclassification (e.g. as ‘shift worker vs. ‘day worker’). At one level, this means failing to adequately account for the number of years of exposure, while at the more ‘acute’ level it means inadequate specification of the shift pattern (both current and in the past). It is widely recognized that the impact of shift work on sleep and circadian rhythms will depend upon the particular shift patterns that are worked e.g. the intensity of night work, the sequence of shifts (presence or absence of rotation, forward or backward rotation, speed of rotation), distribution of rest periods and the days of the week that are worked i.e. just weekdays or including weekends125). A related issue is the reliance by many studies on subjective reports of exposure. For example, the self-reported assessment of shift work schedule has been shown to be potentially unreliable when compared to objective work schedule records, increasing the risk that workers are misclassified with respect to their shift work status126).
In common with other research fields, a major challenge for shift work researchers is taking adequate account of confounds. Shift workers may be predisposed to poorer health due to pre-existing factors such as (lower) socio-economic status and (poorer) health behaviours, which may confound shift work status even within relatively homogeneous samples127, 128). Other potential confounds/mediators/moderators that should be considered when examining the associations between shift work and health include preference for mornings versus evenings (‘chronotype’), neuroticism, extraversion, exposure to light at night, and sleep timing both on work days and non-work days129).
The choice of an appropriate control group is crucial. For example, when studying the effects of night work, shift workers are usually compared with day workers, but if the day workers are working shifts themselves (e.g. mornings and evening shifts, which are associated with their own set of challenges for workers’ health) then the sensitivity of the comparison may be reduced38).
Another, related, challenge for shift work research is the issue of selection effects and in particular the so-called healthy shift worker effect. This refers to the tendency for individuals with poor health (either as a consequence of shift work exposure, or as a pre-existing condition) to quit shift work (the healthy shift worker survivor effect) or avoid it in the first place (the healthy shift worker hire effect). As a result, the effects of shift work in the general population may be underestimated by research because those who participate in the research are a self-selected/survivor population who are better able than most to tolerate the impact of shift work. Accounting for such effects would ideally involve having detailed and accurate measures of relevant pre-employment factors for both shift workers and the comparison group (usually day workers), together with a prolonged period of follow up.
All of these challenges, along with many other besides, mean that while there is a large and suggestive body of evidence linking shift work with various health complaints, there remains a need for further, well-designed, large scale longitudinal studies to clearly and unambiguously demonstrate those relationships. Some of the reviews cited above argue in favour of prospective cohort studies rather than case-control studies as being the most reliable method of examining these issues (e.g. Ijaz et al.46), Travis et al.52)), although others have argued against that view130, 131).
Of course, none of the above is to argue that researchers to date have been in any way negligent. Researchers inevitably have to make compromises in the face of practical and logistical constraints when collecting data ‘in the real world’. The challenge for future research is to build on that past work, identifying and capitalising on those rare opportunities where the constraints are fewer and where the major obstacles can be circumvented.
Final Remarks
Shift work has been associated with a diverse range of significant health problems, from changes in body mass to prostate cancer. The studies reviewed above employed a wide range of methodological approaches, which may account for the disparity in the results obtained. Despite the lack of consistency between studies, a number of broad conclusions can be drawn.
In general, the clearest indications of shift work being the cause of a disease are given when there is a substantial body of evidence from high quality field studies showing an association and there is good evidence from laboratory studies supporting a causal explanation of the link.
The differences between shift work schedules, in addition to different occupational categories, may mean that countermeasures will need to be tailored to workplaces. On the other hand, in order to mitigate the effects of shift work on health we need to understand both the mechanisms behind the development of the diseases and how any proposed countermeasures may be applied in real life. We also need to understand the mechanisms that protect some individuals who are exposed to risk factors from developing the associated disease, in order to understand the healthy worker effect. No single methodological approach allows us to do this on its own.
Overall, we can conclude that there is evidence linking shift work and negative health outcomes. It is also clear that life style/behavioural aspects such as diet and physical activity, as well as many other factors, can contribute to the development/prevention of diseases among shift workers.
