Abstract
Background
White matter (WM) alterations are well documented in schizophrenia. Abnormalities in interhemispheric fibers appear to account for altered WM asymmetry in the illness. However, the regional specificity (e.g., frontal versus occipital) of these alterations and their potential contribution to cognitive dysfunction in schizophrenia remain unknown.
Methods
Forty one patients with schizophrenia and 21 healthy controls (HC) underwent diffusion-weighted imaging on a 3 Tesla MRI machine. Tract-based spatial statistic (FSL) was used to assess whole brain differences in WM. Probabilistic tractography was performed in order to separately measure frontal and occipital WM tracts. Participants also completed tests of verbal memory and processing speed. Repeated measures analyses of covariance and Pearson correlation analyses were performed.
Results
A significant group x cerebral hemisphere interaction was found for fractional anisotropy (FA) (F(1,17) = 7.03; p = .017; ηp2 = 0.29) and radial diffusivity (RD) (F(1,17) = 4.84; p = .042; ηp2 = 0.22) in the frontal tract of patients versus HC. Healthy controls showed higher mean FA and lower mean RD in the left frontal tract compared to patients, who showed the opposite pattern. In patients with schizophrenia, mean FA and RD in the right frontal tract correlated with verbal memory (r = −0.68, p = .046; r = 0.77, p = .015).
Conclusions
Asymmetric WM alterations were found in a frontal tract of patients with schizophrenia. Higher mean FA in the right frontal tract correlated with worse verbal memory performance, suggesting a possible contribution these brain changes to cognitive impairment in schizophrenia.
Keywords: Schizophrenia, White matter, Asymmetry, Cognition, Tractography, Neuroimaging
Highlights
-
•
Asymmetric white matter alterations were found in a frontal tract of patients with schizophrenia.
-
•
Higher mean fractional anisotropy in the right frontal tract correlated with worse verbal memory performance in patients.
-
•
Aberrant white matter asymmetry might contribute to a central form of cognitive dysfunction in schizophrenia.
1. Introduction
Abnormalities in white matter (WM) structural connectivity may contribute to cognitive impairment and clinical symptoms in schizophrenia (Friston and Frith, 1995; Friston, 1998; Dwork et al., 2007; Sui et al., 2015). These abnormalities especially involve medial frontal circuits (Ellison-Wright and Bullmore, 2009; Ellison-Wright et al., 2014; Mamah et al., 2010; Buchsbaum et al., 2006; Wheeler and Voineskos, 2014). Two main regions of reduced WM integrity have been described in the left hemisphere of patients with schizophrenia. The first involves left frontal WM and includes WM interhemispheric fibers of the genu of the corpus callosum (gCC) among others. The second involves the left temporal WM and includes inter-hemispheric fibers via the splenium of the corpus callosum (sCC) (Ellison-Wright and Bullmore, 2009).
Interhemispheric fibers are altered in schizophrenia, possibly causing aberrant connectivity between the two cerebral hemispheres (Ellison-Wright et al., 2014; Wheeler and Voineskos, 2014; Pol et al., 2004; Choi et al., 2011). The corpus callosum (CC) appears to be the most abnormal interhemispheric WM pathway in patients with schizophrenia (Ellison-Wright and Bullmore, 2009; Wheeler and Voineskos, 2014; Raybaud, 2010; van der Knaap and van der Ham, 2011; Holleran et al., 2014). Decreased WM integrity in interhemispheric fibers also has been proposed to account for alterations of the normal brain asymmetry seen in these patients (Innocenti et al., 2003; Park et al., 2004; Ribolsi et al., 2014). Specifically, the hypoconnectivity in the CC has been proposed to contribute to altered asymmetry in persons with schizophrenia (van der Knaap and van der Ham, 2011). This could attenuate the normal leftward asymmetry of WM integrity commonly found in healthy controls (HC) in specific WM fibers (Park et al., 2004; Ribolsi et al., 2014; Miyata et al., 2012; Takao et al., 2010) such as the anterior corpus callosum (gCC) itself. The left-greater-than-right anisotropy asymmetry of the gCC seen in healthy adults is reduced in those with schizophrenia (Park et al., 2004).
Cognitive dysfunction afflicts most persons with schizophrenia, and impairments of simple processing speed and verbal memory are among the most widespread and severe (Heinrichs and Zakzanis, 1998; Mesholam-Gately et al., 2009). Cognitive dysfunction has been related to alterations in WM integrity in schizophrenia (Dwork et al., 2007; Sui et al., 2015), but few studies have examined the possible relationship between the specific abnormalities in brain asymmetries and cognitive dysfunction in schizophrenia (see (Antonova et al., 2004) for a review). Thus, alterations in length of the lateral sulcus, width of Sylvian fissure, and anterior frontal and occipital asymmetries seem to be related to performance on measures of expressive language, attention or verbal memory in patients (De Lisi et al., 1997; Hoff et al., 1992). In HC, WM brain asymmetries have been proposed to serve visuospatial functions, working memory, and especially language (Barrick et al., 2007; Catani et al., 2007; Powell et al., 2006). However, to our knowledge, no study has addressed the implications of the alterations in cerebral WM integrity asymmetry in anterior/frontal cortical projections (gCC) and posterior/occipital cortical projections (sCC) to cognitive dysfunction in persons with schizophrenia.
Diffusion weighted imaging (DWI), and specifically diffusion tractography, are powerful and sensitive tools to characterize WM abnormalities, and more precise than other approaches for identifying alterations in different brain structures in schizophrenia (Kanaan et al., 2006). Thus, tractography is a suitable technique and appropriate tool to study WM integrity of specific tracts. Using DWI, we first aimed to assess the whole brain WM integrity differences between patients with schizophrenia and HC. Secondly, we aimed to examine cerebral asymmetry in anterior frontal cortical projections of the gCC and posterior occipital cortical projections of the sCC in patients and HC by means of probabilistic tractography. This second aim was set based on the bibliography that claims that some of the brain asymmetry alterations found in patients with schizophrenia could be caused by alterations in interhemispheric fibers such as the corpus callosum (van der Knaap and van der Ham, 2011; Innocenti et al., 2003; Park et al., 2004; Ribolsi et al., 2014) along with the localization of the two main areas of alterations described in Ellison-Wright and Bullmore's meta-analysis (Ellison-Wright and Bullmore, 2009). Finally, we aimed to determine whether any observed differences in WM integrity asymmetry contribute to cognitive dysfunction in schizophrenia. We hypothesized that (1) patients with schizophrenia would show reduced WM integrity compared to HC specifically in fronto-temporal and occipital areas, (2) that WM asymmetry of patients would be altered compared to HC, and (3) that these alterations would correlate with cognitive dysfunction in patients with schizophrenia.
