Abstract
As a heterogeneous group of clonal disorders, acute myeloid leukemia with internal tandem duplication of fms-like tyrosine kinase 3 (FLT3-ITD) mutation usually shows an inferior prognosis. In the present study, we found that homoharringtonine (HHT), a protein translation inhibitor of plant alkaloid in China, exhibited potent cytotoxic effect against FLT3-ITD (+) cell lines and primary leukemia cells, and a remarkable synergistic anti-leukemia action was demonstrated in vitro and in vivo in xenograft mouse models when co-treated with the heat shock protein 90 inhibitor IPI504. Mechanistically, HHT combined with IPI504 synergistically inhibited the growth of leukemia cells by inducing apoptosis and G1 phase arrest. This synergistic action resulted in a prominent reduction of total and phosphorylated FLT3 (p-FLT3) as well as inhibition of its downstream signaling molecules such as STAT5, AKT, ERK and 4E-BP1. Furthermore, co-treatment of HHT and IPI504 led to a synergistic or additive effect on 55.56%(10/18) of acute myeloid leukemia cases tested, including three relapsed/refractory patients. In conclusion, our findings indicate that the combination of HHT and HSP90 inhibitor provides an alternative way for the treatment of FLT3-ITD positive acute myeloid leukemia, especially for relapsed/refractory AML.
Background
As a genetically heterogeneous group of diseases, acute myeloid leukemia (AML) shows distinct features in the fields of clinicopathology, cytogenetics and genetics [1], [2]. However, the common pathogenesis of AML are block in cellular differentiation and/or aberrant proliferation in myeloblasts [3]. The FLT3 (FMS-like tyrosine kinase 3) gene with a mutation of internal tandem duplications (ITD) in the juxtamembrane domain occurs approximately 30% in AML with normal karyotype [4]. And accumulating evidence reveals that AML accompanied with FLT3-ITD mutation has poor prognosis and a higher disease relapse rate, which reminds us that FLT3-ITD is a desirable therapeutic target in personalized medicine of AML [5], [6], [7]. Despite improvement of complete remission (CR) induced by conventional chemotherapy, consolidation and/or allogeneic hematopoietic stem cell transplantation (HSCT) in AML, disease relapse and drug resistance are still two key obstacles in long-term remission and overall survival (OS) of patients [8]. Therefore, it is urgent to find novel available strategies or regimens in the treatment of AML patients with FLT3-ITD.
Homoharringtonine (HHT), also known as omacetaxine mepesuccinate, is a classical anti-leukemia drug which has been used for nearly 40 years in China. Although HHT has been studied for many years, the precise targets are still unclear [9], [10]. Our previous study demonstrated that HHT can inhibit the proliferation of leukemia cells by down-regulating the expression of the phospho-eIF4E (p-eIF4E), an indispensable protein for survival and growth in normal cells or malignant cells [11], [12], [13]. Recently, our studies and others’ have shown that HHT is more sensitive to FLT3-ITD (+) AML cell lines and primary samples as compared with AML with wild-type FLT3. However, to date, it exhibits limited anti-leukemic activity with monotherapy.
Previous studies have shown that the heat-shock protein 90 (Hsp90) was a molecular chaperone of FLT3 and highly expressed in FLT3-ITD (+) AML and other tumors [14], [15], [16]. And as a molecular target in leukemic therapy, several small-molecule drugs that target the molecular chaperone Hsp90 have been developed as potential anticancer agents over the past decade [17], [18]. Here, we find out that a novel inhibitor of Hsp90 named as IPI-504, which derives from the well-studied Hsp90 inhibitor 17-allylamino-17-demethoxy-geldanamycin (17-AAG) [19], [20], can effectively induce apoptosis on FLT3-ITD (+) cell lines and patient samples.
Therefore, in the present study, we examined whether HHT would strengthen the anti-leukemic effect when combined with the Hsp90 inhibitor IPI504 in vitro and vivo. Simultaneously, we also explored the potential mechanisms for the synergistic effect of both two drugs.
Materials and Methods
Cell culture
The human AML cell lines THP-1, Kasumi-1 and MV4-11 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MOLM-13 was kindly provided by Prof. Jie Jin (Department of Hematology, The First Affiliated Hospital of Zhejiang University, Hangzhou, China). The cells were cultivated in RMPI 1640 medium (GIBCO, Bethesda, MD, USA) (THP-1, Kasumi-1) and IMDM (GIBCO, Bethesda, MD, USA) (MV4-11, MOLM-13) containing 10% fetal bovine serum (FBS), 100μg/mL streptomycin and 100 units/mL penicillin. All cells were maintained at 37°C with 5% CO2.
Patient Samples and Ethics Statement
The human primary AML cells were isolated from patients with their informed consent according to the Declaration of Helsinki. All experiments were approved by the ethics committee of Second Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China).
Cell Viability and Apoptosis Assay
The assessment of cell viability and apoptosis were performed as before [12]. HHT was purchased from Nanjing Spring & Autumn Biological Engineering Co., Ltd, China. IPI504 was purchased from MCE (MedChemExpress, USA).
Cell Cycle Assay
Different treated cells were harvested and fixed with 75% ethanol overnight at -20°C, then washed with PBS and incubated with propidium iodide (PI) staining solution for 30 min. Cells were detected by flow cytometry and analyzed by ModFit LT5.0 (Verity Software House, USA).
