Abstract
Interventions and strategies to improve health through the management of circadian (re) adaptation have been explored in the field, and in both human and animal laboratory manipulations of shiftwork. As part of an initiative by the Working Time Society (WTS) and International Committee on Occupational Health (ICOH), this review summarises the literature on the management of circadian (re) adaption using bright light treatment. Recommendations to maximise circadian adaptation are summarised for practitioners based on a variety of shiftwork schedules. In slowly rotating night shift schedules bright light appears most suitable when used in connection with the first three night shifts. These interventions are improved when combined with orange glasses (to block blue-green light exposure) for the commute home. Non-shifting strategies involve a lower dosage of light at night and promoting natural daylight exposure during the day (also recommended for day shifts) in acordance with the phase and amplitude response curves to light in humans.
Keywords: Shift work, Treatment, Bright light, Recommendations, Field studies
Consensus Statements.
Recommendations for use of bright light to improve adaptation to shift work.
-
1)
1) For most people, bright light (1,000 lux of white light at eye level) between18:00–04:00 h delays the phase of the circadian system. This means that sleep and alertness rhythms along with many other physiological processes occur later. This means that it can be easier to sleep between 07:00 and 15:00 h than would be otherwise the case (i.e. after nightwork).
-
2)
Bright light between 06:00 and 09:00 h advances the phase of the circadian system or blocks its delay. This means that sleep and alertness rhythms along with many other physiological processes occur earlier or blocks to delay.
-
3)
Orange glasses (blue light blockers that block light below 525 nm) worn between 06:00–09:00 h will block the advancing effects of bright light and assist the circadian system to delay. This can be particularly important if workers are commuting between 05:00–08:00 h.
-
4)
Readaptation after night to daytime schedules can be promoted by exposure to scheduled bright light (or natural daylight) according to the individual’s circadian phase. It is worth noting that the variability in the shift in the circadian system can make it difficult to judge the timing of light exposure too precisely.
-
5)
Working close to a window that allows natural daylight, can promote daytime alertness during day shifts.
-
6)
In practice, lighting interventions should consider 5 known factors that affect light effectiveness: 1. spectrum (the more “blue” the stronger the effect), 2. intensity (the brighter the stronger the effect) (adds up with the more blue), 3. duration of exposure (albeit non-linear), 4. time-of-day of light exposure (phase advance by early morning light/phase delay by evening light), 5. light history (the more daylight, the weaker the impact of articial light in the evening/at night).
Consensus statements review expert panel: Drew DAWSON1(Chair), Charmane EASTMAN2, Mariana FIGUERIO3
1CQUniversity, Australia
2Rush University Medical Center, USA
3Rensselaer Polytechnic Institute, USA
Full consensus among panel members on all statements.
Glossary
Circadian adaptation− “about a day;” any rhythm with a period of approximately 24 h that is alined with a set standard, for example a night shift.
Circadian disruption − synchronized rhythmic variables that have ceased to exhibit the same frequency and/or the same timing of the maximal value of the rhythmic variable.
Entrainment − Coupling of a biological rhythm to an environmental oscillator with the result that both oscillations have the same frequency.
Zeitgebers − Derived from German meaning “time giver” or “synchronizer”, a Zeitgeber is any external time cues (for example light, feeding, exercise and social interactions) that is effective in entraining an organism.
BT − Core body temperature.
DLMO − Onset of melatonn production during dim light conditions in the evening/night.
Context
This manuscript is one of a series of consensus papers developed by the Working Time Society and commissioned by the International Commission on Occupational Health1). The goal of this series is to provide guidance for a broad, international audience comprising researchers, industry members, workers, labor representatives, policy makers, and other stakeholders interested in reducing the potential negative health outcomes associated with nonstandard working hours. They describe the current state of research, identify health and safety risks and make recommendations for effective interventions, and suggest future research directions. Each paper is accompanied by a number of consensus statements, developed through the procedures outlined by Wong et al1).
Introduction
Mammals have developed a specific physiological and behavioural adaptation system in response to environmental changes in illumination across the day2). At the most basic level, circadian regulation is driven by cellular/molecular oscillation through time-specific, molecular feedback loops and clock-controlled genes3, 4). Molecular signals are synchronized by a master central clock, located in the hypothalamus (the suprachiasmatic nucleus (SCN), also called the “biological clock”). This clock is considered vital in order to synchronize peripheral clocks in the organs and facilitate entrainment to exogenous Zeitgebers (time-of-day cues including light, feeding, exercise and social interactions5).
In mammals, entrainment to the 24 h day/night cycle is primarily regulated by light5). Humans are genetically programmed to be diurnally active (day-oriented) to maximize cognitive and physiological ability with high energy mobilization while cell repair, growth and recovery reach a peak during the hours of darkness. However, our modern 24-h society and working conditions are often in conflict with our intrinsic photo-biology. Collectively, we expect services irrespective of time of day (including emergency services, transportation and healthcare), which demands that humans are awake and functioning during the night when we are biologically programmed to sleep. For workers (and other members of society) who are required to be awake during the night, this often results in circadian disruption5, 6).
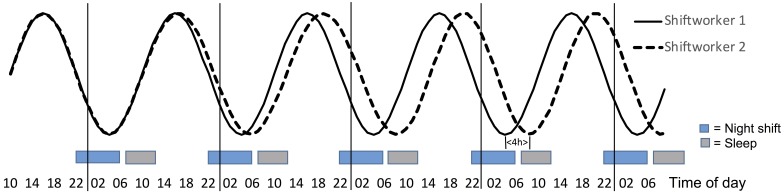
While physiological (e.g, cortisol, glucose, DLMO, BT) measures have been used to illustrate circadian disruption in laboratory shiftwork protocols, changes in alertness also provide a useful indicator and are particularly important for highlighting the safety risks associated with circadian disruption. Figure 1 illustrates a change of circadian phase position for modelled alertness data for a shift worker that either maintains diurnal phase (Shiftworker 1) or shows a more nocturnal phase position (Shiftworker 2) which implies a partial adaptation to night work. While only the circadian component is shown in figure, it is worth noting that the timing and duration of sleep and wake will also influence alertness levels7). The alertness circadian component in Fig. 1 much resembles the expected profile of core body temperature, see Fig. 2. The phase position of Shiftworker 2 is gradually delayed 4 h after a series of 5 night shifts. The phase change is measured by the difference of the minimum level of alertness for Shiftworker 1 (at 04:00h) and Shiftworker 2 (at 08:00h).
Fig. 1.
The modelled circadian component of alertness in connection to 5 eight-hour night shifts (22:00–06:00 h). Shiftworker 1 (black line) shows no phase delay of the rhythm and Shiftworker 2 (dotted line) shows a partial phase delay reaching 4 h by the 5th d with night shift.
