Abstract
Objectives:
No formal comparative effectiveness studies have been conducted to evaluate the effect of eosinophilic esophagitis (EoE) treatment choice on long-term growth in pediatric patients. However, long-term studies of inhaled corticoid steroids in asthma suggest possible effects on linear growth. The aim of this study was to compare longitudinal, anthropometric growth in children with EoE according to treatment approach.
Methods:
We conducted a retrospective, multicenter cohort study of anthropometric growth (height and BMI z-scores) in pediatric (<18 years of age) patients newly diagnosed with EoE across 5 clinical sites between 2005–2014. We compared differences in growth according to treatment approach over a 12 month period. Modification by sex and age was examined and sensitivity analyses were conducted to assess robustness of results given study assumptions.
Results:
In the 409 patients identified, the mean age and proportion male differed by treatment (p=<0.01 and p=0.04, respectively). Baseline growth measures were associated with slight impairment of height at diagnosis (median baseline height z-score of −0.1 [IQR −0.9, 0.8]). In general, treatment approach was not associated with any significant increase or decrease in expected growth over a 12 month period. Subtle decrease in linear growth was observed with treatment using a combined elemental and topical steroid (Δ height z-score (adjusted): −0.04; 95% CI: −0.08, −0.01). Differences in linear growth differed by sex (p for interaction <0.01). For elemental formula in combination with topical steroids, only females exhibited a significant decline in linear growth (Δ height z-score (adjusted): −0.24; 95% CI: −0.32, −0.17). A slight reduction in BMI was observed for patients treated with a combination of elemental diet and dietary elimination (Δ BMI z-score (adjusted): −0.07; 95% CI: −0.13, −0.01).
Conclusions:
Treatment of EoE, in general, is not associated with major anthropometric growth changes in most pediatric patients. Slight linear growth impairment was observed for topical steroid treatment, and sex differences in growth by treatment approach were observed. Future, prospective studies should evaluate the effect of treatment on optimal growth and development and over a longer period of follow-up.
Keywords: anthropometric growth, topical steroids, dietary elimination, elemental diet
INTRODUCTION
Eosinophilic esophagitis (EoE) is an increasingly common, chronic immune and antigen-mediated disorder that contributes to significant morbidity for many patients.1, 2 In children, symptoms may include heartburn, regurgitation, vomiting, food intolerance, poor growth, and failure to thrive.3, 4 Over time and with chronic inflammation, the disease assumes a more fibrostenotic presentation in certain patients,5, 6 such that adolescents and adults with long-standing disease typically present with dysphagia and food impaction.7, 8 Concomitant atopic conditions, including food allergies, are common with EoE,9–14 and food elimination diets are a first line treatment approach for EoE.15–17 Another common treatment approach is use of swallowed or topical corticosteroids (fluticasone inhaler; budesonide respules).18–21 In very young children, for children with impaired growth at diagnosis, or for children and adults refractory to treatment, amino-acid-based, hypoallergenic elemental diets may also be prescribed, either as an exclusive diet, or supplemental to another treatment approach, with nearly universal histologic improvement.22–25
As we have previously shown,26 the first line treatment approach is less informed by patient characteristics than by provider preference, and to date there have been no formal comparative effectiveness trials.27 Moreover, nearly all studies of EoE treatments report short-term outcomes, and little is known about the potential long term consequences of choice of first line treatment. Restrictive elimination diets have the potential to alter food choices that may influence anthropometric growth. Regular use of inhaled corticosteroids for asthma has been demonstrated to reduce linear growth,28 though this has not been seen in children or adolescents with EoE.29, 30 Elemental diets, if adhered to, have the potential to mitigate impaired growth, but with poor adherence may compromise growth if caloric intake is inadequate. Inducing disease remission, irrespective of first line treatment choice, has the potential to increase appetite and food intake, which could increase growth.
