Abstract
Cell responses to mechanical forces are crucial in embryonic development, adult physiology, and numerous diseases including atherosclerosis, hypertension, osteoporosis, muscular dystrophy, myopathies and cancer. These responses are mediated by load-bearing, sub-cellular structures, such as the plasma membrane, cell adhesions and cytoskeleton. Recent work has demonstrated that these structures are dynamic, undergoing assembly, disassembly and movement, even when ostensibly stable. An emerging insight is that transduction of forces into biochemical signals occurs within the context of these processes. This framework helps explain how forces of varying strengths or dynamic characteristics regulate distinct signaling pathways.
There is growing recognition that mechanical factors, such as applied forces or the rigidity of the extracellular matrix (ECM), critically influence the form and function of cells and organisms1–3. Biological regulation has classically been understood through the concepts of solution chemistry, in which enzyme activities, reaction rates and affinities govern cellular processes. However, mechanotransduction, the conversion of mechanical forces into biochemically relevant information, contributes to numerous developmental, physiological and pathological processes and is a major, rapidly advancing area of current research1.
In the vasculature, blood flow exerts fluid shear stresses on the endothelial cells that line the vessels, while blood pressure stretches the vessel wall4. Shear stress is crucial for remodeling the primitive vascular plexus into a hierarchical vascular tree5 and for patterning the cardiac outflow tract in developing mouse embryos6. Hypertension causes thickening of arterial walls and is a major risk factor for cardiovascular diseases4. Atherosclerosis, the chronic cholesterol-dependent inflammation of artery walls, occurs preferentially at regions of disturbed flow such as branch points and areas of high curvature, where both the magnitude and temporal characteristics of the flow are disturbed. The development and pathobiology of bone and muscle also depend strongly on mechanical forces from weight and muscle contraction, while lung physiology and pathology are strongly influenced by forces from inflation1.
Tissue rigidity or stiffness affects many biological processes7. Tumors have long been identified by palpation due to local increases in tissue stiffness. Recently, these changes in the mechanical environment have been shown to be causal for tumor progression8. Fibrotic lung disease begins with a small change in tissue stiffness, which is sensed by cells to induce more severe and irreversible remodeling9. Furthermore, the rigidity of the extracellular environment potently controls the differentiation of mesenchymal stem cells10 and the self-renewal of hematopoietic stem cells11. Developing scaffolds with tunable mechanical properties to control cellular behavior has become a major effort in tissue engineering12.
While applying forces to cells or altering the rigidity of their environment are clearly distinct processes, the underlying mechanisms of mechanotransduction appear to be similar2. A key event in rigidity-sensing is the modulation of cellular contractility. Cells on soft materials exert lower forces than cells on stiff materials, decreasing tension on force-bearing elements. These elements are the same whether forces are generated internally or externally7, thus, many of the cellular responses to distinct mechanical stimuli are similar. Another unifying principle is that the structures that generate and bear cellular forces are involved in sensing forces1. Therefore, cytoskeletal proteins such as actin and tubulin are crucial in mediating mechanical effects in nearly all systems2,13 (Fig.1a and b). Cellular adhesions, both to the extracellular matrix (ECM) and to other cells, are also key, as these structures mechanically connect cells to their surroundings. Correspondingly, candidate genes for diseases that can be considered “mechanotransduction disorders” such as aortic aneurism, heart failure, hypertension or muscular dystrophy include many proteins of the ECM, adhesion complexes and cytoskeleton14–17. There are often drastic changes in the protein composition, dynamics, and mechanics of these structures during metastatic progression18 and stem cell differentiation7.
Figure1. Switch-like models of mechanotransduction.
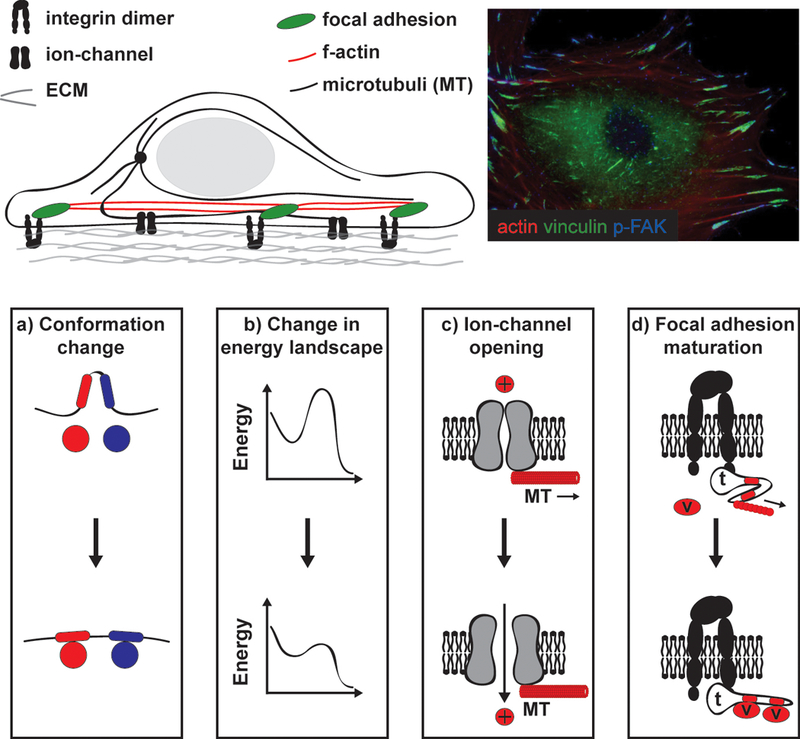
a) Cells are mechanically integrated structures, where the extracellular matrix (ECM) and actin cytoskeleton are connected by integrins and focal adhesion (FA) proteins. Microtubules and many ion channels are also integrated with this network. Forces can be directly applied through the ECM or transmitted through the cytoskeleton to mechanosensitive components, such as FAs, to mediate cellular response to forces. b) Immunostaining of a vascular smooth muscle cell. F-actin filaments (red) link to variably-sized, punctuate FAs visualized by vinculin (green) and phosphorylated focal adhesion kinase (pFAK) staining. The variable amount of pFAK staining in the FAs is indicative of different local signaling environments that are likely linked to distinct mechanical signals. c) A common mechanism of mechanotransduction is force-induced conformational changes. For example, membrane tension can cause ion channel opening. Also, talin connects the integrin cytoplasmic tail to F-actin; tension on talin reveals cryptic vinculin binding sites, subsequent binding of vinculin reinforces the linkage.