2. Materials and methods
2.1. Participants
Forty-one patients with schizophrenia (26 paranoid, 11 undifferentiated, 3 residual, and 1 disorganized) and 21 HC were recruited from the Baltimore site of the Bipolar-Schizophrenia Network on Intermediate Phenotypes study (B-SNIP) as described in Tamminga, et al. (Tamminga et al., 2013). Patients' inclusion criteria were: a) having schizophrenia diagnosis based on clinical data and the Structured Clinical Interview (SCID) for DSM-IV (First et al., 1997) and b) being clinically stable. HC were included if they had no diagnosis in axis I, also based on the SCID. Participants were excluded if they had: a) a known neurological illness or b) current substance use, detected or verified by urine toxicology screens. All the participants gave written informed consent to participate. The study was conducted following Declaration of Helsinki guidelines and was approved by the institutional review boards of all participating institutions.
2.2. Diffusion-weighted images
Diffusion-weighted images were obtained from a scanner placed at Baltimore (USA) as described in Skudlarski, et al. (Skudlarski et al., 2013) (Supplemental Table S1). The scanner used single-shot spin-echo planar imaging (EPI) with a twice-refocused balance echo sequence.
Diffusion image processing was performed using FSL software (Smith et al., 2004). Eddy current correction for distortions (Andersson and Sotiropoulos, 2016) was applied and the non-diffusion image (B0) was extracted. Brain Extraction Tool (BET) (Smith, 2002), was used to remove non-brain tissue. After that, fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD) and mode of anisotropy (MO) maps were generated by using DTIFIT, implemented in the FMRIB's diffusion toolbox.
2.3. Tract-based spatial statistic
Tract-based spatial statistic (TBSS) (Smith et al., 2006) was used to assess group differences in FA, MD, AD, RD and MO. Using TBSS, data were prepared to apply a nonlinear registration of all FA images into standard space. The mean FA image was created using a threshold of 0.2 and thinned to create a “mean FA skeleton” which represents the centers of all tracts common to the group. MD, AD, RD and MO data were analyzed using “tbss non FA” script, applying the original non lineal registration to data and merging all subjects' warped MD, AD, RD and MO data into a 4D file. This file was then projected onto the original mean FA skeleton to create the 4D-projected data. A subsample of 10 patients with schizophrenia and 10 HC was selected for tractography analyses based on the quality of their DWI. Only images with satisfactory quality for tractography were included in the analysis. The aforementioned process was performed in this subsample in order to obtain the whole brain overall FA values as well as the mean FA, MD, AD, RD and MO values of the different areas of the CC. Regions of interest (ROIs) of the gCC, the sCC and the body of the CC (bCC) were obtained from the Johns Hopkins University WM atlas. The obtained values were then exported to SPSS for further analyses.
2.4. Probabilistic tractography
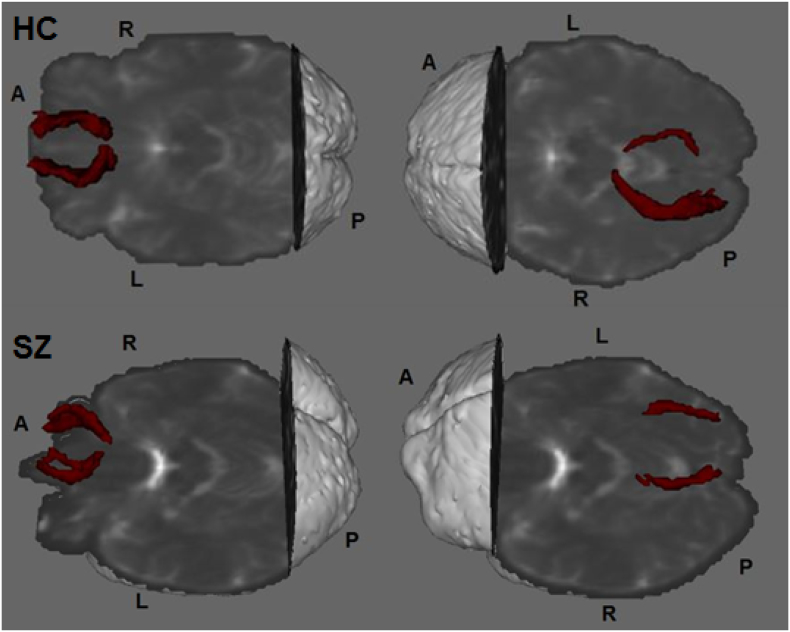
Seeds and waypoint masks for tractography, gCC and frontal area, were extracted from the JHU atlas and Craddock atlas (Craddock et al., 2012), respectively. These were selected based on the whole brain results obtained after performed randomise. Thus, given that white matter differences were found in pathways of a left frontal area that included the gCC as the main interhemispheric fiber, gCC (both right and left separately) and a frontal area (the same area in both right and left hemisphere separately) were set as seed and waypoints masks respectively. After automatic registration between the selected mask and the FA map, gCC masks were manually corrected on each participant's color-coded FA maps based on the MRI Atlas of Human White Matter (Oishi et al., 2010). WM pathways of interest were obtained after applying BedpostX and ProbtrackX (implemented in FSL). Probabilistic connectivity distributions were generated from every voxel in the seed masks, and only those paths that went through the waypoint masks and stopped there were used for analysis. The anterior frontal cortical projections of the gCC were obtained for right and left hemispheres separately. All WM reconstructed trajectories were normalized by dividing by the number of streamlines that went through the waypoint masks and then thresholded at 5% (Fig. 1). The obtained anterior frontal cortical projections of the gCC included WM fibers from different pathways such as the forceps minor, the gCC, the cingulum, the anterior corona radiata and the superior frontal gyrus. Tract-specific FA, MD, AD, RD and MO were obtained for each participant and exported to SPSS. The same process was used to obtain the posterior occipital cortical projections of the sCC interconnecting the sCC and occipital areas including fibers from the forceps major and the optic radiation (Fig. 1). Seeds and waypoints used to obtain this second pathway were set based on the second main area of white matter differences described in Ellison-Wright and Bullmore's meta-analysis (Ellison-Wright and Bullmore, 2009) involving a left temporal area including fibers from the splenium of the corpus callosum and on the whole brain results obtained in the previous analyses.
