Abstract
Objective
To review concepts of a standalone endoscopically assisted lumbar interbody fusion as a simplified method to treat spinal instability.
Methods
MacNab outcomes and complications were analyzed in a series of 48 consecutive patients who underwent standalone lordotic endoscopic wedge lumbar interbody fusion (LEW-LIF) for advanced lumbar disc degeneration, spinal stenosis, and spondylolisthesis.
Results
Forty-two of the 48 patients (77.8%) did well with excellent and good outcomes with a follow up of up to 20 months. Fair outcomes were reported by 4, and poor by another 2 patients, respectively. Six patients had endoscopic decompression procedures at another level. Four patients underwent open transforaminal lumbar interbody fusion revision surgery including the index level between 2 to 6 months postoperatively. An L5 vertebral body fracture was noted in 1 of these 4 patients. Another patient underwent removal of the extruded L3/4 cage. The cage fractured in one additional asymptomatic patient not requiring any intervention. No patient had a wound infection, or permanent sensory, or motor dysfunction. However, 29 patients developed a postoperative irritation of the dorsal root ganglion with burning leg pain typically between postoperative weeks 2 and 6. Symptoms were treated with activity modification, gabapentin, and transforaminal epidural steroid injections in 12 patients (25%).
Conclusion
Standalone LEW-LIF was associated with favorable clinical outcomes in the majority of patients. Patient-related predictors of less favorable outcomes considering normal variations as well as patho-anatomy may aid in the development of next-generation implants.
Keywords: Spinal diseases, Endoscopy, Spinal fusion
INTRODUCTION
Minimally invasive spinal surgeries (MISS) have become commonplace in the treatment of spinal stenosis and instability related symptomatic neurogenic low back and leg pain in patients who have failed nonoperative care. These procedures have drastically reduced hospitalization stays with a lower rate of medical complications [1,2]. MISS advantages include lower blood loss, less postoperative pain with faster return to work and social reintegration [3]. Reduced muscle stripping and less aggressive resection of the lumbar posterior elements. Minimally invasive concepts have been associated with a lower incidence of perioperative complications [4,5], less unintended aftercare, and fewer iatrogenic problems. The net effect translates into substantial cost savings; a factor that is highly relevant to most resource-strained healthcare systems the world over [6]. The growing demand for value-based health care measures to serve the growing aging baby-boomer population [6,7] motivated a standalone interbody fusion surgery [8] which could prove more cost-effective provided clinical outcomes are reliably improved.
The rationale for MISS anterior column reconstruction with the placement of a single oblique interbody fusion cage is straight forward. It allows stabilization of the diseased lumbar spinal motion segment while restoring neuroforaminal height and promoting bony ingrowth [9]. Implant migration [10], subsidence [11], and loss of lordosis [12] are the flipside disadvantages of lumbar interbody fusion cages. Conventional cages require endplate decortication, and impaction into the interspace and posterior supplemental fixation with pedicle screws to avoid these well-recognized problems [7-9]. However, the additional use of pedicle screw instrumentation increases operative time, blood loss, and has also been associated with higher complication rates due to intraoperative nerve root injury [9], damage to the adjacent facet joint complex [13,14], and propagation of symptomatic adjacent segment disease [15].
The advantages of a standalone lordotic endoscopic wedge lumbar interbody fusion (LEW-LIF) with transforaminal implantation of a single oblique interbody fusion device are apparent. This conceivably less burdensome surgery would simplify recovery for most patients with a significant reduction in postoperative complications, and utilization of pain killer to be expected [16-19]. In the United States, the latter problem is of significance because of the opiate abuse epidemic [20,21]. The combination of the overall push by patients, and insurance providers to transition simple lumbar decompression and fusion surgeries into a more cost-effective outpatient settings and the recent rise in endoscopic spinal procedures [19] has led to a substantially increased interest in a simplified version of lumbar spinal fusion that could be done with an endoscope [8] in an ambulatory surgery center (ASC). It is evident that patient acceptance for spinal endoscopy is higher than for other forms of MISS, or open spine surgery due to fewer anesthesia-related problems (postoperative nausea) [22], and equivalent favorable clinical outcomes [16-19,23-28]. In short, it is an elegant method to treat the patients suffering from lumbar radiculopathy in an ASC - often under local anesthesia with sedation, and at a reduced cost [27].
For these reasons, the authors started performing standalone LEW-LIF procedure for advanced lumbar disc degeneration, spinal stenosis, and instability using a single oblique threaded expandable cage. Although the majority of patients did well with the procedure, it became clear after an initial learning curve of this series of 48 patients that certain patient-related and anatomic factors appeared to drive less favorable outcomes. Therefore, the purpose of this study was to analyze further these factors with the intent of identifying appropriate patient selection criteria for the procedure.
MATERIALS AND METHODS
1. Patients and Selection Criteria
In 2007, the Center for Advanced Spine Care of Southern Arizona established an outpatient spinal surgery program for the treatment of lumbar herniated disc and spinal stenosis. With the advancement of videoendoscopic equipment, bony decompression became more feasible broadening the indication to treat spinal stenosis. These advancements provided the foundation for the development of a full-endoscopic decompression procedure popularized by Ruetten and colleagues [16-18] which essentially utilized a combination of the posterolateral transforaminal and the direct posterior interlaminar access to the lumbar neuroforamen and lateral recess allowing combined decompression of neural elements posteriorly and anteriorly. The combination of these advanced percutaneous decompression techniques with an expandable threaded interbody fusion device allowed further expansion of the indication of the outpatient percutaneous transforaminal procedure to treat instability-induced symptoms of degenerated disc disease that have proven refractory to nonoperative treatment. This study included 48 patients (29 females, and 19 males) between the ages of 32 to 88 years and a mean of 64.9 years (Fig. 1). Patients underwent standalone endoscopically assisted decompression and interbody fusion following the LEW-LIF procedure. Surgeries were done by the first and second author using the VariLift-L interbody fusion system (Wenzel Spine, Austin, TX, USA). The inclusion criteria were: (1) clinical signs of lumbar radiculopathy, dysesthesias, and decreased motor function; (2) imaging evidence of foraminal or lateral recess stenosis demonstrated on preoperative magnetic resonance images and computed tomography (CT) scans; (3) grade I spondylolisthesis; (4) unsuccessful nonoperative treatment, including physical therapy and transforaminal epidural steroid injections (TESIs) for at least 12 weeks.