Western Blotting
The immunoblotting was conducted as before [12]. The primary antibodies used were presented as follows, FLT3, p-AKT (Ser473), AKT, p-ERK (T202/Y204), Caspase-3, PARP, 4E-BP1 and p4E-BP1 (S65), which were all purchased from Cell Signaling Technology (Beverly, MA). ERK1/2, STAT5, p-STAT5 (Y694) and β-actin were obtained from Huabio (Hangzhou, China). p-FLT3 (Y591), MCL-1, BCL-XL, BCL-2, CDK-2, CDK-4 and CDK-6 were purchased from Abcam (Cambridge, MA).
Colony Formation Assays
MV4-11 and MOLM-13 cells (600 cells per plate) were seeded in low-melting- point agarose, in the presence of 2.5 nM HHT and/or 5 nM IPI504, then reconstituted with 2×IMDM medium supplemented with 20% FBS. After two weeks, the colonies were visualized by staining with 0.1% crystal violet and then counted under light microscope (cells > 50 will be counted as a colony). The drug-treated colony formation efficiency was calculated through dividing it by control colony numbers.
Xenotransplantation and In Vivo Drug Treatment
All animal procedures were approved by the Institution’s Ethics Committee. NSG (NOD/SCID/IL2Rγ-/-) Mice at 6–7 weeks of age (Beijing Biocytogen Co., Ltd., China) were injected through tail veins with MOLM-13 (2 × 105 cells) stably transduced with a luciferase reporter gene. Fourteen days after injection, the engrafted mice were treated with vehicle (1%DMSO in 200 μL PBS,intraperitoneal), HHT (in 200 μL PBS per mouse, 0.5 mg/kg/d, intraperitoneal), and/or IPI504 (in 200 μL PBS, 50 mg/kg, every other day, intraperitoneal). And engraftment was assessed by intraperitoneal injection of luciferin (2 mg/mouse) (Promega, Madison, WI) after anesthesia (fentanyl/fluniasone and midazolam) and bioimaging of mice was performed at scheduled time points by using an in vivo IVIS 100 bioluminescence/optical imaging system (Xenogen, Alameda, CA).
Statistical Analysis
The statistical analyses were performed with IBM SPSS Statistics 20. The combination index (CI) value was calculated by CalcuSyn software (Biosoft, Cambridge, UK). The IC50 was determined by GraphPad software. Survival analysis was performed by using the Kaplan-Meier method and differences in survival were analyzed by Log-rank test. Other differences were evaluated by t-test analysis of variance and P values <.05 were considered statistically significant. All results are shown as means ± SEM. *P < .05; **P < .01; ***P < .001.
Results
The Cytotoxic Effect of HHT and IPI504 on the AML Cells
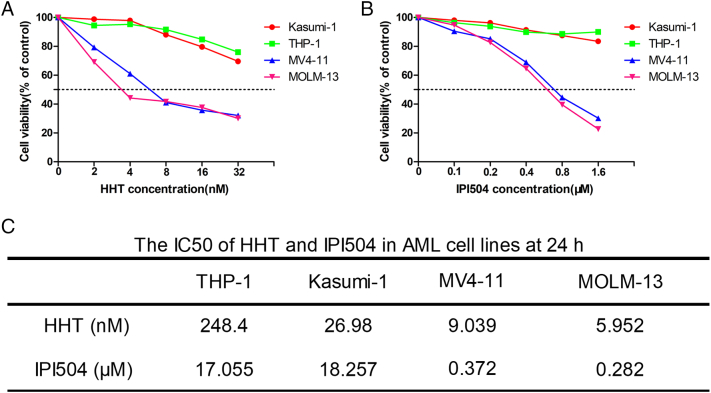
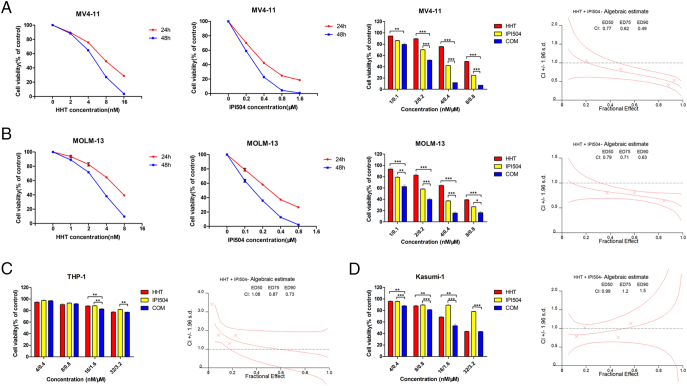
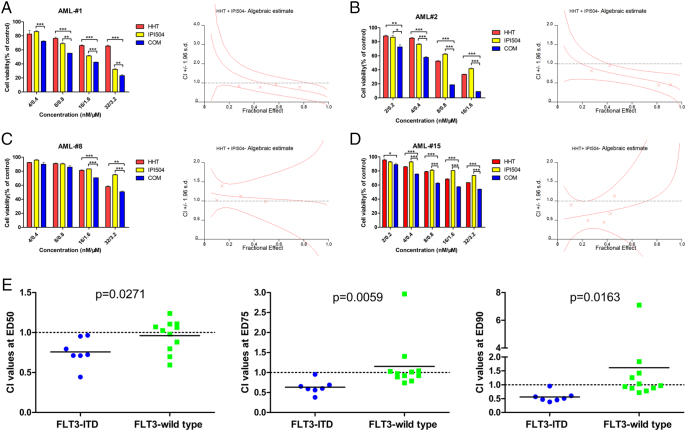
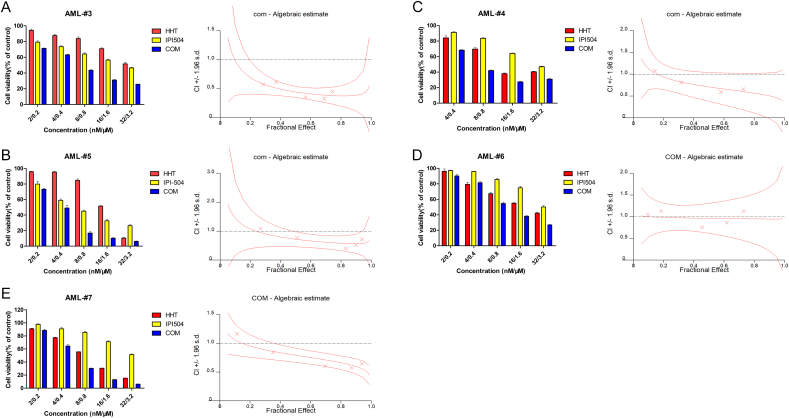
In this study, we used the MTT assay to examine the viability of AML cells treated by HHT and IPI504, alone or combined. Briefly, AML cells were treated with increasing concentrations of HHT (1, 2, 4, 8, 16 and 32 nM) or IPI504 (0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 μM) for 24 h and 48 h. DMSO was used as the control. We observed that FLT3-ITD (+) cell lines MV4-11 and MOLM-13 were significantly more susceptible to HHT or IPI504 than FLT3-ITD negative cell lines Kasumi-1 and THP-1 (Figure 1, A and B). Moreover, both HHT and IPI504 showed higher cytotoxicity in MOLM-13 than that in MV4-11 cells, in which 24 h IC50 values of HHT and IPI504 for MV4-11 and MOLM-13 cells were 9.039 nM and 5.952 nM, 0.372 μM and 0.282 μM, respectively (Figure 1C). HHT or IPI504 treatment inhibited the cell proliferation in a dose and time dependent