Fig. 1.
Anterior frontal cortical projections of the gCC and posterior occipital cortical projections of the sCC in a HC and in a patient with schizophrenia. A, anterior; P, posterior; R, right; L, left; gCC, genu of the corpus callosum; sCC, splenium of the corpus callosum; HC, healthy control; SZ, schizophrenia.
2.5. Cognitive and clinical measures
Participants neuropsychological testing included measures of verbal memory and processing speed. Verbal memory was assessed using a combination of learning and delayed recall from the Hopkins Verbal Learning Test Revised (HVLT-R) (Benedict et al., 1998). Processing speed was assessed using the sum of letters (3 and 6) and patterns (3 and 6) of the Salthouse Perceptual Comparison Test (Salthouse and Babcock, 1991). In addition, the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was used to assess patients' clinical symptoms.
2.6. Statistical analyses
Normality of the variables was assessed with Shapiro-Wilk test and the following analyses were performed depending on the distribution of the variables. Group differences in sex, race, handedness, and DWI sequence were assessed with Chi-square analyses and Fisher's exact test. Student t-tests were used to test for group differences in age and years of education. For neuroimaging analyses, whole WM brain differences in FA, MD, AD, RD and MO between 41 patients and 21 healthy controls were testing using the randomise tool (5000 permutations) with TFCE correction in FSL. Age, sex, race and DWI sequence were included as covariates, due to a software change during the project development. Significant regions were located and labelled anatomically with the MRI Atlas of Human White Matter (Oishi et al., 2010). Statistical threshold was set at p < .05 (FWE-corrected). MANCOVA was used to assess differences between the 10 patients with schizophrenia and 10 HC in gCC, bCC and sCC WM integrity. Repeated measures ANCOVA (including group as inter-subjects factor and laterality as intra-subjects factor) was used to examine group differences in the laterality of both anterior frontal cortical projections of the gCC and posterior occipital cortical projections of the sCC WM indexes (FA, MD, AD, RD and MO). Partial correlations were performed in order to assess the relationship between both right and left anterior frontal cortical projections of the gCC and posterior occipital cortical projections of the sCC WM indexes and cognitive performance separately in patients with schizophrenia and HC. Whole brain overall FA values were entered as a covariate in all the analyses. All the analyses were performed using SPSS v.23.
3. Results
3.1. Sociodemographic and clinical variables
Sociodemographic and clinical variables of the whole sample (41 patients and 21 HC) are shown in Table 1. No differences were observed between patients with schizophrenia and HC in age, handedness, DWI sequence, race, or years of education. The schizophrenia group had a higher proportion of men than the HC group, as expected (Häfner, 1997). In the smaller tractography subsamples (10 patients and 10 HC), no group differences were found in age, years of education, sex, race, handedness, or DWI scan used to acquire images based on Student's t and Fisher's exact tests (Table 2).
Table 1.
Sociodemographic and clinic characteristics of the sample (41 patients with schizophrenia and 21 HC).
| SZ (n = 41) |
HC (n = 21) |
t/χ2 | 95% CI |
p | |||
|---|---|---|---|---|---|---|---|
| M (SD) n (%) | M (SD) n (%) | LL | UL | ||||
| Age | 37.78 (12.13) | 43.29 (11.53) | 1.62 | [−0.90 | 11.91] | 0.091 | |
| Sex | Female | 12 (29.27) | 15 (71.43) | 10.04 | 0.002 | ||
| Male | 29 (70.73) | 6 (28.57) | |||||
| Years of education | 13.33 (2.58) | 14.52 (1.94) | 1.95 | [−0.09 | 2.48] | 0.067 | |
| Handedness | Right | 35 (85.36) | 17 (80.95) | 1.99 | 0.371 | ||
| Left | 4 (9.76) | 4 (19.05) | |||||
| Both | 2 (4.88) | 0 (0.00) | |||||
| Race | Black | 25 (60.98) | 8 (38.10) | 2.92 | 0.087 | ||
| White | 16 (39.02) | 13 (61.90) | |||||
| Age of onset | 21.78 (8.17) | – | |||||
| Illness duration | 16.00 (11.95) | – | |||||
| DWI TR | 6700 ms | 37 (90.24) | 19 (90.48) | 0.001 | 0.977 | ||
| 6300 ms | 4 (9.76) | 2 (9.52) | |||||
| SGAs | Yes | 35 (85.37) | – | ||||
| No | 6 (14.63) | ||||||
| FGAs | Yes | 6 (14.63) | – | ||||
| No | 35 (85.37) | ||||||
| PANSS | Positive | 14.44 (6.17) | – | ||||
| Negative | 15.76 (5.53) | – | |||||
| General | 25.51 (5.75) | – | |||||
SZ, schizophrenia; HC, healthy controls; SD, standard deviation; n, sample size; t, Student t-test; χ2, Chi square; CI, confidence interval; LL, lower limit; UL, upper limit; DWI, diffusion weighted imaging; TR, repetition time; ms, milliseconds; SGAs, second-generation antipsychotics; FGA, first-generation antipsychotics; PANSS, positive and negative syndrome scale.
Table 2.
