Abstract
The omega-3 fatty acid docosahexaenoic acid (DHA) is known to induce apoptosis and cell cycle arrest via the induction of reactive oxygen species (ROS) production and endoplasmic reticulum (ER) stress in many types of cancers. However, the roles of DHA in drug-resistant cancer cells have not been elucidated. In this study, we investigated the effects of DHA in cisplatin-resistant gastric cancer SNU-601/cis2 cells. DHA was found to induce ROS-dependent apoptosis in these cells. The inositol 1,4,5-triphosphate receptor (IP3R) blocker 2-aminoethyl diphenylboninate (2-APB) reduced DHA-induced ROS production, consequently reducing apoptosis. We also found that G-protein-coupled receptor 120 (GPR120), a receptor of long-chain fatty acids, is expressed in SNU-601/cis2 cells, and the knockdown of GPR120 using specific shRNAs alleviated DHA-mediated ROS production and apoptosis. GPR120 knockdown reduced the expression of ER stress response genes, similar to the case for the pre-treatment of the cells with N-acetyl-L-cysteine (NAC), an ROS scavenger, or 2-APB. Indeed, the knockdown of C/EBP homologous protein (CHOP), a transcription factor that functions under ER stress conditions, markedly reduced DHA-mediated apoptosis, indicating that CHOP plays an essential role in the anti-cancer activity of DHA. These results suggest that GPR120 mediates DHA-induced apoptosis by regulating IP3R, ROS, and ER stress levels in cisplatin-resistant cancer cells, and that GPR120 is an effective chemotherapeutic target for cisplatin resistance.
Keywords: apoptosis, cisplatin resistance, DHA, ER stress, GPR120
INTRODUCTION
cis-Diamminedichloroplatinum (II) (cisplatin) is a drug widely used for many types of cancer therapy. A cisplatin-DNA crosslink, which is formed in inter- or intra-DNA strands, interrupts DNA replication and mitosis and severely damages proliferating cells (Eastman, 1983; Sancho-Martinez et al., 2012; Saris et al., 1996). The highly cytotoxic effects of cis-platin are also known to be involved with protein damages and cytoplasm-related apoptotic pathways. In addition, cis-platin induces endoplasmic reticulum (ER) stress in cancer cells (Yu et al., 2008). Cisplatin is known to activate ER-specific caspase-12 through calpain, and increase the expression of GRP78, an ER stress marker, in the cytoplast as well as in intact cells (Mandic et al., 2003). Although cisplatin is a very active substance and a highly effective anticancer drug, its use is limited because of chemoresistance and its nephrotoxicity. Cancer cells acquire resistance to cisplatin via different pathways, and the mechanisms of resistance to cisplatin could be explained by a decrease in cisplatin accumulation, an increase in the intracellular thiol levels, an increase in DNA repair capacity, etc. (Torigoe et al., 2005). Because resistance to cisplatin makes cancer treatment difficult, discovering new drugs for overcoming resistance is very important.
Docosahexaenoic acid (DHA, C22:6n-3), one of the omega-3 long-chain polyunsaturated fatty acids (PUFAs) derived from α-linoleic acid (ALA, 18:3n3), is used to induce apoptosis in cisplatin-resistant gastric cancer cells. Long-chain PUFAs of the omega-3 type have been known to have anti-cancer and anti-inflammatory effects (Stamp et al., 2005; Terry et al., 2003). PUFAs decrease cancer cell growth, induce apoptosis in cancer cells, and increase the efficiency of anti-cancer drugs (Das and Madhavi, 2011; Kato et al., 2002). DHA is also known to inhibit cancer cell proliferation and induce apoptosis through reactive oxygen species (ROS) production and caspase-8 activation in breast cancer cells and colon cancer cells (Chamras et al., 2002; Fasano et al., 2012; Narayanan et al., 2001). DHA supplementation increases the sensitivity to cisplatin in cisplatin-resistant GLC4-CP cancer cells (Timmer-Bosscha et al., 1989). In addition, DHA increases doxorubicin sensitivity through the regulation of drug influx and efflux in human cervical carcinoma cells (Das and Madhavi, 2011). Therefore, it seems to be a good therapeutic agent for increasing the drug sensitivity of cancer cells. Effects of DHA in cisplatin-resistant cancer cells and the underlying mechanisms, however, are not understood.
Since long-chain fatty acids (FAs) such as EPA and DHA show anti-cancer effects, receptors of these FAs, such as G-protein-coupled receptor 120 (GPR120) and G-protein-coupled receptor 40 (GPR40) are considered as good therapeutic targets (Hopkins and Meier, 2017; Senatorov and Moniri, 2018). Many G-protein-coupled receptors (GPCRs) that bind to free fatty acids (FFAs) have been identified over the last several decades. GPR120 is a receptor for long-chain PUFAs such as DHA and EPA, and saturated FAs. GPR120 is highly expressed in the intestine, macrophages, and lungs (Hirasawa et al., 2005; Katsuma et al., 2005; Oh et al., 2010). Omega-3 fatty acid-activated GPR120 inhibits proinflammatory signaling through the inhibition of TAB1-mediated TAK1 activation, consequently ameliorating insulin resistance in obesity (Oh et al., 2010). In addition, GPR120 influences cholecystokinin secretion through the phospholipase C (PLC)-inositol 1,4,5-triphosphate receptor (IP3R) pathway (Shah et al., 2012). Downregulation of GPR120, which is increased during adipocyte differentiation, reduces the transcription levels of PPAR-γ2 and aP2, resulting in the inhibition of adipocyte differentiation (Gotoh et al., 2007). However, the relationship between DHA-induced cytotoxicity and the roles of GPR120 have not yet been elucidated.
The roles of DHA on ER stress and calcium homeostasis are controversial because DHA was shown to induce the unfolded protein response (UPR) and disrupt intracellular calcium homeostasis in some studies, while other studies have reported that it inhibits ER stress (Begum et al., 2013; Begum et al., 2012; Crnkovic et al., 2012; Jakobsen et al., 2008). Thus, by investigating the roles of DHA in cisplatin-resistant gastric cancer cells, we tried to identify the relationship between DHA and ER stress.
In this study, to understand the pro-apoptotic roles of diverse DHA mediators in DHA-mediated cytotoxicity, we investigated the relationship among DHA, ER stress, and apoptosis in cisplatin-resistant cancer cells. We found that IP3R and ROS production, as well as the long-chain FA receptor GPR120, are involved in DHA-induced apoptosis, suggesting that GPR120 could be used as a novel therapeutic target for the treatment of cisplatin-resistant cancer. In addition, CHOP, a key regulator of ER stress-induced apoptosis, plays a critical role in these conditions.
