Abstract
We report a 29-year old nulliparous woman diagnosed with a grade 1 endometrioid adenocarcinoma of the endometrium arising from an atypical polypoid adenomyoma, while being investigated for a suspected threatened miscarriage at 7 weeks gestation. She presented complaining of vaginal bleeding and a small amount of soft tissue in the cervical os was found and sent for histology. An ultrasound scan was performed, which confirmed an intrauterine ongoing pregnancy. The patient had no further episodes of unscheduled bleeding. After the confirmed histological diagnosis an MRI scan was requested, and there were no evidence of myometrial invasion or distant metastasis. The patient was seen at each trimester, remained asymptomatic throughout the pregnancy and had a normal delivery at term. There was no evidence of any residual endometrioid adenocarcinoma in the post-delivery specimen. Six weeks post-natally an endometrial biopsy was performed, which was normal. She is still in remission over a period of 8 years follow-up. Endometrial adenocarcinoma in young pregnant women is a rare clinical circumstance. This case shows that conservative management in young women is possible including in a case of an incidental diagnosis in pregnancy.
Keywords: Endometrial cancer, Fertility sparing-surgery, Hormonal treatment
Highlights
-
•
An example of a successful full term pregnancy in women with early stage endometrial cancer
-
•
Conservative management of endometrial cancer in early pregnancy can be considered in young women wishing to keep fertility.
-
•
Long-term use of progestogen therapy can be safely used to treat early stage endometrial cancer.
1. Introduction
Endometrial carcinoma (EC), in only 25% is in premenopausal women, and among those 3–5% are <40 years of age (Pennant et al., 2016). It is rarely found during pregnancy (Saciragic et al., 2014). The standard therapy of EC is surgery involving total hysterectomy with or without bilateral salpingo-oophorectomy. However, as EC in young women is often well-differentiated, and with minimal or absent myometrial invasion at the time of diagnosis, a feasible alternative for treatment is conservative management with medical hormonal therapy (Gunderson et al., 2012). Conservative treatment is associated with a 55–80% initial complete response, but 50% of the patients relapse over time. To our knowledge, there have been thirty-six cases of EC diagnosed in early pregnancy since the first described by Schumann in 1927 (Schumann, 1927), however none of those ended with a full term pregnancy. In most of these cases, the diagnosis was made at the histology obtained after surgical treatment of miscarriage (Zhou et al., 2015; Yael et al., 2009). We reported a case of grade I endometrioid adenocarcinoma arising within an atypical polypoid adenomyoma (APA), incidentally diagnosed in a patient while investigated for threatened miscarriage. The patient was successfully treated conservatively and had a full term pregnancy.