Sociodemographic and clinic characteristics of the sample (10 patients with schizophrenia and 10 HC).
| SZ (n = 10) |
HC (n = 10) |
t/χ2 | 95% CI |
p/ Fisher's exact | |||
|---|---|---|---|---|---|---|---|
| M (SD) n (%) | M (SD) n (%) | LL | UL | ||||
| Age | 39.00 (8.86) | 45.80 (10.59) | 1.56 | [−2.37 | 15.97] | 0.137 | |
| Sex | Female | 3 (30.00) | 7 (70.00) | 3.20 | 0.179 | ||
| Male | 7 (70.00) | 3 (30.00) | |||||
| Years of education | 12.80 (2.90) | 14.10 (2.33) | 1.11 | [−1.17 | 3.77] | 0.284 | |
| Handedness | Right | 9 (90.00) | 8 (80.00) | 0.39 | 1.000 | ||
| Left | 1 (10.00) | 2 (20.00) | |||||
| Race | Black | 8 (80.00) | 4 (40.00) | 3.33 | 0.170 | ||
| White | 2 (20.00) | 6 (60.00) | |||||
| Age of onset | 25.40 (7.75) | – | |||||
| Illness duration | 13.60 (8.47) | – | |||||
| DWI TR | 6700 ms | 8 (80.00) | 9 (90.00) | 0.39 | 1.000 | ||
| 6300 ms | 2 (20.00) | 1 (10.00) | |||||
| SGAs | Yes | 8 (80.00) | – | ||||
| No | 2 (20.00) | ||||||
| FGAs | Yes | 2 (20.00) | – | ||||
| No | 8 (80.00) | ||||||
| PANSS | Positive | 14.80 (5.55) | – | ||||
| Negative | 13.40 (2.72) | – | |||||
| General | 25.20 (4.47) | – | |||||
SZ, schizophrenia; HC, healthy controls; SD, standard deviation; n, sample size; t, Student t-test; χ2, Chi square; CI, confidence interval; LL, lower limit; UL, upper limit; DWI, diffusion weighted imaging; TR, repetition time; ms, milliseconds; SGAs, second-generation antipsychotics; FGA, first-generation antipsychotics; PANSS, positive and negative syndrome scale.
3.2. Whole brain differences in WM indexes
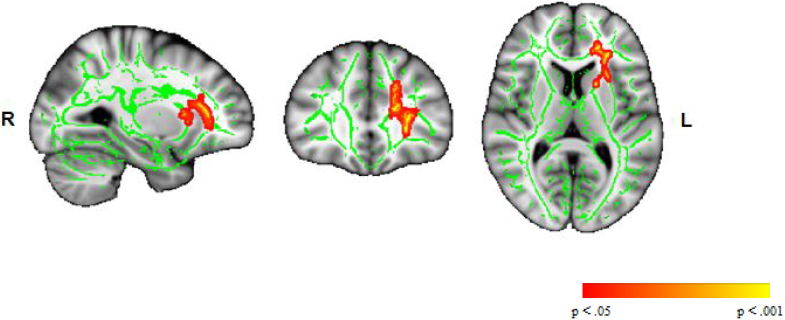
Patients with schizophrenia showed lower mean FA than HC in a well-delimited area of the left frontal lobe (FWE-corrected, p < .05) (Table 3; Fig. 2). No differences were found for MD, AD, RD or MO.
Table 3.
Cluster characteristics of the whole brain differences in mean FA between patients with schizophrenia and HC.
| FSL coordinates |
||||||
|---|---|---|---|---|---|---|
| Cluster size (voxels) | x | y | z | p | Anatomical brain regions | |
| HC > SZ | ||||||
| 871 | 117 | 158 | 81 | 0.023 | Left |
anterior corona radiata anterior limb of the internal capsule (anterior thalamic radiation and inferior fronto-occipital fasciculus) external capsule (inferior fronto-occipital fasciculus) inferior and superior frontal gyrus white matter
|
HC, healthy controls; SZ, schizophrenia.
FSL coordinates indicate: x increases from left to right; y increases from posterior to anterior; and z increases from inferior to superior.
Region in bold represents the maximum coordinate encompassed in the given cluster.
HC > patients with SZ.
Fig. 2.
Whole brain FA differences between patients with schizophrenia and HC. Differences are significant at p < .05 corrected for family-wise error (FWE). WM regions in which patients with schizophrenia showed a lower mean FA compared to HC are shown in red-yellow; the WM skeleton is shown in green. R, right; L, left; FA, fractional anisotropy; WM, white matter; SZ, schizophrenia; HC, healthy controls.
3.3. Differences in WM indexes of the CC
Regarding differences in WM mean FA of the three specific areas of the CC, patients with schizophrenia showed lower mean FA than HC in the gCC (F = 6.48; p = .021). No significant results were found when assessing the differences in other integrity measures of the gCC (MD, AD, RD or MO). No differences were observed in relation to the bCC or the sCC in any of the WM integrity indexes (Table 4).
Table 4.
Differences in mean FA of the CC between patients with schizophrenia and HC.
| SZ (n = 10) |
95% CI |
HC (n = 10) |
95% CI |
F | p | ηp2 | |||
|---|---|---|---|---|---|---|---|---|---|
| M (SE) | LL | UL | M (SE) | LL | UL | ||||
| gCC | 0.152 (0.001) | [0.150 | 0.154] | 0.155 (0.001) | [0.153 | 0.157] | 6.481 | 0.021 | 0.276 |
| bCC | 0.153 (0.002) | [0.148 | 0.158] | 0.154 (0.002) | [0.149 | 0.159] | 0.165 | 0.690 | 0.010 |
| sCC | 0.147 (0.001) | [0.145 | 0.148] | 0.146 (0.001) | [0.144 | 0.147] | 0.923 | 0.350 | 0.052 |
SZ, schizophrenia; HC, healthy controls; SE, standard error; n, sample; CI, confidence interval; LL, lower limit; UL, upper limit; F, MANCOVA; gCC, genu of the corpus callosum; bCC, body of the corpus callosum; sCC, splenium of the corpus callosum; ηp2, partial eta-squared.
3.4. Group differences in tract asymmetry
Repeated measures ANCOVA revealed a statistically significant group x hemisphere interaction for mean FA in anterior frontal cortical projections of the gCC (F(1,17) = 7.03; p = .017; ηp2 = 0.29). That is, the HC group showed higher mean FA in the left than right anterior frontal cortical projections of the gCC (M = 0.49; SE = 0.01 versus M = 0.47; SE = 0.01), while patients with schizophrenia showed higher mean FA in the right than left anterior frontal cortical projections of the gCC (M = 0.51; SE = 0.01 versus M = 0.49; SE = 0.01). In addition, a group x hemispheric laterality effect was also found for mean RD (F(1,17) = 4.84; p = .042; ηp2 = 0.22). In this case, healthy controls showed lower mean RD in the left than right hemisphere tracts (M = 0.54 × 10−3; SE = 0.10 × 10−4 versus (M = 0.56 × 10−3; SE = 0.12 × 10−4). Patients with schizophrenia showed lower mean RD in the right than left hemisphere tract (M = 0.52 × 10−3; SE = 0.12 × 10−4 versus M = 0.54 × 10−3; SE = 0.10 × 10−4). No significant group differences were found for AD, MD or MO (Table 5).
