Abstract
Purpose
OnabotulinumtoxinA has demonstrated efficacy and safety in the treatment of urinary incontinence (UI) associated with neurogenic detrusor overactivity (NDO) and idiopathic overactive bladder (OAB); however, real-world evidence is limited. This postmarketing surveillance study aimed to assess the effectiveness and safety of onabotulinumtoxinA in Korean patients with UI associated with NDO or OAB with an inadequate response or intolerance to anticholinergics.
Methods
Patients received 200 U (NDO) or 100 U (OAB) of onabotulinumtoxinA. Effectiveness (assessed using the validated International Consultation on Incontinence Questionnaire-Short Form [ICIQ-SF]) and safety were assessed for 1–4 months after onabotulinumtoxinA administration.
Results
Overall, 686 patients (NDO, 161; OAB, 525) comprised the safety population; of these, 612 patients were analyzed for effectiveness. There was a significant decrease (P<0.0001) in the mean (standard deviation) ICIQ-SF scores in the NDO (–6.8±5.5) and OAB (–6.0±6.4) groups after onabotulinumtoxinA administration. A decrease of >5 points from baseline in the ICIQ-SF score was observed in 64.9% and 47.3% of patients in the NDO and OAB groups, respectively. Following treatment, 59.9% in the NDO group and 43.0% in the OAB group were dry. There was no effect of age on effectiveness in either group. Only 10 adverse drug reactions (ADRs) were reported in 5.6% of NDO patients and 20 ADRs in 3.2% of OAB patients. Most ADRs in both groups were related to the lower urinary tract such as dysuria (NDO, 1.2%; OAB, 0.6%) and urinary retention (NDO, 0.6%; OAB, 1.5%).
Conclusions
Effectiveness and safety of onabotulinumtoxinA in Korea in a real-world setting was demonstrated.
Keywords: OnabotulinumtoxinA, Urinary incontinence, Neurogenic urinary bladder, Overactive urinary bladder
INTRODUCTION
Urgency urinary incontinence (UUI) affects approximately 1.5% of the global population [1] and approximately 4.5% of the Korean population [2]. Spinal cord injuries and other neurologic disorders such as multiple sclerosis, Parkinson disease, stroke, and spina bifida often cause neurogenic detrusor overactivity (NDO), frequently leading to urinary incontinence (UI) and high detrusor pressures, thereby impairing quality of life (QoL) [3-5] and putting the patient at risk of renal dysfunction [6]. Overactive bladder (OAB) of unknown etiology (idiopathic) manifests as urgency, with or without UUI, and is often accompanied by increased urinary frequency and nocturia [7]. UUI affects approximately one-third of patients with OAB [8] and considerably impacts healthcare resource utilization, QoL, and patient productivity [9].
Anticholinergics have been the first-line treatment for UI associated with NDO and OAB [10,11] for many years, but are known to cause bothersome adverse effects, such as dry mouth and constipation, which lead to poor patient compliance and treatment discontinuation [12-14]. More recently, anticholinergics have also been implicated with an increased risk of dementia [15].
OnabotulinumtoxinA (BOTOX, Allergan plc, Dublin, Ireland) is a locally injected acetylcholine release inhibitor and a neuromuscular blocking agent [16] that requires minimally invasive cystoscopic intradetrusor injections. Trials in the onabotulinumtoxinA development program have demonstrated that it was well tolerated and significantly improved UI, urodynamic parameters, and QoL in patients with an inadequate response to anticholinergics [17-20]. These findings led to the approval of onabotulinumtoxinA for the treatment of UI due to both NDO and OAB associated with symptoms of UUI, urgency, and frequency in patients with an inadequate response or intolerance to anticholinergics [16].
The Korean Ministry of Food and Drug Safety (MFDS) approved onabotulinumtoxinA for the treatment of UI due to detrusor overactivity associated with a neurologic condition (e.g., spinal cord injury, multiple sclerosis) in 2012, and for the treatment of OAB symptoms of UI, urinary urgency, and urinary frequency in 2013; both approvals were in adults with an inadequate response or intolerance to anticholinergics. The Korea National Health Insurance Service has covered onabotulinumtoxinA since October 2015 [21]. However, to date, there is no real-world evidence regarding the use of onabotulinumtoxinA in the Korean population. In accordance with the conditions for approval, this postmarketing surveillance study was conducted in Korea to assess the effectiveness and safety of onabotulinumtoxinA for these indications.
MATERIALS AND METHODS
Study Design
This was an open-label, noncomparative, observational, postmarketing surveillance study conducted at 21 sites from August 31, 2012 to August 30, 2016 (ClinicalTrials.gov identifier: NCT 02010788) in accordance with the Korean MFDS regulations. The protocol was approved by the Institutional Review Board at each hospital. Investigators continuously enrolled all eligible patients to be treated with onabotulinumtoxinA for NDO or OAB at each site to ensure unbiased enrollment of patients during the predefined study period. The decision to treat with onabotulinumtoxinA was determined by the physician and patient, and only patients who provided written consent to participate in the study were included.