MATERIALS AND METHODS
Chemicals and reagents
DHA, MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide), dimethyl sulfoxide (DMSO), 2-aminoethyl diphenylboninate (2-APB), and N-acetyl-L-cysteine (NAC) were purchased from Sigma (USA). 2,7-Dichlorofluorescein diacetate (DCF-DA) was purchased from Molecular Probes (USA). The anti-CHOP and horseradish peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology (USA). The anti-caspase-7, anti-PARP, anti-ATF4, anti-Actin, and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology (USA).
Cell lines and cell culture
SNU-601 cells and SNU-601/cis2 cells, which are human gastric cancer cells, were purchased from the Research Center for Resistant Cells at the Chosun university (Korea)(Xu et al., 2005). The cells were cultured in RPMI 1640 medium (WelGENE, Korea) containing 10% heat-inactivated fetal bovine serum (Gibco) and 1% penicillin/streptomycin mixture (Gibco) at 37℃ under a humidified atmosphere with 5% CO2 conditions. HEK293 cells were routinely maintained in DMEM (WelGENE) containing 10% heat-inactivated fetal bovine serum (Gibco) and 1% penicillin/streptomycin mixture (Gibco).
Preparation of hairpin design and shRNAs
Two pairs of shRNAs and one pair of shRNAs were chosen according to the GPR120 (NM_001195755.1) and CHOP (NM_001109986.1) sequences, following the guideline of Addgene (Cambridge, USA). Each pair contained a specific 21-nt human GPR120 or rat CHOP sense sequence followed by a short spacer (CTCGAG), anti-sense 21-nt sequences, and a transcription termination signal. The target sequences for GPR120-shRNA-2, GPR120-shRNA-6, and CHOP-shRNA are 5′-CCGGCTGGTCATTGTGATCAGTTACCTCGAGGTAACTGA TCACAATGACCAGTTTTTG-3′, 5′-CCGGCCTCATGGTCTCCTT CTTCATCTCGAGATGAAGAAGGAGACCATGAGGTTTTTG- 3′, and 5′-CCGGGAGGAAGAAGAGGAAGATCAACTCGAGTTGATCTTCCTCTTCTTCCTCTTTTTG-3′, respectively. The pairs of shRNAs, which were designed to contain terminal EcoRI and AgeI restriction sites, were subcloned into a vector to generate the pLKO.1-GPR120 and pLKO.1-CHOP shRNA vectors.
Production of lentiviruses for cell infection
Lentiviruses were produced by transfecting HEK293 cells with a four-plasmid system containing pLKO.1-shRNA, pMDLg/pRRE, pMD2-VSVG, and pRSV-Rev. The transfection was performed with iN-fect (iNtron, Korea), according to the manufacturer’s instructions. The lentiviral supernatants were collected at 24 and 48 h post-transfection by filtration. The filtered lentiviral supernatants were stored in a deep freezer at −70℃. The SNU-601/cis2 cells were plated in a 100-mm cell culture dish at density of 1 × 106 cells. After 16 h, the cells were infected with 1 ml of the lentiviral supernatants containing 8 μg/ml polybrene. About 24 h post-infection, the culture medium was replaced with fresh medium containing 0.8 μg/ml puromycin. After 48 h, puromycin-containing medium was replaced with normal growth medium for 24 h; the puromycin-selection step was repeated once more. After puromycin selection, the expression levels of GPR120 and CHOP were quantified by RT-PCR analysis using the total RNAs isolated from each cell line.
Measurement of cell viability
The MTT assay was used to determine the number of viable cells. SNU-601 and SNU-601/cis2 cells were seeded into 96-well plates and incubated for 16 h. The cells were treated with various concentrations of cisplatin and DHA. When needed, the cells were pre-treated with the IP3R inhibitor 2- APB or the ROS scavenger NAC for 2 h before the DHA treatment. After washing the cells twice with PBS, 300 μl or 500 μl of fresh medium containing 0.5 mg/ml MTT was added to each well and incubated for 2 h at 37℃. The insoluble formazan was dissolved using 300 μl of DMSO. The absorbance of the soluble substrate was measured at 570 nm using an ELISA Reader (UYM 340; ASYS Hitech, Austria).
Immunoblot analysis
Proteins from the cells were harvested using the RIPA lysis buffer [150 mM NaCl, 1% Triton X-100, 1% Sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, 2 mM EDTA] containing 1% phosphatase inhibitors and protease inhibitors. The protein concentrations were quantified using the Bradford method (Bio-rad, Hercules, USA). The proteins were boiled in 1× sample buffer [500 mM Tris-HCl (pH 6.8), 10% SDS, 20% glycerol, 0.05% bromophenol blue, and 1% β-mercaptoethanol] for 10 min at 100℃, and were then separated on SDS-polyacrylamide gels. The protein bands were then transferred onto Immobilon-P membranes (Millipore Corp., USA) and incubated with the respective antibodies at 4℃ overnight. Horseradish peroxidase-conjugated antibodies were added to the membranes, followed by incubation at room temperature for 2 h; the band signals were detected using a LAS-3000 Luminescent Image Analyzer (Fujifilm, Japan). To re-probe the blots, the membranes were treated with a stripping buffer [100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl (pH 6.8)] at 60℃ for 30 min, washed thrice with TBST buffer for 10 min (each wash), and re-probed with other antibodies.
Detection of intracellular ROS levels
Intracellular ROS production was detected using the intracellular fluorescence probe DCF-DA. The cells were first treated with 10 μM DCF-DA for 2 h at 37℃. When needed, the ROS scavenger NAC, or the IP3R inhibitor 2-APB was used for the co-treatment of the cells with DCF-DA, and then, DHA or cisplatin was added to the cells. The cells were incubated for the time-periods indicated in Figs. 1–5. The fluorescence intensity of DCF was detected under a fluorescence microscope (ECLIPSE TS-200; Nikon) using a wavelength of 488 nm for excitation (magnification 200×). The ImageJ program was used for the quantification of the fluorescence intensities.
Fig. 1. DHA treatment induces apoptosis in cisplatin-resistant gastric cancer cells.