Table 5.
Repeated measures ANCOVA assessing asymmetry differences between patients with schizophrenia and healthy controls in the anterior frontal cortical projections of the genu of the corpus callosum.
| Variable | SZ |
HC |
F | Sig. | ηp2 | |
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | |||||
| AFCPgCC FA | Right | 0.51 (0.01) | 0.47 (0.01) | 7.03 | 0.017 | 0.29 |
| Left | 0.49 (0.01) | 0.49 (0.01) | ||||
| AFCPgCC RD | Right | 0.52 × 10−3 (0.12 × 10−4) | 0.56 × 10−3 (0.12 × 10−4) | 4.48 | 0.042 | 0.22 |
| Left | 0.54 × 10−3 (0.10 × 10−4) | 0.54 × 10−3 (0.10 × 10−4) | ||||
| AFCPgCC MD | Right | 0.75 × 10−3 (0.12 × 10−4) | 0.77 × 10−3 (0.12 × 10−4) | 1.11 | 0.308 | 0.06 |
| Left | 0.76 × 10−3 (0.10 × 10−4) | 0.77 × 10−3 (0.10 × 10−4) | ||||
| AFCPgCC AD | Right | 0.12 × 10−2 (0.16 × 10−4) | 0.12 × 10−2 (0.16 × 10−4) | 4.12 | 0.058 | 0.20 |
| Left | 0.12 × 10−2 (0.14 × 10−4) | 0.12 × 10−2 (0.14 × 10−4) | ||||
| AFCPgCC MO | Right | 0.42 (0.03) | 0.35 (0.03) | 1.50 | 0.238 | 0.08 |
| Left | 0.42 (0.02) | 0.39 (0.02) | ||||
AFCPgCC, anterior frontal cortical projections of the genu of the corpus callosum; SZ, Schizophrenia; HC, healthy controls; SE, standard error; ηp2 : partial eta square.
No interaction effect was found for FA when assessing asymmetry of the posterior occipital cortical projections of the sCC (F = 4.07; p = .06). Therefore, the other indexes were not analyzed.
3.5. Correlations between WM tracts and cognition
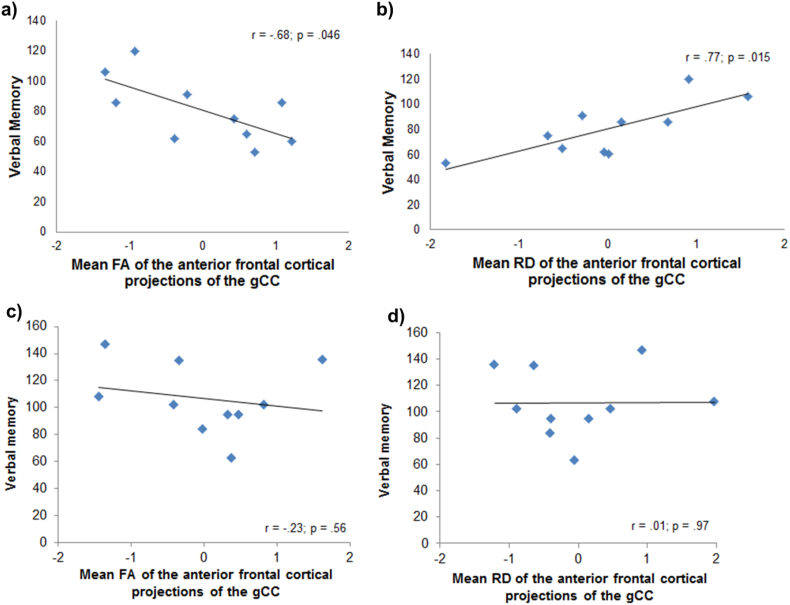
Correlation analyses were performed only for those WM indexes in which a significant group x hemispheric laterality effect was found. In patients with schizophrenia, verbal memory performance correlated inversely with mean FA of the right gCC tract (r = −0.68, p = .046,) (Fig. 3a), indicating higher mean FA correlated with worse verbal memory performance in patients with schizophrenia. Further, verbal memory performance correlated positively with mean RD of the right anterior frontal cortical projections of the gCC (r = 0.77, p = .015) (Fig. 3b), indicating that higher mean RD correlated with better verbal memory. No significant correlations were found regarding processing speed. No significant results were found in HC group (Fig. 3c and d).
Fig. 3.
White matter indices and cognition correlations controlling for overall mean FA. FA, fractional anisotropy; gCC, genu of the corpus callosum.
a) Correlation between verbal memory performance and the mean FA of the right anterior frontal cortical projection of the gCC in patients with schizophrenia; b) Correlation between verbal memory performance and the mean RD of the right anterior frontal cortical projection of the gCC in patients with schizophrenia; c) Correlation between verbal memory performance and the mean FA of the right anterior frontal cortical projection of the gCC in healthy controls; d) Correlation between verbal memory performance and the mean RD of the right anterior frontal cortical projection of the gCC in healthy controls.
4. Discussion
This study aimed firstly to elucidate whole brain WM integrity differences between persons with schizophrenia and HC, secondly to test for group differences in WM asymmetry of both anterior frontal (gCC) and posterior occipital (sCC) cortical projections of the corpus callosum, and thirdly to assess their relationship with cognitive dysfunction in patients.
As expected, whole brain analyses revealed that patients with schizophrenia had lower mean FA than healthy controls in a well-delimited region of the left frontal lobe. Specifically, these group differences included the anterior corona radiata, anterior limb of the internal capsule (left anterior thalamic radiation and part of the left inferior fronto-occipital fasciculus), the gCC, the cingulum, and WM of the left inferior and superior frontal gyri. These fiber tracts clearly overlap one of the two WM networks that were found to be most affected by schizophrenia in a meta-analysis of other studies (Ellison-Wright and Bullmore, 2009). Specifically, that network consists of WM tracts traversing the left frontal WM region, including inter-hemispheric fibers via the gCC, the cingulum bundle, the left inferior fronto-occipital fasciculus, and the left anterior thalamic radiation (Ellison-Wright and Bullmore, 2009). However, we did not find significant group differences in the second area identified by Ellison-Wright & Bullmore (Ellison-Wright and Bullmore, 2009) involving left temporal tracts that include WM fibers running thorough the sCC and others. This could be due to our smaller sample size or methodological differences [different software (FSL vs. DTIstudio…), different technique (tractography vs. TBSS vs. voxel based morphometry…), or the different approach (whole brain vs. region of interest)] between this study and those studies included in the meta-analysis.
