Abstract
Intrapulmonary solitary fibrous tumors (SFTs) are extremely rare neoplasms. We report a case of an intrapulmonary SFT and describe the findings of computed tomography (CT) and F-18 fluorodeoxyglucose positron emission tomography. The case indicates that a benign intrapulmonary SFT can present as a ground-glass nodule in the early stages of disease and may appear as a well-defined, lobular, homogeneously enhanced mass with slow growth on chest CT images. To our knowledge, this is the first report describing the natural course of an intrapulmonary SFT over 16 years based on the findings of chest CT and F-18 fluorodeoxyglucose positron emission tomography/CT.
Keywords: Solitary fibrous tumor (SFT), Intrapulmonary, Computed tomography (CT)
Introduction
Solitary fibrous tumors (SFTs) are uncommon mesenchymal neoplasms that are usually seen in the thoracic cavity; however, it is now recognized that these tumors can occur throughout the body. The reported intrathoracic locations have included the pleural space and mediastinum [1], [2], [3]. Intrapulmonary SFTs are much less common than their pleural counterparts [4]. Primary intrapulmonary SFTs are usually benign, low-grade malignant tumors. However, intrapulmonary SFTs sometimes behave like malignant tumors and can metastasize to distant organs. Its clinical behavior has not been well characterized because of the low-frequency of occurrence [5]. A complete resection is recommended to preclude recurrence and, for diagnosis, a thorough laboratory investigation including genetic analysis should be performed [6].
This report presented the features of an intrapulmonary SFT through computed tomography (CT) in the early stages of the disease. Furthermore, the natural course of the disease over 16 years as it presented in the right upper lobe has been discussed, with adenocarcinoma as a comorbidity. In addition, we also reported here the findings of a 2-hour dual-time-point positron emission tomography (PET)/CT with F-18 fluorodeoxyglucose (FDG). To our knowledge, the morphologic changes of intrapulmonary SFTs overtime as observed on CT images have not been reported previously in an English journal.
Case report
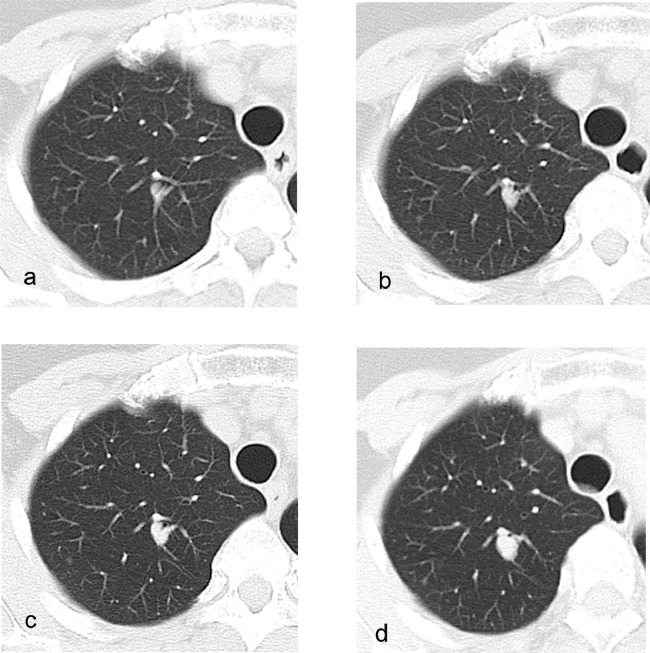
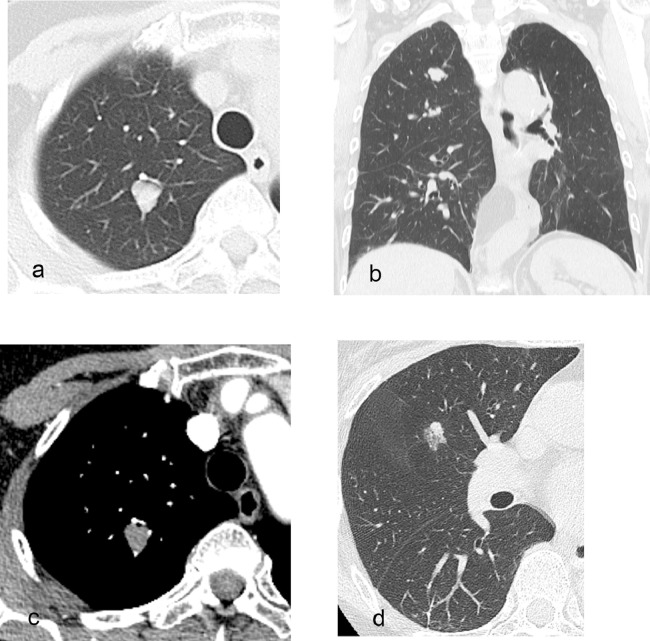
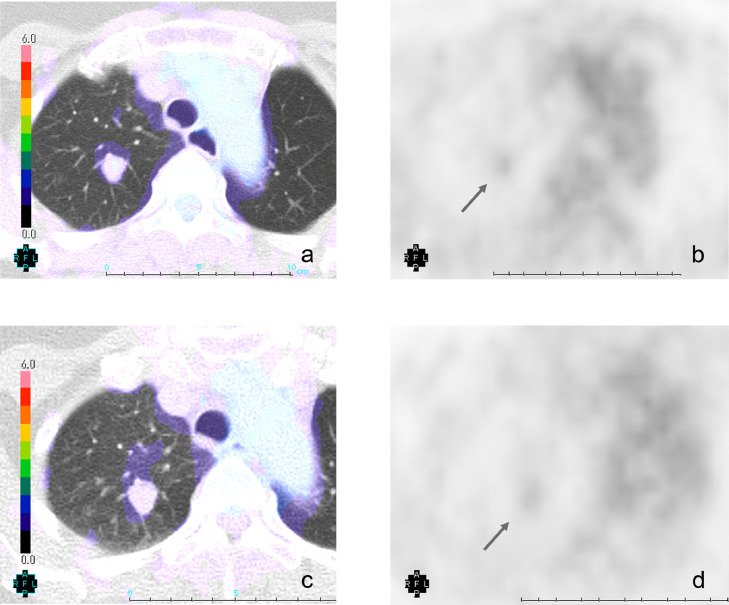
