Abstract
A growing number of youth suffer from obesity and in particular severe obesity for which intensive lifestyle intervention does not adequately reduce excess adiposity. A treatment gap exists wherein effective treatment options for an adolescent with severe obesity include intensive lifestyle modification or metabolic and bariatric surgery, while the application of obesity pharmacotherapy remains largely underutilized. These youth often present with numerous obesity-related comorbid diseases, including hypertension, dyslipidemia, prediabetes/type 2 diabetes, obstructive sleep apnea, non-alcoholic fatty liver disease, musculoskeletal problems, and psychosocial issues such as depression, anxiety, and social stigmatization. Current pediatric obesity treatment algorithms for pediatric primary care providers focus primarily on intensive lifestyle intervention with escalation of treatment intensity through four stages of intervention. Although a recent surge in the number of Food and Drug Administration-approved medications for obesity treatment has emerged in adults, pharmacotherapy options for youth remain limited. Recognizing treatment and knowledge gaps related to pharmacological agents and the urgent need for more effective treatment strategies in this population, discussed here are the efficacy, safety, and clinical application of obesity pharmacotherapy in youth with obesity based on current literature. Legal ramifications, informed consent regulations, and appropriate off-label use of these medications in pediatrics are included, focusing on prescribing practices and prescriber limits.
Keywords: pediatric obesity, pharmacotherapy, medical-legal regulations, weight loss medications, children and adolescents with severe obesity, bariatric surgery
INTRODUCTION
Purpose: Rationale for the Formulation of an Opinion Statement on the Use of Medications to Treat Pediatric Obesity
An independent panel of 12 pediatric obesity medicine and obesity surgery specialists identified an urgent and time-sensitive gap of evidence-based guidance on the clinical use of obesity pharmacotherapy in adolescents. Until such a time when additional scientific evidence is generated justifying the creation of clinical guidelines, this group of experts felt it necessary to develop an initial clinical roadmap for the practical application of obesity pharmacotherapy in pediatric tertiary care centers in the form of an opinion statement. The paper, in essence, reflects the views of the authors. The overall goals were to build a framework to (1) review published data about pediatric obesity pharmacotherapy (what is known and what is not known); (2) have an intelligent, well-informed discussion regarding the fact that, that though these medications are approved in adults, there are limited safety, efficacy and follow up data in youth with obesity but that the consequences as well as risks of obesity may outweigh the potentially unknown risks of medications in these youth; (3) recommend that these obesity medications currently be used only by well-trained experts in a team interdisciplinary environment with conscientious monitoring; and (4) advocate for more research resources for pharmacological intervention trials in youth with obesity to provide data for the use of these medications.
State-specific rules and regulations beyond routine standard of care on weight management practices in adults and children were determined by contacting individual State Medical Boards through either phone calls and/or email exchanges with the respective State’s Department of Health prosecuting attorney or representative (Table S1).
Currently, almost one out of five youth (18.5%) are afflicted with obesity (1) and 9.5% of adolescents have severe obesity (BMI≥120% of the 95th percentile or ≥35kg/m2) (2). Treatment of childhood obesity is complicated by its intricate and multifactorial etiology with a myriad of contributing factors including but not limited to genetics, developmental effects, fetal programming and epigenetics, environment, behavioral and psychosocial issues, physical activity, medications, eating patterns, illness, and cultural and family norms (3). Adolescent overweight and obesity are associated with deteriorating cardiometabolic health, increased cardiovascular mortality, future disease burden into adulthood (4–7) and strongly predict diabetes mortality up to the seventh decade (8). Furthermore, children and adolescents with obesity have lower health-related quality of life compared with children and adolescents with normal weight, a quality of life similar to those with cancer, (9) and suffer from detrimental psychosocial stigmatization (10, 11, 12). Finally, obesity tracks strongly from childhood to adolescence and later adulthood, and reversion from having severe obesity to having moderate obesity or normal weight during childhood is rare (5, 13, 14).
Many studies show that only 2–15% of adolescents with severe obesity respond to lifestyle modification therapy and achieve clinically significant and durable weight or BMI reduction (15, 16, 17, 18, 19). Therefore, prompt recognition, evaluation, and treatment of obesity are warranted, including swift initiation of intensive lifestyle intervention, with appropriate application of pharmacotherapy and/or surgical modalities as needed. Pharmacotherapy and metabolic and bariatric surgery (MBS) for youth with obesity fall under the domain of Stage 3 and Stage 4 Tertiary Care intervention respectively. In this staged approach, pharmacological and/or MBS are further integrated with lifestyle modification in a stepwise progression.