In terms of specific interhemispheric fibers, we found that mean FA in the gCC, but not the sCC or bCC, was decreased in schizophrenia. Others have found reduced FA or WM volume in the sCC of patients with schizophrenia (Foong et al., 2000; Francis et al., 2016; Agartz et al., 2001), but the finding that integrity of WM in the gCC was more affected than WM in the sCC or the bCC is widely supported by previous studies (Skudlarski et al., 2013; Knöchel et al., 2012; Kubicki et al., 2008; Price et al., 2007). One possible explanation for these findings relates to the developmental course of the CC. Due to the heterogeneity, fiber tracts mature at different ages (Kochunov et al., 2013; Kochunov et al., 2012). The active growth period of the CC extends to the mid-20s, (Lebel et al., 2008; Pujol et al., 1993), and within subdivisions, the gCC maturation peaks later than the sCC (Kochunov et al., 2013; Kochunov et al., 2012; Bartzokis et al., 2012). This difference might relate to the typical age of schizophrenia onset in the mid-20s (Häfner, 1997), thereby affecting anterior segments of the CC, such as the gCC, which are less fully developed than posterior segments at illness onset. Further, the gCC contains mostly small fibers that are thinly myelinated, making this area more vulnerable to breakdown and loss (Bartzokis et al., 2012).
The most notable finding of this study pertains to group differences in the asymmetry of anterior frontal cortical projections of the gCC. As expected, HC showed higher FA and lower RD in the left than right hemisphere tract, pointing to a leftward asymmetry of this tract in HC. Patients with schizophrenia showed the reverse pattern, namely: higher mean FA and lower mean RD in the right compared to left anterior frontal cortical projections of the gCC. Furthermore, the magnitude of this asymmetry reversal in patients correlated with worse performance on tests of verbal learning/memory – a hallmark of cognitive dysfunction in schizophrenia.
Supporting the present results, abnormalities in human brain asymmetries have been linked with general alterations in language commonly found in patients with schizophrenia (Pearlson and Folley, 2008; Mitchell and Crow, 2005; Crow, 1997). Some authors also proposed that language impairments in this pathology might be a result of an incomplete hemispheric specialization (Crow, 1997). Crow (Crow, 1997; Crow et al., 1989; Crow, 1995) even proposed that these abnormalities may relate directly to the origin of schizophrenia, and that some cardinal features of the disease, such as hallucinations or delusions, could be understood as errors in the interpretation and organization of language. This perspective suggests that both cerebral asymmetry and language dysfunction might play central roles in the disease (Crow, 1997; Crow et al., 1989).
Although altered WM asymmetries have been documented in schizophrenia (Park et al., 2004; Miyata et al., 2012), this is, to the best of our knowledge, the first study to assess the asymmetry of the anterior frontal cortical projections of the gCC and their relationship with verbal memory impairment in schizophrenia. Consisting with our results, common left greater than right asymmetry of WM integrity found in HC has been shown to be reduced in patients with schizophrenia (Ribolsi et al., 2014) in some WM fibers traversing the anterior CC, the optic radiation (Park et al., 2004), the uncinated fasciculus (Kubicki et al., 2002), the posterior limb of the internal capsule (Miyata et al., 2012), or the cingulum bundle (Park et al., 2004) among others. Moreover, the observed leftward asymmetry in medial prefrontal WM in HC was found to be abnormal in a sample of patients with schizophrenia, pointing to a reduction of this asymmetry in the same area in which the anterior frontal cortical projections of the gCC are located (Park et al., 2004).
The origin of brain asymmetry is a matter of enduring scientific interest, and many experts believe that both genetic and environmental factors contribute to its development (Jahanshad et al., 2010; Toga and Thompson, 2003). Thus, up to 30% of the leftward asymmetry of some WM fibers (i.e., the inferior fronto-occipital fasciculus and anterior thalamic radiation or forceps major) has been attributed to genetic factors. Conversely, environmental factors have shown to account for about 10% of the variance in the asymmetry of other regions such as the forceps minor (Jahanshad et al., 2010). In addition, asymmetries of the right frontal and temporal lobes volumes appear to be more influenced by genetic factors than contralateral volumes of the same regions, which seem to be more affected by environmental features, and the occipital lobe seems to show lower heritability overall (Geschwind et al., 2002). Taking this into account, asymmetry alterations of those brain regions that demonstrate heritability might be used as intermediate phenotypes to guide the search for new genes related to the disease (Lenroot et al., 2009).
While speculative, results regarding the relationship between a higher FA of the right anterior frontal cortical projections of the gCC and worse verbal memory in patients with schizophrenia might result from an abnormality in axonal pruning that affects cognitive development. Others have suggested that deficient axonal pruning might decrease information transmission efficiency by producing redundant brain networks (Alba-Ferrara and De Erausquin, 2013). Thus, one could hypothesize that deficient pruning contributes to increased mean FA and decreased cognitive efficiency in these patients, as found in the present study. In short, the obtained results suggest that abnormalities in WM integrity of right frontal areas, such as anterior frontal cortical projections of the gCC, could reflect a neuroanatomic substrate of verbal memory impairment in schizophrenia.
We also found that higher mean RD in right anterior frontal cortical projections of the gCC correlated with a better verbal memory performance in patients. RD has been related to demyelination process (Alexander et al., 2007). Typically, increases in FA are accompanied by decreases in all other diffusion indices (MD, AD, and RD). In schizophrenia, both mean FA and RD follow an atypical development pattern. Therefore, and inversely to the relationship found between mean FA and verbal memory, higher mean RD shows a relationship with better information transmission efficiency.