A 67-year-old woman without symptoms and no smoking history had undergone yearly follow-up chest CT scans for 16 years after surgeries for lung adenocarcinomas in the right middle lobe and in the left upper lobe. In the initial CT scan at our institution 16 years ago, there was a ground-glass nodule (GGN) with a diameter of 7 mm in the right upper lobe (Fig. 1a) and a pure GGN with a diameter of 4 mm in the bottom of the right upper lobe (not shown). Each pulmonary lesion gradually grew to be a solid tumor with a diameter of 12 mm (Fig. 1b–d). Approximately 15 years after the initial imaging, the tumor became a mixed GGN with a diameter of 12 mm (not shown). Approximately 6 months following this observation, the patient underwent a dual-time-point PET/CT. The PET/CT revealed homogeneous faint FDG accumulation in the solid tumor and GGN in the right upper lobe (not shown). The maximum standardized uptake value (SUVmax) was 1.33 at 1 hour (early phase) and 1.29 at 2 hour (delayed phase) for the solid tumor and 0.82 at 1 hour (early phase) and 0.87 at 2 hour (delayed phase) for the GGN. Over the course of the previous year, the growth rate of the solid tumor increased and subsequently reached 14 mm in diameter and became more lobulated in shape on CT (Fig. 2a–b). On preoperative noncontrast CT, these tumors had no calcification and no fat component. After an intravenous bolus of contrast material, the solid tumor was enhanced slightly and homogeneously (Fig. 2c). The GGN became a mixed GGN with a diameter of 15 mm (Fig. 2d). The patient underwent a second dual-time-point PET/CT several days later. The PET/CT revealed homogeneous faint FDG accumulation in the solid tumor and the SUVmax was 1.56 at 1 hour (early phase) and 1.32 at 2 hour (delayed phase) for the solid tumor (Fig. 3a–d).
Fig. 1.
CT features of an intrapulmonary solitary fibrous tumor over the past 16 years.
Chest CT images of a woman with intrapulmonary solitary fibrous tumor (SFT) in the right upper lobe. The intrapulmonary SFT appears as a ground-glass nodule with a diameter of 7 mm in the initial CT scans (a) The tumor became a solid tumor over time; the images show the tumor (b) 3 years after the initial imaging, (c) 7 years after the initial imaging, and (d) 15 years after the initial imaging.
Fig. 2.
CT features of the intrapulmonary solitary fibrous tumor and adenocarcinoma before surgery.
The solitary fibrous tumor (SFT) had grown into a more lobulated shape with a diameter of 14 mm on CT (a, b). The SFT was enhanced slightly and homogeneously on contrast-enhanced CT (c). The ground-glass nodule had become a mixed ground-glass nodule with a diameter of 15 mm, which strongly suggests adenocarcinoma (d).
Fig. 3.