manner. Notably, co-treatment with HHT and IPI504 exhibited a significant synergistic effect on FLT3-ITD positive cell lines (Figure 2, A and B) and primary AML cells (Figure 3, A and B), but no significant synergism was found in FLT3-wild type cell lines (Figures 2, C and D; 3, C and D). The IC50 values of HHT and IPI504 for the primary samples were shown in Supplementary Table 1 and the clinical characteristics of primary samples were presented in Supplementary Table 3. The dose–effect curves were determined by calcusyn analyses. The primary samples combination index (CI) values were calculated according to the median effect method of Chou and Talala [21], which was presented in Supplementary Table 2. After 18 primary samples co-treated by HHT and IPI504 for 48 h, the CI values between the FLT3-ITD positive and the wild type were compared in Figure 3E. The CI value less than 1.0 meant a synergistic effect. All results were performed by three independent experiments. Taken together, these results suggested that HHT and the Hsp90 inhibitor IPI504 exhibit potent cytotoxic effect and significant synergism when combined with both drugs in AML cell lines and primary samples.
Figure 1.
HHT and IPI-504 inhibit the growth of AML cell lines. (A and B) The cell viability analyzed by MTT assays in AML cell lines treated with increasing concentrations of HHT and IPI504 at 24 h. (C) The IC50 of HHT and IPI504 in AML cell lines at 24 h. The data are presented as mean ± SEM from at least three independent experiments.
Figure 2.
HHT and IPI-504 synergistically inhibit the growth of AML cell lines. The cell viability induced by HHT, IPI504 and HHT + IPI504 in a fixed ratio (1:100) in (A) MV4-11 cells, (B) MOLM-13 cells, (C) THP-1 cells and (D) Kasumi-1 cells at 24 h or 48 h, and the CI values at ED50, ED75 and ED90 were presented. (mean ± SEM, n=3, *P < .05, **P < .01, ***P < .001).
Figure 3.
HHT and IPI50 inhibit the growth of primary AML cells. (A and B) The FLT3-ITD (+) primary AML cells and (C and D) FLT3-wild type primary AML cells were treated with HHT, IPI504 and HHT+IPI504 for 48 h. The rate of cell viability was measured by MTT assay. (E) The combination index (CI) values of 18 patient samples at the ED50, ED75 and ED90 were showed (FLT3-ITD (+) n= 7; FLT3-wt n=11, solid bars represent the mean CI values for each group). The CI values were calculated by Calcusyn according to the median effect method of Chou and Talalay [21]. (mean ± SEM, n=3, *P < .05, **P < .01, ***P < .001)
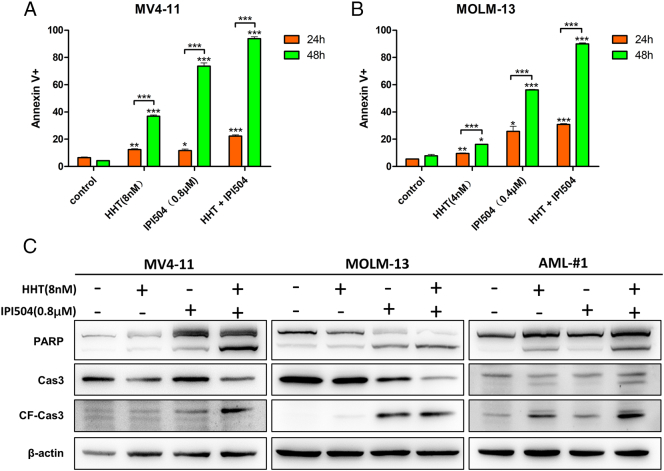
HHT and IPI504 Induce Apoptosis in AML Cell Lines Harboring FLT3-ITD
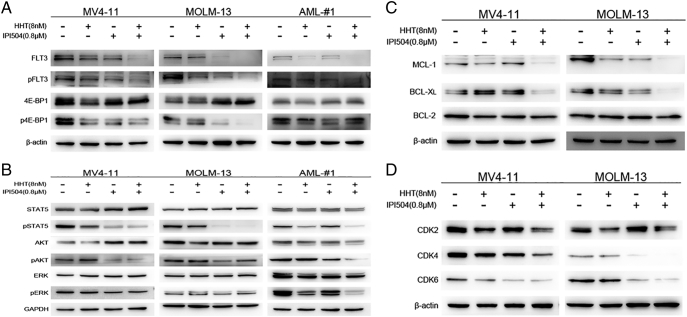
The FLT3-ITD (+) cell lines MV4-11 and MOLM-13 were treated by HHT and/or IPI504 for 24 h and 48 h and then apoptosis was analyzed. We observed that combination of HHT and IPI504 resulted in a prominent augmentation in apoptosis of MV4-11 and MOLM-13 cells compared with the control (DMSO) or both drugs alone (P < .05) (Figure 4, A and B). Next, we performed western blot to analyze the relevant signaling molecules of apoptotic pathway. As shown in Figure 4C, the expression levels of cleaved PARP and cleaved caspase-3 were greatly increased both in MV4-11 and MOLM-13 cells after co-treated by HHT and IPI504 for 24 h. And the expression of pro-survival protein MCL-1 and BCL-XL were remarkably attenuated after cells exposed by HHT and IPI504 for 24 h. However, there was no obvious change in the expression level of BCL-2 (Figure 6C).