We failed to find a significant relationship between processing speed and WM integrity of the anterior frontal cortical projections of the gCC. This might be explained by the fact that these WM fibers are located in the medial prefrontal regions of the brain. Given that processing speed tasks engage many cognitive processes, it is reasonable to expect that performance on them will depend on more widely distributed WM abnormalities in periventricular and other subcortical tracts than anterior frontal cortical projections of the gCC (Magistro et al., 2015; Penke et al., 2010; Vernooij et al., 2009). This added to the previously mentioned relationship between brain asymmetry alterations and language in schizophrenia could explain why only verbal memory seemed to be related with the WM integrity asymmetry of this tract.
Results obtained in the present study must be seen in the context of its limitations. First, the small sample, especially in tractography analyses, limits generalization to schizophrenia in general. As noted above, these findings should be viewed as preliminary but potentially useful insofar as they suggest some direction for future research. Second, while tractography is thought to be a powerful tool for studying WM integrity in schizophrenia and other populations, an important limitation of it relates to the existence of crossing fibers that influence FA and, therefore, might contribute to the present findings. The bending, merging and crossing of fibers in some voxels together with low spatial resolution at 3 T could lead to inexact measures of FA (Dell'Acqua and Catani, 2012). Third, possible medication and illness duration effects are not taking into account in the analyses. Future research, and specifically longitudinal studies, should investigate the influence of medication and illness duration on the relationship between WM asymmetry alterations and cognition in schizophrenia. Finally, due to the reduced sample, we were unable to assess the possible differences between left- and right-handed patients groups in terms of brain asymmetry and its relationship with cognition. Future studies should assess if handedness has a specific role in the relationship between brain asymmetry alterations and cognition in patients with schizophrenia.
Despite these limitations, the present findings suggest that aberrant WM asymmetry might contribute to a central form of cognitive dysfunction in schizophrenia, one that entails both language processing and episodic memory. This could help to design more specific cognitive rehabilitation interventions that account for this putative neuroanatomical basis of impaired verbal learning and memory. Finally, combination of reversed asymmetry in specified WM tracts and impaired verbal learning/memory might serve as a useful marker of schizophrenia if future research shows that it has both sensitivity and specificity for diagnosis.
Role of the funding source
This study was funded by NIH/NIMH (National Institute of Health, USA/National Institute of Mental Health, USA) grant 5R01MH077852–02. The funding source had no involvement in the development of the present study.
Preliminary results of the present study were presented at the Basque Neuroscience Meeting (Neurogune), 27 June 2016.
Financial disclosures
A.G.G. receives a Predoctoral Research Training Grant from the Basque Government.
L.Z.E. receives funding from the University of Deusto and the Department of Education and Science of the Basque Government (Equipo A) and the Spanish Ministry of Economy, Industry and Competitiveness (PSI2017–83657-P; to L.Z.E).
J.P. receives funding from the University of Deusto and the Department of Education and Science of the Basque Government (Equipo A) and the Spanish Ministry of Economy, Industry and Competitiveness (PSI2012–32441; to N.I·B).
N.I.B. receives funding from the University of Deusto, the Department of Education and Science of the Basque Government (Equipo A) and the Spanish Ministry of Economy, Industry and Competitiveness (PSI2012–32441; to N.I.B).
D.J.S. receives funding from Johns Hopkins University.
N.O. receives funding from the University of Deusto and the Department of Education and Science of the Basque Government (Equipo A).
Employment:
O.R. receives funding from The University of Deusto.
Declarations of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101781.
Appendix A. Supplementary data
Supplementary material
References
- Agartz I., Andersson J.L., Skare S. Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport. 2001;12:2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara L., De Erausquin G.A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci. 2013;7:1–5. doi: 10.3389/fnint.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova E., Sharma T., Morris R., Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr. Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Barrick T.R., Lawes I.N., Mackay C.E., Clark C.A. White matter pathway asymmetry underlies functional lateralization. Cereb. Cortex. 2007;17:591–598. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- Bartzokis G., Lu P.H., Heydari P., Couvrette A., Lee G.J., Kalashyan G. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol. Psychiatry. 2012;72:1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Benedict R.H., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test–revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12:43–55. [Google Scholar]
- Buchsbaum M.S., Friedman J., Buchsbaum B.R., Chu K., Hazlett E.A., Newmark R. Diffusion tensor imaging in schizophrenia. Biol. Psychiatry. 2006;60:1181–1187. doi: 10.1016/j.biopsych.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Catani M., Allin M.P., Husain M., Pugliese L., Mesulam M.M., Murray R.M., Jones D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H., Kubicki M., Whitford T.J., Alvarado J.L., Terry D.P., Niznikiewicz M. Diffusion tensor imaging of anterior commissural fibers in patients with schizophrenia. Schizophr. Res. 2011;130:78–85. doi: 10.1016/j.schres.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock R.C., James G.A., Holtzheimer P.E., Hu X.P., Mayberg H.S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 2012;33:1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T.J. A darwinian approach to the origins of psychosis. Br. J. Psychiatry. 1995;167:12–25. doi: 10.1192/bjp.167.1.12. [DOI] [PubMed] [Google Scholar]
- Crow T. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- Crow T.J., Ball J., Bloom S.R., Brown R., Bruton C.J., Colter N. Schizophrenia as an anomaly of development of cerebral asymmetry: a postmortem study and a proposal concerning the genetic basis of the disease. Arch. Gen. Psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- De Lisi L.E., Sakuma M., Kushner M., Finer D.L., Hoff A.L., Crow T.J. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr. Bull. 1997;23:255–271. doi: 10.1093/schbul/23.2.255. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua F., Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr. Opin. Neurol. 2012;25:375–383. doi: 10.1097/WCO.0b013e328355d544. [DOI] [PubMed] [Google Scholar]
- Dwork A.J., Mancevski B., Rosoklija G. White matter and cognitive function in schizophrenia. Int. J. Neuropsychopharmacol. 2007;10:513–536. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Nathan P.J., Bullmore E.T., Zaman R., Dudas R.B., Agius M. Distribution of tract deficits in schizophrenia. BMC Psychiatry. 2014;14:1–13. doi: 10.1186/1471-244X-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Benjamin L.S., Williams J.B. American Psychiatric Pub; 1997. Structured Clinical Interview for DSM-IV Axis II Personality Disorders: SCID-II. [Google Scholar]