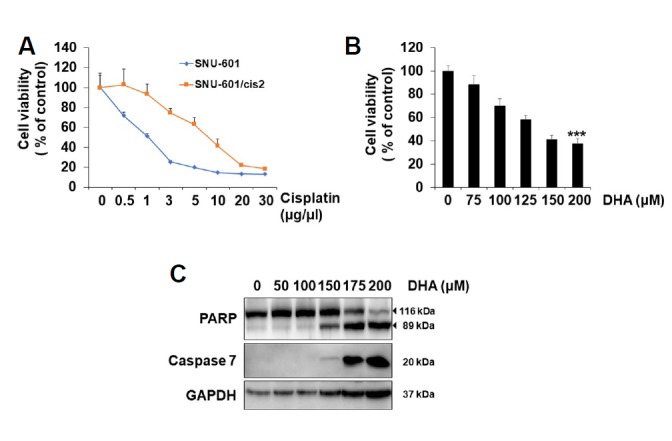
(A) SNU-601 cells and SNU-601/cis2 cells were treated with various concentrations of cis-platin for 48 h as indicated, and their viabilities were determined using the MTT assay. (B) SNU-601/cis2 cells were treated with various concentrations of DHA for 24 h as indicated, and their viabilities were determined using the MTT assay. Significant differences have been indicated as ***p < 0.001. (C) SNU-601/cis2 cells were treated with various concentrations of DHA for 24 h. Then, the cell lysates were subjected to SDS-PAGE, followed by immunoblot analyses using antibodies specific for caspase-7, PARP, and GAPDH.
Fig. 2. DHA treatment induces ROS-dependent apoptosis through IP3R activation in SNU-601/cis2 cells.
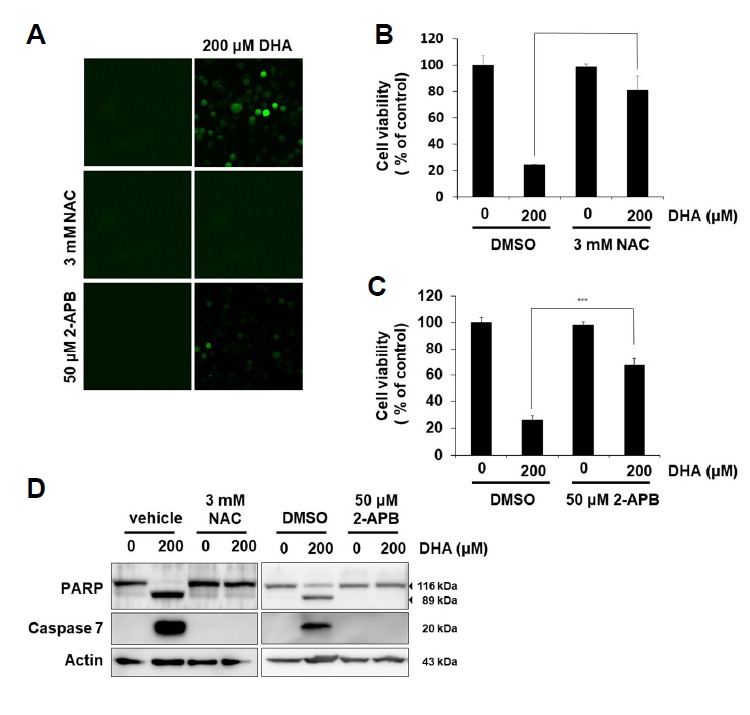
(A) SNU-601/cis2 cells pre-treated with 10 μM DCF-DA for 2 h were treated with 3 mM NAC or 50 μM 2-APB for 2 h, and then treated with 200 μM DHA for 4 h. Intracellular ROS generation was observed by fluorescence microscopy (400×). (B, C) SNU-601/cis2 cells pre-treated with 3 mM NAC or 50 μM 2-APB for 2 h were treated with 200 μM DHA for 24 h. Cell viability was determined using the MTT assay. Significant differences have been indicated as ***p < 0.001. (D) SNU-601/cis2 cells were treated with DHA alone or in combination with 3 mM NAC or 50 μM 2-APB for 24 h. Immunoblot analyses were performed using antibodies specific for PARP, caspase-7, and actin.
Fig. 3. Downregulation of GPR120 diminishes DHA-mediated apoptosis in SNU-601/cis2 cells.
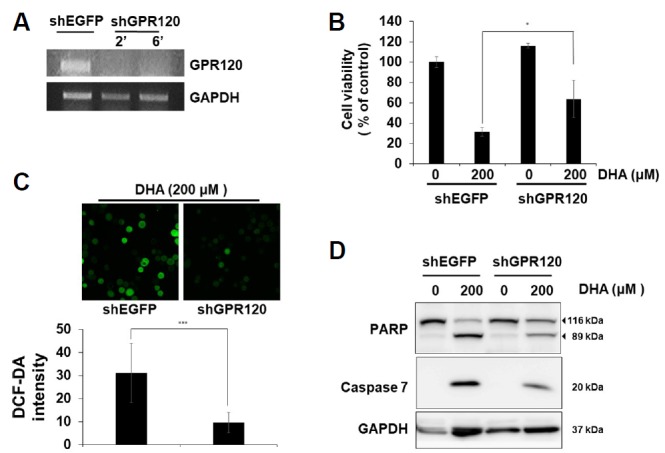
SNU-601/cis2 cells were transfected with shRNAs specific for GPR120 or EGFP as a control. (A) Transcription levels of GPR120 were measured by RT-PCR analysis using total RNAs isolated from each cell line. (B) The cells were treated with 200 μM DHA for 24 h, and their viabilities were measured using the MTT assay. Significant differences have been indicated as *p < 0.05. (C) Cells pre-treated with 10 μM DCF-DA for 2 h were treated with 200 μM DHA for 4 h. The production of intracellular ROS was observed by fluorescence microscopy (top, 400×). Quantification shows the intensity of ROS generation (bottom). The ImageJ program was used for quantifying the fluorescence intensities. Significant differences have been indicated as ***p < 0.001. (D) The cells were treated with 200 μM DHA for 24 h and cell lysates were subjected to SDS-PAGE, followed by immunoblot analyses using antibodies specific for PARP, caspase-7, and GAPDH.
Fig. 4. DHA-induced CHOP expression is involved with GPR120, IP3R, and ROS in SNU-601/cis2 cells.