While much progress has been made toward understanding mechanotransduction, a complete picture is lacking. Mechanotransduction is typically depicted as a series of rapid switch-like events, activated in response to step-like applications of force, which eventually lead to cellular responses. This level of detail, however, is insufficient to explain the cellular responses to dynamic mechanical stimuli often found in physiological settings.
In this review, we first outline the basic features of the switch-like model of mechanotransduction. We divide this process into mechanotransmission, mechanosensing and mechanoresponse, and then highlight this model’s limitations. Next, we describe some recent advances in our understanding of the dynamic processes regulating load bearing sub-cellular structures and the behavior of single molecules in response to applied forces. With guidance from mathematical models of adhesion assembly, these examples are used to develop a more complete model of mechanotransduction based on the concept that forces alter the rates of key sub-cellular processes to affect cell function. This perspective allows us to understand how cells respond to multiple, time-varying mechanical stimuli. We end by suggesting that mechanically the cell may function as a multi-bandpass filter where stimuli with different temporal characteristics activate distinct signaling pathways that affect cell state and disease progression.
Switch-like Models of Mechanotransduction
Descriptions of mechanotransduction typically begin with the forces acting on cellular elements and end with the integrated response from the cell or tissue. These can be divided into three steps:
Mechanotransmission
A force must be transmitted to mechanosensitive elements before it can be sensed. For example, the adhesion receptors that mediate cell-cell and cell-ECM contacts are strongly implicated in mechanotransduction19. Equally important are the cytoskeletal structures that adhesion receptors universally connect to, which allow adhesions to resist deformation from applied forces. The cytoskeleton is comprised of filaments, including F-actin, intermediate filaments and microtubules, that are relatively stiff on the micrometer dimensional scale and stable on minute-to-hour time scales20. This mechanical continuity allows forces to propagate relatively long distances along filaments in the cell, a process called mechanotransmission. Fluid shear stress, for instance, is exerted on the apical domain of endothelial cells, yet the displacements in vimentin filaments are largest at select areas, often near cell-cell and cell-ECM adhesions21. This result correlates with data implicating junctional proteins in responses to shear22. Twisting of magnetic beads bound to integrins also revealed long distance effects, which were primarily transmitted by F-actin23, although some studies have proposed a role for microtubules24 and intermediate filaments25. Using a laser trap to directly apply forces as small as 5.5 pico Newton (pN) to actin stress fibers triggered influx of calcium ions, presumably due to the activation of plasma membrane mechanosensitive ion channels26. Mechanotransmission and subsequent responses also can be extremely fast, on the order of 100’s of milliseconds24,27, consistent with direct mechanical effects.
Mechanosensing
Transmitted forces ultimately impinge upon mechanosensitive macromolecules to alter their conformation and hence their function. While the biological consequences of such events are specific to each system, the underlying physical response is similar; forces promote changes in conformation that accommodate the applied force. The best studied examples at the structural level are bacterial mechanically gated ion channels28, which open in response to increased lateral tension in the plasma membrane during osmotic swelling (Fig.1c). Similar ion-channels are present in all organisms and are essential for survival under changing osmotic conditions29.
There is also evidence that unfolding of protein domains under tension mediates responses to applied forces. The first reported instance was fibronectin (FN), which self-assembles into fibrils in the ECM. Formation of FN fibrils requires cell-generated force30. Conversely, purified FN undergoes self-association when stretched in vitro31,32; fibril assembly is mediated by unfolding of domains revealing cryptic binding sites. Another well studied example is talin-1, which connects integrins to F-actin, thereby transmitting forces between actomyosin filaments and the ECM33. Talin-1 binds vinculin, which also links to F-actin and is recruited to adhesions in response to applied forces. Curiously, many of the vinculin binding sites on talin reside within the interior of bundles comprised of 4–5 α-helices, thus, are inaccessible33. Both biochemical and cellular studies provide evidence that tension unfolds these bundles to expose vinculin binding sites, thereby enabling vinculin recruitment34,35 (Fig.1c). Another protein in the cytoplasmic region of integrin-mediated adhesions is the adapter protein p130Cas. When phosphorylated on tyrosines by src family kinases, p130Cas binds several guanine nucleotide exchange factors (GEFs) that activate small GTPases36. Stretching cells enhances phosphorylation of these tyrosines, leading to GEF binding and Rap1 activation37–40. Studies with purified proteins showed that stretching increases the susceptibility of p130Cas to phosphorylation without changing the intrinsic activity of src family kinases40. Although it is unclear how forces might be transmitted across p130Cas in vivo, these studies illustrate the concept that forces can affect substrate availability through effects on protein conformation.
For physiologically significant mechanosensing, these initial conformation changes must be followed by a second step in which the new conformation triggers downstream events. This step can be fairly direct, as for the mechanosensitive ion channels discussed above. In other instances, changes in protein conformation, especially the opening of domains that contain cryptic sites, leads to binding of new proteins that mediate downstream events. Possibilities include reinforcement of the linkage, as in the case of talin and vinculin34, or the recruitment and activation of new signaling proteins, as in the case of p130Cas40. This general sort of model has been invoked in a wide range of mechanotransduction events in many systems1. Understanding in detail how protein domains change conformation under force and how subsequent events transpire has been the major direction in this field.