In the present study, we sought to evaluate the association between first line treatment approach and longitudinal growth in a pediatric cohort comprised of newly diagnosed eosinophilic esophagitis patients. We hypothesized that we would observe differences in longitudinal growth, with topical steroids demonstrating increased body mass index (BMI) and possibly impaired height, and with dietary elimination approaches eliciting reduced BMI and no impact on height. We hypothesized that elemental diets would result in increased height and possibly reduced BMI, as result of catch up growth in height.
METHODS
Study design and population
We conducted a multicenter retrospective cohort study of first line treatment and longitudinal growth measured by height and BMI over a 12 month follow-up period. Data were abstracted from electronic medical records through the Carolinas EoE Collaborative (CEoEC), a multi-site referral and research network of academic and community practices in North and South Carolina with expertise in managing EoE. Five CEoEC sites contributed to the study, including the University of North Carolina Hospitals (Chapel Hill, NC), Asthma and Allergy Specialists (Charlotte, NC), the Division of Pediatric Gastroenterology, Greenville Health System (Greenville, SC), Wake Forest Baptist Medical Center (Winston-Salem, NC), and the Medical University of South Carolina Children’s Hospital (Charleston, SC). Cases of EoE were defined clinically and per consensus guidelines,3, 4 including clinical symptoms of esophageal dysphagia, esophageal tissue biopsies showing ≥15 eosinophils per high power field (eos/hpf), and exclusion of other competing causes of esophageal eosinophilia. We included patients aged 0–18 years who were newly diagnosed with EoE between 2005–2014, and who had records indicating active management with pharmacologic or dietary interventions. As proton pump inhibitor (PPI) treatment before diagnosis was not included in the diagnostic guidelines until 2007, 23 percent of cases had no documentation of having been treated with a PPI prior to diagnosis. However, we planned sensitivity analyses related to this (see below), and the most recent guidelines have removed the PPI trial requirement for diagnosis of EoE.1, 31, 32 For inclusion in the study, the date of diagnosis had to have occurred within 90 days of the date of the diagnostic endoscopy. This study was approved by the IRB from each of the participating sites.
Data abstraction
Each CEoEC site was provided with a standardized, web-based data abstraction tool from which data could be downloaded and aggregated for assessment at a central location. All sites were provided with a data dictionary and researchers at the five sites all had expertise in EoE. All abstractors received training in abstracting patient data from the electronic medical record. Patient demographic factors abstracted included sex, race, ethnicity, and insurance status. Other baseline patient information abstracted included date of diagnosis, maximum tissue eosinophil count per high power field (eos/hpf) at initial diagnosis, and history of other atopic diseases such as asthma or allergic rhinitis. All data regarding initial treatment approach were collected. First line treatment approach was categorized into one of six categories: 1) topical steroid only, 2) dietary elimination only (both six-food and targeted), 3) elemental diet only, 4) topical steroids provided with supplemental elemental formula, 5) dietary elimination conducted with supplemental elemental formula, and 6) concomitant topical steroid and dietary elimination combined. From initial and follow-up clinic, dietitian, or endoscopic procedure visits, age, height and weight were abstracted. Up to 12 months of follow-up visit data were abstracted. Where follow-up upper endoscopy procedures were performed, we collected data on tissue eosinophil counts to evaluate treatment response as measured by histologic findings. Body mass index (kg/m2) was calculated from height and weight and sex and age-standardized z-scores for height and BMI were determined using the external reference data provided through the Centers for Disease Control.33 The height and BMI z-score represents an age and sex-standardized measure of height or BMI relative to an external reference for growth. A flat slope across the follow-up period indicates a lack of change in z-score over time and roughly corresponds to consistency in height or BMI percentile across time. Z-scores, unlike percentiles, offer better statistical properties as they are not truncated at the low and upper ends of the distribution.34 If a given child is following their centile growth curve, they will maintain roughly the same z-score. When a child crosses centiles, as a result of impaired growth or accelerated growth, their z-score will decline or increase accordingly.