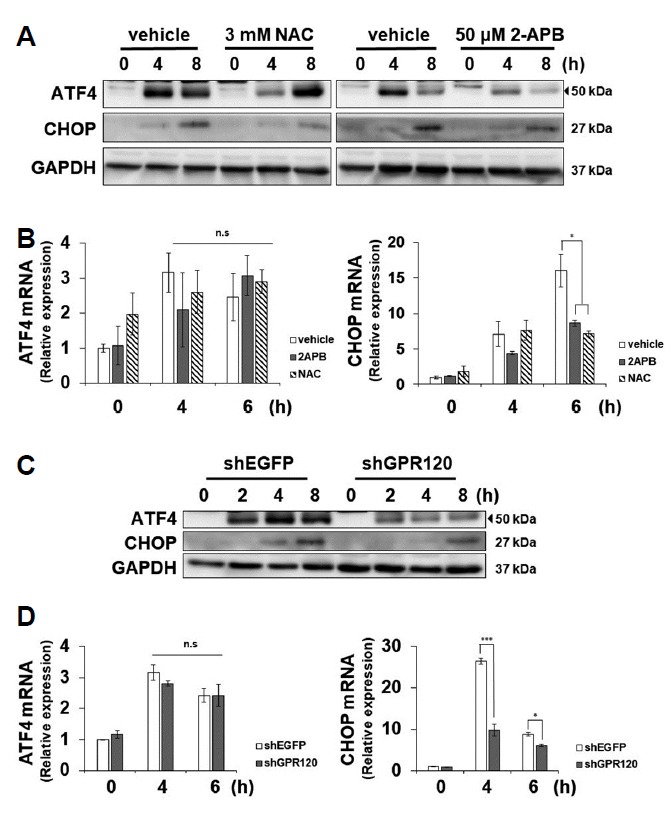
(A, B) Cells pre-treated with 3 mM NAC or 50 μM 2-APB for 2 h were treated with 200 μM DHA for various time-periods, as indicated. The cell lysates were subjected to SDS-PAGE, followed by immunoblot analyses using antibodies specific for ATF4, CHOP, and GAPDH (A). Total RNAs were isolated and the relative levels of ATF4 and CHOP mRNAs were measured by real-time quantitative PCR. Significant differences have been indicated as *p < 0.05, n.s; not significant (B). (C, D) SNU-601/cis2 cells transfected with shRNAs specific for GPR120 or EGFP were treated with 200 μM DHA for various time-periods, as indicated. The cell ly-sates were subjected to SDS-PAGE, followed by immunoblot analyses using antibodies specific for ATF4, CHOP, and GAPDH (C). Total RNAs were isolated and the relative levels of ATF4 and CHOP mRNAs were measured by real-time quantitative PCR. Significant differences have been indicated as *p < 0.05, ***p < 0.001. n.s; not significant (D).
Fig. 5. CHOP is involved in DHA-mediated apoptosis in SNU-601/cis2 cells.
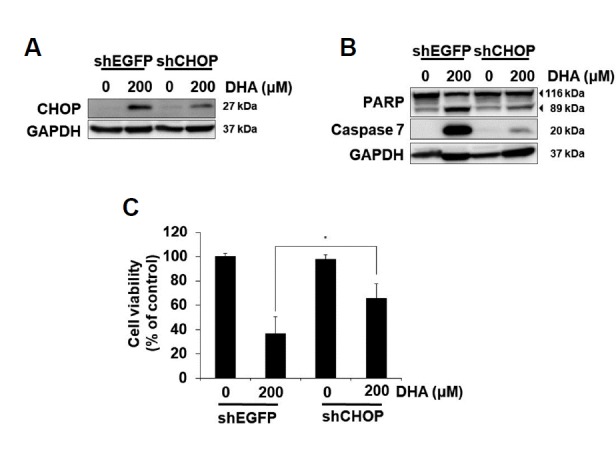
SNU-601/cis2 cells transfected with shRNAs specific for CHOP or EGFP were treated with 200 μM DHA for 12 h (A) or 24 h (B, C). The cell lysates were subjected to SDS-PAGE, followed by immunoblot analyses using antibodies specific for CHOP and GAPDH (A) and PARP, caspase-7, and GAPDH (B). The cell viability was measured using the MTT assay. Significant differences have been indicated as *p < 0.05 (C).
Real-time quantitative PCR
Real-time quantitative PCR was performed using HiPi Real-time PCR 2× Master Mix (SYBR Green, ELPiS, Korea), as described previously (Shin et al., 2018).
Statistical analysis
The values in this study are representative of at least three independent experiments. All results are shown as the means ± S.D. Statistical analysis of the data between the experimental groups was performed using the Student’s t-test. P values less than 0.05 were considered statistically significant.
RESULTS
DHA treatment induces apoptosis in cisplatin-resistant gastric cancer cells
Cisplatin has been widely used for many types of cancer therapies. However, in many cases, intrinsic or acquired resistance to cisplatin has decreased its efficacy (Torigoe et al., 2005). Therefore, research for overcoming cisplatin resistance is very important. SNU-601/cis2 gastric cancer cells, which were selected by cisplatin resistance, showed 10 times higher resistance to cisplatin than the original non-selected SNU-601 cells in the cell viability assay. The IC50 value of cis-platin for SNU-601/cis2 was 10 times higher than that of the control cells (Fig. 1A)(Xu et al., 2005). Although it is known that DHA induces apoptosis in many types of cancer cells, the effects of DHA on drug-resistant cancer cells have not yet been elucidated. Therefore, we studied whether DHA induces apoptosis in SNU-601/cis2 cells. When the SNU-601/cis2 cells were treated with various concentrations of DHA, the cell viability decreased considerably in a dose-dependent manner (Fig. 1B). The western blotting results showed that DHA treatment increased the expression levels of the active form of caspase-7 and cleaved form of PARP, suggesting that DHA induces apoptosis in cisplatin-resistant SNU-601/cis2 cells (Fig. 1C).