Mechanoresponse
Ultimately, sensed mechanical signals influence information processing through complex cellular signaling and transcriptional networks that are not specifically force-dependent. In many cases, these responses feed back to alter the mechanosensitive structures that initiated the responses. Both integrin- and cadherin-mediated adhesions enlarge and strengthen in response to tension19. Distinct from the very rapid, direct recruitment described above, signaling pathways that occur over minutes (e.g. activation of the small GTPase RhoA stimulates formation of actin stress fibers41) and gene expression pathways that operate over hours or days (e.g. induction of vinculin through serum response factor42,43) change the composition and structure of adhesions and the cytoskeleton.
Similar principles apply at the tissue level. High blood pressure, for instance, results in thickening of artery walls to bear the increased tension, and hypertrophy of the left ventricle of the heart to enable stronger pumping against high back pressure44. Analogously, bone deposition increases under weight-bearing exercise1. These integrated responses depend on the intensity and time course of stimulation in ways that differ from the initial responses. For example, a single, brief interval of high blood pressure during exercise will stretch vessel walls and tax the heart but does not trigger compensatory arterial and cardiac remodeling as does sustained hypertension44.
Limitations of Switch-like Models
In switch-like models of mechanotransduction, applied forces are instantaneously transmitted to load-bearing sub-cellular structures and induce conformation changes in mechanosensitive proteins. Different forces are sensed largely by conformation changes in protein domains that are stronger or weaker, and thus respond to forces of different magnitudes45. This view, however, appears incomplete. For example, the frequency of applied cyclic stretch or compression can have major effects. Steady stretch and cyclic stretch of equivalent magnitude induce distinct genes in endothelial cells46 and induce differential phosphorylation of sites on FAK in rabbit aortas stretched ex vivo47. The frequency of applied cyclic stretch also determines endothelial alignment48. In aortic smooth muscle cells, stimulation of integrin activation and subsequent cellular alignment by cyclic stretch depends strongly on stretch duration and frequency49.
These observations are particularly relevant in the vascular system, where shear stress and circumferential stretch in arteries undergo strong, time-dependent variations during the cardiac cycle. Furthermore, these variations are different in various parts of the vasculature50 and correlate with location of atherosclerotic lesion formation4. Interestingly, recent evidence demonstrates that particular frequencies of mechanical stimulation preferentially activate the inflammatory pathways implicated in atherosclerosis, even when the total power applied to the cell is conserved (Feaver, et. al, submitted). The characteristic time between the peaks in wall stretch and fluid shear stress may also critically regulate endothelial activation51. These characteristics of mechanotransduction are not readily captured by switch-like models.
Sub-cellular Structures Are Dynamic
These time-dependent aspects of mechanotransduction can be attributed to the highly dynamic characteristics of the cellular components that bear and respond to force. Cell adhesions to the ECM go through a complicated, force-sensitive maturation process41. Nascent cell-ECM adhesions are very small structures (<1μm in diameter) at the edges of lamellipodia that usually disassemble within tens of seconds, or else mature into slightly larger focal complexes (FCs) that persist for only minutes41. A fraction of these FCs mature into larger focal adhesions (FAs) that persist for tens of minutes. However, even within stable FAs, proteins constantly exchange, with lifetimes from tens of seconds to at most a few minutes52. Thus, even stationary FAs undergo rapid internal dynamics.
Detailed analyses from fluorescence speckle microscopy53 and other high resolution techniques54 have revealed complex interplay between cytoskeletal and FA dynamics. Actin filaments constantly polymerize at the leading edge of the cell and flow backwards over the FAs, with the speed of this flow influenced by the nature of the linkages between the cytoskeleton, the integrins and the ECM. Actin flow is faster over areas with few FAs or where the FAs undergo treadmilling toward the center of the cell (so-called sliding FAs); whereas actin flow is slower in areas with stable FAs53. These and other results55 led to the notion of a “clutch” that controls force transmission between the flowing actin and the integrins (Fig 2). Furthermore, in areas with stable FAs, integrins are immobile and actin flows at 0.1–0.2 μm/min; however, several FA proteins display velocities between these limits53. These results indicate the presence of multiple proteins that act as clutches, or force-sensitive linkages, within FAs.
Figure 2. The focal adhesion clutch.
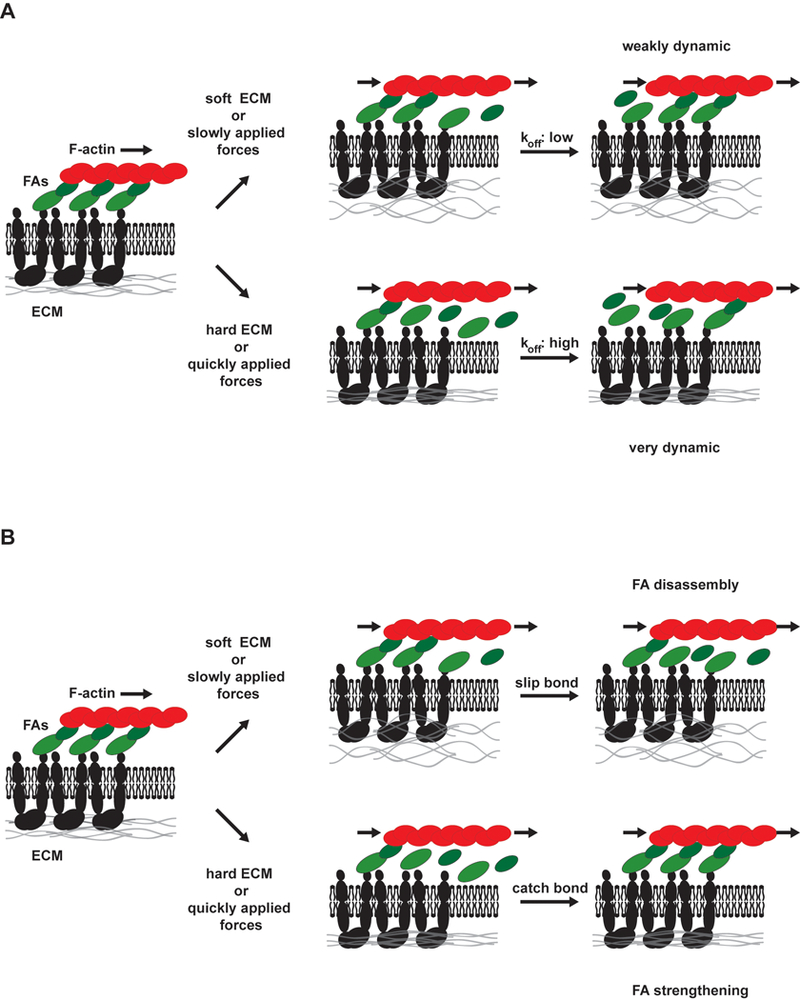
Due to forces from actin polymerization and myosin-dependent contractility, actin filaments flow rearward over FAs toward the nucleus. Through FA proteins that link actin to integrins, force is applied to the ECM. Force-dependent changes in FA exchange rates (represented as green arrows, see Box 1 for details) alter dynamics and size of FAs. On soft surfaces or where external force is applied slowly, on- and off-rates are moderately fast and FAs have moderate lifetimes. However, on stiff surfaces or when external forces are applied quickly, distinct behaviors can be observed. In the absence of FA strengthening, molecular linker dissociation rates increase (slip bond behavior). However, these proteins are sometimes replaced through re-binding, resulting in large exchange rates and short-lived FAs. FA strengthening is associated with catch bonds that slow FA protein dissociation under force, with exposure of cryptic binding sites to recruit new proteins that reinforce the adhesion, and with conformational changes in FA proteins that activate signaling pathways to recruit additional molecular linkers. Any way, large, long-lived FAs are the result.