Patients
Based on the investigator’s clinical judgment, patients were to be enrolled into this real-world observational study per the licensed indications for onabotulinumtoxinA in Korea (i.e., ≥18 years old with UI due to NDO [e.g., spinal cord injury, multiple sclerosis] or with symptoms of urgency, UI, or frequency due to OAB with an inadequate response to or intolerance to anticholinergics).
Treatment
Enrolled patients received 200 U (NDO) or 100 U (OAB) of onabotulinumtoxinA administered as 30 or 20 intradetrusor injections, respectively. Anesthesia and prophylactic antibiotic use were per the Korean label and investigator’s clinical practice.
Endpoints and Assessments
Effectiveness was assessed using the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) that was completed by patients before and 1–4 months after onabotulinumtoxinA administration. The ICIQ-SF is a brief and robust measure to assess the impact of symptoms of UI on QoL and outcome of treatment [22]; it comprises 3 scored items and an unscored self-diagnostic item and helps evaluate the prevalence, frequency, and cause of UI and its impact on QoL and everyday life. A decrease in total score following administration of onabotulinumtoxinA indicates symptom improvement.
Safety was assessed throughout the follow-up period (1–4 months) after onabotulinumtoxinA administration. An adverse drug reaction (ADR) was defined as an adverse event (AE) for which causal relationship to onabotulinumtoxinA was at least a possibility in the physician’s judgment, i.e., the relationship could not be ruled out.
Statistical Analysis
Based on the Standard for Re-examination of New Drugs defined by the Korean MFDS [23], 600 patients were to be enrolled in the study. The mean, standard deviation (SD), median, and range for each ICIQ-SF change were reported. Paired ttests and analysis of variance were used to calculate P-values. All ADRs were standardized and grouped by system organ class and preferred terms based on the World Health Organization Adverse Reaction Terminology.
RESULTS
Demographics and Baseline Characteristics
A total of 739 patients (NDO, 173; OAB, 566) entered the study (Fig. 1). Of these, 53 patients (NDO, 12; OAB, 41) were excluded because they did not receive onabotulinumtoxinA, were unable to undergo follow-up examinations, were prescribed onabotulinumtoxinA for other indications, or received an incorrect dose. The safety analysis population comprised 686 patients (NDO, 161; OAB, 525); of these, 612 patients (NDO, 134; OAB, 478) comprised the effectiveness analysis population that included patients with a completed ICIQ-SF questionnaire at baseline and follow-up.
Fig. 1.
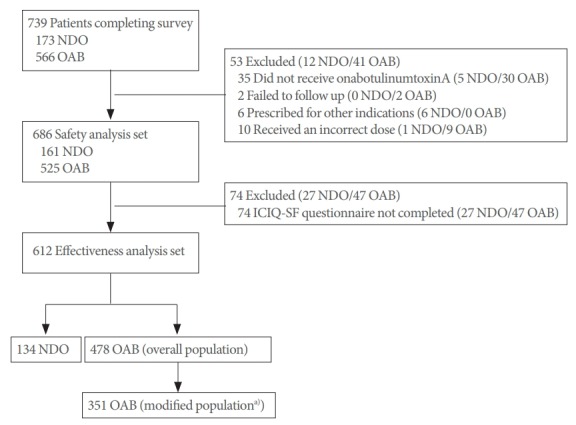
Study flow chart. ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form; NDO, neurogenic detrusor overactivity; OAB, overactive bladder; UI, urinary incontinence. a)Patients with UI at baseline and completed ICIQ-SF questionnaire at baseline and follow-up.
Demographics and baseline characteristics are listed in Table 1. In the NDO group, all but one patient (160 of 161 [99.4%]) had previously used anticholinergics, while 1 of 161 patient (0.6%) had previously used sacral neuromodulation therapy, and 12 of 161 (7.5%) had previously used onabotulinumtoxinA. Similarly, in the OAB group, most patients (515 of 525 [98.1%]) had previously used anticholinergics, 9 of 525 (1.7%) had previously used sacral neuromodulation therapy, and 20 of 525 (3.8%) had previously used onabotulinumtoxinA. Of the patients with OAB, 376 of 525 (71.6%) had UUI.
Table 1.