Dual-time-point F-18 FDG PET/CT and F-18 FDG PET images of intrapulmonary solitary fibrous tumor.
F-18 2 h dual-time-point FDG PET/CT revealed homogeneous faint FDG accumulation in the solid tumor. The SUVmax of the solid tumor was 1.56 at 1 hour (early phase; a, b) and 1.32 at 2 hour (delayed phase; c, d).
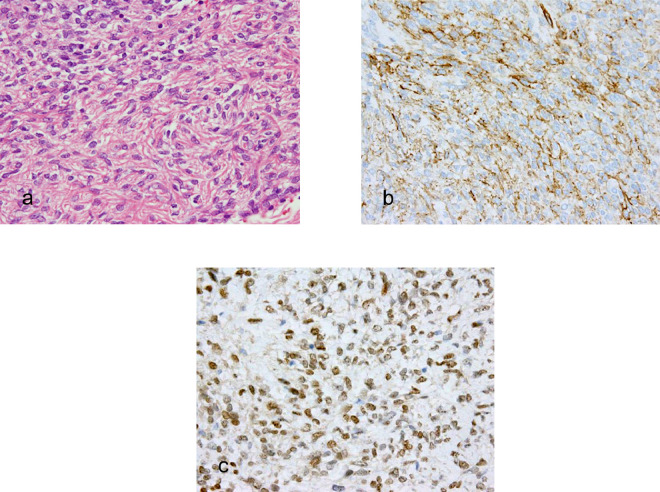
Subsequently, a CT fluoroscopy-guided cutting needle biopsy was performed for the solid tumor in the right upper lobe. A histologic examination revealed that the solid tumor comprised a patternless distribution of spindle cells within a collagenous stroma (Fig. 4a). In an immunohistochemical assessment, the tumor cells showed diffuse positivity for CD34 (Fig. 4b) and diffuse nuclear positivity for signal transducer and activator of transcription 6 (STAT6; Fig. 4c). A provisional diagnosis of SFT was made for the solid tumor.
Fig. 4.
Histopathological and immunohistochemical staining of solitary fibrous tumor.
(a) The photomicrograph shows the specimen comprised a patternless distribution of spindle-shaped cells within a collagenous stroma (H and E, 400×). The tumor cells were positive for CD34 (b) and showed diffuse nuclear positivity for STAT6 (c).
A right upper lobectomy and partial resection of the right middle lobe were simultaneously performed for the solid tumor and the GGN. Histopathologic diagnoses of intrapulmonary SFT and lung adenocarcinoma were made for the solid tumor and the GGN, respectively. The patient is currently alive without evidence of recurrence 18 months after local surgery for the intrapulmonary SFT and lung adenocarcinoma.
Discussion
SFTs are uncommon mesenchymal neoplasms consisting of cellular and collagenous components. Until recently, a diagnosis of SFT was confirmed by characteristic positive immunohistochemical staining for CD34 (90% to 95% of cases) and CD99 (70% of cases), and negative staining for S-100 [2], [7]. However, CD34 is itself nonspecific. Currently, nuclear expression of STAT6 has become the "gold standard" [8]. The majority of SFTs arise from the visceral pleura and some arise from the parietal pleura; however, intrapulmonary SFTs are extremely rare [4], [9], [10].
In general, small SFTs of the thorax are well-defined, homogeneously hyperdense masses. For benign pleural SFTs, the tumor may show both mobility and deformability, with resultant changes in location and shape observed on serial imaging [1]. On contrast-enhanced CT, there is considerable overlap in the type of enhancement; 100% of malignant and 60% of benign pleural SFTs exhibit heterogeneous enhancement [11]. On F-18 FDG PET [2], [5], benign SFTs exhibit low-grade activity and malignant SFTs tend to be hypermetabolic and homogeneous.
Several radiological reports have described CT and MRI findings in patients with intrapulmonary SFT [1], [2], [5], [12], [13]. Chick et al [1] investigated the radiological findings of SFTs and described intrapulmonary SFTs as well-circumscribed masses with variable enhancement patterns on contrast-enhanced CT. Such CT findings can led to a wide range of differential diagnoses including hamartomas, carcinoid tumors, and bronchogenic carcinomas. In the present case, the intrapulmonary SFT was a GGN with a diameter of 7 mm in the initial CT scan. The SFT had gradually grown without mobility and had become more lobulated in shape. Preoperative contrast-enhanced CT showed a well-circumscribed lobulated mass with weak homogeneous enhancement. Two PET/CT scans in the past year revealed a faint accumulation of FDG. Identification of intrapulmonary SFT with images only may be difficult when the neoplasms are intrapulmonary [1]. However, the image findings in our case, especially the slow growing speed and faint FDG uptake, are useful predictors of a benign intrapulmonary SFT.