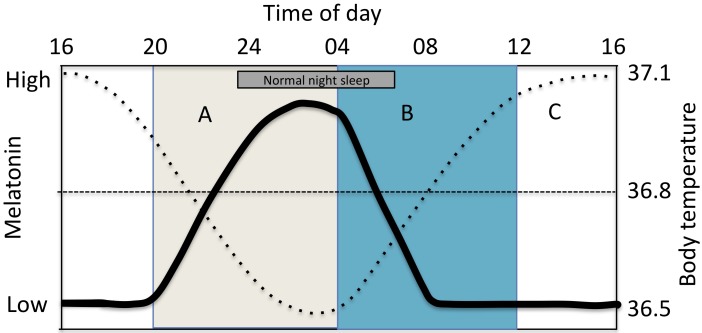
Fig. 2.
Circadian rhythm of melatonin=fat line and core body temperature (BT)=dotted line. Light given after nadir of the BT rhythm (B) promote a phase advance of the rhythm (when BT increases). Light given before the nadir of the BT rhythm (A) promotes a phase delay (when BT is decreases). Little effect on phase occurs at other times (C) but alertness increases.
Adaptation to nonstandard work hours requires (re) aligning internal clocks or oscillators with, for example, night work and day sleep. Given the powerful influence of light in the entrainment of circadian rhythms, this is a challenging task with high individual variability. For example, if you ask shift workers working on an oil platform how long it takes them to adapt (subjectively) to a nightshift schedule, the reported average is approximately three nights9), with considerable differences in physiological indicators of adaptation between individuals10). In general night shift workers show a rather slow rate of adaptation to day sleep11). Consequently, many shiftworkers suffer chronically from circadian rhythm disorders that include significant insomnia and/or excessive sleepiness at inappropriate times12). In extreme cases, the condition may be classified as a medical disorder (shift work disorder (SWD) (ICD10: G47.26). According to this nosology, SWD can be diagnosed when the sleep problem is primarily work-related, and if work hours overlap normal time for sleep based on subjective accounts and screening, and if the symptoms persist for at least a month. SWD prevalence is reportedly high in shiftworking populations, particularly night working nurses (44%) and rotating shift workers (10–23%)12).
Given the ongoing demand for workers on non-standard schedules and the negative effects of shift work on substantial numbers of workers, there is an arguable need to improve circadian adaptation, and reduce the impact of circadian disruption on health and safety. Consequently, a number of studies have considered the potential of the most powerful zeitgeber, light, as an intervention8, 9, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34). Interventions using light for shift workers invoke both35) direct alerting effects and36) indirect effects mediated via effects on the phase and amplitude of the circadian system. Light can therefore be used for both the immediate alerting effects during times when fatigue and sleepiness are likely to be highest (Fig. 1) and/or to promote either a phase delay or phase advance, or to suppress melatonin secretion at specific times of the day to help workers better cope with demands associated with shiftwork.
Interest in use of light to support adaptation to shift work has increased significantly in recent years35). Thus, there is a need to summarize extant findings and to provide recommendations to support organsiations and regulators looking to implement best-practice. In the present review, we emphasize evidence drawn from intervention studies in the field. However, the broader recommendations also draw on broader work from human and animal laboratory studies.
Methods
This specific review addresses only publications on light treatment strategies which affect circadian adaptation to shiftwork. Other treatment strategies—including the use of melatonin, sleep aids, exercise and organizing shift scheduling according to circadian principles—are presented elsewhere in this journal edition.
This text is based on English published articles sourced from PubMed and Scopus. For the search on shiftwork interventions Scopus generated 74 hits in April 2018 on the search terms “shift work”, “circadian” “intervention” and “light” (131 in PubMed). Omitting the term “intervention” generated 777 hits (529 in PubMed) demonstrating a high interest in the area with 30–60 new publications yearly since 2008.
Results and Discussion
Summary: We identified 8 field studies which specifically reported the impact of light interventions and 5 studies using shiftworkers in a combined field and lab-based design. A further 14 studies considered the impact of light interventions in lab-based simulated shiftwork protocols. All studies reported significant effects of light interventions or combined with other interventions such as use by dark goggles, melatonin medication or changed sleep/wake schedules. The general conclusion from these studies is that light interventions forms an effective tool to manipulate the circadian system and, ostensibly, to reduce some of the negative consequences. It should be noted that field studies may have less control of treatment compliance and placebo effects are difficult to avoid. Many insights have been derived from studies exploring light effects using strictly enforced dim light designs, forced bed rest and highly controlled conditions. Even though they are not reported here many of the designs used in light interventions in the field are based on knowledge derived from such experimental work including animal models of shiftwork. For bright light interventions one conclusion from a Cochrane review37) was that there is a lack of randomized control trials and also studies that include circadian measures. It is thus import to point out that the major part of studies that has been performed using bright light and cited in the present review might be considered having a low quality of evidence. Other identified weaknesses are interventions not being blinded and lack of information of potential bias. Also, the particular role of placebo effects in bright light studies needs further to be evaluated (Table 1).
Table 1. Summary of studies (alphabetic order) using a design performed in the field, in the laboratory (Experimental) or in a combined field/experimental setup including outcome variables on circadian adaptation. BL: bright light.