Statistical analyses
Descriptive statistics were used to summarize the data and examine distributions. Subgroup comparisons were evaluated using the Chi-square test for categorical and the Kruskal-Wallis test for continuous measures. Fisher’s exact test was used where categorical data were too sparse for Chi-square estimation. Observed height and BMI z-scores over time, smoothed by treatment type, were examined to establish the general linear form of the data (Supplementary Figures 1–2). Adjusted linear mixed models with random effects for by-subject intercept and slope across follow-up time and with an unstructured covariance matrix were used to evaluate whether there were differences in the rate of height and BMI z-score rate of change according to first line treatment approach. Models included adjustment for factors that could act as confounders. These included factors that were antecedents of both treatment approach and growth, including baseline esophageal tissue eosinophil count (as a proxy for disease severity), age at diagnosis, insurance status, and CEoEC site. We used a complete case analysis approach where cases with missing data on study covariates were dropped from the analyses. We plotted the predicted z-score change (growth) across follow-up for each treatment group and 1) estimated the rate of change for each treatment, 2) whether the rate of change differed overall by treatment approach, and 3) whether the rate of change for each treatment approach was significantly different from a dietary elimination approach only (referent).
Sensitivity and secondary analyses
As a sensitivity analysis, we evaluated whether there were differences in growth by those patients with and without PPI treatment prior to diagnosis, to evaluate the robustness of our results with application of this additional diagnostic criterion.3, 35 Additionally, we conducted analysis whereby we restricted our sample to those that demonstrated histologic improvement (<15 eos/hpf) with treatment, as a means of isolating treatment effect from disease effect on growth. As a secondary analysis, we evaluated differences in the association between treatment type and height and BMI z-score change by age at diagnosis and by sex. We also evaluated whether there were differences in treatment response for those patients with a baseline height or BMI z-score at ≤25th percentile among the patients examined.
RESULTS
Study sites abstracted data on 684 children diagnosed with EoE. After applying criteria for study inclusion (n=183 excluded) and dropping observations with incomplete data (n=72), we identified 409 children for study inclusion across the five CEoEC sites (Supplementary Figure 3). The median age at diagnosis was 8.3 years (IQR 4.0, 12.2), 73% were male, and 61% had a coexisting atopic condition documented. The median baseline height z-score for children newly diagnosed with EoE was −0.1 (IQR −0.9, 0.8), while the baseline BMI z-score was 0.3 (IQR −0.6. 1.0). Of the 409 children included, 48.4% were treated with a topical corticosteroid only, 31.8% with a dietary elimination approach only, 2.4% with an elemental diet only, 3.2% with elemental diet and topical steroid, 3.2% with elemental diet and dietary elimination approach, and 11.0% with a topical steroid and dietary elimination approach.
Examination of baseline patient characteristics across treatment type identified differences in sex and age distribution (p=0.03 and p<0.01, respectively). Children treated with topical steroids were generally older (median age of 9.1 for topical steroids only treatment) and children treated with elemental diet were generally younger (median age of 1.7 years) (Table 1). More males were treated with topical steroids (80.5% versus <68% for all other treatment approaches). Baseline tissue eosinophil count was somewhat higher for patients treated with topical steroids or elemental diet (median count of 50 eos/hpf for topical steroids and elemental diet versus median of ≤40 eos/hpf for other treatment approaches; p<0.01 across treatment groups).
Table 1.