Figure 4.
HHT and IPI504 synergistically induced apoptosis in AML cell lines harboring FLT3-ITD. (A and B) The FLT3-ITD mutant cell lines MV4-11 and MOLM-13 were exposed by HHT (8 nM or 4 nM) and/or IPI504 (0.8 μM or 0.4 μM) for 24 h and 48 h. Cells were harvested and co-stained with Annexin V and PI before apoptosis was analyzed by flow cytometry (mean ± SEM, n=3, *P < .05, **P < .01, ***P < .001). (C) After treated with HHT (8 nM) and/or IPI504 (0.8 μM) for 24 h, MV4-11, MOLM-13 and primary cell lysates were subjected to western blot analysis using the PARP, caspase3 and cleaved caspase3 antibodies, β-actin was displayed as a loading control.
Figure 6.
HHT + IPI-504 synergistically inhibit major targets and oncogenic signaling pathways. (A-D) Western blots exhibiting expression levels of total FLT3, phosphorylated FLT3 (pFLT3) and its downstream signaling proteins or targets in MV4-11, MOLM-13 or primary sample treated with vehicle, HHT (8 nM) and/or IPI504 (0.8 μM) for (A, C and D) 24 hours and (B) 6 hours, with GAPDH or β-actin being the loading control.
Effects of HHT Combined with IPI504 on Cell Cycle and Colony Formation
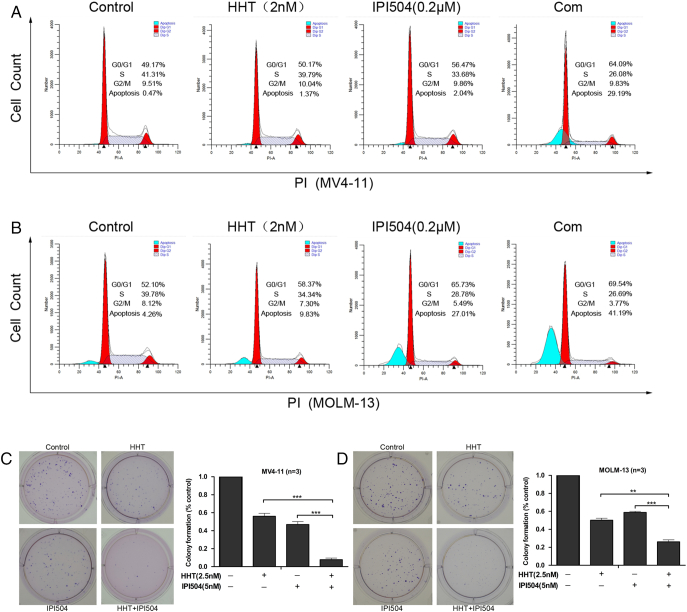
Next, we explored the effects of HHT, IPI504 or the combination of both on the cell cycle and colony formation in FLT3-ITD (+) cells. MV4-11 and MOLM-13 cells were treated with the indicated concentrations of HHT and/or IPI504 for 48 hours. As displayed in Figure 5, A and B, treatment of cells by low dose of HHT at 2 nM or IPI504 at 0.2 μM, and it did not affect G0/G1 and S phase cells and apoptosis obviously. However, co-treatment with HHT at 2 nM and IPI504 at 0.2 μM caused a prominent G0/G1 phase arrest, S phase decreasing and apoptosis increasing. We observed that in MV4-11 and MOLM-13, G0/G1 phase cells increased by 14.92% and 17.44%, respectively, S phase cells decreased by15.23% and 13.09%, respectively. Consistent with above observations, this co-treatment also increased apoptotic cells by 28.72% and 36.93% in MV4-11 and MOLM-13 cells, respectively. We also performed colony formation assay to evaluate the long-term effects of HHT and IPI504. As shown in Figure 5, C and D, low dose of HHT (2.5 nM) or IPI504 (5 nM) alone exhibited 44.00% or 49.76%and 53.00% or 41.20% of colony formation inhibition, respectively, for MV4-11 or MOLM-13 cells, whereas combination of HHT and IPI504 at the same dose caused 99.2% or 73.87% of colony formation inhibition, suggesting that the combination of HHT and IPI504 at low dose significantly enhance cell killing ability.