| Author | Year | Country | Location (field/experimental) | Brief intervention summary | Primary outcomes | Conclusion (BL support circadian adaptation Y/N) | Key limitations |
|---|---|---|---|---|---|---|---|
| Baehr et al. | 1999 | USA | Combined field/ experimental | 3 h of BL (≈ 5000 lux) and 5 h of ordinary indoor room light or “dim” light (<500 lux) during eight 8 h N shifts (n=33). BL was timed according to PRC and estimated Tmin to delay or advance rhythms. | TST on the first night sleep after 2 N increased by 40 min compared with baseline, WASO was reduced and sleep efficiency was increased by the intervention. Salivary melatonin levels were higher on the first and fourth night shift compared with baseline. | Yes | Very few sleep periods selected for study. |
| Bjorvatn et al. | 1999 | Norway | Field | BL 30 min (10.000 lux) scheduled individually (n=7) the first 4 Ns of the N-shift period (oil platform) and first 4 d at home following the shift period. | BL modestly facilitated the subjective adaptation to night work, but was pronounced during the re-adaptation back to day life at home. Sleepiness was reduced and the quality of day was rated better after exposure to BL. | Yes | Unbalanced design, lack of objective data (risk of bias) |
| Bjorvatn et al. | 2007 | Norway | Field | Shift workers (n=17) received a placebo, melatonin (3 mg, 1 h before bedtime), or BL (30-min, individually scheduled) during the first 4 d on the N shift and during the first 4 d on the day shift. | Melatonin modestly reduced sleepiness at work during the day shift and increased sleep by 15–20 min per d. BL gave values in between those of melatonin and the placebo, (few significant results). | Yes | Short BL exposure time |
| Boivin et al. | 2012 | Canada | Field | Intermittent exposure to wide- spectrum (n=14) BL at N + orange-tinted goggles at sunrise, and maintenance of a regular sleep/darkness episode in the day. | UaMT6s levels at home was higher during daytime sleep at the end compared to the start of the work week being more increased in intervention group and more stable PVT performance. | Yes | Small sample |
| Boivin et al. | 2002 | Canada | Combined field/ experimental | Treatment group (n=10) received 6 h of intermittent BL in the workplace (~3,243 lux + tinted goggles in morning). Control group (n=9) observed in their habitual work environments. | Salivary circadian rhythms of core body temperature and salivary melatonin cycles were delayed by an average of −9.32 h (−4.09 in controls) and −11.3 h (−5.08 in controls). | Yes | Lack of control of duration of BL for each subject. |
| Boivin et al. | 2012 | Canada | Field | Treatment group (n=8) received 6 h of intermittent full-spectrum BLduring N shifts + wore dark goggles during the morning at commute home. | The treatment group had daytime sleep episodes that lasted 7.1 h versus 6.6 h for workers in the control group (n=9). | Yes | Lack of control of duration of BL for each subject. |
| Bougrine et al. | 2004 | France | Experimental | BL (2,500–3,000 ulx) (n=4) at 02:00–5:00 in N during 3-5 Ns and during recovery days at 10:00–12:00 or 12:00–15:00. | Urinary melatonin revealed a phase delay of −5 to −10 h during night shift and phase advanced 5 h across 3 recovery days and close to baseline for all but one subject. | Yes | SS were young, few and unexperienced to night work. |
| Burke et al. | 2013 | USA | Experimental | Four conditions (n=36): dim light (~1.9 lux)-placebo, dim light+ melatonin (5 mg), BL (~3,000 lux)-placebo, and BL+melatonin. Melatonin adm 5.75 h prior to bedtime and 3 h of BL exposure started 1 h prior to wake time. | Morning BL combined with melatonin induced a greater phase advance (26 min) than either treatment alone (≈0.25 h). Bright light alone and melatonin alone induced similar phase advances. | Yes | The effect of forced early awakenings not studied |
| Canazei et al. | 2016 | Austria | Experimental | Three light spectra, high, moderate and low blue spectral content, in simulated N (n=31). | Exposure to the ”high” spectrum reduced HR and increased vagal cardiac parameters and similar effects on sustained attention, working memory and subjective alertness. | Yes | Lack of exposure details. |
| Chang et al. | 2012 | USA | Experimental | Single high-intensity (~10,000 lux) light pulse (0.2 h, 1 h, 2.5 h and 4 h) centered in a 16 h wake period at night (n=36). | BL resets the circadian pacemaker in a dose-dependent, non-linear manner. 0.2 h duration was over 5 times more effective at phase delaying the circadian pacemaker as compared to 4.0 h duration | Yes | Very high exposure levels |
| Crowley et al. | 2015 | USA | Experimental | Sleep/dark was advanced 1 h/d for 3 d including three BL conditions (n=50) (~5,000 lux) at waking: (1) four intermittent 30-min exposures (2) four 15-min exposures (3) one 30-min exposure. Participants took 0.5 mg melatonin 5 h before baseline bedtime on treatment day 1, and an hour earlier each treatment day. | Compared to group 2 h (phase shift=2.4 h), smaller shifts were observed in group 1 h (shift=1.7 h) and 0.5 h (shift=1.8 h). The single 30-min BL was as effective as 1 hour of BL spread over 3.25 h, and produced 75% of the phase shift observed with 2 h BL. | Yes | Only combined BL and melatonin studied. |
| Crowley et al. | 2003 | USA | Experimental | 6 intervention groups (n=67) ranged in use of normal/dark sunglasses outside in daytime+BLat N+melatonin before daytime sleep. During 5 simulated N shifts, BL showed a moving (delaying) pattern of intermittent BL (~5,000 lux, 20/40 (min on/off), 4–5 light pulses/N) or dimlight (~150 lux). | With BL during the night shift, almost all of the participants achieved complete re-entrainment, and the phase delay shift was so large that darker sunglasses and melatonin could not increase its magnitude. | Yes | Only combined BL and melatonin studied. |
| Dawson et al. | 1991 | Australia | Experimental | 3 intervention groups in simulated 3N (n=36) exposed to BL (4,000–7,000 lux, 24:00–04:00), received melatonin (2 mg at 08:00, 11:00, 14:00) or placebo (dim light or sucrose tablets). | BL shifted DLMO 8.8h and less with melatonin (4.2 h) and control (4.7 h). Sleep quality improved in BL and melatonin treatment and performance improved at work with BL. | Yes | Longterm strongly desynchronized states not considered. |
| Dawson et al. | 1995 | USA | Experimental | 2 intervention groups in simulated 3N (n=13) exposed to BL (6,000 lux, 24:00–04:00) the first N and then dim loght other shifts. The control groups received dim light throughout. | The BL group showed a delay in BT of 355 min (control 143 min). Day sleep increased 62 min in the BL group, especially the 7th–8th h of sleep was improved in quality. | Yes | Objective on-shift alertness improved in the BL group. |
| Dumont et al. | 2014 | Canada | Experimental | 3 groups (n=38) exposed to specific profiles of daytime light (150–1,800 lux) during simulated N to produce partial phase advance (advance group), partial phase delay (delay group), or a relatively stable circadian phase (stable group). | There was no difference between the three groups, of the decrease in melatonin production for nighttime and for the 24 h | Yes | SS were young and unexperienced to night work. |
| Dumont et al. | 2009 | Canada | Experimental | 3 daytime light exposure profiles (n=38) designed to produce phase delay (1,800 lux from 08:00–09:00 + late day sleep) at N work, or a phase advance (400 lux from 08:00–09:00 + early day sleep) to or an unchanged circadian phase (1,800 lux) from 08:00–09:00 and slept in dim light (20 lux from 09:00–17:00. | Salivary dim light melatonin onset (DLMO) showed a significant phase advance of 2.3 h in the advance group and a significant phase delay of 4.1 h in the delay group. The stable group showed a smaller but significant phase delay of 1.7 h. | Yes | Lack of long-term consequenses. |
| Figueiro et al. | 2016 | USA | Experimental | Acute effects of exposure to red (630 nm) and white (2,568 K) lights (n=17). | Compared to dim light exposure, power in the alpha and alpha-theta regions was decreased after exposure to red light. Melatonin levels were significantly suppressed by white light only. | Yes | Lack of exposure details. |