While cadherin-dependent adhesions have not been studied in as much detail, available evidence indicates similarities to FAs2. Several studies have shown that cell-cell contacts bear significant forces56,57 and, like FAs, they undergo dynamic, myosin-dependent elongation58. Applied forces56 and stiffer substrata59 enhance cell-cell contact assembly indicating that these adhesions also exhibit force-dependent adhesion strengthening. There is also evidence for actin flow along cell-cell contacts60. While most of the molecular players are different, vinculin is recruited to both structures in a myosin-dependent manner, which contributes to adhesion strengthening61,62. These data suggest that the dynamic properties of cell-cell contacts are regulated by processes physically similar to FA regulation.
Single Molecule Response to Dynamic Forces
Studies of single molecules or pairs of molecules under applied force have rarely revealed simple, switch-like behaviors. Of particular relevance, recent work on the effects of force application on the rates of bond dissociation shows that bonds mediating protein-protein interactions can either decrease or increase their average lifetime in response to applied force, referred to as slip or catch bonds, respectively63,64 (Box 1). Importantly, both the strength of the force and the rate of application affect the rates of protein conformation changes63,65 (Box 1). The development of assays involving several molecules (i.e. cross-linking protein adhered to F-actin) have revealed competition between force-activated unbinding and conformation changes66 (Box 1). A current challenge is determining how these molecular processes are integrated to mediate complex phenomenon like mechanotransduction.
Box 1: Physics of Force-activated Bond Dissociation
The non-covalent bonds that mediate protein-protein interactions have finite lifetimes, ranging from milliseconds to days63. Applied force typically shortens the lifetime of a bond (like applying force to remove tape). In a molecular context, these are referred to as slip bonds63,64. There are also molecules whose bond lifetimes increase, although not infinitely so, in response to applied forces (like the “finger trap” children’s toy)64. These are called catch bonds and, though relatively rare, are often found in critical cytoskeletal and cellular adhesion structures.
Protein conformation changes can be understood as a type of slip bond where the dissociation is internal and due to non-covalent bonds between amino acids in a single protein instead of between two proteins63,65. Moreover, both processes can be represented in terms of an energy landscape where two energy minima are separated by a high energy state that slows the rate of transition. Applied force acts both “catalytically” to accelerate rates by lowering the energy requirement for the transition, and by changing the free energy of the states, typically stabilizing the more open conformation or unbound conformations. Thus, the likelihood of a conformation change depends on both the magnitude of the force and its duration63. These conformation changes mediate subsequent mechanotransduction events by revealing binding sites for signaling and cytoskeletal proteins. In the diagram, boxes represent binding sites, while circles represent proteins. Also red denotes signaling proteins, while blue represents cytoskeletal proteins.
Furthermore, the bonds mediating protein conformations and protein-proteins interactions tend to have similar affinities and force sensitivities. As protein dissociation will terminate tension application, these processes will effectively compete. This process was recently studied using a single molecule system that contained both a dissociable bond and a protein that could undergo force-dependent conformational opening66. Protein dissociation and conformational changes were both observed, but, interestingly, the frequency of conformational changes was enhanced at higher loading rate rates.
Box 2: Mechanical Properties of the Cytoskeleton
Materials such as rubber or polyacrylamide gels are elastic solids, meaning they deform rapidly in response to applied stress, but spring back to their original shape when the stress ends. By contrast, liquids like water or honey flow in response to applied stress such that deformation increases irreversibly and linearly with time. Many materials, including cells77 and in vitro mixtures of cytoskeletal components20, show behaviors between these two limits and are referred to as viscoelastic. Their behavior more closely resembles elastic materials on short time scales and viscous liquids on long time scales. A common example is silly putty, which flows like liquid when slowly squeezed but bounces like an elastic ball when thrown against the floor. On a molecular level, viscoelasticity is due to the presence of stress relaxation, usually through bond dissociation. At times shorter than the dissociation time, stress cannot be relaxed and the materials acts like an elastic solid, while on longer times, the bonds dissociate and the materials flow. The details of viscoelasticity in cytoskeletal networks are still controversial, but it is likely that dissociation of or conformational changes in cytoskeletal cross-linking proteins are involved77.
Models of Dynamic FAs
FAs offer a convenient system for understanding the relationship between dynamics and mechanotransduction that may be more generally applicable. From a mechanical perspective, FAs are dynamic, deformable links between an elastic ECM and the force-generating actin cytoskeleton13,41. Myosin-generated forces are transmitted through the actin cytoskeleton to FA proteins. The applied forces affect the dissociation rates of the FA proteins from integrin receptors, each other, and F-actin. Forces are then transmitted through bound molecules to deform the ECM (Fig. 2).