In conclusion, intrapulmonary SFTs should be included in the differential diagnosis of a well circumscribed, lobulated, homogeneously enhancing pulmonary nodule on CT with faint FDG accumulation on PET/CT. Additionally, it is important to be aware that rare cases of SFTs located in the lung are occasionally encountered as GGNs in the early stages of disease.
Footnotes
Declarations of interest: None.
Contributor Information
Takayoshi Shinya, Email: midnight-2005@nifty.com.
Yoshihisa Masaoka, Email: yoshi-masaoka@okayama-u.ac.jp.
Shin Tanabe, Email: me19046@s.okayama-u.ac.jp.
Takehiro Tanaka, Email: takehiro@md.okayama-u.ac.jp.
Shinji Otani, Email: ohtani0814@yahoo.co.jp.
Takao Hiraki, Email: takaoh@tc4.so-net.ne.jp.
Susumu Kanazawa, Email: susumu@cc.okayama-u.ac.jp.
References
- 1.Chick J.F., Chauhan N.R., Madan R. Solitary fibrous tumors of the thorax: nomenclature, epidemiology, radiologic and pathologic findings, differential diagnoses, and management. AJR Am J Roentgenol. 2013;200(3):W238–W248. doi: 10.2214/AJR.11.8430. [DOI] [PubMed] [Google Scholar]
- 2.Ginat D.T., Bokhari A., Bhatt S., Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol. 2011;196(3):487–495. doi: 10.2214/AJR.10.4948. [DOI] [PubMed] [Google Scholar]
- 3.Gengler C., Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48(1):63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 4.Patsios D., Hwang D.M., Chung T.B. Intraparenchymal solitary fibrous tumor of the lung: an uncommon cause of a pulmonary nodule. J Thorac Imaging. 2006;21(1):50–53. doi: 10.1097/01.rti.0000186995.92705.64. [DOI] [PubMed] [Google Scholar]
- 5.Ozeki N., Kawaguchi K., Taniguchi T., Yokoi K. Primary pulmonary solitary fibrous tumour with brain metastases. Eur J Cardiothorac Surg. 2014;45(2):386–388. doi: 10.1093/ejcts/ezt289. [DOI] [PubMed] [Google Scholar]
- 6.Sagawa M., Ueda Y., Matsubara F., Sakuma H., Yoshimitsu Y., Aikawa H. Intrapulmonary solitary fibrous tumor diagnosed by immunohistochemical and genetic approaches: report of a case. Surg Today. 2007;37(5):423–425. doi: 10.1007/s00595-006-3422-3. [DOI] [PubMed] [Google Scholar]
- 7.Guillou L., Fletcher J.A., Fletcher C.D.M., Mandahl N. Extrapleural solitary fibrous tumour and hemangiopericytoma. In: Fletcher C.D.M., Unni K.K., Mertens F., editors. Pathology and genetics of tumours of soft tissue and bone. IARC Press; Lyon: 2002. pp. 86–90. editors. [Google Scholar]
- 8.Yoshida A., Tsuta K., Ohno M., Yoshida M., Narita Y., Kawai A. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38(4):552–559. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 9.Goodlad J.R., Fletcher C.D. Solitary fibrous tumour arising at unusual sites: analysis of a series. Histopathology. 1991;19(6):515–522. doi: 10.1111/j.1365-2559.1991.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 10.Khalifa M.A., Montgomery E.A., Azumi N., Gomes M.N., Zeman R.K., Min K.W. Solitary fibrous tumors: a series of lesions, some in unusual sites. South Med J. 1997;90(8):793–799. doi: 10.1097/00007611-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Rosado-de-Christenson M.L., Abbott G.F., McAdams H.P., Franks T.J., Galvin J.R. From the archives of the AFIP: localized fibrous tumor of the pleura. Radiographics. 2003;23(3):759–783. doi: 10.1148/rg.233025165. [DOI] [PubMed] [Google Scholar]
- 12.Cardillo G., Carbone L., Carleo F., Masala N., Graziano P., Bray A. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg. 2009;88(5):1632–1637. doi: 10.1016/j.athoracsur.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi K., Taniguchi T., Usami N., Yokoi K. Intrapulmonary solitary fibrous tumor. Gen Thorac Cardiovasc Surg. 2011;59(1):61–64. doi: 10.1007/s11748-010-0600-4. [DOI] [PubMed] [Google Scholar]