DHA treatment induces ROS-dependent apoptosis through IP3R activation in SNU-601/cis2 cells
Since it has been reported that DHA induces apoptosis through an increase in ROS and caspase-8 activity in MCF-7 cells (Kang et al., 2010), we tested whether DHA-mediated apoptosis in SNU-601/cis2 is related with ROS generation. When SNU-601/cis2 cells pre-treated with the ROS scavenger NAC for 2 h were treated with DHA for 4 h, DHA-mediated ROS generation was clearly reduced (Fig. 2A). Pre-treatment with NAC also rescued the viability of the DHA-treated cells (Fig. 2B). DHA-mediated cleavage of PARP and activation of caspase-7 disappeared notably after NAC treatment (Fig. 2D, left), suggesting that DHA-mediated apoptosis was mediated by the generation of ROS. Next, we investigated whether DHA-mediated ROS generation is related to IP3R activation, because it was reported that DHA treatment destabilized Ca2+ homeostasis through IP3 production (Aires et al., 2007). IP3R activation, which induces calcium release from the ER, results in the lowering of the calcium in the ER lumen, causing the accumulation of misfolded proteins in the ER (Ivanova et al., 2014). We tested whether the inhibition of IP3R by 2-APB diminishes DHA-induced phenotypes, such as increased ROS generation, reduced cell viability, and increased apoptosis. Pre-treatment with 2-APB decreased DHA-induced ROS generation (Fig. 2A, bottom), rescued the decrease in cell viability (Fig. 2C), and attenuated the DHA-mediated cleavage of PARP and activation of caspase-7 (Fig. 2D, right), suggesting that DHA could induce ROS-dependent apoptosis through IP3R activation in cisplatin-resistant SNU-601/cis cells.
Downregulation of GPR120 diminishes DHA-mediated ROS generation and apoptosis
Among many receptors of FFAs identified so far, GPR120 and GPR40 are known to be activated by medium- or long-chain fatty acids such as EPA and DHA (Hirasawa et al., 2005; Itoh et al., 2003). GPR120 is known to be expressed in the stomach, lungs, liver, macrophages, and adipocytes (Hirasawa et al., 2005; Katsuma et al., 2005; Oh et al., 2010), and it has been suggested that GPR120 changes the intracellular calcium homeostasis through PLC activation (Shah et al., 2012). PLC activates IP3R through the generation of IP3 and causes the release of calcium from the ER. When we checked the expression of GPR120 in SNU-601/cis2 cells, the GPR120 transcript was well observed in normal growth conditions; GPR120 knockdown, which was performed by infecting the cells with lentiviruses expressing shRNAs, abolished the expression of GPR120 (Fig. 3A). Interestingly, GPR120 knockdown increased the cell viability in DHA-treated cells (Fig. 3B). Next, we examined whether GPR120 knockdown influences DHA-mediated ROS generation and apoptosis. GPR120 knockdown notably attenuated DHA-mediated ROS generation (Fig. 3C). In this condition, the expression levels of both the cleaved form of PARP and the active form of caspase-7 were reduced (Fig. 3D), suggesting that GPR120, which is a receptor of DHA, plays a role in both DHA-induced ROS generation and apoptosis.
DHA-mediated induction of the UPR is involved with GPR120, IP3R, and ROS in SNU-601/cis2 cells
It is known that DHA-mediated apoptosis is involved in ER stress in colon cancer cells (Jakobsen et al., 2008). ER stress and oxidative stress are closely involved in cell homeostasis and apoptosis, and some forms of ROS disrupt the protein folding capacity and cause ER stress (Cao and Kaufman, 2014). Since the relationship between GPR120 and ER stress has not yet been elucidated, we tested whether DHA-mediated activation of IP3R and the generation of ROS are related with ER stress. DHA treatment induced ER stress, as expected, and pretreatment with NAC or 2-APB clearly reduced the DHA-induced expression of CHOP at both the transcription levels and steady-state protein levels in SNU-601/cis2 cells, as shown by the immunoblot and real-time PCR results (Figs. 4A and 4B). The steady-state expression levels of the ATF4 protein decreased after combined treatment with 2-APB and DHA (Fig. 4A, right). However, they decreased after 4 h and increased after 8 h in NAC and DHA-treated cells (Fig. 4A, left). The real-time PCR results show that pretreatment with 2-APB or NAC had no influence on ATF4 transcription (Fig. 4B, left). These results suggest that ATF4 expression is controlled by post-transcriptional-level regulation in our experimental conditions. Next, we investigated whether GPR120 is involved in DHA-induced ER stress. Downregulation of GPR120 by using specific shRNA decreased the DHA-mediated expression of ATF4 and CHOP (Figs. 4C and 4D). Similar to the results seen in Figs. 4A and 4B, downregulation of GPR120 reduced the expression of CHOP at both the transcription and steady-state protein levels, but reduced the expression of ATF4 only at the protein level. Collectively, DHA-mediated induction of the UPR is involved with GPR120, IP3R, and ROS in SNU-601/cis2 cells.
DHA-mediated induction of CHOP plays an important role in the apoptosis of SNU-601/cis2 cells
Since CHOP, a downstream target of ATF4, is known to play a critical role in ER stress-induced apoptosis (Cao and Kaufman, 2014; Ron and Walter, 2007) and it was induced by DHA treatment in our experiments, we investigated whether CHOP is involved in DHA-mediated apoptosis. DHA-mediated increase in CHOP expression was reduced by the transfection of shCHOP RNAs in SNU-601/cis2 cells (Fig. 5A). CHOP knockdown significantly reduced DHA-mediated apoptosis, as seen by the decrease in PARP cleavage and caspase-7 activation (Fig. 5B). Similarly, the DHA-mediated reduction in cell viability was significantly rescued by the downregulation of CHOP (Fig. 5C). These results suggest that DHA-mediated induction of CHOP plays an important role in the apoptosis of SNU-601/cis2 cells.
DISCUSSION
PUFAs are known to induce apoptosis and growth arrest in gastric cancer cells partially through lipid peroxidation (Dai et al., 2013). The omega-3 FA DHA is known to induce apoptosis and cell cycle arrest through ROS generation and ER stress in many types of cancer, but its role in drug-resistant cancer cells has not yet been elucidated. In this study, during an attempt to find substances that reduce the viability of cisplatin-resistant gastric cancer SNU-601/cis2 cells, we found that DHA induced ROS-, GPR120-, and CHOP-dependent apoptosis. The IP3R blocker 2-APB reduced DHA-induced ROS generation and consequently reduced apoptosis. We also found that GPR120, a receptor of long chain FAs, is expressed in SNU-601/cis2 cells, and that the knockdown of GPR120 using specific shRNAs alleviated DHA-mediated ROS production, ER stress, and apoptosis. With regards to ER stress, GPR120 knockdown showed similar results as those in case of pre-treatment with NAC or 2-APB. Indeed, the knockdown of CHOP, a transcription factor that functions under ER stress conditions, markedly reduced DHA-induced apoptosis, indicating that CHOP plays an essential role in mediating the anti-cancer effects of DHA. These results suggest that GPR120 mediates DHA-induced apoptosis by regulating the IP3R, ROS, and ER stress levels in cisplatin-resistant cancer cells, and that GPR120 is an effective chemotherapeutic target for cisplatin resistance.