Several models that address how cells respond to the mechanical properties of the ECM propose that rigidity determines how quickly forces act upon the integrin-actin linkages13,67,68. The models can be classified based on whether rapidly applied forces increase or decrease adhesion turnover by promoting adhesion breakage or strengthening. In all cases, FA kinetics are determined by the balance of protein association and dissociation. On soft substrates or in response to slowly applied forces, the on and off rates of molecules into and out of the adhesions, and the lifetimes of the whole adhesions are moderately fast68 (Fig. 2). In models without FA strengthening, exposing cells to large, rapidly applied forces or plating cells on rigid substrata increases dissociation of linker molecules like vinculin or talin from the integrin or the actin (slip bond behavior). However, with large numbers of unoccupied sites, there is also rapid rebinding. This model leads to FAs with faster exchange of linker molecules and shorter whole adhesions lifetimes (Fig. 2)68. In models with FA strengthening, applied force either results in decreased protein dissociation (catch bond behavior) and/or a conformation change that induces new protein recruitment67,69. Either way, force leads to large, reinforced FAs with slower exchange rates for linker molecules and longer lifetimes (Fig 2).
Interestingly, FA dynamics consistent with both classes of models have been observed53,68,70. In some cases, the difference is cell type-dependent. However, there is also evidence for spatial specificity within single cells, such that FA strengthening is restricted to the front of migrating cells71. This result makes intuitive sense since, if adhesions always strengthened under force, cells could not migrate. A polarized mechanism that strengthens adhesions at the front while allowing those at the rear to break under tension will produce forward movement when the cell contracts.
The polarized signaling pathways that determine whether adhesions strengthen or weaken under force are unknown. However, a recent study using a novel biosensor that reports the tension across the FA protein vinculin shed some light on the mechanism. It showed that vinculin is under high tension in FAs that assemble, whereas it is under low tension in FAs that disassemble under cellular contractile force in migrating cells72. Furthermore, vinculin is required for adhesion strengthening under force. These results suggest that the pathways that determine adhesion strengthening vs. weakening under force regulate whether the force is transmitted across vinculin or across other linkages.
A Dynamic Model of Mechanotransduction
The examples listed above suggest that a dynamic treatment of mechanotransduction is necessary. A key concept is that applied forces can regulate the rates of biochemically detectable processes, such as protein unbinding and protein conformational changes. While switch-like models emphasize the serial nature of the steps of mechanotransduction, a dynamic model reveals a more integrated picture where mechanotransmission, mechanotransduction, and mechanoresponse are intimately related and capable of affecting each other.
Dynamic Mechanotransmission
Broken linkages cannot transmit forces. Thus, the stability of the load-bearing sub-cellular structures dictates paths of force transmission and their duration. On short times (sub-second to tens of seconds), mechanotransmission is governed by the physics of force-activated bond dissociation63,64. While some cellular structures may simply be strong enough to bear the relevant forces, others will not. For example, bonds between actin and its cross linking protein α-actinin are slip bonds, and other calponin homology domain actin binding proteins are likely to behave similarly73. Other critical linkages in mechanotransduction, such as fibronectin-integrin (α5β1)74,75 and actin-myosin bonds76, show catch bond behavior. These considerations suggest that only certain dynamic, sub-cellular structures may be stabilized in response to applied force to allow force transmission to mechanosensitive areas or molecules.
On larger length scales, the dynamic nature of cytoskeletal protein-protein bonds directly leads to viscoelasticity77 (Box 2). Applied forces can result in either re-enforcement23 or fluidization78 of the cytoskeleton. While the exact mechanisms are still debated, re-enforcement is associated with maintenance of physical linkages, stiffening of the actin network and increased cell contractility23,79. Fluidization involves disruption of the cytoskeleton, either from breakage of mechanical linkages80,81 or force induced, biochemically controlled disassembly82. In terms of mechanotransmission, these properties are extremely important as forces will be propagated along re-enforced, elastic filaments, but quickly dissipate in a fluidized, viscous environment. Furthermore, viscoelastic effects can enable certain frequencies of mechanical stimulus to be selectively transmitted over greater distance in cells83. The efficiency of transmission for different frequencies is determined by the rates of bond dissociation that cause cellular viscoelasticity. These effects have been observed in force-induced movements of FAs84 and mitochondria85. Mechanical stimuli containing frequencies that are transmitted efficiently are likely to promote greater mechanoresponses.
Dynamic Mechanosensing
The basis of mechanosensing is thought to be force-sensitive changes in the rates of conversion between different protein conformations. These transitions depend on the strength and duration of force application (Box 1). For instance, when forces are applied to talin, 50 pN induces conformational changes within 25 ms, whereas at 20 pN, the same changes require 200 ms34. For successful mechanosensing, forces must be transmitted for sufficient time to induce conformational changes and subsequent biochemical detection. However, force also accelerates slip bond breakage, which will terminate the force transmission. Thus, there is competition between conformational change and bond breakage. Transmission pathways with catch bonds will therefore be more sensitive.
The rate at which forces are applied influences force transmission and subsequent signaling. The rate of force application through F-actin to the actin cross-linking proteins α-actinin or filamin determined the relative frequency of dissociation of the actin-linker bonds versus conformational changes66. Conformational changes are more likely to occur at higher rates of force application. Other experiments showed that fibronectin coated beads are less likely to dissociate from integrins when forces are applied quickly86. The critical effect of rate of force application underscores the importance of these dynamic aspects of mechanotransduction
Dynamic Mechanoresponse
The downstream mechanoresponse pathways are not innately force sensitive but often regulate cytoskeletal and adhesion structures that therefore feed back to influence mechanotransduction. The cytoskeletal protein zyxin, for instance, specifically localizes to areas of strain-induced stress fiber thinning, and recruits α−actinin and VASP, which promote actin polymerization and stress fiber repair87,88. The MAL/serum response factor pathway is activated by actin polymerization in response to force or other stimuli, and regulates expression of numerous cytoskeletal genes including vinculin, filamin and actin42,43. Cyclic strain also enhances the expression of ECM proteins and induces the assembly of ECM structures89. Thus, on time scales on the order of minutes to days, cells use signaling or transcriptional programs to alter or maintain force transmission pathways.