Fig. 1.
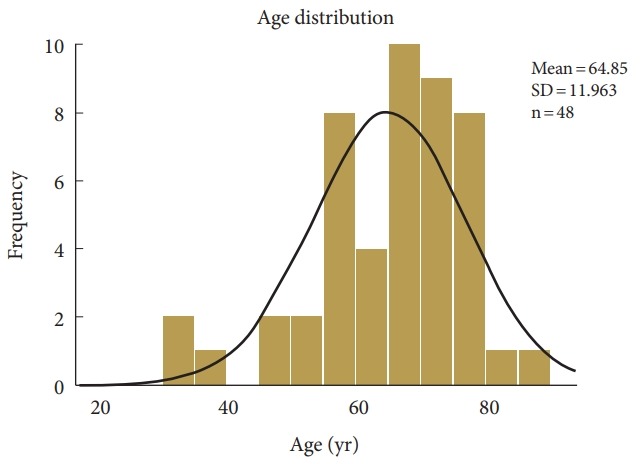
Age distribution of patients undergoing standalone lordotic endoscopic wedge lumbar interbody fusion. Patient’s age ranged from 32 to 88 years of age and averaged 64.9 years. The expected normal age distribution is indicated by the black line. SD, standard deviation.
Patients considered not suitable for the transforaminal endoscopically assisted intervertebral fusion procedure were stratified according to the following exclusion criteria: (1) Segmental instability with greater than grade II spondylolisthesis or translational motion of greater than 8 mm on preoperative extension-flexion radiographs, (2) severe central stenosis (less than 100 mm2) [29], (3) extensive facet arthropathy, (4) infection, and (5) metastatic disease.
All patients in this consecutive case series provided informed consent and IRB approval was obtained (CEIFUS 106-19).
2. Surgical Technique
The surgical procedure employs the endoscopic transforaminal approach using the “outside-in” technique, in which the working sheath is placed into the lower portion of the neuroforamen, thus, aiding in the retraction and avoiding the exiting nerve root. During the interbody fusion procedure and for preparation of the endplate the cannula tip or the endoscope can be positioned in the disc space utilizing “inside-out” techniques for discectomy and endplate preparation. The modified endoscopic decompression technique applied by the author [8,24-27] has initially been popularized by Yeung, Hoogland and Schubert et al. [23-27,29,30] It employs foraminal decompression and expansive foraminoplasty. The interbody fusion procedure has been described in great detail [27]. The described procedure was performed in prone position under general anesthesia with adjunctive use of local anesthesia using 0.25% bupivacaine in all patients. In short, the working cannula is placed into the safe zone of Kambin’s triangle bordered by the traversing nerve root medially, the exiting nerve root laterally, and the lower adjacent pedicle distally [31,32]. Endoscopic osteotomes, motorized drills, Kerrison rongeurs, and percutaneous trephines were employed through the inner 4.1-mm inner working channel of the spinal endoscope for the foraminoplasty, which was done via removal of bone from the hypertrophied superior and inferior articular process. The entire superior articular process (SAP) was resected starting rostral to distal via osteotomy and detached from the superomedial pedicle wall.
In some cases, the foraminoplasty was expanded by partial resection of the superomedial pedicle wall of the distal pedicle. This was often necessary to prepare the introitus for the interbody fusion cage to promote parallel alignment of the implant with the vertebral interspace and to avoid rostral migration of the cage insertion point into the axilla between the exiting and traversing nerve root. Cannulated paddle shavers were used to remove disc tissue and to decorticate the endplates without going through the subchondral bone to minimize implant subsidence. The interbody fusion was done using the VariLift-L interbody fusion system with one oblique positioned cage. The round expandable implants are available in sizes from 10 to14 mm with the final expanded outer diameter typically being 3 to 4 mm larger than its starting size before the expansion maneuver. The implant’s outer diameter is 4 mm smaller at the tip than at the end of the implant’s body. Under fluoroscopic guidance, the sizing tap is inserted into the intervertebral disc space by turning it clockwise. The posterolateral insertion trajectory in the axial plane is typically between 40° to 60° of the midsagittal vertical plumb line but essentially dictated by the patient’s vertical alignment of the intervertebral discs with the iliac crest. The expandable implant should be sized snug. After successful placement and expansion of the implant, local bone- and cancellous allograft was inserted into the cage’s central graft chamber via the inserter.
3. Clinical Follow-up
Typically, patients returned for clinical follow-up at 2, and 6 weeks postoperatively, and at 3, 6, 12, and 24 months, respectively. Primary clinical outcome measures were reductions in the visual analogue scale (VAS) [33] for leg pain ranging from no pain (0) to worst pain (10) both done by the patient and by the treating surgeon (KUL) using the MacNab criteria [34]. Briefly, follow-up outcome results were classified as excellent (little pain, able to perform desired activities with few limitations); good (occasional pain or dysesthesia, able to perform daily activities with minor limitations and did not need pain medication); fair (level of pain somewhat improved but continued to need pain medication; or poor (function worsened or needed additional surgery to address symptoms. At each and the final follow-up visit, the clinical outcome of each patient was graded using these modified MacNab and criteria [34]. Ultimately, the standardized MacNab outcome measures were used to determine the clinical impact of any patient-related factors that could contribute to less favorable outcomes.
4. Complication, Cage Subsidence, and Outcome Analysis
Failure to cure following a well-executed standalone LEW-LIF surgery with minimal or no pain relief was not considered a complication. Likewise, sequelae including extravasation of irrigation fluid into the spinal canal or in the subcutaneous tissues causing spinal headaches, or increased incisional pain, or pain from contusion of the ilium during the L5/S1 transforaminal approach, or dysesthetic leg pain from dorsal root ganglion (DRG) irritation were also not considered complications. All complications during the stay at the ASC were recorded. As all patients enrolled in this study were discharged to return home after surgery. At each follow-up visit, patients were asked whether they were treated for any postoperative complications in an emergency room and if any of these visits resulted in an admission to a hospital. Patients were also monitored during regularly scheduled follow-up visits for any signs of unbeknownst or asymptomatic postoperative complications, such as new onset of motor weakness, abnormal sensory function or acute onset of low back pain. In the case of fair and poor postoperative MacNab outcomes, patients’ postoperative plain film surveillance studies were scrutinized for implant subsidence, migration, or extrusion. Also, patients preoperative advanced imaging studies were analyzed for any other contributing patient-related factors in case of less favorable clinical outcomes if no implant-related problems could be identified. Examples included multilevel- or severe disease as evidenced by facet hypertrophy, central spinal stenosis, and anterior column tethering with sentinel osteophytes of the ring apophysis.