Although many reports have suggested that DHA has ER-associated functions, including the regulation of intracellular calcium/lipid homeostasis-mediated ER stress (Jakobsen et al., 2008), the mediators that function downstream of DHA have not been identified specifically. There are several evidences showing that GPR120 is involved in signaling pathways that regulate the intracellular calcium levels, indirectly suggesting that GPR120 mediates the effects of DHA. For example, GPR120 increases the intracellular calcium levels through the activation of PLC (Shah et al., 2012), and short-splice variants of GPR120 have been reported to induce intracellular calcium increase (Watson et al., 2012). Therefore, it is possible that ligands of GPR120, such as DHA, modulate intracellular calcium homeostasis and ER stress in GPR120-expressing cells. Indeed, in this study, we showed that GPR120 knockdown reduced DHA-mediated ROS induction and apoptosis (Fig. 3). These results indicate that GPR120 is a receptor that mediates the effects of DHA, and that ROS generation, apoptosis, and intracellular calcium homeostasis are related with the function of GPR120.
Cisplatin resistance, which reduces the efficiency of chemotherapy, is known to be acquired by reducing the accumulation of cisplatin in the cells, increasing DNA repair ability, and/or increasing the levels of cisplatin-binding nucleophilic species and proteins, etc. (Galluzzi et al., 2012; Usanova et al., 2010; Wang et al., 2004). DHA, one of the major components of omega-3 PUFAs, is known to increase the chemosensitivity of multidrug-resistant cancer cells, as well as drug-sensitive cancer cells (Corsetto et al., 2017). DHA-mediated changes in the composition of the plasma membrane, especially, the incorporation of DHA into membrane phospholipids and lipid rafts, increase the ratio of drug influx to efflux, resulting in increased chemosensitivity (Gelsomino et al., 2013; Zijlstra et al., 1987). Similar results were observed in leukemia cells from mice fed with the coconut oil diet, which contains high levels of PUFAs (Burns et al., 1979). Although there are many explanations for the chemosensitizing effects of PUFAs, including lipid peroxidation, ROS generation, and gene expression, no report has indicated that GPR120-IP3R pathway-mediated ROS induction is involved with the effects of DHA, as shown in this study. The treatment of cisplatin-resistant gastric cancer cells with shGPR120, an inhibitor of IP3R (2-APB), and an antioxidant (NAC) reduced the effects of DHA, demonstrating the importance of this pathway. Our results support the claim that DHA-mediated activation of GPR120 induces ROS-mediated apoptosis in cisplatin-resistant gastric cancer cells, and that DHA could serve as a useful adjuvant for the treatment of multidrug-resistant cancer.
Many factors disrupting calcium homeostasis induce an adaptive response in the ER. The ER is a major organelle for protein synthesis/maturation and intracellular calcium homeostasis. Depletion of calcium from the ER lumen, as well as the accumulation of misfolded or unfolded proteins in the ER lumen lead to an ER stress response known as the UPR (Harding et al., 2002; Rutkowski and Kaufman, 2004; So, 2018). The interrelationship between calcium homeostasis, oxidative stress, and the ER stress is so complicated that it is hard to ascertain which event occurs upstream of the others. For example, IP3R-mediated Ca2+ release activates Ca2+/calmodulin-dependent protein kinase CaMKII, leading to the induction of NADPH oxidase and NADPH oxidase-mediated ROS generation and ER stress (Cao and Kaufman, 2014; Kim et al., 2016; Pedruzzi et al., 2004). Furthermore, CHOP-induced ERO1α regulates IP3R activity, which generates the positive feed-forward cycle of the ER and oxidative stress processes (Cao and Kaufman, 2014; Li et al., 2009). As shown in Figs. 2, 4, and 5, our results suggest that DHA-induced ROS generation seems to work upstream of the Ca2+-mediated induction of the ER stress response and apoptosis; the fact that not only scavenging ROS using NAC, but also inhibiting the function of IP3R prevented the elevation of the ER stress and apoptosis supports this idea (Figs. 2 and 4). The steady-state expression levels of the ATF4 and CHOP proteins were increased by DHA treatment (Figs. 4 and 5A). Since the expression of ATF4 and CHOP is closely related with the phosphorylation of eIF2α, which is regulated by the four eIF2α kinases PERK, PKR, HRI, and GCN2 that function in the integrated stress response (ISR) (Taniuchi et al., 2016; Wek et al., 2006), it is likely that the four ISR kinases activated under conditions of DHA treatment contribute towards the elevation of the ER stress response and the induction of CHOP. Indeed, it has been reported that ROS-induced PERK activation plays an important role in the induction of apoptosis in diabetic cardiomyopathy (Liu et al., 2013). Mitochondrial dysfunction-induced ROS also increases the expression of ATF4 through GCN2-mediated eIF2α phosphorylation in human gastric cancer cells (Wang et al., 2016). When SH- SY5Y cells were treated with H2O2 to induce oxidative stress, eIF2α phosphorylation was induced by PKR (Zhang et al., 2016). ATF4 and CHOP also upregulate the transcription of genes related to protein synthesis, which leads to oxidative stress and cell death (Han et al., 2013). Collectively, these reports suggest that increased ROS generation could induce ER stress via direct action on the eIF2α kinases.
The mitochondria-ER association through mitochondria-associated ER membranes (MAMs) makes the ROS-apoptosis relationship more complicated. The Ca2+ channels of the ER, including IP3R, are enriched in MAMs, which might make the generation of calcium currents between the two organelles easy (Lee and Min, 2018; Szabadkai et al., 2006). The ER stress sensor PERK plays an important role in maintaining the ER-mitochondrion juxtaposition, which is required for ROS-based mitochondrial apoptosis (Verfaillie et al., 2012). CHOP, a pro-apoptotic factor of UPR, seems to function as a key player in DHA-mediated apoptosis in cisplatin-resistant gastric cancer cells. CHOP, a downstream target of ATF4, plays a pro-apoptotic role under ER stress conditions depending on the cellular context. Overexpression of CHOP decreases the expression of the anti-apoptotic factor Bcl-2 (Oyadomari and Mori, 2004) and increases the transcription of genes involved in protein synthesis, thereby inducing oxidative stress and apoptosis (Han et al., 2013). Considering that DHA-induced ROS generation is related to the induction of ER stress as well as IP3R activity as shown by our results, and that DHA-mediated cell cycle arrest, apoptosis, and the ER stress occur via the calcium-dependent induction of oxidative stress (Crnkovic et al., 2012), DHA-mediated ROS generation, ER stress, and IP3R activation seem to work as a positive feedback loop, thereby effectively exerting anti-cancer effects.