| Hébert et al. | 2002 | Canada | Field | 4.3 h BL per day in one week (outside or light boxes indoors) compared to a dim week with dark goggles at 1.4 hr per day outside. 500 lux was presented for 3 h in the middle of N (n=12). | Less melatonin suppression after the BL history week (41%) than after the dim week (53%) compared to baseline condition (p<0.05). | Yes | Large individual differences. Possibly too short light history exposure (1 wk). |
| James et al. | 2004 | Canada | Combined field/ experimental | Intermittent exposure to full-spectrum BL (≈2,000 lux) in the first 6 h of 12 N shifts across three weeks + tinted lenses in morning (n=9). | Shift of peak cortisol expression was found (experimental = 11:38 and control = 1:15 h) after bedtime. | Yes | Cortisol measures taken at rather long intervals (every 4 h) for calculating phase angels. |
| Koller et al. | 1994 | Austria | Field | Description of N workers being ‘non-shifters’ (n=9), and ‘shifters’ (n=5), deviation from midN more than 6 h of the melatonin acrophase. | Light avoidance behavior during morning hours, as observed in 5 out of 14 night workers, coincided significantly with a phase delay of melatonin acrophase. Light avoidance correlated with an earlier sleep onset and a tendency to longer sleep hours. | Yes | Non-experimental design, results could be explained by masking from sleep, differences in sleep duration |
| Lee et al. | 2009 | USA | Experimental | Intervention (n=23) aiming to a partial phase position using 15 min per hour intermittent BL pulses (≈3500 lux; ≈1100 mW/cm2) during N shifts + blue blockers at traveling home + sleep in the dark. | Tmin of the experimental subjects was 04:24 h at baseline and 7:36 h after the 2:nd night shift. Tmin of controls was at 04:00 h at baseline and drifted to 4:36+1.4 h. | Yes | Difficult evaluate single countermeasure effects. |
| Lowden et al. | 2004 | Sweden | Field | BL (2,500 lx) during breaks or normal light during four consecutive weeks in N work compared to four control weeks (n=18). | Reduction of sleepiness in the BL condition on the first two nights at 04:00 and 06:00 h. Day sleep in the BL condition was lengthened, melatonin more suppressed (mostly at 02:00 h). Daytime melatonin during the readaptation after night work remained unaffected. | Yes | SS were young and unexperienced to night work. |
| Mitchell et al. | 1997 | USA | Combined field/ experimental | 3 h of BL (≈5,000 lux) and 5 h of ordinary indoor room light or “dim” light (<500 lux) during eight 8 h N shifts. BL was timed according to PRC and estimated Tmin to delay or advance rhythms (n=32). | Combined BL+ delayed sleep strongly shifted all SS (7.7 h). Combined BL + advanced sleep caused small phase shifts (2.6 h). It appears timing of sleep/dark is as important as the timing of BL. | Yes | Very few sleep periods selected for study. |
| Rahman et al. | 2013 | Canada | Combined field/ experimental | Quickly rotating shift workers (n=9) received blue blockers during N shifts (7 N in 11 d). (intervention) or standard indoor light (baseline) on N shifts. | TST on the first night sleep after 2 N increased by 40 min compared to baseline, WASO was reduced and sleep efficiency was increased. Melatonin levels were higher on the first and fourth N compared with baseline. | Yes | Very few sleep periods selected for study. |
| Santhi et al. | 2008 | USA | Experimental | 3 N with combined treatment: (1) Morning Sleep + BL 23:00–03:00, and (2) Evening Sleep + BL 03:00–07:00 (n=21). | Evening sleep: 2.27 h phase advance; morning sleep: 4.98 h phase delay. At the third shift evening sleep group showed 37% fewer episodes of long response times: 22 vs. 35 and quicker responses on PVT than their morning sleep group. | Yes | Clear effects on performance only third N. |
| Sasseville et al. | 2009 | Canada | Field | Permanent N workers (n=28) wore blue-blockers glasses, either just before leaving the workplace at the end of their shift (summer group) or 2 h before the end of the N shift (fall-winter group) outdoors until 16:00. | Compared to baseline sleep efficacy increased by 1.9% and 4.6%, and lowered sleep fragmentation by 1.7% and 4.2% in the summer and fall–winter group, respectively. | Yes | Summer and fall-winter group given different instructions of use of glasses. |
| Smith et al. | 2009 | USA | Experimental | 3 simulated N (23:00–07:00), 2 d off, 4 N, and 2 more days off (n=19). Experimental ss received four 15-min BL pulses during each N shift+sunglasses outside+dark bedrooms at scheduled times. | Experimental group improved performance and reached a partial phase position for DLMO at 3:22 ± 2.0 h, and controls at 23:24 ± 3.8 h. | Yes | Evaluations lacking on days off. |
This literature review raises many unanswered questions and highlights several critical areas of which we no little or nothing. If we are to make comprehensive recommendations regarding light interventions to support circadian adaptation this should include an acknowledgement of what we do not know and may well be critical in evaluating the use of bright light to improve adaptation to shift work.
At the broadest level, we do not yet know whether, or how, circadian misalignment mediates any of the the long-term health consequences of shift work. Critically, we do not really know whether the lag time for adaptation as a result of circadian ‘inertia’ works to harm, or protect, health. Circadian ‘inertia’ could arguably serve a protective role, reducing the degree of aggregate phase shift and minimizing the degree of circadian disruption over time. If the aggregate amount of phase disruption over time were to mediate the negative health effects, accelerating adaptation might potentially increase aggregate circadian phase disruption and result in more, rather than less damage.
Similarly, we do not know how ‘phase tolerant’ the circadian system is with respect to disruption. Are the health consequences linked to the degree of phase disruption in a linear or monotonic manner or is there a threshold level of phase disturbance that we can tolerate before our physiology is negatively impacted?
It is also the case that the results from intervention studies using light mainly predict short-term effects on circadian outcomes, which limits the ability to provide recommendations for medium- to long-term health and safety. In new lighting installations, economy and energy savings still greatly influence lighting choices; unfortunately less emphasis is placed on long-term health and safety at work. It may be difficult to generalize results from a short-term study across a single sequence of shifts to the likely long-term impact impact. Moreover, outcomes based on a limited subset of measures (e.g. melatonin phase) may not reflect changes in other health-related issues. One such example is if circadian adaption to night work is accelerated how do long-term changes in food intake and metabolic function respond?
To date, there are no long-term randomized controlled trials that have evaluated how different phase positions and altered rates of circadian adaptation affect long-term health. The variety of symptoms, differential speed of adaptation for regulatory biological processes and broader individual differences make intervention strategies complex to evaluate and it is difficult to make general recommendations. In general, if circadian misalignment is considered unhealthy one obvious solution could be to forbid or reduce unhealthy shift schedules/shiftwork. Obviously, this is not feasible in most industries and cultures. Not surprisingly, while clearly sub-optimum, strategies for improving circadian adaptation need to be considered—if only from a harm minimization perspective.
Based on the published literature to date, it is our view that there is insufficient evidence in the existing literature for light interventions in shiftworkers to provide detailed definitive advice on best practices. Many new insights are born from animal and experimental studies. Even though such data could have great influences on recommendations for lighting at work we still lack studies to test this in the field. There is also a risk that individuals oppose new tests of light regiments in the field or have high expectations.