For the clinical outcome analysis, cross tabulation statistics and measures of association were computed for two-way tables using IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA). Descriptive statistic measures were used to calculate the mean, range, and standard deviation as well as percentages. Crosstabulation methods were used to assess for any statistically significant association between clinical outcome data based on the modified MacNab criteria and VAS scores. Pearson chi-square and Fisher exact test were employed as statistical measures of association. Expected cell counts, continuity corrections, and likelihood ratios were calculated for some analyses. The VAS reductions were compared and tested for statistically significant difference between preoperative and postoperative values using the paired t-test.
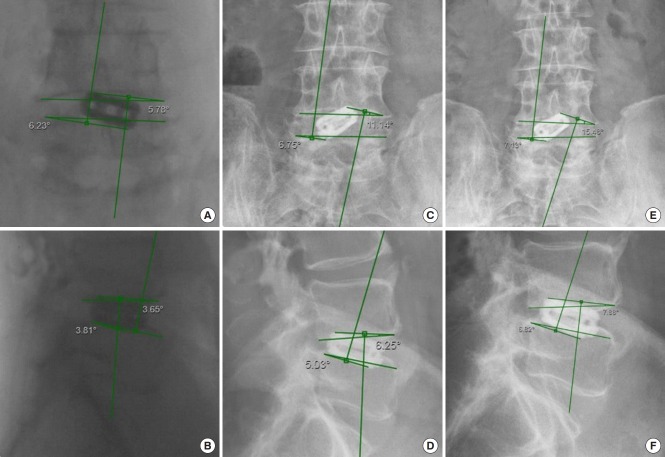
Angular cage subsidence (tilt) was determined on intraoperative fluoroscopic and postoperative anteroposterior and lateral views using medical imaging technology Merge picture archiving and communication system (PACS) by Watson Health Imaging, Chicago, IL, USA. Angular measurements were done between horizontal lines drawn along the inferior and superior endplates of the vertebral bodies of the fused lumbar motion segment and additional lines drawn to the most external contact point of the cage with the endplates superiorly and inferiorly on either end of the cage (Fig. 2). For clarity of data presentation only coronal plane, angular subsidence data were included for presentation in this publication. The vertical subsidence was measured exclusively on intraoperative fluoroscopic and postoperative lateral views as the distance between horizontal lines drawn at the posterior margin of the superior and inferior endplate of each motion segment and the horizontal lines draw at the most external point of the cage at each end contacting the endplate or protruding into the vertebral body (Fig. 3).
Fig. 2.
Intraoperative fluoroscopic posterior-anterior (PA) (A), and lateral (B) view of an 88-year-old male who underwent L4/5 standalone lordotic endoscopic wedge lumbar interbody fusion and was asymptomatic at final follow-up. Change in implant position was measured against 2 horizontal lines drawn along the interior L4 and superior L5 endplate. The cylindrical threaded interbody fusion cage subsided both vertically but also by tilting mostly through the inferior endplate of the rostral vertebral body. Progressive implant subsidence was estimated by measuring the angles between the horizontal lines and additional lines drawn to the outer diameter of the circular cage in contact with the inferior L4 endplate superiorly and the L5 endplate inferiorly. These angles indicated progressive subsidence of the standalone threaded fusion cage in both the coronal (A, C, E) and in the sagittal plane (B, D, F): Intraoperative (A, B) lateral (LAT) subsidence into L4 = 3.65°, into L5 = 3.81°, PA subsidence L4 = 5.78°, into L5 = 6.23°; Three months postoperatively (C, D): LAT subsidence into L4 = 6.25°, into L5 = 5.03°, AP subsidence L4 = 11.14°, into L5 = 6.75°; Eleven months postoperatively (E, F): LAT subsidence into L4 = 7.88°, into L5 = 6.82°, AP subsidence L4 = 7.13°, into L5 = 15.48°.
Fig. 3.
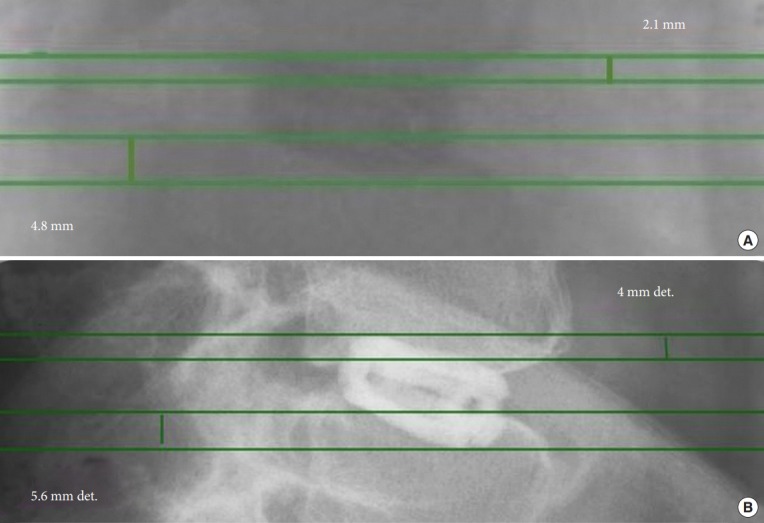
Intraoperative fluoroscopic (A), and 11 months postoperative lateral (B) view of an 88-year-old male who underwent L4/5 standalone lordotic endoscopic wedge lumbar interbody fusion and was asymptomatic at final follow-up. His imaging studies suggested minimal lateral vertical subsidence. The vertical collapse due to implant subsidence was estimated on lateral radiographs by measuring the distance between horizontal lines placed at the most posterior aspect of the inferior L4 and superior L5 endplates. The distance to additional horizontal lines drawn at the most superior rostral and most inferior distal part of the cage was measured. The progressive distance between these superior and inferior lines were used as an estimate of cage subsidence: intraoperative (A) lateral (LAT) subsidence into L4 = 2.1 mm, into L5 = 4.8 mm, and LAT subsidence at 11 months postoperatively into L4 = 4.0 mm, (B) LAT subsidence into L5 = 5.6 mm.