- Foong J., Maier M., Clark C.A., Barker G.J., Miller D.H., Ron M.A. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J. Neurol. Neurosurg. Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A.N., Mothi S.S., Mathew I.T., Tandon N., Clementz B., Pearlson G.D. Callosal abnormalities across the psychosis dimension: bipolar schizophrenia network on intermediate phenotypes. Biol. Psychiatry. 2016;80:627–635. doi: 10.1016/j.biopsych.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J. The disconnection hypothesis. Schizophr. Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome. Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Geschwind D.H., Miller B.L., DeCarli C., Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H., van der Heiden W. Epidemiology of schizophrenia. Can. J. Psychiatr. 1997;42:139–151. doi: 10.1177/070674379704200204. [DOI] [PubMed] [Google Scholar]
- Heinrichs R.W., Zakzanis K.K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoff A.L., Riordan H., O'Donnell D., Stritzke P., Neale C., Boccio A. Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr. Bull. 1992;18:257–272. doi: 10.1093/schbul/18.2.257. [DOI] [PubMed] [Google Scholar]
- Holleran L., Ahmed M., Anderson-Schmidt H., McFarland J., Emsell L., Leemans A. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology. 2014;39:944–954. doi: 10.1038/npp.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti G., Ansermet F., Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol. Psychiatry. 2003;8:261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Jahanshad N., Lee A.D., Barysheva M., McMahon K.L., de Zubicaray G.I., Martin N.G. Genetic influences on brain asymmetry: a DTI study of 374 twins and siblings. Neuroimage. 2010;52:455–469. doi: 10.1016/j.neuroimage.2010.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R.A., Shergill S.S., Barker G.J., Catani M., Ng V.W., Howard R. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. Neuroimaging. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Flszbein A., Opfer L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Knöchel C., Oertel-Knöchel V., Schönmeyer R., Rotarska-Jagiela A., van de Ven V., Prvulovic D. Interhemispheric hypoconnectivity in schizophrenia: Fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Kochunov P., Williamson D., Lancaster J., Fox P., Cornell J., Blangero J., Glahn D. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol. Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P., Glahn D.C., Rowland L.M., Olvera R.L., Winkler A., Yang Y. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol. Psychiatry. 2013;73:482–491. doi: 10.1016/j.biopsych.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., Westin C., Maier S.E., Frumin M., Nestor P.G., Salisbury D.F. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., Styner M., Bouix S., Gerig G., Markant D., Smith K. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr. Res. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Schmitt J.E., Ordaz S.J., Wallace G.L., Neale M.C., Lerch J.P. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistro D., Takeuchi H., Nejad K.K., Taki Y., Sekiguchi A., Nouchi R. The relationship between processing speed and regional white matter volume in healthy young people. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0136386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D., Conturo T.E., Harms M.P., Akbudak E., Wang L., McMichael A.R. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. Neuroimaging. 2010;183:144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately R.I., Giuliano A.J., Goff K.P., Faraone S.V., Seidman L.J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Mitchell R.L., Crow T.J. Right hemisphere language functions and schizophrenia: the forgotten hemisphere? Brain. 2005;128:963–978. doi: 10.1093/brain/awh466. [DOI] [PubMed] [Google Scholar]
- Miyata J., Sasamoto A., Koelkebeck K., Hirao K., Ueda K., Kawada R. Abnormal asymmetry of white matter integrity in schizophrenia revealed by voxelwise diffusion tensor imaging. Hum. Brain Mapp. 2012;33:1741–1749. doi: 10.1002/hbm.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A.V., van Zijl P.C., Mori S. Academic Press; 2010. MRI Atlas of Human White Matter. [Google Scholar]
- Park H., Westin C., Kubicki M., Maier S.E., Niznikiewicz M., Baer A. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson G.D., Folley B.S. Schizophrenia, psychiatric genetics, and darwinian psychiatry: an evolutionary framework. Schizophr. Bull. 2008;34:722–733. doi: 10.1093/schbul/sbm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L., Munoz Maniega S., Murray C., Gow A.J., Hernandez M.C., Clayden J.D. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J. Neurosci. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol H.E.H., Schnack H.G., Mandl R.C., Cahn W., Collins D.L., Evans A.C., Kahn R.S. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Powell H.R., Parker G.J., Alexander D.C., Symms M.R., Boulby P.A., Wheeler-Kingshott C.A. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Price G., Cercignani M., Parker G.J., Altmann D.R., Barnes T.R., Barker G.J. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007;35:458–466. doi: 10.1016/j.neuroimage.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Vendrell P., Junqué C., Martí-Vilalta J.L., Capdevila A. When does human brain development end? evidence of corpus callosum growth up to adulthood. Ann. Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010;52:447–477. doi: 10.1007/s00234-010-0696-3. [DOI] [PubMed] [Google Scholar]
- Ribolsi M., Daskalakis Z.J., Siracusano A., Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front. Hum. Neurosci. 2014;8:1–11. doi: 10.3389/fnhum.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T.A., Babcock R.L. Decomposing adult age differences in working memory. Dev. Psychol. 1991;27:763. [Google Scholar]
- Skudlarski P., Schretlen D.J., Thaker G.K., Stevens M.C., Keshavan M.S., Sweeney J.A. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am. J. Psychiatry. 2013;170:886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sui J., Pearlson G.D., Du Y., Yu Q., Jones T.R., Chen J. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol. Psychiatry. 2015;78:794–804. doi: 10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao H., Abe O., Yamasue H., Aoki S., Kasai K., Ohtomo K. Cerebral asymmetry in patients with schizophrenia: a voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) study. J. Magn. Reson. Imaging. 2010;31:221–226. doi: 10.1002/jmri.22017. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Ivleva E.I., Keshavan M.S., Pearlson G.D., Clementz B.A., Witte B. Clinical phenotypes of psychosis in the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) Am. J. Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- van der Knaap L., van der Ham I. How does the corpus callosum mediate interhemispheric transfer? A review. Behav. Brain Res. 2011;223:211–221. doi: 10.1016/j.bbr.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Vernooij M.W., Ikram M.A., Vrooman H.A., Wielopolski P.A., Krestin G.P., Hofman A. White matter microstructural integrity and cognitive function in a general elderly population. Arch. Gen. Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- Wheeler A.L., Voineskos A.N. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 2014;8:1–18. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material