Light effect mechanisms
In the last 30 yr we have identified that the human circadiam system responds to light in a similar manner to most other diurnal species but at much higher levels that was historically used for lab-based animal studies. We are now well aware of the capacity of bright light to reset human circadian rhythms but that this effect is far from straightforward. The magnitude of the circadian effect of light depends on the level of illumination ‘(bright light shifts more than dim light), spectral distribution (the blue end of the spectrum shifts more than the orange end) and the direction depends on circadian time of administration (light during the early biological night will delay the system and light during the latter part of the biological night will advance the system38). The central oscillator or pacemaker is influenced by light via photic stimulation of ganglion cells in the retina and the light-dark cycle of the solar day being the strongest synchronizer, timekeeper or Zeitgeber. The retinal ganglion cells (iPRGC’s) are most sensitive to blue wavelengths sensitive to the range of 450–490 nm, an optical radiation range that the human brain perceives as blue included in daylight, white light, monochromatic blue light, and exposure signaling to the brain will suppress melatonin production. Other wavelengths within the visual light spectrum will also suppress plasma melatonin but only at much higher light intensities39). Based on the research reviewed one possible strategy to promote adaptation to nightwork is to use coloured lenses to block blue wavelengths while traveling home from work (e.g. dark or orange lenses)10, 12, 20, 24, 26, 27, 32, 40). A suggested strategy for use of dark glasses is therefore included in the key statements below.
Thus, light as a zeitgeber (timekeeper) can either maintain, delay or phase advance the circadian rhythm. In Fig. 2 central circadian rhythms are plotted. If light is given on the declining limb of the core body temperature cycle between late afternoons until late at night the rhythm is delayed (period A). This condition promotes eveningness that includes evening alertness, delayed onset of the melatonin rhythm and a delayed sleep onset. A phase advance occurs when light is given on the increasing limb of the core body temperature cycle (period B) and increased morningness is obtained. The condition promotes early morning awakenings, morning alertness and suppression of morning melatonin and melatonin suppression being the the central phase-resetting factor. The conditions during period B are of particular interest to shift workers and are also included in the key statements below. Light during period C will promote alertness and suppress melatonin41). Bright light during period C will strengthen and maintain diurnal orientation and seem to less alter evening melatonin production even if there is a blue light exposure in the evening44, 45). It has been shown that sub groups will be more sensitive for light at night when exposed for a more dim light during the day, further demonstrating the influence of prior light history25). In summary, the effect of timing of light exposure on phase shifting has been presented as the Phase Response Curve41) and commonly used to guide recommendations of light treatment in shiftwork40, 42, 43).
Nightwork
Many researchers have suggested that faster circadian adaptation to night work might improve alertness at work and recovery sleep at home. Useful practical guidelines to estimate individual oriented timing of bright light or melatonin treatment have been described35, 42) that also include guidance on the treatment of circadian rhythm sleep-wake disorders. For example, in certain circumstances such as before space missions there could be a need to induce pre-adaptation to a nominal ‘night shift’ schedule before start of work. Light exposure (3,000−12,000 lux) could then be administered prior to launch in order to produce a gradual shift of the circadian system and sleep timing suited to the mission timing44).
At night, shift workers are often exposed to workplace lighting levels lower than 100 lux21, 45). The suppressing effect of light exposure on melatonin is large at higher illuminances (e.g. >200 lux, decreases to 50% by a reduction to 100 lux and with only very small suppression effects at light levels lower than 80 lux45). Melatonin secretion at night is progressively reduced by the circadian adaptation to night work during consecutive shifts mainly caused by the phase delay of peak values towards daytime. Use of bright light to reduce sleepiness during night shifts is most effective during the first nights in a series of consecutive shifts28). In laboratory studies light treatment during the first three night shifts out of five nights seems to be sufficient for adaptation of the melatonin rhythm for most subjects if working in otherwise dim light conditions15). However, based on the data it is still too early to make a highly specific recommendations to promote an immediate adaptation (short term effect) since we lack studies of the long term effects and potential threats to health.
Light exposure outside work may produce either phase advances or delays of melatonin rhythms even if light exposure levels are maintained low at work22). One study included night working nurses that were subjected to intermittent 6 h of bright light (3,200 lux) at work14). Nurses delayed the midpoint of melatonin secretion by 11.3 h whereas the control group only delayed 5.1 h. Delay of melatonin excretion towards the day sleep period has shown to improve sleep length by half an hour13). The treatment groups of nurses were likely helped by wearing dark goggles to reduce light exposure during morning hours and other groups like police officers and oil platform workers also seem to benefit from equivalent treatments (e.g. blue blocking orange lenses)10, 32).
A treatment of around 2,000 lux in a similar fashion as above during night work has been shown to significantly facilitate the adaptation of cortisol excretion and generate lower levels of cortisol at habitual bedtime, possibly supporting sleep26). It seems that a combined use of bright light at night and dark goggles reducing light intensity and short-wavelength light transmission during the day is effective to accelerate circadian adaptation to permanent night work and improve daytime sleep and nighttime performance30).
The timing of light at night is also critical and should be scheduled before the zero phase position (i.e. in humans, the time of the core temperature minimum. If this is not done correctly there is the risk of conflicting light influences i.e. competing delay and advance signals that can result result in a singularity point at which phase and amplitude become indeterminable29). This also points to the importance of considering individual differences as is reflected in ‘proxy’ measures of circadian phase, i.e. measures of diurnal preference or chronotype46). Ideally, in scheduling timed light exposure, it is useful to know the phase of the individual’s circadian rhythm. But it is rarely possible to include physiological measures outside the laboratory. If that is not possible, a rough rule of thumb suggests that, on average, the nadir of the circadian rhythm for core body temperature is located 2 h prior to habitual wake up42).
This is also the period when workers show the greatest sleepiness levels if staying awake the whole night. When administering light at night it is useful to be aware that exposure close to the nadir of body temperature yields the best results in improving worker alertness42). Indirect exposure to light such as performing a reading task while being exposed to light interventions will help mitigate fatigue and onset of sleepiness47). Practical advice on how light should be administered, including important considerations, have also been provided by Burgess et al.44) and Smith et al35). The authors summarize that a phase shift is increased by increased light intensity and duration. And since the circadian system has a periodicity longer than 24 h the periodicity is a force that slightly delays the circadian rhythm day by day. This is the reason why phase delays are easier to promote than phase advances. Furthermore, Burgess et al. and other groups have recommended that a light treatment is most effective when work includes several consecutive night shifts combined by use of dark sunglasses while traveling home from work48) and when day sleep is maintained in darkness.
In Fig. 2 the crossover between period A and B coincides with the time of lowest core body temperature (nadir = Tmin)48). According to differences in light sensitivity across the day the magnitude of circadian shift change is dependent on how close light is presented to Tmin. In practice a phase change will thus primarily depend on light intensity, duration of light exposure and closeness to Tmin48).
Altering both the timing of sleep and bright light exposure could also facilitate circadian adaption31). In an experimental study Santhi and colleagues exposed one group of subjects to bright light during the first half of the night shift, and delayed sleep until to the morning; and exposed a second group of subjects to bright light during the latter half of the night and advanced sleep time until the evening. The results showed that evening sleepers showed less impaired attention at night and became less sleepy. The authors concluded that an earlier sleep period could be a successful treatment for workers with shiftwork disorder31).