5. Postoperative Rehabilitation and Unintended Aftercare
Postoperative rehabilitation and supportive care were routinely instituted for all patients. These included active exercise programs prescribed in the postoperative instructions but without formal physical or occupational therapy. Patients were asked whether they developed any new pain syndromes or hitherto unknown conditions that negatively impacted their walking endurance. Patients with dysethetic leg pain due to irritation of the DRG were treated with a combination of oral medication including nonsteroidal anti-inflammatory drugs, gabapentin, or pregabalin, TESI containing a mixture of 1 mL of Depo Medrol Sterile Aqueous Suspension (which contains Methylprednisolone Acetate 20 mg/1 mL and 1 mL of 1% lidocaine), activity modification to a light walking schedule and reduced physical activity program. Patients were advised that narcotic pain medication is not an effective treatment for dysesthetic leg pain due to postoperative DRG irritation. Any unintended aftercare measures were recorded.
RESULTS
Of the 48 patients, 44 patients underwent surgery to alleviate spondylolisthesis-related symptoms (Table 1). Another 2 patients underwent endoscopic decompression fusion surgery for central and lateral recess stenosis. An additional 2 patients had the surgery for adjacent segment disease. Thirty-six patient had transforaminal cage implantation using a left-sided approach. The remaining 12 patients underwent interbody fusion using the right-sided approach. Another 12 of the 48 patients underwent bilateral transforaminal decompression with an additional foraminoplasty on the opposite side from cage implantation. Thirty-six of the 48 patients underwent endoscopically assisted interbody fusion at the L4/5 level (Table 2). This procedure was performed in another 8 patients at the L5/S1 level, and in one other patient at the L3/4 level, respectively. Three additional patients were treated with a hybrid decompression fusion procedure consisting of L4/5 LEW-LIF surgery combined with an L3/4 laminoforaminotomy microdiscectomy in one patient, and at the L5/S1 level in another 2 patients (Table 2).
Table 1.
Descriptive statistics of demographics, preoperative diagnosis, and laterality of surgery
| Variable | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| Sex | ||||
| Female | 29 | 60.4 | 60.4 | 60.4 |
| Male | 19 | 39.6 | 39.6 | 100.0 |
| Total | 48 | 100 | 100 | |
| Preoperative diagnosis | ||||
| Adjacent segment disease | 2 | 4.2 | 4.2 | 4.2 |
| Spondylolisthesis | 44 | 91.7 | 91.7 | 95.8 |
| Stenosis | 2 | 4.2 | 4.2 | 100.0 |
| Total | 48 | 100 | 100 | |
| Laterality of decompression | ||||
| Bilateral | 12 | 25.0 | 25.0 | 25.0 |
| Left | 25 | 52.1 | 52.1 | 77.1 |
| Right | 11 | 22.9 | 22.9 | 100.0 |
| Laterality of transforaminal implantation | ||||
| Left | 36 | 75.0 | 75.0 | 75.0 |
| Right | 12 | 25.0 | 25.0 | 100 |
| Total | 48 | 100 | 100 |
Table 2.
Level distribution of endoscopic transforaminal procedures
| Variable | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| L3/4 fusion | 1 | 2.1 | 2.1 | 2.1 |
| L4/5 fusion | 36 | 75.0 | 75.0 | 77.1 |
| L4/5 fusion & L3/4 laminoforaminotomy microdiscectomy | 1 | 2.1 | 2.1 | 79.2 |
| L4/5 fusion & L5/S1 laminoforaminotomy microdiscectomy | 2 | 4.2 | 4.2 | 83.3 |
| L5/S1 | 8 | 16.7 | 16.7 | 100 |
| Total | 48 | 100 | 100 |
Clinical outcomes according to MacNab criteria were favorable in the majority of patients with 29 of them having reported excellent, and another 13 patients having reported good outcomes at final follow-up (Table 3). Fair results were reported by 4, and poor results by another 2 patients. Twenty-nine of the 48 patients experienced dysethetic leg pain due to DRG irritation between postoperative week 2 to 6 (green-shaded area in Fig. 4). Typically, symptoms were mild and resolved with the supportive care measures and TESI, as described above, was only required in 12 of the 29 patients. Eleven of the 48 patients had additional follow-up surgeries typically within 2 to 6 months (orange-shaded area in Fig. 4) postoperatively following the standalone LEW-LIF procedure. Most follow-up surgeries performed postoperatively were single-level unilateral endoscopic transforaminal decompression procedures at adjacent symptomatic levels (6 patients; Table 4).
Table 3.
Clinical outcomes by MacNab
| Outcome | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| Excellent | 29 | 60.4 | 60.4 | 60.4 |
| Good | 13 | 27.1 | 27.1 | 87.5 |
| Fair | 4 | 8.3 | 8.3 | 95.8 |
| Poor | 2 | 4.2 | 4.2 | 100 |
| Total | 48 | 100 | 100 |
Fig. 4.
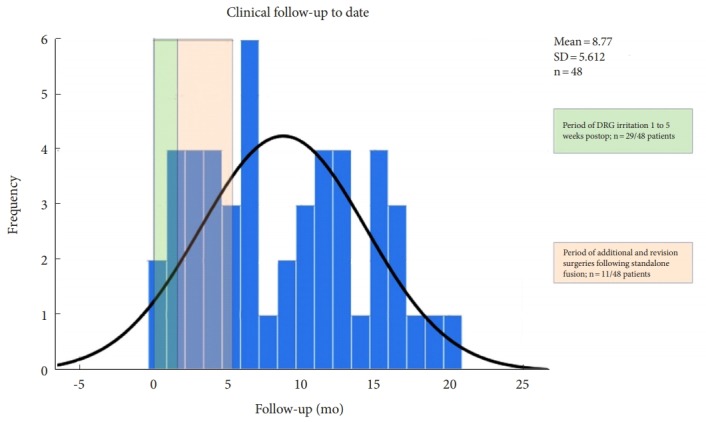
Follow-up to date of patients undergoing standalone lordotic endoscopic wedge lumbar interbody fusion. The green-shaded area highlights the time period when postoperative dorsal root ganglion (DRG) irritations were treated with transforaminal epidural steroid injections. The orange-shaded area signifies the postoperative interval in which additional- and revision surgeries were performed. The expected normal distribution of follow-up data is indicated by the black line. Most postoperative interventions occurred early on within the first 5 months following surgery. SD, standard deviation.
Table 4.