Demographics and baseline patient characteristics (safety population)
| Characteristic | NDO (n = 161) | OAB (n = 525) | Total (n = 686) |
|---|---|---|---|
| Age (yr) | 51.4 ± 14.2 | 62.5 ± 14.5 | 60.0 ± 15.2 |
| < 50 | 63 (39.1) | 99 (18.9) | 162 (23.6) |
| ≥ 50, < 60 | 48 (29.8) | 101 (19.2) | 149 (21.7) |
| ≥ 60, < 70 | 34 (21.1) | 122 (23.2) | 156 (22.7) |
| ≥ 70 | 16 (9.9) | 203 (38.7) | 219 (31.9) |
| Sex | |||
| Male | 124 (77.0) | 112 (21.3) | 236 (34.4) |
| Female | 37 (23.0) | 413 (78.7) | 450 (65.6) |
| Duration since diagnosis (yr)a) | 11.3±10.9 | 4.8±5.4 | - |
| Underlying neurologic conditionb) | |||
| Multiple sclerosis | 5 (3.1) | - | - |
| Spinal cord injury | 156 (96.9) | - | - |
| Stroke | 1 (0.6) | - | - |
| Symptomsc) | |||
| UUI | - | 376 (71.6)d) | - |
| Urgency | - | 348 (66.3) | - |
| Frequency | - | 385 (73.3) | - |
| Previous use of anticholinergics | 160 (99.4) | 515 (98.1) | 675 (98.4) |
| Previous use of SNM | 1 (0.6) | 9 (1.7) | 10 (1.5) |
| Previous use of onabotulinumtoxinA | 12 (7.5) | 20 (3.8) | 32 (4.7) |
Values are presented as mean±standard deviation or number (%).
NDO, neurogenic detrusor overactivity; OAB, overactive bladder; UUI, urgency urinary incontinence; SNM, sacral neuromodulation; UI, urinary incontinence.
Data for “duration since diagnosis” were available for 505 patients in the OAB group.
One patient had both spinal cord injury and stroke.
For patients with OAB, same patients can appear in different categories.
This population (patients with OAB and UI) comprised the modified effectiveness population.
Study Drug Administration
In the NDO group, all patients received onabotulinumtoxinA 200 U via 30 injection sites, whereas in the OAB group, all patients received 100 U via 20 injection sites (Table 2). Administration of local anesthesia (NDO, 97 of 134 [72.4%]; OAB, 302 of 478 [63.2%]) and general anesthesia (NDO, 26 of 134 [19.4%]; OAB, 124 of 478 [25.9%]) was comparable between etiologies. Prophylactic antibiotics were administered to almost all patients in both groups (NDO, 129 of 134 [96.3%]; OAB, 428 of 478 [89.5%]).
Table 2.
Study drug administration details (safety population)
| Variable | NDO (n = 161) | OAB (n = 525) | Total (n = 686) |
|---|---|---|---|
| Treatment setting | |||
| Outpatient | 116 (72.1) | 212 (40.4) | 328 (47.8) |
| Inpatient | 45 (28.0) | 313 (59.6) | 358 (52.2) |
| Use of anesthesia during onabotulinumtoxinA administrationa) | |||
| Local | 97 (72.4) | 302 (63.2) | 399 (65.2) |
| General | 26 (19.4) | 124 (25.9) | 150 (24.5) |
| Use of prophylactic antibiotics during onabotulinumtoxinA administrationa) | 129 (96.3) | 428 (89.5) | 557 (91.0) |
Values are presented as number (%).
NDO, neurogenic detrusor overactivity; OAB, overactive bladder.
NDO, n=134; OAB, n=478; total, n=612.
Effectiveness
ICIQ-SF scores
In the NDO group, we observed a significant decrease in the mean (±SD) ICIQ-SF score from 14.3±5.0 before onabotulinumtoxinA administration to 7.5±5.8 after administration (difference, 6.8±5.5; P<0.001), reflecting an improvement in symptoms. The mean (±SD) time to follow-up averaged 47.5±23.8 days (Table 3).
Table 3.
Change in ICIQ-SF scores from baseline (effectiveness population)
| NDO (n=134) | OAB |
||
|---|---|---|---|
| Overall population (n=478) | Modified populationa) (n=351) | ||
| OnabotulinumtoxinA administration | |||
| Before | 14.3 ± 5.0 | 12.2 ± 6.6 | 14.4 ± 4.7 |
| After | 7.5 ± 5.8 | 6.2 ± 6.3 | 7.4 ± 6.3 |
| Difference (after–before) | –6.8 ± 5.5 | –6.0 ± 6.4 | –7.0 ± 6.5 |
| P-value (paired t-test) | < 0.001 | < 0.001 | < 0.001 |
Values are presented as mean±standard deviation.
ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form; NDO, neurogenic detrusor overactivity; OAB, overactive bladder; UI, urinary incontinence.
OAB patients with UI at baseline and completed ICIQ-SF questionnaire at baseline and follow-up.
Similarly, in the OAB group, there was a significant decrease in the mean (±SD) ICIQ-SF score from 12.2±6.6 before onabotulinumtoxinA administration to 6.2±6.3 after administration (difference, 6.0±6.4; P<0.001), after a mean (±SD) follow-up of 64.6±123.4 days (Table 3).