Reflecting an increased understanding of the underpinnings of the complex energy regulatory pathways, there are now six Food and Drug Administration (FDA)-approved medications for the indication of obesity in adults (orlistat, phentermine, phentermine/topiramate extended-release [ER], lorcaserin, bupropion sustained-release [SR]/naltrexone SR, and liraglutide)(20). Obesity medications are FDA-approved in adults (20) 18 years and older (with the exception of orlistat (≥12 years) and phentermine (>16 years)) for BMI ≥27 kg/m2 with the presence of at least one obesity-related comorbidity such as hyperlipidemia, sleep apnea, Type 2 diabetes mellitus, or hypertension or a BMI ≥30 kg/m2 irrespective of comorbidities. This major milestone in the development and recent approval of obesity pharmacotherapy paves a pathway for potential future pediatric obesity clinical trials and the burgeoning field of pediatric obesity medicine that is still in its infancy. Pharmacotherapy for obesity in conjunction with lifestyle therapy has the potential to target physiological hunger, cravings, appetite, and hedonic eating behaviors, and elicit weight loss through the peripheral and central nervous systems, including reward-motivation pathways and executive decision-making function, that control food intake and satiety through efferent and afferent signaling cascades (20). However, long-term use, durability of effect, and safety profile might prompt concerns especially when applied to the pediatric population for whom treatment with pharmacotherapy is likely chronic. Therefore, determining overall risk/benefit ratios can be an arduous task for the clinician particularly when no current consensus or structured guideline exists for pediatric obesity pharmacotherapy. The rapidly growing number of youth with this serious and intractable chronic disease hastens the need for more aggressive treatment including pharmacotherapy. Moreover, the therapeutic application of obesity pharmacotherapy in pediatrics is not standardized across all practitioners though there is consensus among pediatric obesity medicine specialists who routinely utilize available adult FDA-approved obesity medications and recognize the benefits of treatment in certain phenotypes of severe obesity, which has not yet been studied in clinical trials. Consequently, we offer practical considerations regarding the responsible use of obesity medications by trained pediatric obesity medicine specialists in pediatric obesity care centers, taking into account existing evidence (unfortunately, currently limited), legal ramifications, and pertinent prescriber and prescribing-related concerns. We also highlight a revised and updated clinical approach to the treatment of pediatric obesity (Figure 1). Though we outline the difficulty in the treatment of pediatric obesity once the diagnosis has been established, preventive measures to combat obesity cannot be understated.
Figure 1. Proposed Clinical Approach to Obesity Treatment for Adolescents with Obesity.
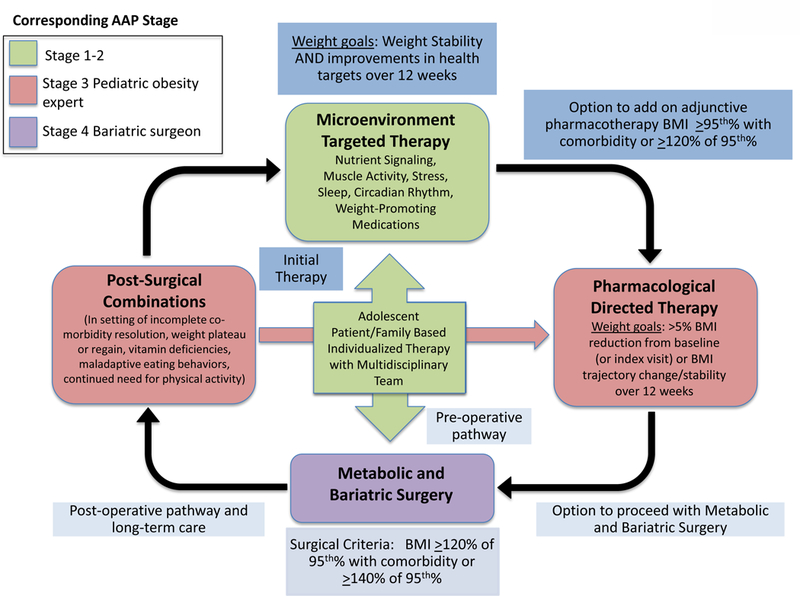
Progress through algorithm as clinically required for a patient with risk factors/ready to make change. Start with family-based therapy (can encompass basic education to more intensive therapy based on resources, time constraints, and psychosocial support) followed by microenvironment-targeted therapy with the help of ancillary services such as a dietitian, exercise specialist, nutritionist, and therapist if initial therapy is unsuccessful. Modifiable micro-environmental factors (20), such as nutrient signaling, muscle activity, sleep, stress, circadian rhythm, and iatrogenic causes (weight promoting medications which are frequently prescribed (60)), influence neuro-hormonal pathways affecting food intake and satiety. Prior to more aggressive intervention, these factors should be assessed and altered if perturbing the physiology leading to excess body fat accumulation. This may include physician and ancillary team evaluation providing more intense structure to weight management and medical evaluation with assessment of health targets and cardiovascular risk factors (usually prompting a corresponding AAP Stage 2–3 referral for intervention (24)). If microenvironment-targeted therapy fails, the option to add on adjunctive obesity pharmacotherapy falls under the domain of corresponding AAP Stage 3–4 intervention, either preceded or followed by MBS (corresponding to AAP Stage 4 intervention). Because obesity is a life-long disease, patients often may experience weight regain post-bariatric surgery and continue to need aftercare more closely especially in the adolescent population. As a result, they should continue to resume aftercare and may require lifestyle and/or combination pharmacological intervention later on in life.