Types of additional surgeries following prior standalone endoscopic transforaminal fusion
| Type of additional surgery | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| L3-S1 TLIF | 1 | 2.1 | 2.1 | 2.1 |
| L4-S1 TLIF | 3 | 6.3 | 6.3 | 8.3 |
| L4/5 laminoforaminotomy | 1 | 2.1 | 2.1 | 10.4 |
| L5-S1 lamiotomy rhizotomy | 1 | 2.1 | 2.1 | 12.5 |
| L5/S1 laminoforaminotomy rhizotomy | 1 | 2.1 | 2.1 | 14.6 |
| L5/S1 laminotomy rhizotomy | 2 | 4.2 | 4.2 | 18.8 |
| Patients without additional surgery | 37 | 77.1 | 77.1 | 95.8 |
| Revision L3/4 fusion | 1 | 2.1 | 2.1 | 97.9 |
| Right laminoforaminotomy rhizotomy | 1 | 2.1 | 2.1 | 100 |
| Total | 48 | 100 | 100 |
TLIF, transforaminal lumbar interbody fusion.
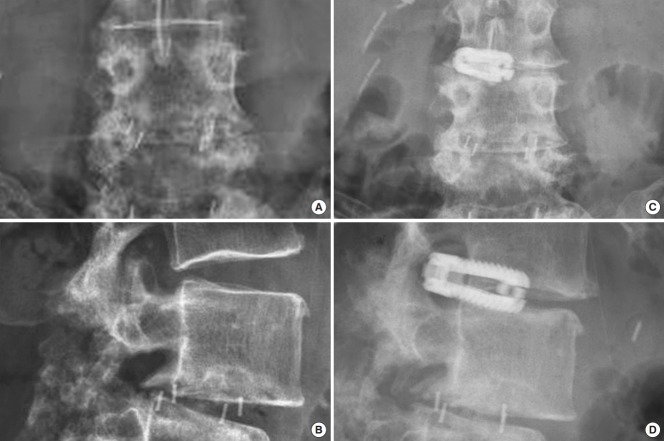
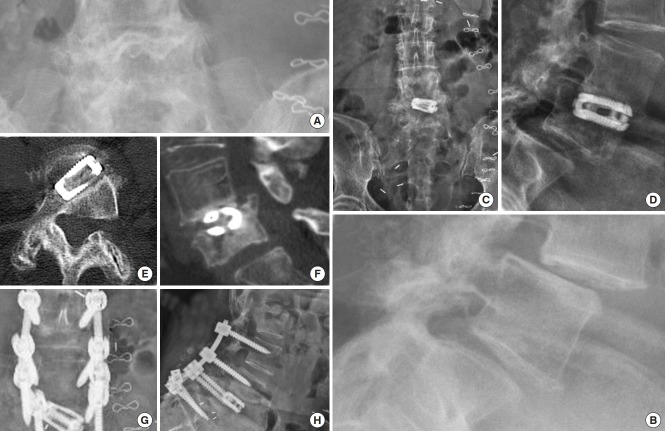
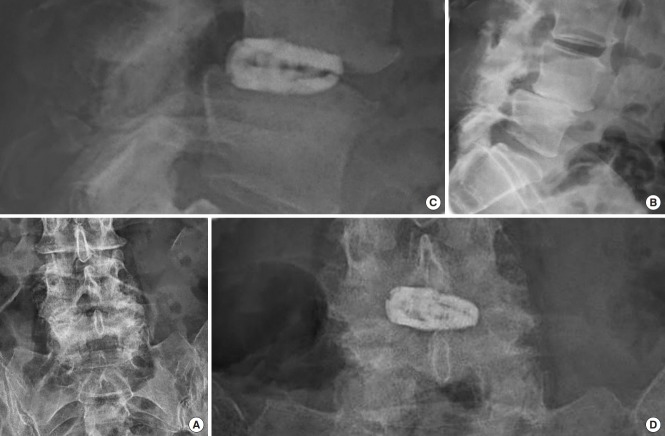
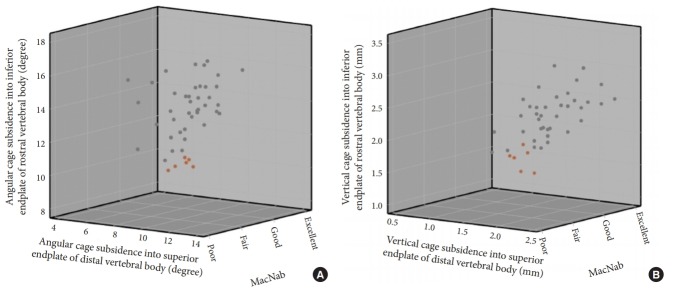
An additional four patients required revision surgery including the same index level. One female patient underwent removal of the standalone expandable fusion cage and placement of cancellous bone allograft into the interspace after it posteriorly extruded following a fall when walking her dog (Fig. 5). An additional female patient sustained an anterior beak fracture of the L5 vertebral body 3 weeks postoperatively after having undergone an L4/5 standalone LEW-LIF for grade II spondylolisthesis (Fig. 6). This patient had an additional fracture fragment rotated posteriorly into the spinal canal causing an acute onset of low back and leg pain requiring revision L3–S1 decompression fusion surgery to stabilize the 2-level lumbar spondylolisthesis. Three-month routine plain film radiographs of an asymptomatic female patient who underwent standalone LEW-LIF for grade I L4/5 spondylolisthesis showed a fracture of the implant expansion mechanism with the collapse of the intervertebral fusion space (Fig. 7). She required no treatment. Additional potentially confounding pain generators were observed in the 48 study patients (Table 5). Multilevel stenosis adjacent segment disease, deformity, and osteoporosis in one patient were associated with less favorable outcomes at a statistically significant level (Table 6) (p=0.001). Analysis of vertical and angular cage subsidence in all 48 patients showed that all cages subsided and preferentially tilted into the inferior endplate of the rostral vertebral body. Scatter plotting of vertical and angular subsidence in relation to clinical outcomes as measured by MacNab criteria showed best in 3-dimensional plots in an easyto-understand manner that fair and poor outcomes were associated with increased cage subsidence into the superior endplate of the distal vertebral body (Fig. 8).
Fig. 5.