Baseline patient characteristics by treatment approach
| Topical steroids (n=198) | Dietary elimination (n=130) | Elemental diet (n=10) | Elemental and steroids (n=13) | Elemental and dietary elimination (n=13) | Steroids and dietary elimination (n=45) | p | |
|---|---|---|---|---|---|---|---|
| Male sex (%) | 81.3 | 69.2 | 60.0 | 69.2 | 69.2 | 64.4 | p=0.04 |
| Non-Hispanic ethnicity (%) | 2.7 | 4.6 | 0.0 | 0.0 | 0.0 | 9.3 | p=0.33 |
| White race (%) | 84.2 | 84.6 | 80.0 | 69.2 | 91.7 | 80.0 | p=0.91 |
| Insurance (%) | |||||||
| Private | 69.2 | 66.9 | 60.0 | 61.5 | 61.5 | 60.0 | p=0.95 |
| Public | 28.8 | 30.8 | 40.0 | 38.5 | 38.5 | 35.6 | |
| Self-Pay | 2.0 | 2.3 | 0.0 | 0.0 | 0.0 | 4.4 | |
| Atopic disease (%) | 57.6 | 63.9 | 60.0 | 38.5 | 63.6 | 64.4 | p=0.52 |
| Height z-score at diagnosis (median; IQR) | −0.16 (−0.97, 0.85) | 0.14 (−0.85, 0.73) | −1.52 (−2.94, 0.40) | 0.74 (−0.50, 1.37) | 0.06 (−1.30, 0.39) | 0.27 (−0.43, 1.28) | p=0.39 |
| BMI z-score at diagnosis (median; IQR) | 0.06 (−0.85, 0.95) | 0.36 (−0.25, 1.02) | −1.10 (−1.51, 1.31) | 0.72 (0.15, 1.33) | 0.10 (−1.58, 0.86) | 0.55 (−0.36, 1.32) | p=0.20 |
| Age at diagnosis (median; IQR) | 9.1 (5.0, 12.9) | 7.0 (4.4, 11.3) | 4.2 (3.1, 5.5) | 1.7 (0.8, 5.9) | 2.3 (1.7, 3.0) | 9.4 (5.5, 14.3) | p<0.01 |
| Maximum eos/HFP at diagnosis (median; IQR) | 50 (30, 100) | 40 (25, 60) | 35 (20, 50) | 50 (30, 90) | 35 (28, 65) | 40 (34, 70) | p<0.01 |
Longitudinal growth by treatment type
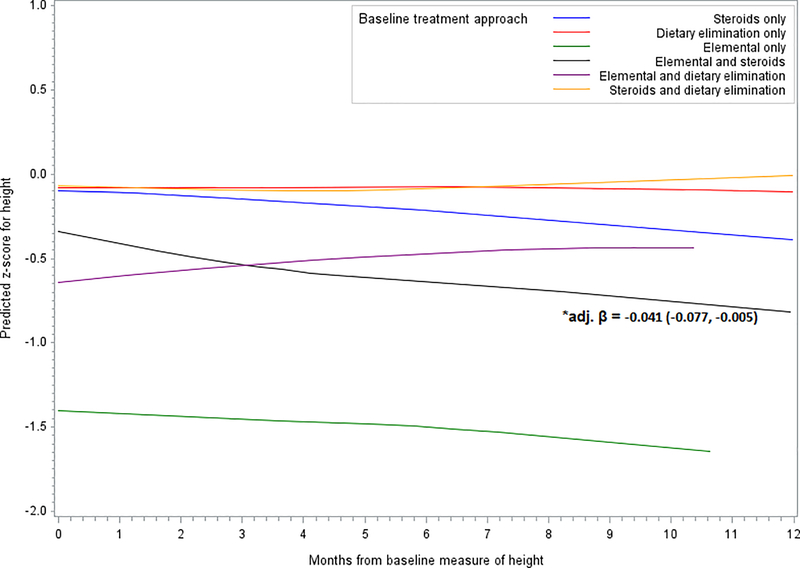
Over the 12 month follow-up period, there was a significant decrease in height z-score among patients treated with a combination of elemental formula and topical steroids (Δ height z-score (adjusted): −0.04; 95% CI: −0.08, −0.01). A slight, although not significant, decrease of height score was also observed for those treated with topical steroids only (Δ height z-score (adjusted): −0.003; 95% CI: −0.02, 0.01). Patients treated with dietary elimination supplemented with elemental formula were, on average, shorter at baseline than children treated with steroids or diet alone. These patients trended toward an increase in height z-score with treatment (Δ height z-score (adjusted): 0.03; 95% CI: −0.01, 0.07) (Figure 1, Supplementary Table 1). No other significant decreases or increases in height z-score were observed for any of the other treatment approaches (Figure 1, Supplementary Table 1). Overall, the difference in height z-score between treatment approaches was non-significant (p=0.10). However, relative to dietary treatment only, there was a significant difference (p=0.03) in the change in height z-score for patients treated with the combined approach of an elemental diet with topical steroids (height z-score difference: −0.04). Relative to dietary treatment alone, no other significant differences were observed for height z-score.