Another promising strategy showing potentially positive results for circadian adaptation is partial adaptation to a night working schedule. Eastman and colleagues have proposed the use of partial adaptation of phase position in night work by gradually pushing Tmin towards day-sleep by giving light treatment at the beginning of the shift (2 h) and then delaying light exposure by one hour for each following shift48). For permanent night workers it is recommended to maintain a phase delay on days off by keeping bedtime to around 04:00 h. Administering of lower illumination levels and timed bright light exposure can weaken the adaptive response and result in partial phase adaptation of the circadian system27, 34), see Fig. 1. For a night worker, 10 min daylight exposure while traveling home from night work could significantly reduce the degree of night work adaptation of the melatonin rhythm9). The importance of light while traveling home has further been pinpointed as giving the direction of the phase position in simulated field studies48).
Partial shifting strategies
Since retinal ganglion cells are most sensitive to blue wavelengths at the lower end of the spectrum it has been suggested to reduce the blue wavelengths at night to avoid phase changes by nocturnal illumination. Therefore, use of orange tinted glasses (blue-blockers) or using more yellow/red illumination at night will only mildly affect melatonin excretion30). Using a red light exposure is found to maintain alertness and performance at night without suppressing or changing circadian melatonin phase24). Filtering out blue wavelengths that normally would suppress melatonin could be used as a health and safety promoting strategy49). The strategy might benefit shift workers on fast schedule rotations for example if three night shifts in a row is closely followed by morning shifts28). Similar actions could be considered for groups who have increased risk of metabolic disturbances for example having a state of elevated insulin resistance where the ability to reduce elevated blood sugar levels is failing and with an increased risk of developing diabetes. Melatonin has been identified as a key factor in energy metabolism and insulin resistance since it alters release of insulin through the regulation of the expression of transporter glucose type 4 (GLUT 4) or triggers phosphorylation of insulin receptors50).
Readjustment after night work
In fast rotating shift schedules but also in 12-h night work schedules there is a need to help readaptation to a day-oriented circadian rhythm. In a study of oil platform workers, it was reported that after 14 consecutive night shifts, workers estimated it took 5 d to return to normal diurnal circadian rhythms8). In addition, alertness was more quickly regained in the experimental group than in controls after administering 30-min light therapy at 10,000 lux starting at 14:00 h (considered to be before nadir of the core body temperature rhythm) on the first rest day, followed by delaying exposure by 2 h per day, for the remaining 4 d. For most subjects, complete circadian readaptation during rest days was obtained within three days if they were exposed to bright light during the day. Exposure to daylight during daytime may also be a preferable strategy to obtain bright light rather than given by artificial light sources but light exposure should not be given too early to avoid inadvertent phase advances (i.e before lunch).
Morning and daywork
Ideally, circadian and homeostatic processes (internal drives to obtain for example stable sleep patterns) interact synergistically to ensure uninterrupted sleep of good quality and duration51) whereas sleep/wake rhythms and circadian rhythms after initial night shifts show conflicting patterns. Unfortunately, modern society has decreased exposure to daytime natural lighting and extended exposure to indoor artificial lighting. As a consequence, modern industrialized (shift) workers can have phase shifted sleep/wake and melatonin rhythms52, 53). Bright light interventions can potentially improve adaptation to daywork including early morning work. However, the optimum dosing regimens have not yet been well defined and there is still debate over how best to standardize clinical interventions. Some recent studies have aimed to clarify the circadian effects of various light levels at daytime. For example, 2 h compared to 0.5 h of bright light treatment in the morning (3,510–6,880 lux) gave a stronger phase advance in onset of melatonin in the evening. Still, the 0.5 h condition produced 75% of the advance achieved by 2 h of bright light exposure19). This suggests that the benefits of bright light exposure are quite non-linear with respect to illumination level (lux). Lower lighting levels have been shown to be less effective than higher levels. For example, 90–150 lux does not seem to alter the onset of evening melatonin even though it seems to promote an earlier sleep period54). Moreover, some studies claim that a 30 min daily dosage of natural daylight for healthy individuals is enough to maintain a stable diurnal rhythm55). In the laboratory, a stable diurnal rhythm may be encountered using a strict and stable electric lighting environment56). Thus, typical office lighting (150–300 lux) gives about half the circadian ‘drive’ compared with outdoor exposures of 10,000 lux57). In an experiment investigating day work in windowless offices, to spend some time close to a window (1,000–4,000 lux) significantly reduced daytime sleepiness58) through a direct stimulating effect independent of melatonin secretion. There is not yet a consensus on how much light is to be recommended but it has been suggested that office workers in order to maintain healthy mood and sleep states should have ≥0.3 of circadian efficient lighting (≈ 300 lux white light reaching the eyes) before noon (0.7=max effect resembling outdoor daylight exposure, >1,000 lux)59).
New challenges
Recently, new initiatives have emphasized the healthy visual and non-visual biological effects of lighting—forming the concept of “human-centric lighting”60). Before taking action on implementing light designs in shiftwork settings it is important to consider the basic aim of light strategies and limitations. It is at times assumed that circadian misalignment to work hours could be pathophysiological. However, an alignment to work hours by use of light could increase circadian disruption (promote greater circadian phase changes) that could be even more harmful. Conserns have been raised that circadian misalignment could have negative impact on health that include dysregulation of feeding behaviours, changes in appetite stimulating hormones, glucose metabolism and mood61). Circadian misalignment has been described as key aspects in the development of chronic illnesses including cardiovascular disease, diabetes, obesity and cancer as well as psychiatric disorders.
A partial phase position (Fig. 1) clearly promotes alertness at work and promotes a better sleep since the lowest alertness level coincides with day sleep following a night shift. However, such benefits have to be evaluated against a possible harmful misalignment as compared to a diurnal phase orientation.
Many non nightshift workers in industrialized societies are assigned working time arrangements and adopt lifestyles that phase shift their circadian system. This results in what has been referred to as ‘social jetlag’ when compared to those living in a natural light/dark environment62). Therefore, while shift workers are the most extreme version of workers affected by circadian disruption, many day workers can also show circadian misalignments and a need for advice, treatment and support to adapt successfully to work hours. Finally, increased levels of trans-meridian travel will also increase the number of people affected with circadian misalignment and who could benefit from the use of bright light interventions43, 63).
Due to individual differences use of light interventions may be most effective for identified groups that experience a reduced health status, reduced alertness at work and circadian strain. Light interventions also have to be weighted against other possible countermeasures not evaluated in this review that at times are more feasible. Among these are changed work hours, napping, use of oral melatonin, sleep hygiene etc.
An interesting development is the strategy of combined treatment. Burke et al.16) combined morning bright light treatment and evening melatonin resulting in greater phase advancing effects for evening melatonin onset. Phase delays have also been best promoted by combined bright light, dark sunglasses and melatonin treatment20).