Many patients had an improvement in symptoms, indicated by a decrease of greater than 5 points (NDO, 87 of 134 [64.9%]; OAB, 226 of 478 [47.3%]), and a much smaller proportion of patients had an improvement of only 1–5 points (NDO, 24 of 134 [17.9%]; OAB, 120 of 478 [25.1%]) in the ICIQ-SF score from baseline (Table 4).
Table 4.
Change in ICIQ-SF score by range (effectiveness population)
| NDO (n=134) | OAB |
||
|---|---|---|---|
| Overall population (n=478) | Modified populationa) (n=351) | ||
| Decrease by > 5 points | 87 (64.9) | 226 (47.3) | 191 (54.4) |
| Decrease by 1–5 points | 24 (17.9) | 120 (25.1) | 95 (27.1) |
| Increase by 1–5 points | 21 (15.7) | 122 (25.5) | 59 (16.8) |
| Increase by ≥ 5 points | 2 (1.5) | 10 (2.1) | 6 (1.7) |
| Total | 134 (100) | 478 (100) | 351 (100) |
Values are presented as number (%).
ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form; NDO, neurogenic detrusor overactivity; OAB, overactive bladder; UI, urinary incontinence.
OAB patients with UI at baseline and completed ICIQ-SF questionnaire at baseline and follow-up.
Subanalysis of ICIQ-SF scores by age and sex
In all groups, a subanalysis of ICIQ-SF scores before and after onabotulinumtoxinA administration by age revealed that there was a significant decrease (P<0.001) in scores after drug administration in all age groups, indicating no effect of age on effectiveness. Similarly, in all groups, there was a significant decrease (P<0.001) in ICIQ-SF scores after onabotulinumtoxinA administration in both males and females.
Degree of urine leaks
Following treatment, 59.9% of patients in the NDO group and 43.0% in the OAB group were dry. Furthermore in the NDO group, patients reporting “Leaks before you can get to the toilet,” “Leaks when you are asleep,” “Leaks when you are physically active/exercising,” “Leaks when you have finished urinating and are dressed,” “Leaks for no obvious reason,” and “Leaks all the time” decreased (Fig. 2), whereas in the OAB group, patients reporting “Leaks before you can get to the toilet,” “Leaks when you cough or sneeze,” “Leaks when you are asleep,” “Leaks when you are physically active/exercising,” “Leaks when you have finished urinating and are dressed,” “Leaks for no obvious reason,” and “Leaks all the time” decreased (Fig. 3).
Fig. 2.
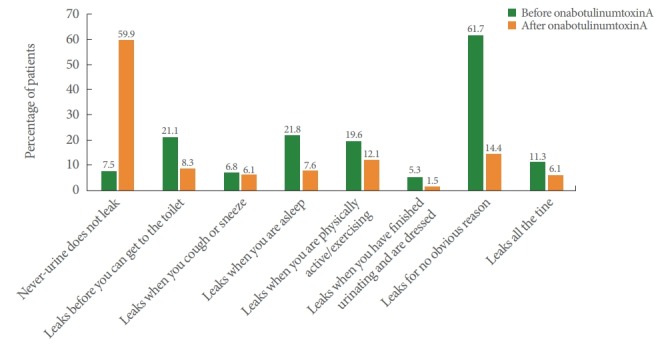
Patient-reported effectiveness outcomes in patients with neurogenic detrusor overactivity (effectiveness population). The same patient can be counted in multiple categories.
Fig. 3.
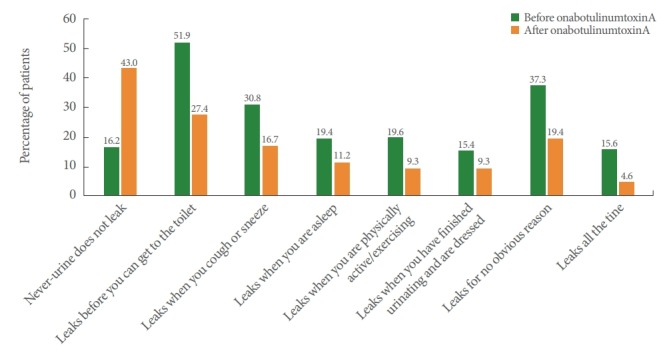
Patient-reported effectiveness outcomes in patients with overactive bladder (effectiveness population). The same patient can be counted in multiple categories.
Analysis of ICIQ-SF by baseline UI
Since the ICIQ-SF is designed specifically for patients with UI, an additional modified OAB population analysis was performed specifically in 351 patients who reported UI at baseline on their ICIQ-SF, as several patients in the overall OAB effectiveness population did not report UI as a symptom on their baseline ICIQ-SF in this real-world study (Fig. 1). The decrease in ICIQ-SF scores in the overall OAB effectiveness population and the OAB modified effectiveness population was comparable: −6.0±6.4 points and −7.0±6.5 points, respectively (Tables 3 and 4).