Figure 1.
Predicted change in z-score for height by treatment
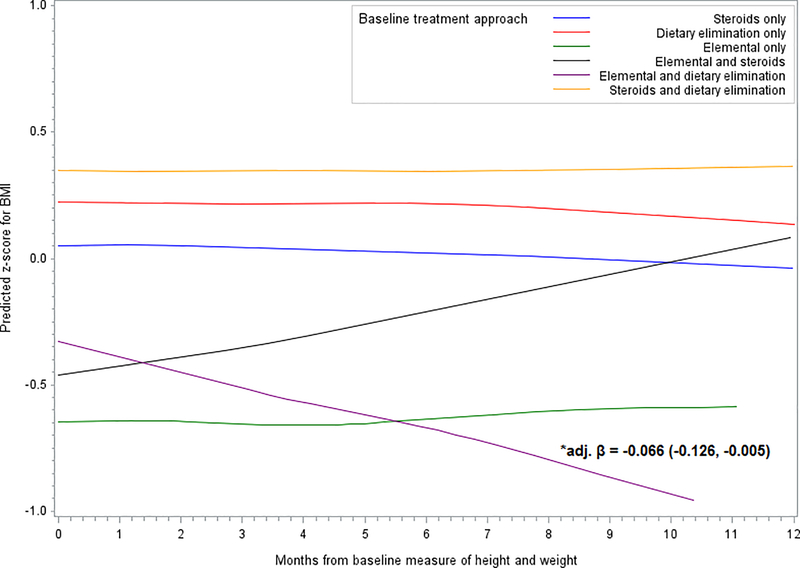
For longitudinal BMI z-score change there were no significant differences in z-score for any of the treatments, with the exception of the combined elemental formula with dietary elimination approach, where there was a significant decrease in BMI z-score observed across the 12 month period (Δ BMI z-score (adjusted): −0.07; 95% CI: −0.13, −0.01) (Figure 2, Supplementary Table 1). Treatment with topical steroids combined with elemental formula trended toward an increase in BMI z-score, with a predicted z-score reaching the estimated z-score of diet and steroids alone at the end of the 12 month follow-up period (Δ BMI z-score (adjusted): 0.05; 95% CI: −0.01, 0.11) (Figure 2, Supplementary Table 1). There was no significant difference in BMI z-score change between treatment types overall (p=0.14) and no significant differences in BMI z-score relative to dietary elimination treatment alone.
Figure 2.
Predicted change in z-score for BMI by treatment
Secondary and sensitivity analyses
There was no significant difference in height or BMI z-score change between those patients treated with or not treated with a PPI prior to diagnosis (p=0.54 and 0.92, respectively). Restricting analysis to those patients with a treatment response (<15 eos/hpf on follow-up endoscopy) indicated no substantively different observations from the full sample (Supplementary Table 2). Secondary analyses evaluating interaction by age at diagnosis and by patient sex identified significant differences in height z-score by sex only (p for interaction <0.01 for sex and p for interaction=0.43 for age at diagnosis). Use of an exclusive elemental formula diet resulted in a non-significant decrease in height z-score for males (Δ height z-score (adjusted): −0.047; 95% CI: −0.114, 0.021), but the sample size was too small to estimate height z-score change in females treated with an elemental diet only (Supplementary Figure 4A–B). Use of an elemental formula diet in combination with steroids resulted in a decrease in height z-score for females (Δ height z-score (adjusted): −0.24; 95% CI: −0.32, −0.17) (Supplementary Figure 4A–B). There was no significant interaction between BMI z-score change and age at diagnosis (p=0.45) or sex (p= 0.52).
When examining patients at ≤25th percentile for height (−0.91) or BMI z-score (−0.67) at baseline, we observed no significant difference between use of a topical steroid as compared to dietary treatment only (p=0.45 for height and p=0.37 for BMI for difference in slope).