Figure 5.
Effects of HHT, IPI504, HHT+ IPI504 on cell cycle and colony formation. (A and B) The representative flow cytometry analysis for PI staining in MV4-11 and MOLM-13 cell lines treated with HHT, IPI504 or HHT+IPI504 at the indicated concentrations for 48 h are depicted. (C and D) Representative images of colonies formation for MV4-11 and MOLM-13 cells under HHT (2.5 nM), IPI504 (5 nM) and HHT+IPI504 treatment about 14 days. Quantification of colony counts (% of control) with different group treatment as indicated. (mean ± SEM, n=3, *P < .05, **P < .01, ***P < .001)
In western blotting assay, MV4-11 and MOLM-13 cells treated with the indicated concentration of HHT and IPI504 for 24 hours displayed an apparent down-regulation in cyclin dependent kinase 2, 4 and 6 (CDK-2, CDK-4, CDK-6) proteins, which represented the G1 phase arrest of cell cycle (Figure 6D). Taking all the results into consideration, it further confirmed the synergistic anti-proliferative effects of HHT combined with IPI504 in FLT3-ITD (+) AML.
HHT and IPI504 Down-Regulated the FLT3-ITD Mutant and Its Oncogenic Signaling Pathways
It is well known that the mutation of FLT3-ITD causes aberrant activation of downstream kinases such as STAT5, AKT and MAPK/ERK [22], [23], [24]. To determine whether the combination of HHT and IPI504 would synergistically affect FLT3-ITD mutant protein, leukemia cells were treated with HHT at 8 nM or IPI504 at 0.8 μM for 24 hours and then collected for analysis of FLT3-ITD using western blot. We found the combination of HHT and IPI504 synergistically reduced the protein levels of both total FLT3 and phosphorylated FLT3 (p-FLT3) in MV4-11, MOLM-13 and primary sample cells (Figure 6A). To determine whether the combination of HHT and IPI504 would affect FLT3-ITD downstream molecules such as STAT5, AKT and MAPK/ERK, MV4-11 and MOLM-13 cell lines were treated with HHT (8 nM) plus IPI504 (0.8 μM) for 6 h and then collected for western blotting analysis. We observed that the combination of HHT and IPI504 greatly reduced phosphorylated protein levels of STAT5 (p-STAT5), AKT (p-AKT) and ERK1/2 (p-ERK1/2), but it did not affect their total protein levels. In line with this, similar results were also observed in primary AML samples (Figure 6B). Because 4E-binding protein 1 (4E-BP1) played a key role in protein synthesis, whose phosphorylation loosened its inhibition on eukaryotic translation initiation factor 4E (eIF4E), which facilitated protein translation initiation [25], we next evaluated the effect of this combination on p4E-BP1 and found out that phosphorylated 4E-BP1 (p4E-BP1) was obviously attenuated in MV4-11 and MOLM-13 cells treated by HHT (8 nM) combined with IPI504 (0.8 μM) for 24 h (Figure 6A). Therefore, we have reasons to believe that HHT and IPI504 synergistically abrogate proliferation and induce apoptosis through regulating the major molecular target FLT3 and then lead to an inhibition in its downstream signaling cascades on FLT3-ITD mutant acute myeloid leukemia.
Combination of HHT and IPI504 Enhances Anti-Leukemia In Vivo
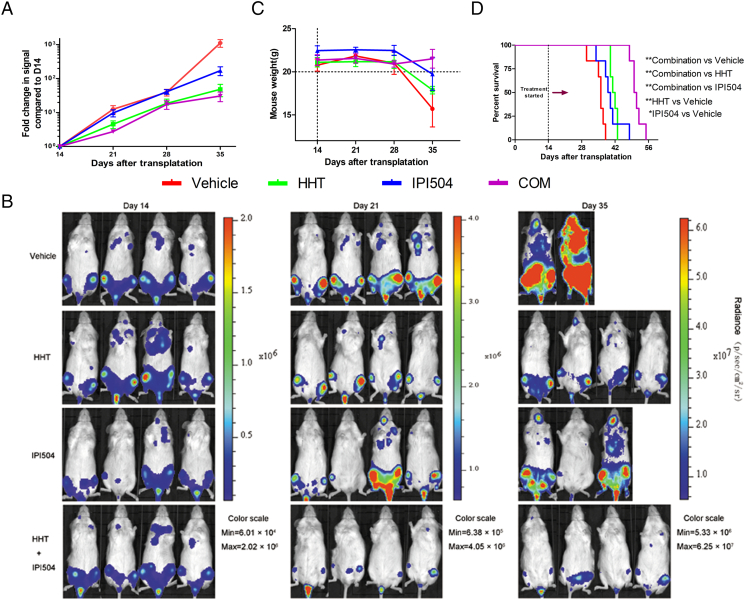
To validate the synergic anti-leukemia effect of HHT and IPI504 in vivo, we engrafted NSG mice with MOLM-13 cell line expressing the luciferase reporter gene. After 14 days, leukemic mice were intraperitoneally injected daily with vehicle, HHT (0.5 mg/kg per day), IPI504 (50 mg/kg, every other day), or the combination. Compared with those receiving vehicle, HHT or IPI504 monotherapy, mice treated with HHT and IPI504 showed less engraftment of MOLM-13 and a slower increase of leukemic signal up to 35 days (Figure 7, A and B), which was consistent with the results in vitro. Meanwhile, the survival time of mice treated with HHT+IPI504 was longer than those receiving vehicle, HHT or IPI504 monotherapy. And we also analyzed the statistical significances between each survival curve and vehicle by Log-rank test which P value were 0.004 (HHT compared to vehicle), 0.017 (IPI504 compared to vehicle) and 0.001 (HHT+IPI504 compared to vehicle), respectively (Figure 7D). Notably, only the group of mice treated with the combination of HHT and IPI504 showed an increasing body weight up to 35 days (Figure 7C), suggesting that the combination of HHT and IPI504 might improve the quality of life for AML. As described above, our data further proves the synergic anti-leukemia effect of HHT plus the Hsp90 inhibitor IPI504 in vivo, which provides a firm base in future clinical therapy for FLT3-ITD (+) acute myeloid leukemia.