Safety
In the NDO group, 27 AEs were reported in 19 of 161 (11.8%) patients. Of all AEs reported, those with a causal relationship in the opinion of the physician to onabotulinumtoxinA were considered ADRs; 10 ADRs were reported in 9 of 161 (5.6%) patients (Table 5). These ADRs included dysuria (2 of 161 [1.2%]), urinary tract infection (2 of 161 [1.2%]), myalgia (2 of 161 [1.2%]), urinary retention (1 of 161 [0.6%]), difficulty in micturition (1 of 161 [0.6%]), constipation (1 of 161 [0.6%]), and testis disorder (1 of 161 [0.6%]). Urinary retention was managed per local clinical practice, with 3 of 20 patients (15%) not catheterizing at baseline initiating clean intermittent catheterization (CIC) after onabotulinumtoxinA treatment. Testis disorder, an unexpected ADR, was considered to have a “conditional/unclassified (i.e., more data required for appropriate evaluation)” relationship with onabotulinumtoxinA.
Table 5.
ADRs in patients with NDO (safety population, n=161)
| Incidence of ADRs |
||
|---|---|---|
| No. (%) | No. of events | |
| Urinary system disorders | 3 (1.9) | 4 |
| Urinary retention | 1 (0.6) | 1 |
| Dysuria | 2 (1.2) | 2 |
| Difficulty in micturition | 1 (0.6) | 1 |
| Gastrointestinal system disorders | 1 (0.6) | 1 |
| Constipation | 1 (0.6) | 1 |
| Resistance mechanism disorders | 2 (1.2) | 2 |
| Urinary tract infecion | 2 (1.2) | 2 |
| Musculoskeletal system disorders | 2 (1.2) | 2 |
| Myalgia | 2 (1.2) | 2 |
| Reproductive disorders, male | 1 (0.6) | 1 |
| Testis disorder | 1 (0.6) | 1 |
| Total | 9 (5.6) | 10 |
ADRs, adverse drug reactions; NDO, neurogenic detrusor overactivity.
In the OAB group, 51 AEs were reported in 40 of 525 patients (7.6%). Twenty ADRs were reported in 17 of 525 patients (3.2%). These included urinary retention (8 of 525 [1.5%]), dysuria (3 of 525 [0.6%]), and hematuria, pyuria, urodynia, urinary frequency, urinary hesitation, nausea, cystitis, pyelonephritis, and perineal pain in a male (1 of 525 [0.2%] each) (Table 6). Urinary retention was managed per local clinical practice, with 15 of 416 patients (3.6%) initiating CIC after onabotulinumtoxinA treatment. Pyelonephritis was the only serious ADR, reported in 1 of 525 patients (0.2%). All ADRs in both groups were mild or moderate in severity.
Table 6.
ADRs in patients with OAB (safety population, n=525)
| Incidence of ADRs |
||
|---|---|---|
| No. (%) | No. of events | |
| Urinary system disorders | 14 (2.7) | 16 |
| Urinary retention | 8 (1.5) | 8 |
| Dysuria | 3 (0.6) | 3 |
| Hematuria | 1 (0.2) | 1 |
| Pyuria | 1 (0.2) | 1 |
| Urodynia | 1 (0.2) | 1 |
| Urinary frequency | 1 (0.2) | 1 |
| Urinary hesitation | 1 (0.2) | 1 |
| Gastrointestinal system disorders | 1 (0.2) | 1 |
| Nausea | 1 (0.2) | 1 |
| Resistance mechanism disorders | 2 (0.4) | 2 |
| Cystitis | 1 (0.2) | 1 |
| Pyelonephritis | 1 (0.2) | 1 |
| Reproductive disorders, male | 1 (0.2) | 1 |
| Perineal pain male | 1 (0.2) | 1 |
| Total | 17 (3.2) | 20 |
ADRs, adverse drug reactions; OAB, overactive bladder.
DISCUSSION
This is the first postmarketing surveillance study in Korea to assess the effectiveness and safety of onabotulinumtoxinA for the treatment of UI associated with NDO or OAB in patients who have an inadequate response or intolerance to anticholinergics.
The ICIQ-SF was used to assess effectiveness in this study; this questionnaire is a practical and reliable method for the evaluation of patients with UUI, with a significant correlation having been found between ICIQ-SF scores and urodynamic parameters [24]. The mean change in ICIQ-SF scores compared to baseline was significant (P<0.001) in all NDO and OAB groups. Although a responder definition has not been established for the ICIQ-SF, a previous study graded the ICIQ-SF scale as slight (1–5), moderate (6–12), severe (13–18), and very severe (19–21) [25]. Based on this grading, in the current study, patients with NDO, on average, improved from severe to moderate status after a mean follow-up period of 47.5 days, and patients with OAB, on average, improved from borderline moderate to borderline slight after a mean of 64.6 days. In yet another study in women with stress UI who received pelvic floor muscle training, QoL was assessed using both the ICIQ-SF and the Patient Global Impression of Improvement (PGI-I) questionnaires, and a decrease of approximately 5 points from baseline on the ICIQ-SF questionnaire corresponded to a “much better” grade on the PGI-I questionnaire. Based on this correlation, approximately half of the patients in the current study were “much better” or “very much better” after onabotulinumtoxinA administration [26].