The light-at-night theory (LAN), suggesting negative health effects of light exposure at night might temper our enthusiasm to undertake light treatment at night. While the International Agency for Cancer Research has classified shift work that involves circadian disruption as a probable human carcinogen64), there still is not evidence that light treatment of exposed shift workers or light pollution in the environment is the definitive causal mechanism for the elevated risk. In addition, the mechanisms for producing negative health effects have yet to be provided65, 66). There are very few studies on the long-term health effects of light treatment in shiftwork as well as integrating the visual ergonomics and safety concerns at the workplace as pointed out by guest editors of the Chronobiology International67). From a health perspective, long-term effects should be studied of systems that provide circadian adaptation to individuals who have limited daylight exposure due to work scheduling (e.g., night, early morning and evening shifts), work environment (e.g., windowless buildings) or other factors (e.g. season, geographical location). New lighting techniques today make it feasible to provide dynamic lighting systems that may provide 24-h light profiles and human centric lighting68). It is advised that workers should be offered a non-glare illumination that could induce unwanted side effects.
It is important to note that this is a new and rapidly developing field of research. Due to the variety of shift work effects on worker health and safety, individual differences and the weak experimental designs used in some studies, and the obvious lack of long-term population studies, the recommendations in the present paper should be viewed as guidance rather than definitive.
References
- 1.Wong I, Dawson D, Van Dongen H. (2019) International consensus statements on non-standard working time arrangements and occupational health and safety. Ind Health 57, 135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golombek DA, Casiraghi LP, Agostino PV, Paladino N, Duhart JM, Plano SA, Chiesa JJ. (2013) The times they’re a-changing: effects of circadian desynchronization on physiology and disease. J Physiol Paris 107, 310–22. [DOI] [PubMed] [Google Scholar]
- 3.Partch CL, Green CB, Takahashi JS. (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24, 90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore RY. (1997) Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med 48, 253–66. [DOI] [PubMed] [Google Scholar]
- 5.Wright KP, Jr, Bogan RK, Wyatt JK. (2013) Shift work and the assessment and management of shift work disorder (SWD). Sleep Med Rev 17, 41–54. [DOI] [PubMed] [Google Scholar]
- 6.Vetter C. (2018) Circadian disruption: what do we actually mean? Eur J Neurosci 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Åkerstedt T, Connor J, Gray A, Kecklund G. (2008) Predicting road crashes from a mathematical model of alertness regulation—The Sleep/Wake Predictor. Accid Anal Prev 40, 1480–5. [DOI] [PubMed] [Google Scholar]
- 8.Bjorvatn B, Kecklund G, Åkerstedt T. (1999) Bright light treatment used for adaptation to night work and re-adaptation back to day life. A field study at an oil platform in the North Sea. J Sleep Res 8, 105–12. [DOI] [PubMed] [Google Scholar]
- 9.Koller M, Härma M, Laitinen JT, Kundi M, Piegler B, Haider M. (1994) Different patterns of light exposure in relation to melatonin and cortisol rhythms and sleep of night workers. J Pineal Res 16, 127–35. [DOI] [PubMed] [Google Scholar]
- 10.Boivin DB, Boudreau P, Tremblay GM, Boivin DB, Boudreau P, Tremblay GM. (2012) Phototherapy and orange-tinted goggles for night-shift adaptation of police officers on patrol. Chronobiol Int 29, 629–40. [DOI] [PubMed] [Google Scholar]
- 11.Flo E, Pallesen S, Magerøy N, Moen BE, Grønli J, Hilde Nordhus I, Bjorvatn B. (2012) Shift work disorder in nurses--assessment, prevalence and related health problems. PLoS One 7, e33981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorvatn B, Stangenes K, Oyane N, Forberg K, Lowden A, Holsten F, Åkerstedt T. (2007) Randomized placebo-controlled field study of the effects of bright light and melatonin in adaptation to night work. Scand J Work Environ Health 33, 204–14. [DOI] [PubMed] [Google Scholar]
- 13.Boivin DB, Boudreau P, James FO, Kin NM. (2012) Photic resetting in night-shift work: impact on nurses’ sleep. Chronobiol Int 29, 619–28. [DOI] [PubMed] [Google Scholar]
- 14.Boivin DB, James FO. (2002) Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms 17, 556–67. [DOI] [PubMed] [Google Scholar]
- 15.Bougrine S, Mollard R, Ignazi G, Coblentz A. (1995) Appropriate use of bright light promotes a durable adaptation to night-shifts and accelerates readjustment during recovery after a period of night-shifts. Work Stress 9, 314–26. [DOI] [PubMed] [Google Scholar]
- 16.Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, Wright KP., Jr2013) Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep 36, 1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canazei M, Pohl W, Bliem HR, Weiss EM. (2017) Acute effects of different light spectra on simulated night-shift work without circadian alignment. Chronobiol Int 34, 303–17. [DOI] [PubMed] [Google Scholar]
- 18.Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE, Czeisler CA. (2012) Human responses to bright light of different durations. J Physiol 590, 3103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley SJ, Eastman CI. (2015) Phase advancing human circadian rhythms with morning bright light, afternoon melatonin, and gradually shifted sleep: can we reduce morning bright-light duration? Sleep Med 16, 288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. (2003) Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms 18, 513–23. [DOI] [PubMed] [Google Scholar]
- 21.Dumont M, Paquet J. (2014) Progressive decrease of melatonin production over consecutive days of simulated night work. Chronobiol Int 31, 1231–8. [DOI] [PubMed] [Google Scholar]
- 22.Dumont M, Blais H, Roy J, Paquet J. (2009) Controlled patterns of daytime light exposure improve circadian adjustment in simulated night work. J Biol Rhythms 24, 427–37. [DOI] [PubMed] [Google Scholar]
- 23.Baehr EK, Fogg LF, Eastman CI, Erin K, Fogg LF, Charmane I. (1999) Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol 277, R1598–604. [DOI] [PubMed] [Google Scholar]
- 24.Figueiro MG, Sahin L, Wood B, Plitnick B. (2016) Light at night and measures of alertness and performance: implications for shift workers. Biol Res Nurs 18, 90–100. [DOI] [PubMed] [Google Scholar]
- 25.Hébert M, Martin SK, Lee C, Eastman CI. (2002) The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res 33, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James FO, Walker CD, Boivin DB. (2004) Controlled exposure to light and darkness realigns the salivary cortisol rhythm in night shift workers. Chronobiol Int 21, 961–72. [DOI] [PubMed] [Google Scholar]
- 27.Lee C, Smith MR, Eastman CI. (2006) A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int 23, 859–75. [DOI] [PubMed] [Google Scholar]
- 28.Lowden A, Åkerstedt T, Wibom R. (2004) Suppression of sleepiness and melatonin by bright light exposure during breaks in night work. J Sleep Res 13, 37–43. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. (1997) Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms 12, 5–15. [DOI] [PubMed] [Google Scholar]