DISCUSSION
While multiple studies have examined short term outcomes of either dietary or topical steroid treatment for EoE,30, 36, 37 very few have examined long-term outcomes and even fewer have focused specifically on growth outcomes in a pediatric population. One observational study comparing ≥6 months use of budesonide versus fluticasone propionate in relation to adrenal insufficiency identified that adrenal insufficiency was associated with a reduced BMI z-score, however no formal assessment of longitudinal growth was conducted.38 A 24 month, single center study of long-term fluticasone propionate effectiveness identified a non-significant increase in weight and height z-scores with treatment when comparing baseline to follow-up growth, but differences by age or sex were not assessed.39 Because of this gap in knowledge, and because there are reasons that treatment could either improve or worsen growth, we performed a multicenter study to address this question. This was the largest study conducted to date of the influence of treatment on growth in EoE, and one of the largest EoE cohorts assembled to date, thus allowing for examination of possible effect modification by sex and age. The results suggested that while there are baseline differences in height and BMI z-score, children who are diagnosed with EoE are, by and large, not malnourished on average. While there were also some differences by treatment approach, children with EoE generally tended to maintain their expected growth regardless of treatment modality. Subtle changes were noted for those patients treated with elemental diets in combination with other treatment modalities and significant differences in longitudinal change in height were observed by sex.
The finding that children maintain growth, irrespective of treatment approach, is generally consistent with short-term pediatric outcomes studies of topical steroids30, 36, 37 and long-term pediatric outcomes of swallowed fluticasone for treatment of EoE.39 We did observe some indications of decreased height with topical steroid treatments (non-significant decrease for topical steroids alone, and significant decrease for topical steroids in combination with elemental diet). Concomitantly, we observed an increase in BMI with topical steroids (non-significant decrease for topical steroids alone, and non-significant decrease for topical steroids in combination with elemental diet). The slight, suggestive increase in BMI may reflect the observed change in stature. While some studies suggest that dietary elimination strategies may impair growth,40 we did not see strong evidence of impaired growth in this study sample. It may be that restrictive eating is tempered by increased food volume as a result of an improvement in symptoms, and one prior study reported weight gain in growth-impaired children treated with an elemental diet.41 It may also be that these observations would change with a longer period of follow-up. Our study suggested there may be sex differences in the effect of treatment on growth. To our knowledge, this is the first study to examine sex differences.
Limitations of the current study are the retrospective design which necessitated use of medical records for abstraction, with an associated lack of standardization across sites in obtaining height and weight. However, the heights and weights recorded are from medical clinic intake, not from patient self-report, and were the values used for clinical management. Additionally, while our statistical modeling approach allows for incomplete data follow-up on subjects, this assumes that loss to follow-up is uninformative. This may not be a reasonable assumption if healthier patients are less likely to follow-up. However, the sensitivity analysis whereby we restricted to those patients that were not refractory to treatment (healthier patients) identified no substantive differences from the observations reported in the full sample. Despite the relatively large sample size, there were very few patients treated with elemental formulas, either exclusively or in combination with other treatments. Elemental diet treatments may have been less commonly used in this sample given costs of treatment (insurance providers often do not offer coverage for elemental diet treatments) and the mean age of diagnosis (elemental diets may be less appealing to older children). Furthermore, in this sample, patients were generally not malnourished and thus, elemental diets may not have been considered. This limits the precision and interpretability of the estimates observed for elemental diet treatment approaches. As this study was retrospective, we did not have complete data on adherence to treatment. There was also likely selection bias related to the initial clinical decision regarding treatment modality.
Strengths of the study include the multi-center design spanning both academic and private centers and the resultant large patient cohort. We also employed a comprehensive and standardized data extraction protocol, with exhaustive accounting of clinical follow-up and rigorous data management. The analysis approach focused on height and BMI z-scores to eliminate variability based on age and sex, and included multiple sensitivity and sub-analyses to address potential limitations and identify possible modification of effects by age and sex.