Preoperative lumbar anteroposterior (AP) (A) and lateral (B) plain films of a 68-year-old female with lateral recess stenosis and retrolisthesis at L3/4 due to adjacent segment disease following a prior L4/5 transforaminal lumbar interbody fusion. The patient underwent uneventful standalone lordotic endoscopic wedge lumbar interbody fusion at L3/4. She did well initially but fell at 6 weeks postoperatively when walking her dog. She developed sudden onset of new back pain and right leg anteromedial thigh pain consistent with L3 radiculopathy. Postoperative AP (C) and lateral (D) plain films showed posterolateral dislocation of the cage with extrusion through the transforaminal surgical tract. The patient improved immediately after removal of the implant and placement of bone graft into the interspace.
Fig. 6.
Preoperative lumbar anteroposterior (A) and lateral (B) plain films of a 72-year-old female with two level L4/5 and L5/S1 spondylolisthesis. She underwent successful L4/5 standalone lordotic endoscopic wedge lumbar interbody fusion as the first of a planned 2-stage surgery to include the L5/S1 level at a later point after sufficient recovery from the L4/5 fusion. She fell 4 weeks postoperatively and represented with acute onset of leg- and low back pain after an initial postoperative period with good pain relief. Imaging workup (C-F) showed a displaced L5 anterior beak fracture and a displaced fracture of the rostral posterior wall with complete cage subsidence causing severe spinal stenosis. She underwent L3–S1 instrumented fusion with placement of L5–S1 polyetheretherketone interbody fusion cages. The postoperative computed tomography scan serendipitously showed good graft filling of the internal graft chamber of the cage.
Fig. 7.
Preoperative lumbar anteroposterior (AP) (A) and lateral (B) plain films of a 63-year-old female with spondylolisthesis who underwent standalone lordotic endoscopic wedge lumbar interbody fusion at L4/5 for spondylolisthesis. The patient improved immediately postoperatively and never had any pain through final follow-up. At 3 months postoperatively, fracture of the cage was noticed routine lateral (C) and AP (D) X-rays. Since the patient was asymptomatic, no further treatment was instituted.
Table 5.
Confounding factors observed in patients who underwent transforaminal endoscopic standalone fusion
| Confounding factor | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| Adjacent segment | 2 | 4.2 | 4.2 | 4.2 |
| Adjacent segment disease, scoliosis | 1 | 2.1 | 2.1 | 6.3 |
| Multilevel stenosis | 10 | 20.8 | 20.8 | 27.1 |
| Multilevel stenosis, osteoporosis | 1 | 2.1 | 2.1 | 29.2 |
| Multilevel stenosis, scoliosis | 4 | 8.3 | 8.3 | 37.5 |
| Patients without confounding factors | 22 | 45.8 | 45.8 | 83.3 |
| Postlaminectomy syndrome | 2 | 4.2 | 4.2 | 87.5 |
| Postlaminectomy syndrome, adjacent level disease | 2 | 4.2 | 4.2 | 91.7 |
| Postlaminectomy syndrome, multilevel stenosis | 3 | 6.3 | 6.3 | 97.9 |
| Postop adjacent segment disease | 1 | 2.1 | 2.1 | 100 |
| Total | 48 | 100 | 100 |
Table 6.
Crosstabulation confounding factors versus MacNab outcomes
| Confounding factor | MacNab outcomes |
||||
|---|---|---|---|---|---|
| Excellent | Good | Fair | Poor | Total | |
| Multilevel stenosis, scoliosis | 3 | 1 | 0 | 0 | 4 |
| Patients without confounding factors | 17 | 5 | 0 | 0 | 22 |
| Adjacent segment | 1 | 1 | 0 | 0 | 2 |
| Adjacent segment disease, scoliosis | 1 | 0 | 0 | 0 | 1 |
| Multilevel stenosis | 3 | 4 | 2 | 1 | 10 |
| Multilevel stenosis, osteoporosis | 0 | 0 | 0 | 1 | 1 |
| Postlaminectomy syndrome | 0 | 0 | 2 | 0 | 2 |
| Postlaminectomy syndrome, adjacent level disease | 1 | 1 | 0 | 0 | 2 |
| Postlaminectomy syndrome, multilevel stenosis | 2 | 1 | 0 | 0 | 3 |
| Postoperative adjacent segment disease | 1 | 0 | 0 | 0 | 1 |
| Total | 29 | 13 | 4 | 2 | 48 |
Pearson chi-square: 57.278804; asymptotic significance (2-sided)=0.001. Likelihood ratio: 32.738249; asymptotic significance (2-sided)=0.206.
Fig. 8.
Three-dimensional (3D) scatter plot of angular (A) and vertical (B) subsidence of standalone lordotic endoscopic wedge lumbar interbody fusion cages into the inferior endplate of the rostral, and the superior endplate of the distal vertebral body below versus clinical outcomes using MacNab criteria. These 3D scatter plots show that the 6 patients fair and poor clinical outcomes suffered from preferential vertical and angular subsidence into the superior endplate of L5 suggesting stress concentration at the implant-bone interface is occurring at the expanded part the threaded interbody fusion.
DISCUSSION
This study showed the feasibility of a standalone endoscopically assisted interbody fusion surgery with the LEW-LIF procedure. The majority of patients (37 of 48 patients, 77.08%) had excellent and good outcomes. No significant complications were observed with this outpatient procedure. None of the patients had wound infections, or permanent sensory or motor dysfunction or required admission to a hospital for any reason in the immediate postoperative period. As a result of having mastered the learning curve of the procedure with a case series of 48 patients, the overall rate of failure to cure (fair and poor outcomes) of 10.42% was still lower in comparison to reported success rates with open spinal fusion with only 5 patients requiring unintended aftercare with revision surgery directly related to implant subsidence, extrusion, and fracture. Patient-related factors were relevant in another 6 patients who developed pain during the postoperative recovery period due to symptoms from another level. Risk factors for less favorable outcomes for the standalone LEW-LIF procedure without the use of any supplemental posterior fixation include multilevel stenosis, prior spine surgery with postlaminectomy syndrome and adjacent level disease. Once these 6 patients were treated with additional single-level endoscopic decompression procedures at another, and not the index fusion level, their functional outcome rating improved. In other words, their initially reported fair and poor outcomes were most likely related to pain generators from other levels as demonstrated by the one patient with 2-level spondylolisthesis (Fig. 6).