References
- 1.Wong IS, Dawson D, Van Dongen HPA. (2019) International consensus statements on non-standard working time arrangements and occupational health and safety. Ind Health, 57, 135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulhôa MA, Marqueze EC, Burgos LG, Moreno CR. (2015) Shift work and endocrine disorders. Int J Endocrinol 2015, 826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES. (2016) Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315, 1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kecklund G, Axelsson J. (2016) Health consequences of shift work and insufficient sleep. BMJ 355, i5210. [DOI] [PubMed] [Google Scholar]
- 5.McHill AW, Wright KP., Jr2017) Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev 18 Suppl 1, 15–24. [DOI] [PubMed] [Google Scholar]
- 6.Wright KP, Jr, Bogan RK, Wyatt JK. (2013) Shift work and the assessment and management of shift work disorder (SWD). Sleep Med Rev 17, 41–54. [DOI] [PubMed] [Google Scholar]
- 7.Touitou Y, Reinberg A, Touitou D. (2017) Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci 173, 94–106. [DOI] [PubMed] [Google Scholar]
- 8.Mohawk JA, Green CB, Takahashi JS. (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35, 445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. (2013) Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol 23, 1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teclemariam-Mesbah R, Ter Horst GJ, Postema F, Wortel J, Buijs RM. (1999) Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J Comp Neurol 406, 171–82. [PubMed] [Google Scholar]
- 11.Qian J, Scheer FAJL. (2016) Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab 27, 282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14, 2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291, 490–3. [DOI] [PubMed] [Google Scholar]
- 14.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buhr ED, Yoo SH, Takahashi JS. (2010) Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekine M, Chandola T, Martikainen P, Marmot M, Kagamimori S. (2006) Work and family characteristics as determinants of socioeconomic and sex inequalities in sleep: The Japanese Civil Servants Study. Sleep 29, 206–16. [DOI] [PubMed] [Google Scholar]
- 17.Costa G, Sartori S, Åkerstedt T. (2006) Influence of flexibility and variability of working hours on health and well-being. Chronobiol Int 23, 1125–37. [DOI] [PubMed] [Google Scholar]
- 18.Rotenberg, Portela LF, Marcondes WB, Moreno CRC, Nascimento CP (2000) Gender and diurnal sleep in night workers at a Brazilian industry. Arbeitswissenschaft Betrieblichen 17, 305–9. [Google Scholar]
- 19.Åkerstedt T, Wright KP., Jr2009) Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin 4, 257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake CL, Wright KP., Jr 2011) Shift Work, Shift Work Disorder, and Jet Lag. In M.H. Kryger, T. Roth, W.C. Dement (Eds). Principles and Practice of Sleep Medicine, 5th Ed. 784–98. [Google Scholar]
- 21.Åkerstedt T, Kecklund G, Gillberg M. (2007) Sleep and sleepiness in relation to stress and displaced work hours. Physiol Behav 92, 250–5. [DOI] [PubMed] [Google Scholar]
- 22.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. (2004) Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 27, 1453–62. [DOI] [PubMed] [Google Scholar]
- 23.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. (2000) Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L, Kogevinas M, Kogi K, Lie JA, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V, Straif K. (2011) Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med 68, 154–62. [DOI] [PubMed] [Google Scholar]
- 25.Siepka SM, Takahashi JS. (2005) Methods to record circadian rhythm wheel running activity in mice. Methods Enzymol 393, 230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zee PC, Vitiello MV. (2009) Circadian rhythm sleep disorder: irregular sleep wake rhythm type. Sleep Med Clin 4, 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan ZL, Bao AM, Tao M, Liu YJ, Zhou JN. (2007) Circadian rhythm of salivary serotonin in patients with major depressive disorder. Neuroendocrinol Lett 28, 395–400. [PubMed] [Google Scholar]
- 28.Szuba MP, Guze BH, Baxter LR., Jr1997) Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry 42, 1130–7. [DOI] [PubMed] [Google Scholar]
- 29.Wirz-Justice A, Kräuchi K, Brunner DP, Graw P, Haug HJ, Leonhardt G, Sarrafzadeh A, English J, Arendt J. (1995) Circadian rhythms and sleep regulation in seasonal affective disorder. Acta Neuropsychiatr 7, 41–3. [DOI] [PubMed] [Google Scholar]