Cell alignment in response to applied force is a form of adaptation that involves local regulation of dynamic cytoskeletal elements, largely through the regulation of Rho family GTPases90. In two-dimensional cultures, uniaxial static stretch (“stretch and hold”) induces actin stress fiber and FA alignment parallel to the applied force, consistent with the general notion of adhesion strengthening90. By contrast, cyclic stretch induces alignment perpendicular to the applied force91 in a frequency-dependent manner49.
A mathematical model recently proposed that forces applied faster than the characteristic rates of remodeling in load bearing sub-cellular structures induce cell alignment perpendicular to the direction of strain to minimize stretching of these elements92. By contrast, when forces are applied more slowly than the remodeling rate, cells can internally remodel and align parallel to applied stress. This concept may also explain a fascinating effect in which inhibiting Rho kinase or mDia shifts the direction that cells align under cyclic stretch from perpendicular to parallel93. Inhibiting Rho signaling also is known to deplete cells of stable FAs and stress fibers, resulting in more dynamic sub-cellular structures41. The switch in direction of alignment may be explained if the higher cytoskeletal remodeling rate now exceeds the characteristic rate of the cyclic stretch, which, according to the mathematical model, would yield alignment in the direction of strain. This model provides insight into how the internal dynamics of the cytoskeleton can determine responses to dynamic mechanical stimuli.
Adhesion Strengthening and Rigidity Sensing
The principles enumerated above can provide at least a first explanation for how cells sense the mechanical properties of their substrata (Fig. 2). A key point is that forces on ECM-integrin-cytoskeletal linkages build up more rapidly in cells on rigid than on compliant surfaces67,68. These more rapidly applied forces are more effective at triggering conformational changes in cytoskeletal proteins rather than bond dissociation66. Additionally, if these linkages contain catch bonds, whose conversion to high affinity state is predicted to be enhanced at high loading rates94, they will be further stabilized. As a result, mechanotransmission will be more efficient and longer lived. As a consequence of domain unfolding under force, additional proteins are recruited to support critical linkages. Adhesion strengthening will then occur through the mechanisms we describe above.
Sensing Dynamic, Applied Forces
One set of physiologically important mechanotransduction events involves the stretching of artery walls by blood pressure. Hypertension increases static stretch while cyclic pumping during the cardiac cycle causes time-varying stretch4, both of which lead to transmission of forces to vascular cells and activation of multiple signaling pathways95. However, cyclic stretch and static stretch of the same amplitude (and likely similar force application rates) induce both common and unique mechanoresponses46. For example, 10% cyclic stretch of endothelial cells increases expression of VEGF receptor (VEGFR)2 and the angiopoietin receptor Tie-2, but not VEGFR1. By contrast, 10% static stretch increases expression of VEGFR2 and VEGFR1, but not Tie-246. We propose that these various responses can be explained by the dynamic processes intrinsic to the FAs and the cytoskeleton that sense the applied force (Fig. 3). Responses selectively activated by static stretch are likely mediated by protein conformational changes in strong proteins in relatively stable structures, requiring long applications of force to unfold. Responses selectively activated by cyclic stretch likely involve weaker proteins in dynamic structures that adapt to the statically applied forces but are constantly stimulated by dynamic signals. Responses that are activated by both are likely mediated by weak proteins in relatively stable structures.
Figure 3. Dynamic aspects of mechanotransduction.
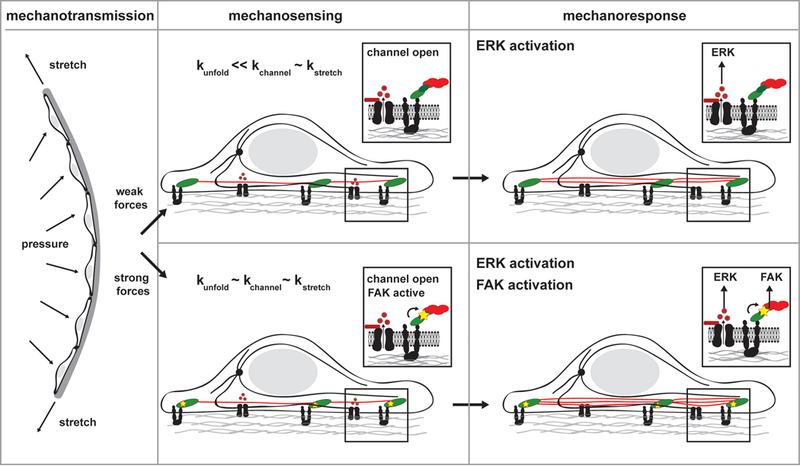
The endothelial cells that line blood vessel walls are subject to both static and cyclic stretch. Even when the signal strengths are matched, dynamically distinct mechanical stimuli can activate common or unique signaling pathways based on the strength of the proteins, specifically resistance to conformation changes, and the dynamic nature of the structures bearing load. In dynamic structures, like nascent adhesions, and stable structures, like mature adhesions and stress fibers, cyclic stretch does not apply forces for sufficient time to induce conformation changes in strong proteins, but weak proteins will signal. In response to static stretch dynamic structures can readily adapt, and there is no long term signaling. In stable structures the long force application causes conformational changes in both weak and strong proteins. Thus signaling pathways that are preferentially activated by cyclic stretch are likely induced by weak proteins that localize exclusively to dynamic structures. Pathways selectively activated by static stretch are expected to contain mechanosensors that are strong proteins in stable structures. And pathways activated by both types of signals are likely due to weak proteins that localize to both dynamic and stable structures. Stars indicate active proteins.