The results of this study suggest that DHA is a very important molecule for anti-cancer therapy, especially in GPR120-expressing cancer cells. Since GPR120 is reported to be expressed in macrophages, lungs, and the stomach (Hirasawa et al., 2005; Katsuma et al., 2005; Oh et al., 2010), it would be useful to examine the efficacy of DHA in cancer cells derived from these tissues. DHA is also known to decrease inflammation; it inhibits the production of inflammatory cytokines such as IL-1β, TNF-α, and IL-6 in human endothelial cells and human macrophages (Khalfoun et al., 1997; Weldon et al., 2007). In in vivo models, the oral administration of DHA and EPA was shown to decrease acute inflammation through the inhibition of ROS generation and cytokine secretion (Mori et al., 2003). Since inflammation plays an important role for the maintenance of the cancer microenvironment and metastasis, controlling DHA-modulated cancer inflammation will greatly improve cancer treatment. Collectively, our results demonstrate that the anti-cancer effects of DHA are mediated by GPR120 via Ca2+, ROS, and CHOP. Therefore, GPR120 could serve as a novel therapeutic target for anti-cancer therapy not only for general cancers, but also for cisplatin-resistant cancers.
ACKNOWLEDGMENTS
This paper was supported by Konkuk University in 2014.
REFERENCES
- Aires V., Hichami A., Filomenko R., Ple A., Rebe C., Bettaieb A., Khan N.A. Docosahexaenoic acid induces increases in [Ca2+]i via inositol 1,4,5-triphosphate production and activates protein kinase C gamma and -delta via phosphatidylserine binding site: implication in apoptosis in U937 cells. Mol Pharmacol. 2007;72:1545–1556. doi: 10.1124/mol.107.039792. [DOI] [PubMed] [Google Scholar]
- Begum G., Harvey L., Dixon C.E., Sun D. ER stress and effects of DHA as an ER stress inhibitor. Translational Stroke Research. 2013;4:635–642. doi: 10.1007/s12975-013-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum G., Kintner D., Liu Y., Cramer S.W., Sun D. DHA inhibits ER Ca2+ release and ER stress in astrocytes following in vitro ischemia. J Neurochem. 2012;120:622–630. doi: 10.1111/j.1471-4159.2011.07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C.P., Luttenegger D.G., Dudley D.T., Buettner G.R., Spector A.A. Effect of modification of plasma membrane fatty acid composition on fluidity and methotrexate transport in L1210 murine leukemia cells. Cancer Res. 1979;39:1726–1732. [PubMed] [Google Scholar]
- Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamras H., Ardashian A., Heber D., Glaspy J.A. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J Nutr Biochem. 2002;13:711–716. doi: 10.1016/s0955-2863(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Corsetto P.A., Colombo I., Kopecka J., Rizzo A.M., Riganti C. Omega-3 long chain polyunsaturated fatty acids as sensitizing agents and multidrug resistance revertants in cancer therapy. Int J Mol Sci. 2017;18:2770. doi: 10.3390/ijms18122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnkovic S., Riederer M., Lechleitner M., Hallstrom S., Malli R., Graier W.F., Lindenmann J., Popper H., Olschewski H., Olschewski A., et al. Docosahexaenoic acid-induced unfolded protein response, cell cycle arrest, and apoptosis in vascular smooth muscle cells are triggered by Ca(2)(+)-dependent induction of oxidative stress. Free Radic Biol Med. 2012;52:1786–1795. doi: 10.1016/j.freeradbiomed.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Shen J., Pan W., Shen S., Das U.N. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013;12:71. doi: 10.1186/1476-511X-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U.N., Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis. 2011;10:159. doi: 10.1186/1476-511X-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II) Biochemistry. 1983;22:3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Fasano E., Serini S., Piccioni E., Toesca A., Monego G., Cittadini A.R., Ranelletti F.O., Calviello G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta. 2012;1822:1762–1772. doi: 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- Gelsomino G., Corsetto P.A., Campia I., Montorfano G., Kopecka J., Castella B., Gazzano E., Ghigo D., Rizzo A.M., Riganti C. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol Cancer. 2013;12:137. doi: 10.1186/1476-4598-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh C., Hong Y.H., Iga T., Hishikawa D., Suzuki Y., Song S.H., Choi K.C., Adachi T., Hirasawa A., Tsujimoto G., et al. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun. 2007;354:591–597. doi: 10.1016/j.bbrc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Han J., Back S.H., Hur J., Lin Y.H., Gildersleeve R., Shan J., Yuan C.L., Krokowski D., Wang S., Hatzoglou M., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H.P., Calfon M., Urano F., Novoa I., Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Hopkins M.M., Meier K.E. Free fatty acid receptors and cancer: from nutrition to pharmacology. Handb Exp Pharmacol. 2017;236:233–251. doi: 10.1007/164_2016_48. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Ivanova H., Vervliet T., Missiaen L., Parys J.B., De Smedt H., Bultynck G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta. 2014;1843:2164–2183. doi: 10.1016/j.bbamcr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Jakobsen C.H., Storvold G.L., Bremseth H., Follestad T., Sand K., Mack M., Olsen K.S., Lundemo A.G., Iversen J.G., Krokan H.E., et al. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J Lipid Res. 2008;49:2089–2100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K.S., Wang P., Yamabe N., Fukui M., Jay T., Zhu B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Hancock R.L., Mohammadpour H., McGregor B., Manalo P., Khaiboullina S., Hall M.R., Pardini L., Pardini R.S. Influence of omega-3 fatty acids on the growth of human colon carcinoma in nude mice. Cancer Lett. 2002;187:169–177. doi: 10.1016/s0304-3835(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Katsuma S., Hatae N., Yano T., Ruike Y., Kimura M., Hirasawa A., Tsujimoto G. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005;280:19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- Khalfoun B., Thibault F., Watier H., Bardos P., Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400B:589–597. [PubMed] [Google Scholar]
- Kim H.S., Lim J.M., Kim J.Y., Kim Y., Park S., Sohn J. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int J Cancer. 2016;138:1432–1441. doi: 10.1002/ijc.29879. [DOI] [PubMed] [Google Scholar]