- 30.Bonnell EK, Huggins CE, Huggins CT, McCaffrey TA, Palermo C, Bonham MP. (2017) Influences on dietary choices during day versus night shift in shift workers: a mixed methods study. Nutrients 9, E193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bushnell PT, Colombi A, Caruso CC, Tak S. (2010) Work schedules and health behavior outcomes at a large manufacturer. Ind Health 48, 395–405. [DOI] [PubMed] [Google Scholar]
- 32.Field AP, Gillett R. (2010) How to do a meta-analysis. Br J Math Stat Psychol 63, 665–94. [DOI] [PubMed] [Google Scholar]
- 33.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG. (2012) Shift work and vascular events: systematic review and meta-analysis. BMJ 345, e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frost P, Kolstad HA, Bonde JP. (2009) Shift work and the risk of ischemic heart disease—a systematic review of the epidemiologic evidence. Scand J Work Environ Health 35, 163–79. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Zhang L, Zhang Y, Zhang B, He Y, Xie S, Li M, Miao X, Chan EY, Tang JL, Wong MC, Li Z, Yu IT, Tse LA. (2014) Meta-analysis on night shift work and risk of metabolic syndrome. Obes Rev 15, 709–20. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Rong Y, Huang X, Lai H, Luo X, Zhang Z, Liu Y, He M, Wu T, Chen W. (2015) Shift work and the relationship with metabolic syndrome in Chinese aged workers. PLoS One 10, e0120632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RCH, van der Beek AJ, van Steeg H, van Kerkhof LWM. (2016) The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am J Prev Med 50, e147–57. [DOI] [PubMed] [Google Scholar]
- 38.van Drongelen A, Boot CRL, Merkus SL, Smid T, van der Beek AJ. (2011) The effects of shift work on body weight change—a systematic review of longitudinal studies. Scand J Work Environ Health 37, 263–75. [DOI] [PubMed] [Google Scholar]
- 39.Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O, Deng J, Bi H, Lu Z. (2015) Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 72, 72–8. [DOI] [PubMed] [Google Scholar]
- 40.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. (2016) Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev 30, 11–24. [DOI] [PubMed] [Google Scholar]
- 41.Silva-Costa A, Rotenberg L, Nobre AA, Schmidt MI, Chor D, Griep RH. (2015) Gender-specific association between night-work exposure and type-2 diabetes: results from longitudinal study of adult health, ELSA-Brasil. Scand J Work Environ Health 41, 569–78. [DOI] [PubMed] [Google Scholar]
- 42.Hansen AB, Stayner L, Hansen J, Andersen ZJ. (2016) Night shift work and incidence of diabetes in the Danish Nurse Cohort. Occup Environ Med 73, 262–8. [DOI] [PubMed] [Google Scholar]
- 43.He C, Anand ST, Ebell MH, Vena JE, Robb SW. (2015) Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health 88, 533–47. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Yeung KL, Chan WC, Kwok CCH, Leung SL, Wu C, Chan EYY, Yu ITS, Yang XR, Tse LA. (2013) A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann Oncol 24, 2724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, Huang S, Chen J. (2013) Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol 37, 197–206. [DOI] [PubMed] [Google Scholar]
- 46.Ijaz S, Verbeek J, Seidler A, Lindbohm ML, Ojajärvi A, Orsini N, Costa G, Neuvonen K. (2013) Night-shift work and breast cancer—a systematic review and meta-analysis. Scand J Work Environ Health 39, 431–47. [DOI] [PubMed] [Google Scholar]
- 47.Viswanathan AN, Schernhammer ES. (2009) Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett 281, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erren TC, Pape HG, Reiter RJ, Piekarski C. (2008) Chronodisruption and cancer. Naturwissenschaften 95, 367–82. [DOI] [PubMed] [Google Scholar]
- 49.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. (2005) Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer 41, 2023–32. [DOI] [PubMed] [Google Scholar]
- 50.Lin X, Chen W, Wei F, Ying M, Wei W, Xie X. (2015) Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med 16, 1381–7. [DOI] [PubMed] [Google Scholar]
- 51.Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. (2013) Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 138, 291–301. [DOI] [PubMed] [Google Scholar]
- 52.Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang XS, Roddam AW, Gathani T, Peto R, Green J, Key TJ, Beral V. (2016) Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst 108, djw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, Laden F, Willett WC, Hankinson SE, Schernhammer ES. (2017) Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am J Epidemiol 186, 532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schernhammer ES. (2017) RE: Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst 109, 1–2. [DOI] [PubMed] [Google Scholar]