Importantly, 59.9% of patients with NDO and 43.0% of patients with OAB reported that urine had stopped leaking and that they achieved “dry” status after onabotulinumtoxinA injection. This real-world rate is much higher than the range of approximately 23%–38% reported in previous pivotal trials [17-20]. Previous studies showed a greater proportion of patients with NDO (36%–38%) than those with OAB (23%) achieving “dry” status; this is in line with the observations in the current study.
Safety was as expected; it must be noted that, similar to previous clinical trials, most ADRs in this study were local and mostly related to the urinary tract [17-19]. However, in comparison to previous clinical trials, the incidence of ADRs was much lower, most likely due to the observational nature of the trial. While protocols for previous trials included specific requirements for measuring postvoid residual urine volume and guidance for following and reporting urinary retention, there were no requirements for the collection of postvoid residual urine in the current study. The investigators did a routine follow-up after onabotulinumtoxinA injection, which could include a postvoid residual evaluation depending on local practice and/or if the patient reported symptoms. Therefore, the ADRs reported in this study are possibly more consistent with real-world rates versus previous clinical trials. Although the follow-up periods were relatively short, it is also important to note that most AEs, including those related to the urinary tract, occurred within the first 12 weeks in the pivotal registration trials [17-19]. This is well within the follow-up period of the current study; therefore, it is quite likely that this study captured the complete effectiveness and safety profile of onabotulinumtoxinA in a real-world setting.
This study did have some limitations: diagnostic criteria for OAB were not objective; however subjective, symptom-based diagnoses have been shown to have utility and are used frequently in clinical settings. Therefore, this study is perhaps more reflective of clinical practice than trials that use objective diagnostic criteria such as a bladder diary. As the previous registrational studies used a 3- to 7-day bladder diary before baseline and at each follow-up visit to confirm a 100% reduction in UI [17-19], whereas the current study used the ICIQ-SF to assess UI, a direct comparison of effectiveness in this real-world study compared to the pivotal phase 3 studies is not possible. Despite these limitations, this post-marketing surveillance study provides valuable and powerful information from a realworld setting. Patient selection was based on the investigator’s judgment; there were no strict inclusion and exclusion criteria. Thus, these data show that, in a real-world situation, the CIC incidence rate following onabotulinumtoxinA administration in the bladder is lower than that reported in previous phase 3 studies (which may not reflect real-world practice). It is unclear why the real-world incidence of CIC is lower; this might be due to the strict requirement in phase 3 studies to commence CIC based on post-void residual thresholds or due to different patient selection criteria in real-world practice. In addition, we believe that in order to assess the impact of incontinence comprehensively, it is necessary to measure not only the severity of symptoms but also the extent to which these symptoms impair the patient’s QoL. This study also showed that the ICIQ-SF is a brief, robust, useful, and sensitive questionnaire that can assess the symptoms and impact of incontinence and can be used universally in clinical practice and research. While the ICIQ-SF questionnaire has been used previously to assess the efficacy of onabotulinumtoxinA for the treatment of incontinence associated with detrusor overactivity or OAB [27-29], its use in a realworld setting in South Korea is unique. Moreover, the study demonstrated that onabotulinumtoxinA is an effective and safe treatment option for NDO and OAB, specifically in real-world clinical settings in the Korean population.
In this postmarketing surveillance study, the effectiveness and safety profile of onabotulinumtoxinA for the treatment of UI associated with NDO or OAB in a real-world Korean population was found to be comparable or improved compared to that previously reported in randomized clinical trials in global populations. Notably, the proportion of “dry” patients following onabotulinumtoxinA administration was higher in this study compared to previous reports. This study also supports the use of the ICIQ-SF questionnaire to assess the impact of symptoms of UI on QoL and outcome of treatment in patients with NDO or OAB.