- Lee S., Min K.T. The Interface Between ER and Mitochondria: Molecular Compositions and Functions. Mol Cells. 2018;41:1000–1007. doi: 10.14348/molcells.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Mongillo M., Chin K.T., Harding H., Ron D., Marks A.R., Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.W., Zhu H.T., Chen K.L., Dong X., Wei J., Qiu C., Xue J.H. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol. 2013;12:158. doi: 10.1186/1475-2840-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandic A., Hansson J., Linder S., Shoshan M.C. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Mori T.A., Woodman R.J., Burke V., Puddey I.B., Croft K.D., Beilin L.J. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- Narayanan B.A., Narayanan N.K., Reddy B.S. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int J Oncol. 2001;19:1255–1262. doi: 10.3892/ijo.19.6.1255. [DOI] [PubMed] [Google Scholar]
- Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J.C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rutkowski D.T., Kaufman R.J. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sancho-Martinez S.M., Prieto-Garcia L., Prieto M., Lopez-Novoa J.M., Lopez-Hernandez F.J. Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacol Ther. 2012;136:35–55. doi: 10.1016/j.pharmthera.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Saris C.P., van de Vaart P.J., Rietbroek R.C., Blommaert F.A. In vitro formation of DNA adducts by cisplatin, lobaplatin and oxaliplatin in calf thymus DNA in solution and in cultured human cells. Carcinogenesis. 1996;17:2763–2769. doi: 10.1093/carcin/17.12.2763. [DOI] [PubMed] [Google Scholar]
- Senatorov I.S., Moniri N.H. The role of free-fatty acid receptor-4 (FFA4) in human cancers and cancer cell lines. Biochem Pharmacol. 2018;150:170–180. doi: 10.1016/j.bcp.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B.P., Liu P., Yu T., Hansen D.R., Gilbertson T.A. TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am J Physiol Cell Physiol . 2012;302:C210–219. doi: 10.1152/ajpcell.00209.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.I., Jeon Y.J., Lee S., Lee Y.G., Kim J.B., Kwon H.C., Kim S.H., Kim I., Lee K., Han Y.S. Apoptotic and anti-inflammatory effects of eupatorium japonicum thunb. in rheumatoid arthritis fibroblast-like synoviocytes. Biomed Res Int. 2018;2018:1383697. doi: 10.1155/2018/1383697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So J.S. Roles of endoplasmic reticulum stress in immune responses. Mol Cells. 2018;41:705–716. doi: 10.14348/molcells.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp L.K., James M.J., Cleland L.G. Diet and rheumatoid arthritis: a review of the literature. Semin Arthritis Rheum. 2005;35:77–94. doi: 10.1016/j.semarthrit.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Szabadkai G., Bianchi K., Varnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi S., Miyake M., Tsugawa K., Oyadomari M., Oyadomari S. Integrated stress response of vertebrates is regulated by four eIF2alpha kinases. Sci Rep. 2016;6:32886. doi: 10.1038/srep32886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P.D., Rohan T.E., Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77:532–543. doi: 10.1093/ajcn/77.3.532. [DOI] [PubMed] [Google Scholar]
- Timmer-Bosscha H., Hospers G.A., Meijer C., Mulder N.H., Muskiet F.A., Martini I.A., Uges D.R., de Vries E.G. Influence of docosahexaenoic acid on cisplatin resistance in a human small cell lung carcinoma cell line. J Natl Cancer Inst. 1989;81:1069–1075. doi: 10.1093/jnci/81.14.1069. [DOI] [PubMed] [Google Scholar]
- Torigoe T., Izumi H., Ishiguchi H., Yoshida Y., Tanabe M., Yoshida T., Igarashi T., Niina I., Wakasugi T., Imaizumi T., et al. Cisplatin resistance and transcription factors. Curr Med Chem Anticancer Agents. 2005;5:15–27. doi: 10.2174/1568011053352587. [DOI] [PubMed] [Google Scholar]
- Usanova S., Piee-Staffa A., Sied U., Thomale J., Schneider A., Kaina B., Koberle B. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol Cancer. 2010;9:248. doi: 10.1186/1476-4598-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie T., Rubio N., Garg A.D., Bultynck G., Rizzuto R., Decuypere J.P., Piette J., Linehan C., Gupta S., Samali A., et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.F., Chen M.S., Chou Y.C., Ueng Y.F., Yin P.H., Yeh T.S., Lee H.C. Mitochondrial dysfunction enhances cisplatin resistance in human gastric cancer cells via the ROS-activated GCN2-eIF2alpha-ATF4-xCT pathway. Oncotarget. 2016;7:74132–74151. doi: 10.18632/oncotarget.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Ke S., Chen G., Gao Q., Wu S., Wang S., Zhou J., Yang X., Lu Y., Ma D. Effect of lung resistance-related protein on the resistance to cisplatin in human ovarian cancer cell lines. Oncol Rep. 2004;12:1365–1370. [PubMed] [Google Scholar]
- Watson S.J., Brown A.J., Holliday N.D. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol. 2012;81:631–642. doi: 10.1124/mol.111.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R.C., Jiang H.Y., Anthony T.G. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Weldon S.M., Mullen A.C., Loscher C.E., Hurley L.A., Roche H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2007;18:250–258. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Xu H., Choi S.M., An C.S., Min Y.D., Kim K.C., Kim K.J., Choi C.H. Concentration-dependent collateral sensitivity of cisplatin-resistant gastric cancer cell sublines. Biochem Biophys Res Commun. 2005;328:618–622. doi: 10.1016/j.bbrc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Yu F., Megyesi J., Price P.M. Cytoplasmic initiation of cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2008;295:F44–52. doi: 10.1152/ajprenal.00593.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.S., Zhou S.F., Wang Q., Guo J.N., Liang H.M., Deng J.B., He W.Y. Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2alpha pathway in Alzheimer’s disease. Neuroscience. 2016;325:1–9. doi: 10.1016/j.neuroscience.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Zijlstra J.G., de Vries E.G., Muskiet F.A., Martini I.A., Timmer-Bosscha H., Mulder N.H. Influence of docosahexaenoic acid in vitro on intracellular adriamycin concentration in lymphocytes and human adriamycin-sensitive and -resistant small-cell lung cancer cell lines, and on cytotoxicity in the tumor cell lines. Int J Cancer. 1987;40:850–856. doi: 10.1002/ijc.2910400625. [DOI] [PubMed] [Google Scholar]