- 55.Rao D, Yu H, Bai Y, Zheng X, Xie L. (2015) Does night-shift work increase the risk of prostate cancer? A systematic review and meta-analysis. Onco Targets Ther 8, 2817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Ji A, Zhu Y, Liang Z, Wu J, Li S, Meng S, Zheng X, Xie L. (2015) A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget 6, 25046–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stocker LJ, Macklon NS, Cheong YC, Bewley SJ. (2014) Influence of shift work on early reproductive outcomes: a systematic review and meta-analysis. Obstet Gynecol 124, 99–110. [DOI] [PubMed] [Google Scholar]
- 58.Bonde JP, Jørgensen KT, Bonzini M, Palmer KT. (2013) Miscarriage and occupational activity: a systematic review and meta-analysis regarding shift work, working hours, lifting, standing, and physical workload. Scand J Work Environ Health 39, 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer KT, Bonzini M, Harris EC, Linaker C, Bonde JP. (2013) Work activities and risk of prematurity, low birth weight and pre-eclampsia: an updated review with meta-analysis. Occup Environ Med 70, 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Melick MJ, van Beukering MD, Mol BW, Frings-Dresen MH, Hulshof CT. (2014) Shift work, long working hours and preterm birth: a systematic review and meta-analysis. Int Arch Occup Environ Health 87, 835–49. [DOI] [PubMed] [Google Scholar]
- 61.Gaskins AJ, Rich-Edwards JW, Lawson CC, Schernhammer ES, Missmer SA, Chavarro JE. (2015) Work schedule and physical factors in relation to fecundity in nurses. Occup Environ Med 72, 777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knutsson A, Bøggild H. (2010) Gastrointestinal disorders among shift workers. Scand J Work Environ Health 36, 85–95. [DOI] [PubMed] [Google Scholar]
- 63.Lee A, Myung SK, Cho JJ, Jung YJ, Yoon JL, Kim MY. (2017) Night shift work and risk of depression: meta-analysis of observational studies. J Korean Med Sci 32, 1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angerer P, Schmook R, Elfantel I, Li J. (2017) Night work and the risk of depression. Dtsch Arztebl Int 114, 404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyse CA, Celis Morales CA, Graham N, Fan Y, Ward J, Curtis AM, Mackay D, Smith DJ, Bailey MES, Biello S, Gill JMR, Pell JP. (2017) Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann Med 49, 411–20. [DOI] [PubMed] [Google Scholar]
- 66.Neil-Sztramko SE, Gotay CC, Demers PA, Campbell KL. (2016) Physical activity, physical fitness, and body composition of Canadian shift workers: data from the Canadian Health Measures Survey Cycles 1 and 2. J Occup Environ Med 58, 94–100. [DOI] [PubMed] [Google Scholar]
- 67.Warbuton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. (2010) A systematic review of the evidence for Canada’s physical activity guidelines for adults. Int J Behav Nutr Phys Act 7, 1–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, Hébert JR. (2014) Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. J Occup Environ Med 56, 145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17, 1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knutsson A. (2017) Mortality of shift workers. Scand J Work Environ Health 43, 97–8. [DOI] [PubMed] [Google Scholar]
- 71.Buysse DJ. (2014) Sleep health: can we define it? Does it matter? Sleep 37, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Consensus Conference Panel (2015) Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 38, 1161–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nedeltcheva AV, Scheer FA. (2014) Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 21, 293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vallières A, Azaiez A, Moreau V, LeBlanc M, Morin CM. (2014) Insomnia in shift work. Sleep Med 15, 1440–8. [DOI] [PubMed] [Google Scholar]
- 75.Ohayon MM. (2009) Observation of the natural evolution of insomnia in the American general population cohort. Sleep Med Clin 4, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. (1994) Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93, 1930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Axelsson J, Åkerstedt T, Kecklund G, Lindqvist A, Attefors R. (2003) Hormonal changes in satisfied and dissatisfied shift workers across a shift cycle. J Appl Physiol 1985 95, 2099–105. [DOI] [PubMed] [Google Scholar]
- 78.Wright KP, Jr, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA. (2015) Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun 47, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garaulet M, Ordovás JM, Madrid JA. (2010) The chronobiology, etiology and pathophysiology of obesity. Int J Obes 34, 1667–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marqueze EC, Ulhôa MA, Castro Moreno CR. (2014) Leisure-time physical activity does not fully explain the higher body mass index in irregular-shift workers. Int Arch Occup Environ Health 87, 229–39. [DOI] [PubMed] [Google Scholar]