2. Case report
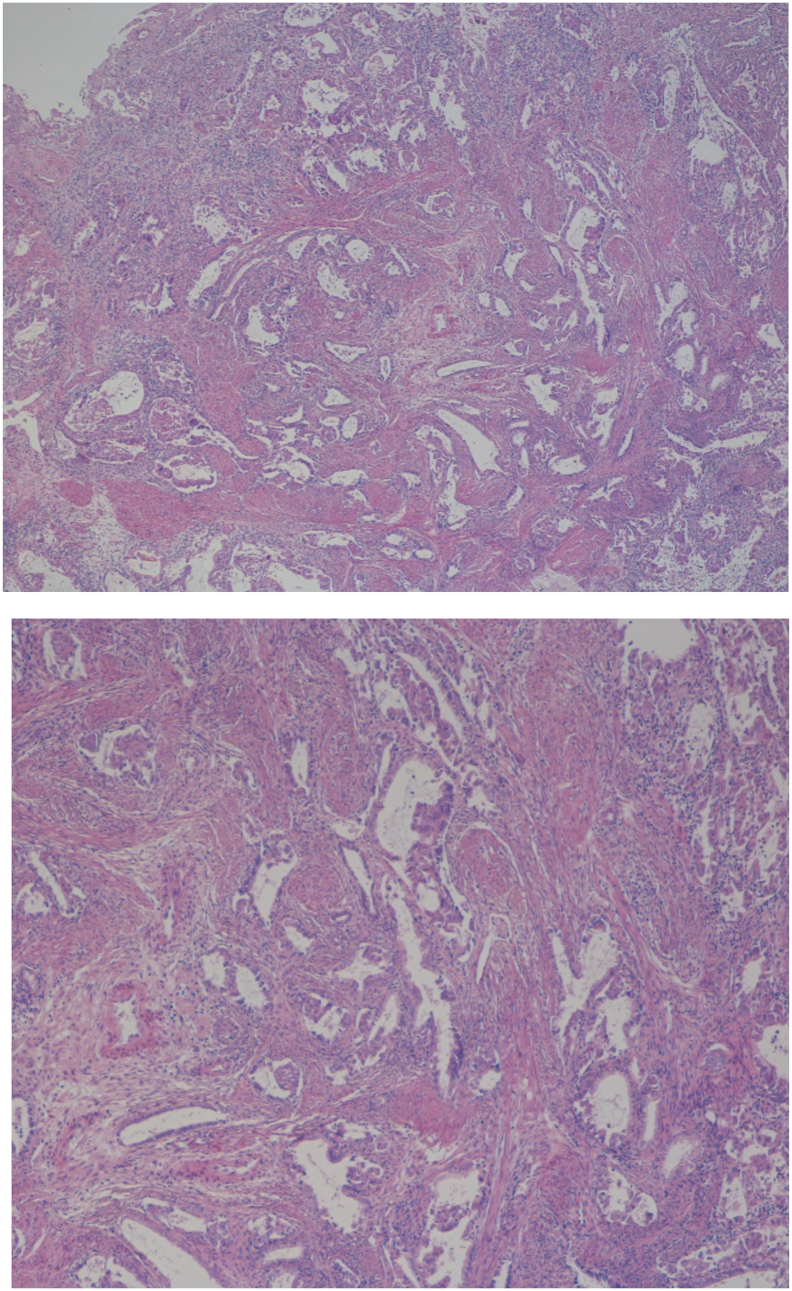
This is a case report of a 29-year-old nulliparous woman presented at the emergency gynaecological unit of the Ipswich Hospital at 7 weeks gestation with symptoms of threatened miscarriage. She was found to have a small amount of soft tissue in the cervical os, which was removed and sent for histology. The final histologic analysis of a paraffin section showed a grade 1 endometrioid adenocarcinoma arising from an APA (Fig. 1, Fig. 2). A further opinion from two other institutions was attained and the diagnosis was confirmed. After that first episode of vaginal bleeding, an ultrasound scan was performed, which confirmed an intrauterine pregnancy of 7 weeks gestation. The patient had no further episodes of unscheduled bleeding. After the confirmed histological diagnosis an MRI scan was requested, and the radiologist could not identify any evidence of myometrial invasion or distant metastasis (Fig. 3). As background history, the patient was nulliparous, with a BMI of 30, no diabetes and no features of polycystic ovarian syndrome. She had no family history of EC or colorectal cancer, but there was a strong family history of breast cancer. The treatment of EC was explained to the patient and the options offered with the view of balancing foetal and maternal outcome. She was counseled about the possibility that in the case of going ahead with a hysterectomy, the endometrioid adenocarcinoma could have been confined in the APA and therefore there was a possibility that no residual disease may be found at the hysterectomy specimen. The option more recommended in her case was to terminate the pregnancy with subsequent assessment of the endometrium and a successive treatment with a high dose of progestogen. This alternative was aimed at offering the patient fertility sparing treatment. We also discussed that the patient could continue this pregnancy, with the understanding that there may be progression of the disease, as well as the chance of miscarriage due to underlying cancer. Both the patient and her family had a strong desire to preserve fertility, and she decided to continue the pregnancy.
Fig. 1.
Atypical polypoid adenomyoma: The lesion is composed of glands showing cytological atypia and architectural complexity set within a fibromuscular stroma.
Fig. 2.
Endometrioid adenocarcinoma: Areas of glandular crowding and fusion. Islands of atypical epithelium are seen infiltrating around thick walled vessels.
Fig. 3.
MRI pelvis at 7 weeks of pregnancy after histological diagnosis.