Acknowledgments
This study was funded by Allergan plc. The authors would like to thank Seung Jun Oh (Seoul National University Hospital), Jang Hwan Kim (Yonsei University Health System), Jung Goo Lee (Korea University Anam Hospital), Jun Chul Kim (Catholic University Bucheon St. Mary’s Hospital), Jung Joo Lee (Pusan National University Hospital), Young Ho Kim (Sooncheonhyang University Bucheon Hospital), Sung Jin Jung (Seoul National University Bundang Hospital), Ha Na Yoon (Ewha Womans’ University Mokdong Hospital), Myung Soo Choo (Asan Medical Center), Hee Jong Jung (Wonkwang University Hospital), Young Seop Jang (Kunyang University Hospital), Seon Ok Kim (Chonnam National University Hospital), Eun Sang Yoo (Gyeongbuk National University Hospital), Ji Yeon Han (Pusan National University Yangsan Hospital), Sung Yong Cho (Seoul National University Boramae Hospital), Geon Cheol Lee (Inje University Ilsan Paik Hospital), Gye Hwan Kim (Gachon Gil Hospital), Seung Ryeol Lee (Bundang CHA Hospital), Joo Tae Seo (Cheil General Hospital), Deok Yoon Kim (Daegu Catholic University Medical Center), Hyun Woo Kim (Catholic University St. Paul Hospital), Yong Tae Kim (Hanyang University Medical Center), Seong Tae Cho (Hallym University Gangnam Sacred Heart Hospital), Hyung Geon Park (Kunkuk University Hospital), Hee Chang Jeong (Yeongnam National University Hospital), Dae Yeon Cho (Inje University Seoul Paik Hospital), Jae Hyun Kim (Sooncheonhyang University Seoul Hospital), Jae Sik Kim (National Traffic Rehabilitation Hospital), Joon Hwa Noh (Gwangju Christian Hospital), Jeong Kyun Yeo (Inje University Seoul Paik Hospital), Jae Hyun Bae (Korea University Ansan Hospital), So Yeon Lee (Myungji Hospital), Won Ki Lee (Hallym University Chuncheon Sacred Heart Hospital), Gyeong Hee Kim (MIZLOVE Female Urology Clinic), Seok Joong Yoon (Chungbuk National University Hospital), Mimi Oh (Korea University Guro Hospital), Tak Geon Yoo (Eulji University Eulji Hospital), Gyo Ik Mo (Korea Worker’s Compensation and Welfare Service Incheon Hospital), Dong Hwan Lee (Catholic University Incheon St. Mary’s Hospital), In Kyung Kim (Tower Urology Clinic), Seok Young Lee (National Health Insurance Ilsan Hospital), Ho Seop Kwak (Dong-Eui Medical Center), Gyeong Mi Lee (Pusan Metropolitan Clinic), Kyu Sung Lee (Samsung Medical Center), Myung Soo Choo (Asan Medical Center), Jun Chul Kim (Catholic University Bucheon St. Mary’s Hospital), and Dong Gil Shin (Pusan National University Hospital) for their contribution.
Footnotes
Grant/Fund Support
This study was sponsored by Allergan plc and the sponsor was involved in the design and conduct of the study, collection and analysis of data, and preparation of the manuscript.
Research Ethics
This study was an open-label, noncomparative, observational, postmarketing surveillance study conducted at 21 sites from August 31, 2012 to August 30, 2016 (ClinicalTrials.gov identifier: NCT02010788) in accordance with the Korean Korean Ministry of Food and Drug Safety regulations. The study was performed according to the Helsinki Declaration and approved by the Institutional Review Boards of the institution where the study was performed. A written informed consent was obtained from all subjects.
Conflict of Interest
KJK and KSL declare no conflicts of interest. BJ and AP are employees of and hold stock options in Allergan plc. This study was sponsored by Allergan plc and the sponsor was involved in the design and conduct of the study, collection and analysis of data, and preparation of the manuscript.