- 81.Morris CJ, Aeschbach D, Scheer FA. (2012) Circadian system, sleep and endocrinology. Mol Cell Endocrinol 349, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moreno CRC, Carvalho FA, Lorenzi C, Matuzaki LS, Prezotti S, Bighetti P, Louzada FM, Lorenzi-Filho G. (2004) High risk for obstructive sleep apnea in truck drivers estimated by the Berlin questionnaire: prevalence and associated factors. Chronobiol Int 21, 871–9. [DOI] [PubMed] [Google Scholar]
- 83.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. (2010) Pathophysiology of sleep apnea. Physiol Rev 90, 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, Kotani K, Nakamura T, Takemura K, Matsuzawa Y. (1997) Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med 241, 11–8. [DOI] [PubMed] [Google Scholar]
- 85.Spiegel K, Tasali E, Penev P, Van Cauter E. (2004) Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141, 846–50. [DOI] [PubMed] [Google Scholar]
- 86.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. (2005) Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab 90, 2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106, 4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leproult R, Van Cauter E. (2010) Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev 17, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thorand B, Zierer A, Baumert J, Meisinger C, Herder C, Koenig W. (2010) Associations between leptin and the leptin/adiponectin ratio and incident Type 2 diabetes in middle-aged men and women: results from the MONICA/KORA Augsburg study 1984–2002. Diabet Med 27, 1004–11. [DOI] [PubMed] [Google Scholar]
- 90.Yun JE, Kimm H, Jo J, Jee SH. (2010) Serum leptin is associated with metabolic syndrome in obese and nonobese Korean populations. Metabolism 59, 424–9. [DOI] [PubMed] [Google Scholar]
- 91.Leproult R, Holmbäck U, Van Cauter E. (2014) Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. (1992) Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol 262, E467–75. [DOI] [PubMed] [Google Scholar]
- 93.Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, Ribeiro D, Arendt J. (1996) Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol 151, 259–67. [DOI] [PubMed] [Google Scholar]
- 94.Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. (1998) Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinol 158, 305–10. [DOI] [PubMed] [Google Scholar]
- 95.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. (2015) Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA 112, E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. (2012) Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4, 129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heath G, Roach GD, Dorrian J, Ferguson SA, Darwent D, Sargent C. (2012) The effect of sleep restriction on snacking behaviour during a week of simulated shiftwork. Accid Anal Prev 45 Suppl, 62–7. [DOI] [PubMed] [Google Scholar]
- 98.Cain SW, Filtness AJ, Phillips CL, Anderson C. (2015) Enhanced preference for high-fat foods following a simulated night shift. Scand J Work Environ Health 41, 288–93. [DOI] [PubMed] [Google Scholar]
- 99.Johnston JD, Ordovás JM, Scheer FA, Turek FW. (2016) Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr 7, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. (2010) Eating and shift work—effects on habits, metabolism and performance. Scand J Work Environ Health 36, 150–62. [DOI] [PubMed] [Google Scholar]
- 101.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, Wright KP., Jr2014) Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci USA 111, 17302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heath G, Coates A, Sargent C, Dorrian J. (2016) Sleep duration and chronic fatigue are differently associated with the dietary profile of shift workers. Nutrients 8, E771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hemiö K, Puttonen S, Viitasalo K, Härmä M, Peltonen M, Lindström J. (2015) Food and nutrient intake among workers with different shift systems. Occup Environ Med 72, 513–20. [DOI] [PubMed] [Google Scholar]
- 104.Perry L, Gallagher R, Duffield C. (2015) The health and health behaviours of Australian metropolitan nurses: an exploratory study. BMC Nurs 14, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costa G. (2010) Shift work and health: current problems and preventive actions. Saf Health Work 1, 112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sargent C, Zhou X, Matthews RW, Darwent D, Roach GD. (2016) Daily rhythms of hunger and satiety in healthy men during one week of sleep restriction and circadian misalignment. Int J Environ Res Public Health 13, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fullick S, Morris C, Jones H, Atkinson G. (2009) Prior exercise lowers blood pressure during simulated night-work with different meal schedules. Am J Hypertens 22, 835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grant C, Coates A, Dorrian J, Kennaway DJ, Wittert G, Heilbronn L, Pajcin M, Della Vedova C, Gupta C, Banks S. (2016) Eating on simulated night shift effects glucose response to breakfast: pilot study. FASEB J, 30, 1160.2. [Google Scholar]