Figure 7.
In vivo effect of HHT and IPI504 combination. (A, B) The trend of bioluminescence intensities and xenogen images of NSG mice transplanted with luciferase-labeled MOLM-13 cells before (day 14) and after treatment with vehicle (n = 6), HHT (n = 6), IPI504 (n = 6), and their combination (n = 6). (C) The change of body weight of mice treated with vehicle, HHT, IPI504 and combination of both. (D) Kaplan-Meier survival curves were analyzed for the mice and Log-rank test was used to calculate the statistical significances with HHT compared to vehicle (P - .004), IPI504 compared to vehicle (P - .017) and HHT+IPI504 compared to vehicle (P - .001), respectively.
Discussion
In this report, we demonstrated that the combination of HHT and the Hsp90 inhibitor IPI504 exhibited a significant anti-leukemic action on FLT3-ITD (+) AML in vitro and in vivo. Mechanistically, the combination of HHT and IPI504 synergistically inhibited FLT3 protein and its downstream STAT5, AKT, ERK and 4E-BP1, leading to apoptosis and cell arresting at G1.
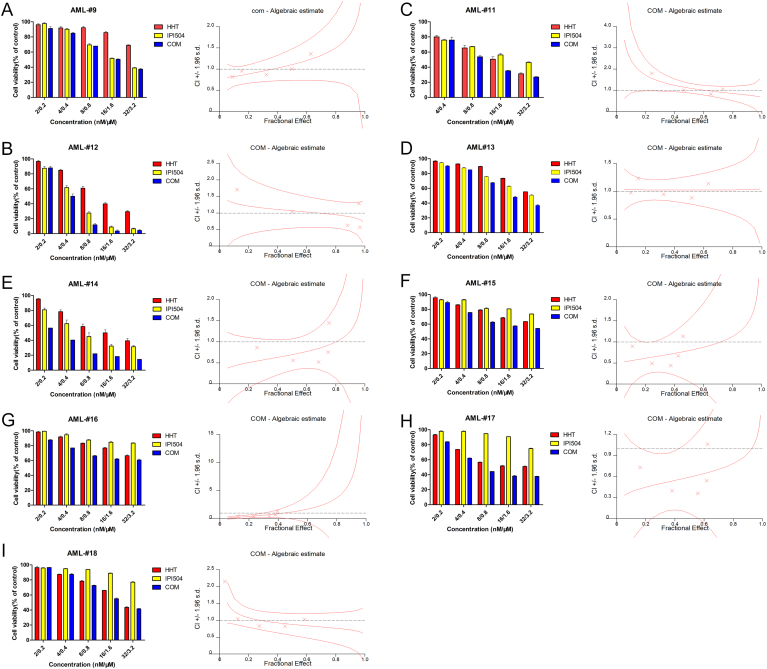
Acute myeloid leukemia with FLT3-ITD mutations are general associated with decreased disease-free survival (DFS) and poor overall survival (OS) [26]. Although FLT3 tyrosine kinase inhibitors (TKI) have been developed to treat FLT3-mutant AML patients, limited efficacy was observed because of relapse and rapid drug resistance [27]. Recently, we and others observed that HHT behaved a sensitive cytotoxic effect on FLT3-ITD (+) AML cells [28], [29]. As many works highlighted, Hsp90 is a molecular chaperone of mutant FLT3 which is involved in holding conformation, stabilization and function of oncoproteins. The Hsp90 inhibitors have been developed to selectively kill FLT3-mutant cells in AML [14], [16], [30], [31], [32]. We therefore speculated that whether anti-leukemic effect might be enhanced when combining HHT with the Hsp90 inhibitor. As expected, HHT and the Hsp90 inhibitor IPI504 synergistically inhibited the proliferation and clonogenic growth of human FLT3-ITD (+) AML cell lines. And we also evaluated the effects on primary AML patient cells. We observed that 39%(7/18) FLT3-ITD mutant patient samples presented a significant synergistic effect after performed by HHT + IPI504 for 48 h. In addition, the combination of HHT with IPI504 also exhibited great synergistic action for several FLT3-wild type AML cells, implying other underlying mechanisms may be involved in the therapy of both two drugs which need further study (Supplementary Figure 2). More importantly, similar results were also observed in three relapsed/refractory AML samples (Supplementary Figure 1, B, D and E). Consistent with in vitro observations, the combination of HHT with IPI504 not only significantly reduced tumor burden but also prolonged tumor-bearing mouse survival time as compared to the vehicle or monotherapy in FLT3-ITD (+) AML xenograft mouse models.
Supplementary Figure 2.
The synergistic anti-leukemic effects of HHT + IPI504 in FLT3-ITD (-) AML samples.
(A -I) The FLT3-ITD (-) primary AML cells were treated with HHT, IPI504 and HHT+IPI504 for 48 h then cell viability was measured by MTT assay. And the CI values were calculated by Calcusyn.
Supplementary Figure 1.