In conclusion, while the current study generally suggests no significant decrease (or increase) in rate of growth by treatment approach, baseline height z-scores were lower than expected and suggest that some EoE patients may, at diagnosis, already be demonstrating impaired growth. For these patients, an increase in growth velocity may be indicated. Additionally, there was some indication that topical steroids may effect linear growth and that treatment effects on growth may be sex-specific. Additional prospective longitudinal studies are needed to evaluate baseline anthropometric factors and growth in newly diagnosed EoE patients to definitely determine the optimal treatment approach in the context of growth and nutrition.
Supplementary Material
Supplementary Figure 1. Observed (smoothed) change in z-score for height by treatment approach
Supplementary Figure 2. Observed (smoothed) change in z-score for BMI by treatment approach
Supplementary Figure 3. Selection of study sample for inclusion in complete case analysis
Supplementary Figure 4A-B. Predicted change in z-score for height by treatment – males and females
What is known:
Comparative studies in assessing treatment impact on growth parameters in EoE are lacking. Inhaled steroids have been demonstrated to impair growth in treatment for asthma; it is unknown whether topical steroids confer similar effects.
What is new:
Across 12 months of follow-up, treatment approach was associated with minor differences in anthropometric growth, with combined elemental and topical steroids decreasing linear growth. The effect of treatment on growth parameters may differ by sex.
Acknowledgments
Funding support: This study was funded, in part, by an investigator-initiated grant from Nutricia and by NIH grant T35 DK007386.
Footnotes
Potential competing interests: Dr. Dellon has received research funding from Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire. He is a consultant for Adare, Banner, GSK, Receptos/Celgene, Regeneron, and Shire.
Dr. Johnston is a consultant for Shire, CSL Behring, Biocryst Pharmaceuticals, Nutricia, Merck, and Novartis
None of the other authors have any potential competing interests to report.
REFERENCES
- 1.Lucendo AJ, Molina-Infante J, Arias A, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017; 5:335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015; 110:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.e6. [DOI] [PubMed] [Google Scholar]
- 4.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras C, Katzka DA. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol 2013; 108:679–92. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014; 79:577–85.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipka S, Kumar A, Richter JE. Impact of Diagnostic Delay and Other Risk Factors on Eosinophilic Esophagitis Phenotype and Esophageal Diameter. J Clin Gastroenterol 2016; 50:134–40. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology 2014; 147:1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutlas NT, Dellon ES. Progression from an Inflammatory to a Fibrostenotic Phenotype in Eosinophilic Esophagitis. Case Rep Gastroenterol 2017; 11:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellano Mdel R, Cimbollek S, Quiralte J. Defining the role of food allergy in a population of adult patients with eosinophilic esophagitis. Inflamm Allergy Drug Targets 2010; 9:257–62. [DOI] [PubMed] [Google Scholar]
- 10.Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol 2010; 10:231–7. [DOI] [PubMed] [Google Scholar]
- 11.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol 2010; 104:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Vogel NM. Food allergy and eosinophilic esophagitis: learning what to avoid. Cleve Clin J Med 2010; 77:51–9. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen RG, Husby S. Eosinophilic oesophagitis: epidemiology, clinical aspects, and association to allergy. J Pediatr Gastroenterol Nutr 2007; 45:281–9. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Cervera J, Arias A, Redondo-Gonzalez O, Cano-Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic esophagitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol 2017; 118:582–90.e2. [DOI] [PubMed] [Google Scholar]
- 15.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of Dietary Interventions for Inducing Histologic Remission in Patients With Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Gastroenterology 2014; 146:1639–48. [DOI] [PubMed] [Google Scholar]
- 16.Doerfler B, Bryce P, Hirano I, Gonsalves N. Practical approach to implementing dietary therapy in adults with eosinophilic esophagitis: the Chicago experience. Dis Esophagus 2015; 28:42–58. [DOI] [PubMed] [Google Scholar]