Some pearls of the standalone endoscopic interbody fusion LEW-LIF surgery emerged from this study. First, a generous foraminoplasty with complete resection of the SAP is necessary to mobilize the spinal motion segment and facilitate the introduction of the implant. A partial pediculectomy and resection of obstructing osteophytes of the ring apophysis may aid in that. In turn, these steps may further decrease the rate of DRG irritation. However, improvements in next-generation implant design and instrumentation may also contribute to a decrease in the incidence of this postoperative sequela, which in 25% of the study population required treatment with a TESI. Second, endplate sparing decortication in preparation of the interbody fusion is critical to avoid excessive subsidence of the implant. Osteoporosis may exacerbate this problem. Paddle shavers used during the decortication maneuvers should rest on the opposite ring apophysis when rotated to avoid breaching the subchondral bone of the endplate. Intraoperative observations showed that the inferior endplate of the rostral vertebral body is more susceptible to injury than the superior endplate of the inferior vertebral body during these maneuvers. Third, maximizing intervertebral height is critical to aid in indirect decompression of the foramen and lateral recess opposite from the access side. Under-sizing the implant may contribute to recurrent symptoms and should be avoided. However, oversizing of the implant should also be avoided not to propagate any vertebral fractures.
Analysis of vertical and angular subsidence of the expandable threaded cylindrical cage showed that more postoperative pain and less favorable outcomes are associated with preferential cage subsidence into the superior endplate of the distal vertebral body. The anterolateral placement of a single cage and its subsequent expansion may have led to stress concentration in the small contact area of the expanded portion of the cage. While it is difficult to say whether this stress concentration at the bone-implant interface at the expanded end of the cylindrical cage contributed to mechanical failure of the implant in one patient (Fig. 7), the extrusion of the implant in another (Fig. 5), and propagation of an anterior beak fracture of the L5 vertebral body in an additional patient (Fig. 6), it is, however, intuitive but cannot be irrefutably concluded and was beyond the scope of this clinical study. Further mechanical in vitro and clinical studies could investigate this perceived stress concentration problem and perhaps lead to improved implant designs.
Besides the obvious that performing a standalone LEW-LIF requires hands-on experience with the procedure and is not for the novice endoscopic spine surgeon, this study also shows that adherence to appropriate patient selection criteria is critical to achieving successful outcomes. The authors conclude that patients with multilevel lumbar disease, deformity, osteoporosis, more than grade I spondylolisthesis are at risk for less favorable outcomes than spondylolisthesis patients displaying minimal or no translational motion on extension/flexion radiographs and single rather than multilevel stenosis involvement. Therefore, conclusive preoperative workup of the symptomatic pain generators is essential for selecting patients for standalone LEW-LIF. The senior author, A. Yeung, with 28 years of experience in transforaminal endoscopic decompression since 1991 contributed to the standalone concept of decompression and stabilization in the face of spondylolisthesis. He mainly focused on endoscopic decompression, while saving fusion for failed endoscopic decompression. Approximately 25% of his case series with spondylolisthesis eventually required fusion as a staged procedure after 5 years. Highly vetted patient selection and stratification of the failures allowed Yeung to reduce his fusion rate to 12.5% (personal communication and recent and publications in press). For similar reasons, the third author, J Ramirez, has developed a standalone endoscopic interbody fusion implant following the LEW-LIF concept . a procedure that is reserved for patients with failed endoscopic decompression and spondylolisthesis (personal communication). The importance of preoperative planning of LEW-LIF is equally essential as for removal of herniated discs or spinal stenosis [35-40].
CONCLUSION
Standalone LEW-LIF with the use of a single oblique threaded expandable interbody fusion cage is not only feasible but resulted in excellent and good MacNab clinical outcomes in the majority of patients. This simplified version of lumbar interbody fusion is attractive as an alternative to open or other forms of MISS in the medically complex patients. Vertical subsidence and tilt was observed in nearly every patient and but was only associated with less favorable outcomes in case of excessive subsidence of the expansion mechanism into the superior endplate of the distal vertebral body.
Acknowledgments
The views expressed in this article represent those of the authors and no other entity or organization.
Footnotes
The first author has no direct (employment, stock ownership, grants, patents), or indirect conflicts of interest (honoraria, consultancies to sponsoring organizations, mutual fund ownership, paid expert testimony). The first author is not currently affiliated with or under any consulting agreement with any vendor that the clinical research data conclusion could directly enrich. This manuscript is not meant for or intended to endorse any products or push any other agenda other than the associated clinical outcomes with standalone lordotic endoscopic wedge lumbar interbody fusion. The motive for compiling this clinically relevant information is by no means created and/or correlated to directly enrich anyone due to its publication. However, this publication was intended to substantiate the LEW-LIF concept to facilitate technology advancements.
SUPPLEMENTARY MATERIAL
Supplementary video clip 1 can be found via https://doi.org/10.14245/ns.1938046.023.v1.