The patient was seen at each trimester, remained asymptomatic throughout the pregnancy and had a normal delivery at term. After the delivery, the decidua and placental villus were collected and sent for histological examination. There was no evidence of any residual endometrioid adenocarcinoma in the post-delivery specimen. She was seen six weeks post-natally and underwent a hysteroscopy, endometrial biopsy and insertion of a Levonorgestrel intrauterine device (LNS-IUD) under general anesthesia. The histology did not show any evidence of EC or hyperplasia at that time. The case was further discussed at our multidisciplinary meeting, and the decision was to perform endometrial biopsy yearly for at least 5 years and to keep the LNS-IUD until patient's desire to conceive. There has been no evidence of relapse over 8 years of follow-up, and endometrial biopsy has been negative for cancer throughout the time of follow-up. She was pregnant again after 3 years from the initial diagnosis with another uneventful delivery. In the second pregnancy, the placenta was again sent for histology, and no evidence of malignancy was found. We have obtained consent for publication of this case from the patient.
3. Discussion
Endometroid adenocarcinoma incidentally diagnosed during pregnancy is a rare event and difficult to explain, as the high progesterone level makes this disease extraordinary. It is impossible to know whether the carcinoma, in all the cases reported to date (Zhou et al., 2015; Yael et al., 2009) and in our case, was present prior to the pregnancy. However, most likely coexistence of EC and pregnancy may imply preexisting neoplasia when the diagnosis is made during the first trimester, whereas a diagnosis done during the second trimester or post-partum may be consistent with an onset of the disease during the pregnancy. Most case reports of first trimester endometrioid adenocarcinoma are also reported as arising in a focal lesion (Saciragic et al., 2014). In our case, the disease was most likely focally present only in the APA, as the endometrial histology post-delivery was negative and as suggested by the histopathology findings at the first specimen. The coexistence of endometrioid adenocarcinoma in APA has been reported between 8.8 and 17.2% of cases. The precise mechanism to explain the development of an adenocarcinoma in APA has not been understood yet (Ma et al., 2018). Moreover, the localized lesion in our patient could explain the implantation and development of the embryo; also the high progesterone levels might have limited the number of mitosis and therefore the process. Both the patient and her family opted to continue her pregnancy. Conservative treatment was thought to be reasonable, as it is considered to be an option in patients with grade 1 stage IA disease in APA (Tanmahasamut and Wongwananuruk, 2010). As a result of the patient's choice to have expectant management, we were able to observe the natural course of the disease. Our patient had no evidence of disease up to 8 years of follow-up and during this time she was treated with LNG-IUD. The majority of case reports published describes a hysterectomy with or without bilateral-salpingo-oophorectomy within the 5 years post-diagnosis and only a minority of them report conservative management or were followed-up for longer than 5 years (Pal et al., 2018). Recurrence rate of APA with coexistence of endometrial cancer has been reported to be 8.8% (Ma et al., 2018). Our experience does not offer evidence to suggest that expectant management should be undertaken without counseling the patient about the risk of recurrence, cancer progression and that conservative management is not the standard treatment for EC in APA. Our patient opted for the use of LNG-IUD, although the documented results are mixed we acknowledged the latest evidence suggesting a response rate rating of 75% especially in grade 1 and 2 early stage EC (Pal et al., 2018). A study by Shabani et al. (Shabani et al., 2007) reported that response to progestins is related to progesterone receptors distribution on the sample. According to the authors, G1 lesions show a higher rate of ER and PR receptors (85%–90%) comparing to G2 and G3 tumours (55%–60%).
Another area of controversy is the duration of progestative treatment, which varies as published by different authors, as well as the surveillance of these patients with serial endometrial biopsies (Colombo et al., 2016; La Russa et al., 2018). Following the first assessment at 6 months, surveillance was done yearly for 5 years with an outpatient pipelle biopsy. Recent research (Park and Nam, 2015) suggested that a follow-up with a biopsy before 6 months after the treatment and diagnosis is not warranted.
We opted for continuous therapy with progesterone in our patient even after the first 12 months, as prophylaxis. However, we are aware that the majority of data published on the use of progestins are about its use for treatment. A large meta-analysis, for example, showed that 72.4% of women responded within 6 months to treatment with progesterone and this percentage increases only to 78% when the treatment is prolonged for the other 6 months (Gunderson et al., 2012). The use of progestins as prophylaxis has been recommended even to prevent recurrence of APA, which has been described to be between 7 and 30% (Heatley, 2006; Raffone et al., 2019).
In conclusion, in case of low grade and early stage disease diagnosed in early pregnancy a conservative treatment is a possible option in young nulliparous women, who are keen on preserving fertility. Further therapy with progestins, as prophylaxis should be tailored according to patients' wishes and couselling about the current published evidence about its use.