- 30.Rahman SA, Shapiro CM, Wang F, Ainlay H, Brown TJ, Casper RF. (2013) Effects of filtering visual short wavelengths during nocturnal shiftwork on sleep and performance. Chronobiol Int 30, 951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. (2008) The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms 23, 341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasseville A, Benhaberou-Brun D, Fontaine C, Charon MC, Hébert M. (2009) Wearing blue-blockers in the morning could improve sleep of workers on a permanent night schedule: a pilot study. Chronobiol Int 26, 913–25. [DOI] [PubMed] [Google Scholar]
- 33.Dawson D, Campbell SS. (1991) Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep 14, 511–6. [DOI] [PubMed] [Google Scholar]
- 34.Dawson D, Encel N, Lushington K. (1995) Improving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administration. Sleep 18, 11–21. [DOI] [PubMed] [Google Scholar]
- 35.Smith MR, Eastman CI. (2012) Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep 4, 111–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haus EL, Smolensky MH. (2013) Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17, 273–84. [DOI] [PubMed] [Google Scholar]
- 37.Slanger TE, Gross JV, Pinger A, Morfeld P, Bellinger M, Duhme AL, Reichardt Ortega RA, Costa G, Driscoll TR, Foster RG, Fritschi L, Sallinen M, Liira J, Erren TC. (2016) Person-directed, non-pharmacological interventions for sleepiness at work and sleep disturbances caused by shift work. Cochrane Database Syst Rev CD010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooley JJ. (2008) Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singapore 37, 669–76. [PubMed] [Google Scholar]
- 39.Thapan K, Arendt J, Skene DJ. (2001) An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535, 261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MR, Fogg LF, Eastman CI. (2009) Practical interventions to promote circadian adaptation to permanent night shift work: study 4. J Biol Rhythms 24, 161–72. [DOI] [PubMed] [Google Scholar]
- 41.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. (2003) A phase response curve to single bright light pulses in human subjects. J Physiol 549, 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjorvatn B, Pallesen S. (2009) A practical approach to circadian rhythm sleep disorders. Sleep Med Rev 13, 47–60. [DOI] [PubMed] [Google Scholar]
- 43.Revell VL, Eastman CI. (2005) How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms 20, 353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess HJ, Sharkey KM, Eastman CI. (2002) Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev 6, 407–20. [PubMed] [Google Scholar]
- 45.Dumont M, Lanctôt V, Cadieux-Viau R, Paquet J, Dumont M, Lanctôt V, Cadieux-viau R, Paquet J. (2012) Melatonin production and light exposure of rotating night workers. Chronobiol Int 29, 203–10. [DOI] [PubMed] [Google Scholar]
- 46.Kantermann T, Sung H, Burgess HJ. (2015) Comparing the morningness-eveningness questionnaire and munich chronotype questionnaire to the dim light melatonin onset. J Biol Rhythms 30, 449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirz-Justice A, Terman M. (2012) Chronotherapeutics (light and wake therapy) as a class of interventions for affective disorders. Handb Clin Neurol 106, 697–713. [DOI] [PubMed] [Google Scholar]
- 48.Eastman CI, Martin SK, Eastman CI, Martin SK. (1999) How to use light and dark to produce circadian adaptation to night shift work. Ann Med 31, 87–98. [DOI] [PubMed] [Google Scholar]
- 49.Casper RF, Rahman S. (2014) Spectral modulation of light wavelengths using optical filters: effect on melatonin secretion. Fertil Steril 102, 336–8. [DOI] [PubMed] [Google Scholar]
- 50.Ulhôa MA, Marqueze EC, Burgos LGA, Moreno CRC. (2015) Shift work and endocrine disorders. Int J Endocrinol 2015, 826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boivin DB, James FO. (2005) Light treatment and circadian adaptation to shift work. Ind Health 43, 34–48. [DOI] [PubMed] [Google Scholar]
- 52.Moreno CRC, Vasconcelos S, Marqueze EC, Lowden A, Middleton B, Fischer FM, Louzada FM, Skene DJ. (2015) Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci Rep 5, 14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. (2013) Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol 23, 1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. (2012) Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One 7, e30037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumont M, Beaulieu C. (2007) Light exposure in the natural environment: relevance to mood and sleep disorders. Sleep Med 8, 557–65. [DOI] [PubMed] [Google Scholar]
- 56.Czeisler CA, Richardson GS, Zimmerman JC, Moore-Ede MC, Weitzman ED. (1981) Entrainment of human circadian rhythms by light-dark cycles: a reassessment. Photochem Photobiol 34, 239–47. [PubMed] [Google Scholar]
- 57.Waterhouse J, Minors D, Folkard S, Owens D, Atkinson G, Macdonald I, Reilly T, Sytnik N, Tucker P. (1998) Light of domestic intensity produces phase shifts of the circadian oscillator in humans. Neurosci Lett 245, 97–100. [DOI] [PubMed] [Google Scholar]
- 58.Kaida K, Takahashi M, Haratani T, Otsuka Y, Fukasawa K, Nakata A. (2006) Indoor exposure to natural bright light prevents afternoon sleepiness. Sleep 29, 462–9. [DOI] [PubMed] [Google Scholar]
- 59.Figueiro MG, Steverson B, Heerwagen J, Kampschroer K, Hunter CM, Gonzales K, Plitnick B, Rea MS. (2017) The impact of daytime light exposures on sleep and mood in office workers. Sleep Health 3, 204–15. [DOI] [PubMed] [Google Scholar]
- 60.Boyce P. (2016) Editorial: Exploring human-centric lighting. Light Res Technol 48, 101. [Google Scholar]
- 61.Baron KG, Reid KJ. (2014) Circadian misalignment and health. Int Rev Psychiatry 26, 139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischer D, Vetter C, Roenneberg T. (2016) A novel method to visualise and quantify circadian misalignment. Sci Rep 6, 38601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowden A, Åkerstedt T. (1998) Retaining home-base sleep hours to prevent jet lag in connection with a westward flight across nine time zones. Chronobiol Int 15, 365–76. [DOI] [PubMed] [Google Scholar]
- 64.Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-vinyals G, Davis S, Frings-Dresen MH, Fritschi L, Kogevinas M, Kogi K, Lie JA, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V, Straif K. (2015) Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report BMJ 68, 154–62. [DOI] [PubMed] [Google Scholar]
- 65.Kantermann T, Roenneberg T. (2009) Is light-at-night a health risk factor or a health risk predictor? Chronobiol Int 26, 1069–74. [DOI] [PubMed] [Google Scholar]
- 66.Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. (2015) Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int 32, 1294–310. [DOI] [PubMed] [Google Scholar]
- 67.Kecklund G, Di Milia L, Axelsson J, Lowden A, Åkerstedt T. (2012) 20th International Symposium on Shiftwork and Working Time: biological mechanisms, recovery, and risk management in the 24-h society. Chronobiol Int 29, 531–6. [DOI] [PubMed] [Google Scholar]
- 68.Lowden A, Åkerstedt T. (2012) Assessment of a new dynamic light regimen in a nuclear power control room without windows on quickly rotating shiftworkers—effects on health, wakefulness, and circadian alignment: a pilot study. Chronobiol Int 29, 641–9. [DOI] [PubMed] [Google Scholar]