REFERENCES
- 1.Xie L, Wu WJ, Liang Y. Comparison between minimally invasive transforaminal lumbar interbody fusion and conventional open transforaminal lumbar interbody fusion: an updated meta-analysis. Chin Med J (Engl) 2016;129:1969–86. doi: 10.4103/0366-6999.187847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein CL, Macwan K, Sundararajan K, et al. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24:416–27. doi: 10.3171/2015.2.SPINE14973. [DOI] [PubMed] [Google Scholar]
- 3.Virdee JS, Nadig A, Anagnostopoulos G, et al. Comparison of peri-operative and 12-month lifestyle outcomes in minimally invasive transforaminal lumbar interbody fusion versus conventional lumbar fusion. Br J Neurosurg. 2017;31:167–71. doi: 10.1080/02688697.2016.1199790. [DOI] [PubMed] [Google Scholar]
- 4.Phan K, Rao PJ, Kam AC, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and metaanalysis. Eur Spine J. 2015;24:1017–30. doi: 10.1007/s00586-015-3903-4. [DOI] [PubMed] [Google Scholar]
- 5.Khan NR, Clark AJ, Lee SL, et al. Surgical outcomes for minimally invasive vs open transforaminal lumbar interbody fusion: an updated systematic review and meta-analysis. Neurosurgery. 2015;77:847–74. doi: 10.1227/NEU.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 6.Adogwa O, Parker SL, Shau DN, et al. Cost per quality-adjusted life year gained of revision neural decompression and instrumented fusion for same-level recurrent lumbar stenosis: defining the value of surgical intervention. J Neurosurg Spine. 2012;16:135–40. doi: 10.3171/2011.9.SPINE11308. [DOI] [PubMed] [Google Scholar]
- 7.O'Lynnger TM, Zuckerman SL, Morone PJ, et al. Trends for spine surgery for the elderly: implications for access to healthcare in North America. Neurosurgery. 2015;77 Suppl 4:S136–41. doi: 10.1227/NEU.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 8.Lewandrowki KU. Surgical technique of endoscopic transforaminal decompression and fusion with a threaded expandable interbody fusion cage and a report of 24 cases. J Spine. 2018;7:409. [Google Scholar]
- 9.Blumenthal SL, Ohnmeiss DD, NASS Intervertebral cages for degenerative spinal diseases. Spine J. 2003;3:301–9. doi: 10.1016/s1529-9430(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Yang H, Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine (Phila Pa 1976) 2005;30:2171–5. doi: 10.1097/01.brs.0000180402.50500.5b. [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Sung KH. Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J. 2006;15:16–22. doi: 10.1007/s00586-004-0817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zdeblick TA, Phillips FM. Interbody cage devices. Spine (Phila Pa 1976) 2003;28(15 Suppl):S2–7. doi: 10.1097/01.BRS.0000076841.93570.78. [DOI] [PubMed] [Google Scholar]
- 13.Babu R, Park JG, Mehta AI, et al. Comparison of superiorlevel facet joint violations during open and percutaneous pedicle screw placement. Neurosurgery. 2012;71:962–70. doi: 10.1227/NEU.0b013e31826a88c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RD, Graziano GP, Vanderhave KL, et al. Facet violation with the placement of percutaneous pedicle screws. Spine (Phila Pa 1976) 2011;36:E1749–52. doi: 10.1097/BRS.0b013e318221a800. [DOI] [PubMed] [Google Scholar]
- 15.Lee CS, Hwang CJ, Lee SW, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–43. doi: 10.1007/s00586-009-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruetten S. Full-endoscopic operations of the spine in disk herniations and spinal stenosis. Surg Technol Int. 2011;21:284–98. [PubMed] [Google Scholar]
- 17.Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18:61–70. [PubMed] [Google Scholar]
- 18.Komp M, Hahn P, Ozdemir S, et al. Operation of lumbar zygoapophyseal joint cysts using a full-endoscopic interlaminar and transforaminal approach: prospective 2-year results of 74 patients. Surg Innov. 2014;21:605–14. doi: 10.1177/1553350614525668. [DOI] [PubMed] [Google Scholar]
- 19.Birkenmaier C, Komp M, Leu HF, et al. The current state of endoscopic disc surgery: review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician. 2013;16:335–44. [PubMed] [Google Scholar]
- 20.Cheatle MD. Facing the challenge of pain management and opioid misuse, abuse and opioid-related fatalities. Expert Rev Clin Pharmacol. 2016;9:751–4. doi: 10.1586/17512433.2016.1160776. [DOI] [PubMed] [Google Scholar]
- 21.Hupp JR. The surgeon’s roles in stemming the prescription opioid abuse epidemic. J Oral Maxillofac Surg. 2016;74:1291–3. doi: 10.1016/j.joms.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh S, Chung F, Imarengiaye C, et al. Pain, nausea, vomiting and ocular complications delay discharge following ambulatory microdiscectomy. Can J Anaesth. 2003;50:514–8. doi: 10.1007/BF03021067. [DOI] [PubMed] [Google Scholar]
- 23.Yeung AT, Yeung CA. Minimally invasive techniques for the management of lumbar disc herniation. Orthop Clin North Am. 2007;38:363–72. doi: 10.1016/j.ocl.2007.04.005. abstract vi. [DOI] [PubMed] [Google Scholar]
- 24.Tsou PM, Alan Yeung C, Yeung AT. Posterolateral transforaminal selective endoscopic discectomy and thermal annuloplasty for chronic lumbar discogenic pain: a minimal access visualized intradiscal surgical procedure. Spine J. 2004;4:564–73. doi: 10.1016/j.spinee.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Tsou PM, Yeung AT. Transforaminal endoscopic decompression for radiculopathy secondary to intracanal noncontained lumbar disc herniations: outcome and technique. Spine J. 2002;2:41–8. doi: 10.1016/s1529-9430(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 26.Yeung AT, Yeung CA. Advances in endoscopic disc and spine surgery: foraminal approach. Surg Technol Int. 2003;11:255–63. [PubMed] [Google Scholar]
- 27.Lewandrowski KU. Readmissions after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis. Int J Spine Surg. 2018;12:342–51. doi: 10.14444/5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am. 2003;34:281–95. doi: 10.1016/s0030-5898(02)00069-x. [DOI] [PubMed] [Google Scholar]
- 29.Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976) 2006;31:E890–7. doi: 10.1097/01.brs.0000245955.22358.3a. [DOI] [PubMed] [Google Scholar]
- 30.Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol. 2005;17:641–61. doi: 10.1007/s00064-005-1156-9. [DOI] [PubMed] [Google Scholar]
- 31.Kambin P, Casey K, O'Brien E, et al. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg. 1996;84:462–7. doi: 10.3171/jns.1996.84.3.0462. [DOI] [PubMed] [Google Scholar]
- 32.Kambin P, O'Brien E, Zhou L, et al. Arthroscopic microdiscectomy and selective fragmentectomy. Clin Orthop Relat Res. 1998;(347):150–67. [PubMed] [Google Scholar]
- 33.Huskisson EC, Jones J, Scott PJ. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol Rehabil. 1976;15:185–7. doi: 10.1093/rheumatology/15.3.185. [DOI] [PubMed] [Google Scholar]
- 34.Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891–903. [PubMed] [Google Scholar]
- 35.Kim MJ, Lee SH, Jung ES, et al. Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol. 2007;68:623–31. doi: 10.1016/j.surneu.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 36.Ahn Y, Lee SH, Park WM, et al. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine (Phila Pa 1976) 2004;29:E326–32. doi: 10.1097/01.brs.0000134591.32462.98. [DOI] [PubMed] [Google Scholar]
- 37.Lewandrowski KU. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg. 2014 Dec 1; doi: 10.14444/1026. 8. . eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J. 2007;16:431–7. doi: 10.1007/s00586-006-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine (Phila Pa 1976) 1988;13:313–20. doi: 10.1097/00007632-198803000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am. 1995;77:32–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video clip 1 can be found via https://doi.org/10.14245/ns.1938046.023.v1.