Definitions
The generally accepted measure for clinical screening of overweight or obesity in children and adolescents is age and sex-adjusted body mass index (BMI) plotted on US Centers for Disease Control & Prevention (CDC) growth charts (21, 22, 23). For the purposes of classification and terminology (Table 1), “obesity” in children and adolescents is defined as a BMI-for-age/sex ≥95th percentile but < 120% of the 95th percentile [Class I obesity], and “severe obesity” is defined as a BMI-for-age/sex ≥ 120% of the 95th percentile [Class II or higher; >35 kg/m2] (24). This terminology was derived as per Flegal et al. in 2009 where extreme percentiles extrapolated from the Centers for Disease Control and Prevention supplied-supplied lambda-mu-sigma parameters did not match well to the empirical data for the 99th percentile(25). A better fit to the empirical data was obtained by using 120% of the smoothed 95th percentile. Furthermore, because we offer obesity pharmacotherapy clinical considerations in the context of adolescents, we define adolescence, according to the World Health Organization definition (26) and the US Department of Health and Human Services (27), for which adolescence is the period of development corresponding to the onset of physiologically normal puberty and ending with adult identity and behavior, spanning the ages 10–19 years. We also define intensive lifestyle modification therapy as per widely accepted guidelines(24, 28, 29, 30) with the general aim of helping patients adopt healthier eating habits, increase physical activity, and decrease sedentary time while changes are made in stepwise progression and treatment (individual, group, and/or family-based) is intensified if needed.
Table 1.
BMI-for-age/sex percentile Classification in Pediatrics1
| Underweight (<5th percentile) |
| Normal weight (5th to <85th percentile) |
| Overweight (85th to <95th percentile) |
| Obesity class 1 (95th percentile to <120% of the 95th percentile) |
| Severe obesity class 2 (120% to <140% of the 95th percentile or BMI 35.0 to <40.0 kg/m2) |
| Severe obesity class 3 ( ≥ 140% of the 95th percentile or BMI ≥ 40.0 kg/m2) |
Skinner AC, Perrin EM, and Skelton JA. Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity 2016.
Measuring Clinical Efficacy of Obesity Pharmacotherapy in Pediatrics: % BMI Reduction
For children and adolescents, clinically meaningful weight loss based on observational and interventional studies has been defined by some as a BMI z-score (or BMI standard deviation, s.d.) reduction between 0.20–0.25 s.d. over 6–12 months. This degree of BMI change has been associated with improvements in cardiovascular and metabolic risk factors (31, 32, 33, 34). However, in children and adolescents with severe obesity, BMI z-score correlates poorly to adiposity with wide variation associated with differences in age, sex and racial influences (5, 35, 36, 37). Accordingly, alternative measures for monitoring weight outcomes have been recommended including percent change in BMI and/or change in BMI percent of the 95th percent (35). A change in BMI z-score of 0.2 is approximately equivalent to a 5% change in BMI. (Similarly, for adults, 3–5% weight loss is considered clinically meaningful (20).)
RECOMMENDATIONS (Table 2)
Table 2.
| (1) Multidisciplinary team recommended |
| (2) BMI ≥95th percentile (or BMI ≥30 kg/m2 whichever is lower), plus the presence of at least one obesity-related comorbidity; OR BMI ≥120% of 95th percentile (or BMI ≥35 kg/m2 whichever is lower), irrespective of co-morbidity |
| (3) No upper BMI threshold for initiation of pharmacotherapy1 |
| (4) Documentation of previous lifestyle therapies or attempts at initial medical encounter sufficient as proof of prior lifestyle interventionc |
| (5) Tanner stage2–3: no lower limit unless evidence suggests developmental risk of specific agent being prescribed |
| (6) Criteria for bariatric surgery are met, yet operation is not appropriate or possible at this time or medications are recommended as adjunct therapy |
| (7) Continuation of medication(s) if there is ≥% BMI reduction from baseline at 12 weeks on the optimal dose or arrest or slowing of weight gain is considered a reasonable clinical outcome; medication(s) should be discontinued if not tolerated by the patient or if dangerous side effects occur or persist despite dose adjustment |
Exclusion criteria and contraindications may require astute clinical skills and complex decision-making prompting consultation of a trained multidisciplinary team.