Future Perspectives
The ability of mechanical perturbations to influence cellular signaling in a frequency-dependent manner can be conceptualized with mechanotransducers that function as bandpass filters, which selectively transmit specific frequencies. The ability of the cytoskeleton to selectively transmit certain frequencies of mechanical stimuli to sub-cellular structures provides one such mechanism83–85. Mechanosensitive elements and mechanoresponse pathways are also rate and frequency sensitive due to their own intrinsic time scales. In this view, the time scale of the applied force must match the critical time scale of a given signaling process in order to affect it. Stimuli that change too quickly are simply averaged, whereas stimuli that vary too slowly are not detected at all. Knowledge of the dynamics of cellular signaling processes could therefore facilitate understanding frequency-dependent cell and tissue responses. As cells contain multiple mechanically sensitive biochemical signaling pathways with a wide variation of critical time scales, they may therefore act as a multi-band pass filters, which pass several ranges of frequencies. These systems would allow cells to distinguish stimuli based on their frequencies or time scales.
Our understanding of mechanical signaling is still slim compared to our understanding of signaling by hormones and growth factors. But the more we learn, the more it seems that mechanical forces can play subtle and precise roles in governing morphogenesis, physiology and disease. We propose that just as conventional signals from soluble regulators act together in regulatory networks where complex temporal and spatial characteristics determine outputs, so mechanical stresses may also convey large amounts of information through precise time and force-dependent modulation. For periodic stimuli, this will take the form of frequency and amplitude features that determine cellular outputs. Elucidating the dynamics of cellular mechanotransduction systems holds the key to understanding these mechanisms.
Footnotes
Author Information: The authors declare no competing financial interests.
References
- 1.Orr AW, Helmke BP, Blackman BR, and Schwartz MA Mechanisms of mechanotransduction. Dev Cell 10, 11 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Chen CS Mechanotransduction - a field pulling together? J Cell Sci 121, 3285 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Geiger B, Spatz JP, and Bershadsky AD Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10, 21 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Hahn C and Schwartz MA Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10, 53 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucitti JL et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yashiro K, Shiratori H, and Hamada H Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450, 285 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Discher DE, Janmey P, and Wang YL Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Levental KR et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190, 693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engler AJ, Sen S, Sweeney HL, and Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Gilbert PM et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dado D and Levenberg S Cell-scaffold mechanical interplay within engineered tissue. Semin Cell Dev Biol 20, 656 (2009). [DOI] [PubMed] [Google Scholar]
- 13.De R, Zemel A, and Safran SA Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol 98, 143 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Saratzis A et al. Abdominal aortic aneurysm: a review of the genetic basis. Angiology 62, 18 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Hershberger RE, Morales A, and Siegfried JD Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genet Med 12, 655 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekharan K and Martin PT Genetic defects in muscular dystrophy. Methods Enzymol 479, 291 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Laurent S, Boutouyrie P, and Lacolley P Structural and genetic bases of arterial stiffness. Hypertension 45, 1050 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Yu H, Mouw JK, and Weaver VM Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol 21, 47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz MA and DeSimone DW Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol 20, 551 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardel ML, Kasza KE, Brangwynne CP, Liu J, and Weitz DA Chapter 19: Mechanical response of cytoskeletal networks. Methods Cell Biol 89, 487 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmke BP, Rosen AB, and Davies PF Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J 84, 2691 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzima E et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426 (2005).Identifies a critical complex, consisting of PECAM1, VE-cadherin and VEGFR2, in the pathway leading to integrin activation by shear flow.
- 23.Matthews BD, Overby DR, Mannix R, and Ingber DE Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 119, 508 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Na S et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A 105, 6626 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N and Stamenovic D Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol 279, C188 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Hayakawa K, Tatsumi H, and Sokabe M Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci 121, 496 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Poh YC et al. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One 4, e7886 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sukharev S, Betanzos M, Chiang CS, and Guy HR The gating mechanism of the large mechanosensitive channel MscL. Nature 409, 720 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Arnadottir J and Chalfie M Eukaryotic mechanosensitive channels. Annu Rev Biophys 39, 111 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Zhong C et al. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141, 539 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, and Fernandez JM The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol 319, 433 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Smith ML et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 5, e268 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler WH, Gingras AR, Critchley DR, and Emsley J Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans 36, 235 (2008). [DOI] [PubMed] [Google Scholar]
- 34.del Rio A et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638 (2009).Demonstrates force-induced binding of vinculin to cryptic sites in talin at the single molecule level.
- 35.Zhang X et al. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol 10, 1062 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Defilippi P, Di Stefano P, and Cabodi S p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 16, 257 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Tamada M, Sheetz MP, and Sawada Y Activation of a signaling cascade by cytoskeleton stretch. Dev Cell 7, 709 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Sawada Y et al. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci 114, 1221 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Sawada Y and Sheetz MP Force transduction by Triton cytoskeletons. J Cell Biol 156, 609 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawada Y et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015 (2006).Shows that stretching of p130Cas leads to the exposure of tyrosines that can be phosphorylated to effect signaling pathways.
- 41.Parsons JT, Horwitz AR, and Schwartz MA Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11, 633 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asparuhova MB, Gelman L, and Chiquet M Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand J Med Sci Sports 19, 490 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Olson EN and Nordheim A Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol 11, 353 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallion JM, Baguet JP, Siche JP, Tremel F, and De Gaudemaris R Left ventricular hypertrophy and arterial hypertrophy. Adv Exp Med Biol 432, 123 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Vogel V Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct 35, 459 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Zheng W, Christensen LP, and Tomanek RJ Differential effects of cyclic and static stretch on coronary microvascular endothelial cell receptors and vasculogenic/angiogenic responses. Am J Physiol Heart Circ Physiol 295, H794 (2008).Providences evidence that statically and dynamically applied stretches lead to the activation of distinct pathways in stretched endothelial cells.