The synergistic anti-leukemic effects of HHT + IPI504 in FLT3-ITD (+) AML samples.
(A -E) The FLT3-ITD (+) primary AML cells were treated with HHT, IPI504 and HHT+IPI504 for 48 h then cell viability was measured by MTT assay. And the CI values were calculated by Calcusyn. The (B), (D) and (E) are relapsed/refractory AML samples.
Evasion of apoptosis, an important hallmark of cancer, is caused by activation of anti-apoptotic molecules of the BCL-2 protein family [33], [34], [35]. And aberrant activation of BCL-2 members such as MCL-1, BCL-XL and BCL-2 were involved in anti-apoptosis and drug resistance in FLT3-ITD mutant AML [36], [37], [38]. Our studies reveal that the combination of HHT with IPI504 potently inhibits the expressions of anti-apoptotic molecules MCL-1 and BCL-XL, which may explain why the combination of HHT with IPI504 efficiently kill relapsed/refractory AML cells. It is as well know that FLT3-ITD mutations lead to loss of autoinhibitory function for FLT3 kinase, which then results in constitutive activation of its downstream signaling pathways, including the JAK/STAT5, PI3K/AKT and RAS/MAPK [23], [26], [39]. In the study, we demonstrate that HHT combined with IPI504 synergistically decrease the expression of total and phosphorylated forms of FLT3 as well as its downstream members such as p-STAT5, p-AKT and p-ERK in human FLT3-ITD (+) AML cell lines and patient samples.
Since JAK/STAT5 signaling is significantly activated in leukemic stem cells of high-risk AML patients [40], the STAT5 activation induced by FLT3-ITD confers drug resistance by protecting the mTOR/4EBP1/MCL-1 pathway [41]. Interestingly, our results show that the combination of HHT with IPI504 could disrupt the mTOR/4EBP1/MCL-1 pathway with inhibition of p-4EBP1, MCL-1 and p-STAT5, suggesting that the combination of HHT with the Hsp90 inhibitor IPI504 may affect leukemic stem cells.
Conclusions
Taken together, in this study, we mainly investigated the synergistic anti- leukemic effect between HHT and the Hsp90 inhibitor IPI504 on FLT3-ITD mutant cells and then further confirmed by primary AML samples as well as orthotopic xenograft models. All of the results provide a solid base for future personal therapy with novel therapeutic regimen HHT + Hsp90 inhibitors in de novo or relapsed/refractory AML patients.
The following are the supplementary data related to this article.
The IC50 of HHT and IPI504 in primary AML samples at 48 h
The characteristics of the patient samples
The CI values of HHT + IPI504 in primary AML samples at 48 h
Declarations
Ethics Approval and Consent to Participate
The human primary AML cells were isolated from patients with their informed consent according to the Declaration of Helsinki. This study was approved by the ethics committee of Second Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China).
Consent for Publication
All the patients involved in the study have agreed to publish their individual data. Authors have reviewed and approved the manuscript for submission.
Conflict of Interest
The authors have no conflicts to declare.
Funding
This work was supported in part by Special project foundation of the State Administration of traditional Chinese Medicine (JDZX2015114), Foundation of Zhejiang province Chinese medicine science and technology planes (2017ZB030), the Natural Science Foundation of Zhejiang Province (LY14H160032,LY19H290003 and LY18H160023), the National Natural Science Foundation of China (81270601, 81328016, 81470306 and 81670138) and Science Technology Department of Zhejiang Province (2016C33096).
Authors' Contributions
R.Z.X and J.P.S conceived of the study, initiated, designed, and supervised the experiments and wrote the manuscript. Z.X.W, H.F.Z and Q.F.Y designed and performed experiments and wrote the manuscript. X.Z.Z, X.D.J, X.Y.L, Y.X, L.L.Y, B.W.W, A.M, L.Z, X.B.X, Y.L and R.L.G performed experiments.
Acknowledgements
We thank Prof. Jie Jin from Department of Hematology, The First Affiliated Hospital of Zhejiang University for providing the MOLM-13 cell line.
Contributor Information
Jianping Shen, Email: zrxyk10@zju.edu.cn.
Rongzhen Xu, Email: zrxyk10@zju.edu.cn.