AUTHOR CONTRIBUTION STATEMENT
· Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: KSL
· Study concept and design: KSL
· Acquisition of data: KJK
· Analysis and interpretation of data: KJK
· Drafting of the manuscript: KJK, BJ, AP
· Critical revision of the manuscript for important intellectual content: KSL
· Statistical analysis: KJK
· Obtained funding: KSL
· Administrative, technical, or material support: KSL
· Study supervision: KSL
REFERENCES
- 1.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–14. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Lee YS, Lee KS, Jung JH, Han DH, Oh SJ, Seo JT, et al. Prevalence of overactive bladder, urinary incontinence, and lower urinary tract symptoms: results of Korean EPIC study. World J Urol. 2011;29:185–90. doi: 10.1007/s00345-009-0490-1. [DOI] [PubMed] [Google Scholar]
- 3.Hicken BL, Putzke JD, Richards JS. Bladder management and quality of life after spinal cord injury. Am J Phys Med Rehabil. 2001;80:916–22. doi: 10.1097/00002060-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Phé V, Chartier-Kastler E, Panicker JN. Management of neurogenic bladder in patients with multiple sclerosis. Nat Rev Urol. 2016;13:275–88. doi: 10.1038/nrurol.2016.53. [DOI] [PubMed] [Google Scholar]
- 5.Ruffion A, Castro-Diaz D, Patel H, Khalaf K, Onyenwenyi A, Globe D, et al. Systematic review of the epidemiology of urinary incontinence and detrusor overactivity among patients with neurogenic overactive bladder. Neuroepidemiology. 2013;41:146–55. doi: 10.1159/000353274. [DOI] [PubMed] [Google Scholar]
- 6.Lawrenson R, Wyndaele JJ, Vlachonikolis I, Farmer C, Glickman S. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology. 2001;20:138–43. doi: 10.1159/000054774. [DOI] [PubMed] [Google Scholar]
- 7.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 9.Tang DH, Colayco DC, Khalaf KM, Piercy J, Patel V, Globe D, et al. Impact of urinary incontinence on healthcare resource utilization, health-related quality of life and productivity in patients with overactive bladder. BJU Int. 2014;113:484–91. doi: 10.1111/bju.12505. [DOI] [PubMed] [Google Scholar]
- 10.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–62. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 11.Madhuvrata P, Singh M, Hasafa Z, Abdel-Fattah M. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol. 2012;62:816–30. doi: 10.1016/j.eururo.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Brostrøm S, Hallas J. Persistence of antimuscarinic drug use. Eur J Clin Pharmacol. 2009;65:309–14. doi: 10.1007/s00228-008-0600-9. [DOI] [PubMed] [Google Scholar]
- 13.Jundt K, Schreyer K, Friese K, Peschers U. Anticholinergic therapy: do the patients take the pills prescribed? Arch Gynecol Obstet. 2011;284:663–6. doi: 10.1007/s00404-010-1720-x. [DOI] [PubMed] [Google Scholar]
- 14.Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011;65:567–85. doi: 10.1111/j.1742-1241.2010.02626.x. [DOI] [PubMed] [Google Scholar]
- 15.Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7:217–24. doi: 10.1177/2042098616658399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dublin (Ireland): Allergan plc; c2019. BOTOX® (onabotulinumtoxinA) Prescribing Information [Internet] [cited 2017 Apr 10]. Available from: https://www.allergan.com/assets/pdf/botox_pi. [Google Scholar]
- 17.Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60:742–50. doi: 10.1016/j.eururo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187:2131–9. doi: 10.1016/j.juro.2012.01.125. [DOI] [PubMed] [Google Scholar]
- 19.Nitti VW, Dmochowski R, Herschorn S, Sand P, Thompson C, Nardo C, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189:2186–93. doi: 10.1016/j.juro.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Chapple C, Sievert KD, MacDiarmid S, Khullar V, Radziszewski P, Nardo C, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2013;64:249–56. doi: 10.1016/j.eururo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Cho YS, Kim KH. Botulinum toxin in spinal cord injury patients with neurogenic detrusor overactivity. J Exerc Rehabil. 2016;12:624–31. doi: 10.12965/jer.1632874.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 23.Cheongju (Korea): Ministry of Food and Drug Safety; 2015. Standard for Re-examination of New Drugs, etc 2015 [Internet] [cited 2018 Mar 26]. Available from: http://www.mfds.go.kr/eng/index.do?nMenuCode=128. [Google Scholar]
- 24.Seckiner I, Yesilli C, Mungan NA, Aykanat A, Akduman B. Correlations between the ICIQ-SF score and urodynamic findings. Neurourol Urodyn. 2007;26:492–4. doi: 10.1002/nau.20389. [DOI] [PubMed] [Google Scholar]
- 25.Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: The ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn. 2009;28:411–5. doi: 10.1002/nau.20674. [DOI] [PubMed] [Google Scholar]
- 26.Nyström E, Sjöström M, Stenlund H, Samuelsson E. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodyn. 2015;34:747–51. doi: 10.1002/nau.22657. [DOI] [PubMed] [Google Scholar]
- 27.Avallone MA, Sack BS, El-Arabi A, Guralnick ML, O’Connor RC. Less is more-A pilot study evaluating one to three intradetrusor sites for injection of OnabotulinumtoxinA for neurogenic and idiopathic detrusor overactivity. Neurourol Urodyn. 2017;36:1104–7. doi: 10.1002/nau.23052. [DOI] [PubMed] [Google Scholar]
- 28.Balzarro M, Rubilotta E, Braga A, Bassi S, Processali T, Artibani W, et al. OnabotulinumtoxinA detrusor injection improves female sexual function in women with overactive bladder wet syndrome. Eur J Obstet Gynecol Reprod Biol. 2018;225:228–31. doi: 10.1016/j.ejogrb.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Chughtai B, Dunphy C, Lee R, Lee D, Sheth S, Marks L, et al. Randomized, double-blind, placebo controlled pilot study of intradetrusor injections of onabotulinumtoxinA for the treatment of refractory overactive bladder persisting following surgical management of benign prostatic hyperplasia. Can J Urol. 2014;21:7217–21. [PubMed] [Google Scholar]