Authors' contribution
IR has written the first draft, IR, RN, KD, HJ,WM,BR have all revised and agreed to the final manuscript. KD has provided histopathology opinion.
Declaration of interest
All the authors have no potential conflict of interest to report.
Synopsis
A case of an incidental diagnosis of endometrial cancer in a patient at 7 weeks of gestation managed conservatively and resulted in a successful delivery.
Conflict of interest
The authors have no conflicts of interest.
References
- Colombo N., Creutzberg C., Amant F. ESMO-ESGOESTRO consensus conference on endometrial Cancer: diagnosis, treatment and follow-up. Ann. Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- Gunderson C.C., Fader A.N., Carson K.A., Bristow R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol. Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Heatley M.K. Atypical polypoid adenomyoma: a systematic review of the English literature. Histopathology. 2006;48:609–610. doi: 10.1111/j.1365-2559.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- La Russa M., Zapardiel I., halaska M.J., Zalewski K., Laky R., Dursun P., Lindquist D., Sukhin V., Polteraurer S., Biliatis I. Conservative management of endometrial cancer: a survey amongst European clinicians. Arch. Gynecol. Obstet. 2018;298:373–380. doi: 10.1007/s00404-018-4820-7. [DOI] [PubMed] [Google Scholar]
- Ma B., Zhu Y., Liu Y. Management of atypical polypoid adenomyoma of the uterus: a single center's experience. Medicine (Baltimore) 2018;97(12):e0135. doi: 10.1097/MD.0000000000010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal N., Broaddus R.R., Urbauer D.L., Balakrishnan N., Milbourne A., Schmeler K.M., Meyer L.A., Soliman P.T., Lu K.H., Ramirez P.T., Ramodetta L., Bodurka D.C., Westin S.N. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet. Gynecol. 2018;131:109–116. doi: 10.1097/AOG.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Nam J.-H. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist. 2015;20:270–278. doi: 10.1634/theoncologist.2013-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennant M.E., Mehta R., Moody P., Hackett G., Prentice A., Sharp S.J., Lakshman R. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG. 2016:404–411. doi: 10.1111/1471-0528.14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffone A., Travaglino A., Saccone G., Insabato L., Mollo A., de Placido G., Zullo F. Endometrial hyperplasia and progression to cancer: which classification system stratifies the risk better? A systematic review and meta-analysis. Arch. Gynecol. Obstet. Feb 27, 2019 doi: 10.1007/s00404-019-05103-1. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Saciragic L., Ball C.G., Islam S., Fung-Kee-Fung M. Incidental endometrial carcinoma diagnosed at first trimester pregnancy loss: a case report. J. Obstet. Gynaecol. Can. 2014;36(11):1010–1013. doi: 10.1016/S1701-2163(15)30415-1. [DOI] [PubMed] [Google Scholar]
- Schumann E.A. Observations upon the coexistence of carcinoma fundus uteri and pregnancy. Trans. Am. Gynecol. Soc. 1927;52:245–251. [Google Scholar]
- Shabani N., Kuhn C., Kunze S., Schulze S., Mayr D., Dian D., Gingelmaier A., Schindlbeck C., Willgeroth F., Sommer H., Jeschke U., Friese K., Mylonas I. Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor a (PR-A) and B (PR-B) in endometrial carcinomas. Eur. J. Cancer. 2007;43:2434–2444. doi: 10.1016/j.ejca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tanmahasamut P., Wongwananuruk T. Treatment of endometrial adenocarcinoma in young women: a case report and review of the literature. Case Rep. Oncol. 2010;3:380–385. doi: 10.1159/000321731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yael H.K., Lorenza P., Evelina S., Roberto P., Roberto A., Fabrizio S. Incidental endometrial adenocarcinoma in early pregnancy: a case report and review of the literature. Int. J. Gynecol. Cancer. 2009;19(9):1580–1585. doi: 10.1111/IGC.0b013e3181a841a7. [DOI] [PubMed] [Google Scholar]
- Zhou F., Qian Z., Li Y., Huang L. Endometrial adenocarcinoma in spontaneous abortion: two cases and review of the literature. Int. J. Clin. Exp. Med. 2015;8(5):8230–8233. [PMC free article] [PubMed] [Google Scholar]