- 47.Lehoux S, Esposito B, Merval R, and Tedgui A Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation 111, 643 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Hsu HJ, Lee CF, and Kaunas R A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One 4, e4853 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B et al. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys J 94, 1497 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelfand BD, Epstein FH, and Blackman BR Spatial and spectral heterogeneity of time-varying shear stress profiles in the carotid bifurcation by phase-contrast MRI. J Magn Reson Imaging 24, 1386 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Dancu MB and Tarbell JM Large Negative Stress Phase Angle (SPA) attenuates nitric oxide production in bovine aortic endothelial cells. J Biomech Eng 128, 329 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Wehrle-Haller B Analysis of integrin dynamics by fluorescence recovery after photobleaching. Methods Mol Biol 370, 173 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Hu K, Ji L, Applegate KT, Danuser G, and Waterman-Storer CM Differential transmission of actin motion within focal adhesions. Science 315, 111 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Brown CM et al. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci 119, 5204 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Maruthamuthu V, Aratyn-Schaus Y, and Gardel ML Conserved F-actin dynamics and force transmission at cell adhesions. Curr Opin Cell Biol 22, 583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Z et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 107, 9944 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruthamuthu V, Sabass B, Schwarz US, and Gardel ML Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A 108, 4708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mege RM, Gavard J, and Lambert M Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol 18, 541 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Ladoux B et al. Strength dependence of cadherin-mediated adhesions. Biophys J 98, 534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kametani Y and Takeichi M Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol 9, 92 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Riveline D et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153, 1175 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.le Duc Q et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 189, 1107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans EA and Calderwood DA Forces and bond dynamics in cell adhesion. Science 316, 1148 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Thomas WE, Vogel V, and Sokurenko E Biophysics of catch bonds. Annual Review of Biophysics 37, 399 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Bustamante C, Chemla YR, Forde NR, and Izhaky D Mechanical processes in biochemistry. Annu Rev Biochem 73, 705 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Ferrer JM et al. Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc Natl Acad Sci U S A 105, 9221 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruinsma R Theory of force regulation by nascent adhesion sites. Biophys J 89, 87 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan CE and Odde DJ Traction dynamics of filopodia on compliant substrates. Science 322, 1687 (2008).Proposes and validates a model describing rigidity-sensitive FA dynamics in terms of force-activated protein dissociation.
- 69.Li Y, Bhimalapuram P, and Dinner AR Model for how retrograde actin flow regulates adhesion traction stresses. Journal of Physics-Condensed Matter 22 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Gardel ML et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol 183, 999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt CE, Horwitz AF, Lauffenburger DA, and Sheetz MP Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol 123, 977 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grashoff C et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263 (2010).Reports a biosensor that measures forces across specific proteins in dynamic FAs and shows that molecular tension across vinculin correlates with FA strengthening.
- 73.Miyata H, Yasuda R, and Kinosita K Jr. Strength and lifetime of the bond between actin and skeletal muscle alpha-actinin studied with an optical trapping technique. Biochim Biophys Acta 1290, 83 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Kong F, Garcia AJ, Mould AP, Humphries MJ, and Zhu C Demonstration of catch bonds between an integrin and its ligand. J Cell Biol 185, 1275 (2009).Demonstrates the linkage between α5β1 integrin and fibronectin acts like a catch bond at the single molecule level.
- 75.Friedland JC, Lee MH, and Boettiger D Mechanically activated integrin switch controls alpha5beta1 function. Science 323, 642 (2009).Shows that force and increased extracellular rigidity switch α5β1 integrin between a relaxed and a tensioned state that is required for mechanically induced FAK signaling.
- 76.Guo B and Guilford WH Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc Natl Acad Sci U S A 103, 9844 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoffman BD and Crocker JC Cell mechanics: dissecting the physical responses of cells to force. Annu Rev Biomed Eng 11, 259 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Trepat X et al. Universal physical responses to stretch in the living cell. Nature 447, 592 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardel ML et al. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci U S A 103, 1762 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee H, Ferrer JM, Lang MJ, and Kamm RD Molecular origin of strain softening in cross-linked F-actin networks. Phys Rev E Stat Nonlin Soft Matter Phys 82, 011919 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaudhuri O, Parekh SH, and Fletcher DA Reversible stress softening of actin networks. Nature 445, 295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C et al. Fluidization and resolidification of the human bladder smooth muscle cell in response to transient stretch. PLoS One 5, e12035 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shafrir Y and Forgacs G Mechanotransduction through the cytoskeleton. Am J Physiol Cell Physiol 282, C479 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Mack PJ, Kaazempur-Mofrad MR, Karcher H, Lee RT, and Kamm RD Force-induced focal adhesion translocation: effects of force amplitude and frequency. Am J Physiol Cell Physiol 287, C954 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Hu S and Wang N Control of stress propagation in the cytoplasm by prestress and loading frequency. Mol Cell Biomech 3, 49 (2006). [PubMed] [Google Scholar]
- 86.Jiang G, Huang AH, Cai Y, Tanase M, and Sheetz MP Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys J 90, 1804 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith MA et al. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev Cell 19, 365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wojtowicz A et al. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res 107, 898 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Chiquet M, Gelman L, Lutz R, and Maier S From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta 1793, 911 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Katsumi A et al. Effects of cell tension on the small GTPase Rac. J Cell Biol 158, 153 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanda K and Matsuda T Behavior of Arterial-Wall Cells Cultured on Periodically Stretched Substrates. Cell Transplantation 2, 475 (1993). [DOI] [PubMed] [Google Scholar]
- 92.De R, Zemel A, and Safran SA Dynamics of cell orientation. Nature Physics 3, 655 (2007).A theory-based study suggesting how cytoskeletal dynamics affects the ability of cells to align to dynamically applied stretches.
- 93.Kaunas R, Nguyen P, Usami S, and Chien S Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A 102, 15895 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prezhdo OV and Pereverzev YV Theoretical aspects of the biological catch bond. Acc Chem Res 42, 693 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Haga JH, Li YS, and Chien S Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 40, 947 (2007). [DOI] [PubMed] [Google Scholar]