- 109.Stevens RG. (1987) Electric power use and breast cancer: a hypothesis. Am J Epidemiol 125, 556–61. [DOI] [PubMed] [Google Scholar]
- 110.Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS, Reinlib L. (2007) Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect 115, 1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tenorio F, Simões MJ, Teixeira VW, Teixeira ÁA. (2015) Effects of melatonin and prolactin in reproduction: review of literature. Rev Assoc Med Bras 1992 61, 269–74. [DOI] [PubMed] [Google Scholar]
- 112.Driesen K, Jansen NW, Kant I, Mohren DC, van Amelsvoort LG. (2010) Depressed mood in the working population: associations with work schedules and working hours. Chronobiol Int 27, 1062–79. [DOI] [PubMed] [Google Scholar]
- 113.Magnusson A, Boivin D. (2003) Seasonal affective disorder: an overview. Chronobiol Int 20, 189–207. [DOI] [PubMed] [Google Scholar]
- 114.Tahghighi M, Rees CS, Brown JA, Breen LJ, Hegney D. (2017) What is the impact of shift work on the psychological functioning and resilience of nurses? An integrative review. J Adv Nurs 73, 2065–83. [DOI] [PubMed] [Google Scholar]
- 115.McClung CA. (2013) How might circadian rhythms control mood? Let me count the ways.... Biol Psychiatry 74, 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richter K, Acker J, Adam S, Niklewski G. (2016) Prevention of fatigue and insomnia in shift workers—a review of non-pharmacological measures. EPMA J 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Albrecht U. (2017) Molecular mechanisms in mood regulation involving the circadian clock. Front Neurol 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saksvik IB, Bjorvatn B, Hetland H, Sandal GM, Pallesen S. (2011) Individual differences in tolerance to shift work—a systematic review. Sleep Med Rev 15, 221–35. [DOI] [PubMed] [Google Scholar]
- 119.Sverke M, Falkenberg H, Kecklund G, Magnusson Hanson L, Lindfors P .(2016) Women’s and men’s working conditions: the importance of organizational factor and psychosocial work environment for work and health-related outcomes. Swedish Work Environment Agency, Stockholm (in Swedish). [Google Scholar]
- 120.Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. (2016) Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci USA 113, E2730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hennekens CH, Buring JE .(1987) Epidemiology in medicine. Little Brown, Boston. [Google Scholar]
- 122.Reynolds AC, Paterson JL, Ferguson SA, Stanley D, Wright KP, Jr, Dawson D. (2017) The shift work and health research agenda: considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med Rev 34, 3–9. [DOI] [PubMed] [Google Scholar]
- 123.Downs SH, Black N. (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52, 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wells G, Shea B, O’Connell D, Perterson J, Welsch V, Losos M, Tugwell P .(2009) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 125.Kecklund G, Sallinen M, Axelsson J .(2016) Optimisig shift scheduling. In: Kryger MH, Roth T, Dement WC (Eds.), Principles and practice of sleep medicine, 6th Ed., 742–749, Elsevier, Philadelphia. [Google Scholar]
- 126.Härmä M, Koskinen A, Ropponen A, Puttonen S, Karhula K, Vahtera J, Kivimäki M. (2017) Validity of self-reported exposure to shift work. Occup Environ Med 74, 228–30. [DOI] [PubMed] [Google Scholar]
- 127.Bøggild H, Suadicani P, Hein HO, Gyntelberg F. (1999) Shift work, social class, and ischaemic heart disease in middle aged and elderly men; a 22 year follow up in the Copenhagen Male Study. Occup Environ Med 56, 640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yadegarfar G, McNamee R. (2008) Shift work, confounding and death from ischaemic heart disease. Occup Environ Med 65, 158–63. [DOI] [PubMed] [Google Scholar]
- 129.Costa G, Haus E, Stevens R. (2010) Shift work and cancer—considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health 36, 163–79. [DOI] [PubMed] [Google Scholar]
- 130.Stevens RG, Hansen J, Schernhammer ES, Davis S. (2013) Response to Ijaz S, et al. “Night-shift work and breast cancer—a systematic review and meta-analysis”. Scand J Work Environ Health 39, 631–2. [DOI] [PubMed] [Google Scholar]
- 131.Hansen J. (2017) RE: Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst 109, 1. [DOI] [PubMed] [Google Scholar]