References
- 1.Medinger M, Passweg JR. Acute myeloid leukaemia genomics. Br J Haematol. 2017;179:530–542. doi: 10.1111/bjh.14823. [DOI] [PubMed] [Google Scholar]
- 2.Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, Beverloo HB, Moorhouse MJ, van der Spek PJ, Lowenberg B. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 3.Olsson I, Bergh G, Ehinger M, Gullberg U. Cell differentiation in acute myeloid leukemia. Eur J Haematol. 1996;57:1–16. doi: 10.1111/j.1600-0609.1996.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 4.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–4269. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiyoi H, Yanada M, Ozekia K. Clinical significance of FLT3 in leukemia. Int J Hematol. 2005;82:85–92. doi: 10.1532/IJH97.05066. [DOI] [PubMed] [Google Scholar]
- 6.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T, Misawa S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 7.Sheikhha MH, Awan A, Tobal K, Liu Yin JA. Prognostic significance of FLT3 ITD and D835 mutations in AML patients. Hematol J. 2003;4:41–46. doi: 10.1038/sj.thj.6200224. [DOI] [PubMed] [Google Scholar]
- 8.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 9.Lu S, Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J Hematol Oncol. 2014;7:2. doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman E, Arlin Z, Ahmed T, Mittelman A, Puccio C, Chun H, Cook P, Baskind P. Homoharringtonine is safe and effective for patients with acute myelogenous leukemia. Leukemia. 1992;6:1185–1188. [PubMed] [Google Scholar]
- 11.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175:415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, Zhou H, Gan Y, Zhang J, Chen J, Gan X, Li H, Zheng W, Meng Z, Ma X. Small-molecule induction of phospho-eIF4E sumoylation and degradation via targeting its phosphorylated serine 209 residue. Oncotarget. 2015;6:15111–15121. doi: 10.18632/oncotarget.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagner PR, Schneider A, Gartenhaus RB. Targeting the translational machinery as a novel treatment strategy for hematologic malignancies. Blood. 2010;115:2127–2135. doi: 10.1182/blood-2009-09-220020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9:4483–4493. [PubMed] [Google Scholar]
- 15.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 16.Minami Y, Kiyoi H, Yamamoto Y, Yamamoto K, Ueda R, Saito H, Naoe T. Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia. 2002;16:1535–1540. doi: 10.1038/sj.leu.2402558. [DOI] [PubMed] [Google Scholar]
- 17.An WG, Schulte TW, Neckers LM. The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ. 2000;11:355–360. [PubMed] [Google Scholar]
- 18.Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003–4009. [PubMed] [Google Scholar]
- 19.Ge J, Normant E, Porter JR, Ali JA, Dembski MS, Gao Y, Georges AT, Grenier L, Pak RH, Patterson J. Design, synthesis, and biological evaluation of hydroquinone derivatives of 17-amino-17-demethoxygeldanamycin as potent, water-soluble inhibitors of Hsp90. J Med Chem. 2006;49:4606–4615. doi: 10.1021/jm0603116. [DOI] [PubMed] [Google Scholar]
- 20.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci U S A. 2006;103:17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Choudhary C, Brandts C, Schwable J, Tickenbrock L, Sargin B, Ueker A, Bohmer FD, Berdel WE, Muller-Tidow C, Serve H. Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood. 2007;110:370–374. doi: 10.1182/blood-2006-05-024018. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J Hematol Oncol. 2011;4:13. doi: 10.1186/1756-8722-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21:2555–2563. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 25.Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, Baselga J, Ramon y Cajal S. 4E-binding protein 1: a key molecular "funnel factor" in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 26.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 27.Kayser S, Levis MJ. FLT3 tyrosine kinase inhibitors in acute myeloid leukemia: clinical implications and limitations. Leuk Lymphoma. 2014;55:243–255. doi: 10.3109/10428194.2013.800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Yin X, Wang H, Huang J, Yu M, Ma Z, Li C, Zhou Y, Yan X, Huang S. The combination effect of homoharringtonine and ibrutinib on FLT3-ITD mutant acute myeloid leukemia. Oncotarget. 2017;8:12764–12774. doi: 10.18632/oncotarget.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam SS, Ho ES, He BL, Wong WW, Cher CY, Ng NK, Man CH, Gill H, Cheung AM, Ip HW. Homoharringtonine (omacetaxine mepesuccinate) as an adjunct for FLT3-ITD acute myeloid leukemia. Sci Transl Med. 2016;8:359ra129. doi: 10.1126/scitranslmed.aaf3735. [DOI] [PubMed] [Google Scholar]
- 30.Blagosklonny MV. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia. 2002;16:455–462. doi: 10.1038/sj.leu.2402415. [DOI] [PubMed] [Google Scholar]
- 31.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 32.Al Shaer L, Walsby E, Gilkes A, Tonks A, Walsh V, Mills K, Burnett A, Rowntree C. Heat shock protein 90 inhibition is cytotoxic to primary AML cells expressing mutant FLT3 and results in altered downstream signalling. Br J Haematol. 2008;141:483–493. doi: 10.1111/j.1365-2141.2008.07053.x. [DOI] [PubMed] [Google Scholar]
- 33.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Minami Y, Yamamoto K, Kiyoi H, Ueda R, Saito H, Naoe T. Different antiapoptotic pathways between wild-type and mutated FLT3: insights into therapeutic targets in leukemia. Blood. 2003;102:2969–2975. doi: 10.1182/blood-2002-12-3813. [DOI] [PubMed] [Google Scholar]
- 37.Rahmani M, Aust MM, Hawkins E, Parker RE, Ross M, Kmieciak M, Reshko LB, Rizzo KA, Dumur CI, Ferreira-Gonzalez A. Co-administration of the mTORC1/TORC2 inhibitor INK128 and the Bcl-2/Bcl-xL antagonist ABT-737 kills human myeloid leukemia cells through Mcl-1 down-regulation and AKT inactivation. Haematologica. 2015;100:1553–1563. doi: 10.3324/haematol.2015.130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat. 2009;12:81–89. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, Libra M, Stivala F, Milella M, Tafuri A. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 40.Cook AM, Li L, Ho Y, Lin A, Li L, Stein A, Forman S, Perrotti D, Jove R, Bhatia R. Role of altered growth factor receptor-mediated JAK2 signaling in growth and maintenance of human acute myeloid leukemia stem cells. Blood. 2014;123:2826–2837. doi: 10.1182/blood-2013-05-505735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogami A, Oshikawa G, Okada K, Fukutake S, Umezawa Y, Nagao T, Kurosu T, Miura O. FLT3-ITD confers resistance to the PI3K/Akt pathway inhibitors by protecting the mTOR/4EBP1/Mcl-1 pathway through STAT5 activation in acute myeloid leukemia. Oncotarget. 2015;6:9189–9205. doi: 10.18632/oncotarget.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The IC50 of HHT and IPI504 in primary AML samples at 48 h
The characteristics of the patient samples
The CI values of HHT + IPI504 in primary AML samples at 48 h