- 17.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 2012; 130:461–7 e5. [DOI] [PubMed] [Google Scholar]
- 18.Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, et al. Swallowed Fluticasone Improves Histologic but Not Symptomatic Responses of Adults with Eosinophilic Esophagitis. Clin Gastroenterol Hepatol 2012; 10:742–9.e1. [DOI] [PubMed] [Google Scholar]
- 19.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, Dose Reduction, and Resistance to High-dose Fluticasone in Patients with Eosinophilic Esophagitis. Gastroenterology 2014; 147:324–33.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straumann A, Degen L, Felder S, Bussmann C, Conus S, Bucher S, et al. Budesonide as induction treatment for active eosinophilic esophagitis in adolescents and adults: A randomized, double-blind, placebo-controlled study (Bee-I trial). Gastroenterology 2008; 134 (Suppl 1):726 (A104). [Google Scholar]
- 21.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared with Placebo in Patients with Eosinophilic Esophagitis. Gastroenterology In press, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–82. [DOI] [PubMed] [Google Scholar]
- 23.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–102. [DOI] [PubMed] [Google Scholar]
- 24.Peterson K, Clayton F, Vinson LA, Fang JC, Boynton KK, Gleich GJ, et al. Utility of an Elemental Diet in Adult Eosinophilic Esophagitis. Gastroenterology 2011; 140 (Suppl 1):AB 1080. [DOI] [PubMed] [Google Scholar]
- 25.Warners MJ, Vlieg-Boerstra BJ, Verheij J, van Rhijn BD, Van Ampting MT, Harthoorn LF, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther 2017; 45:777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang KZ, Jensen ET, Chen HX, Johnston D, Durban R, Frost C, et al. Mo1201 Practice Patterns in Pediatric EoE in the Carolinas EoE Collaborative - A Model of Research in Community and Academic Practices. Gastroenterology; 150:S669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotton CC, Erim D, Eluri S, Palmer SR, Green DJ, Wolf WA, et al. Cost-utility analysis of topical steroids compared to dietary elimination for treatment of eosinophilic esophagitis. Clin Gastroenterol Hepatol In press, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth. Cochrane Database Syst Rev 2014:Cd009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015; 13:66–76.e3. [DOI] [PubMed] [Google Scholar]
- 30.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology 2017; 152:776–86.e5. [DOI] [PubMed] [Google Scholar]
- 31.Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2016; 65:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eluri S, Dellon ES. Proton pump inhibitor-responsive oesophageal eosinophilia and eosinophilic oesophagitis: more similarities than differences. Curr Opin Gastroenterol 2015; 31:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics growth charts: A SAS program for the 2000 CDC growth charts (ages 0 to <20). Centers for Disease Control and Prevention.] Available from https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 34.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child 2014; 99:1020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG Clinical Guideline: Evidenced Based Approach to the Diagnosis and Management of Esophageal Eosinophilia and Eosinophilic Esophagitis (EoE). The American journal of gastroenterology 2013; 108:679–92. [DOI] [PubMed] [Google Scholar]
- 36.Gupta SK, Collins MH, Lewis JD, Farber RH. Efficacy and Safety of Oral Budesonide Suspension (OBS) in Pediatric Subjects With Eosinophilic Esophagitis (EoE): Results From the Double-Blind, Placebo-Controlled PEER Study Gastroenterology 2011; 140 (Suppl 1):S179. [Google Scholar]
- 37.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology 2014; 147:324–33.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal Insufficiency after Chronic Swallowed Glucocorticoid Therapy for Eosinophilic Esophagitis. The Journal of Pediatrics 2016; 170:240–5. [DOI] [PubMed] [Google Scholar]
- 39.Andreae DA, Hanna MG, Magid MS, Malerba S, Andreae MH, Bagiella E, et al. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am J Gastroenterol 2016; 111:1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta H, Groetch M, Wang J. Growth and nutritional concerns in children with food allergy. Curr Opin Allergy Clin Immunol 2013; 13:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology 2006; 4:1097–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Observed (smoothed) change in z-score for height by treatment approach
Supplementary Figure 2. Observed (smoothed) change in z-score for BMI by treatment approach
Supplementary Figure 3. Selection of study sample for inclusion in complete case analysis
Supplementary Figure 4A-B. Predicted change in z-score for height by treatment – males and females