Definition of adolescence age varies: World Health Organization (10–19 years); US Department of Health and Human Services (10–19 years), American Academy of Pediatrics (11–21 years)
Of note, exceptions may apply in select cases involving younger patients where clinical decision-making and consultation of a multidisciplinary team on risks: benefits ratio is necessary.
However in cases of severe clinical severity such as a BMI >120th of 95th percentile with at least one comorbidity or >140th of 95th percentile in the absence of comorbidity, one should consider the long term goals and initial BMI in order to involve a bariatric surgeon early if surgery is likely also needed.
In select cases, exceptions may apply where clinical decision-making may be necessary in the case of serious health compromise (such as moderate-severe obstructive sleep apnea AHI >15 events/hr, pseudotumor cerebri, poorly controlled Type 2 diabetes mellitus) related to severe obesity that warrant more emergent intervention.
Tanner stage 4–5 is advisable, though clinical judgment should be utilized on an individual patient basis if benefits outweigh the risks of treatment. Clinical decision-making may be required when benefits far outweigh risks of obesity pharmacotherapy. Effects of obesity medications on puberty and tanner staging are largely not known.
Pediatric Weight Management Multidisciplinary Team
We recommend that prescription of FDA-approved or off-label use of obesity medications be utilized with support of a trained pediatric multidisciplinary team and monitoring of both treatment adherence and possible adverse events. The multidisciplinary team is also responsible for comprehensive obesity assessment and treatment recommendations for youth with obesity. Exclusions for obesity pharmacotherapy (for example, pregnancy) and cautionary use (for example, as in children with growth deficiency, other endocrinopathies, eating disorders, syndromes affecting bone health) may require astute clinical skills and complex decision-making prompting consultation of a multidisciplinary weight team and other specialists.
Team Member Qualifications
Team member qualifications for a pediatric weight management center are similar to those required for adolescent MBS and are adapted from the American Society of Metabolic and Bariatric Surgery (ASMBS) Pediatric Best Practice Guidelines (38).
Pediatric specialist The trained or certified pediatric provider will hold an American Board of Pediatrics (or American Board of Surgery with Pediatric Surgery sub-specialization) certification or appropriate Family Medicine or Nurse Practitioner or Physician Assistant certification eligible to practice under State-mandated rules and regulations. Currently, fellowship training and board certification in nutrition and in pediatric obesity are lacking. Training in Pediatric Cardiology, Pediatric Endocrinology, Pediatric Gastroenterology, Adolescent Medicine, Pediatric Surgery, General Academic Pediatrics or General Pediatrics with a focus study area such as in nutrition, lifestyles, motivational interviewing(39, 40, 41) and obesity represent the most common routes to expertise in the field of pediatric obesity. Frequent and continuing clinical care of patients with obesity, ongoing continuing medical education in pediatric obesity medicine, scholarly work in obesity research or education, and certification by entities such as the American Board of Obesity Medicine or the American Board of Nutrition can enhance advanced understanding of obesity. In general, these activities currently serve to designate experts in the field of pediatric obesity care. Primary care and subspecialty pediatricians without this expertise should refer to their colleagues with these skills and experience. Given the extensive prevalence of obesity, protocols and guidelines should be developed in the future wherein practitioners without expertise can be trained.
Registered dietitian: experience in treating obesity and working with children and families as recommended by the Academy of Nutrition and Dietetics position statement on pediatric overweight and obesity (42); specialty certification in obesity care is available for registered dietitians (www.cdrnet.org). Experience with patients undergoing MBS may be preferable but not mandatory
Mental health specialist: psychiatrist, psychologist, or other qualified and independently licensed mental health specialist or social worker with specialty training in pediatric, adolescent, and family treatment and experience in treating eating disorders and obesity; certification examination such as through the International Association of Eating Disorders is suggested to enhance coverage for mental health services. Professionals trained in intensive cognitive behavioral therapy for obesity can effectively design and lead lifestyle modification programs.
Coordinator: registered nurse, social worker, or other team member with the responsibility of coordinating the care for each child or adolescent and ensuring implementation of treatment plan and follow-up.
Exercise physiologist, physical therapist, or other individual specially trained to provide safe physical activity prescriptions to adolescents with severe obesity is recommended but not required.
Collaboration with the metabolic and bariatric surgeon: pediatric surgeons who specialize in metabolic and bariatric surgery (MBS) and/or adult bariatric surgeons who choose to include the treatment of adolescents at their center by establishing a comprehensive adolescent obesity program in accordance with current accreditation guidelines put forth by the American College of Surgeons-Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) are recommended if such expertise is accessible, but not required for pharmaceutical obesity therapy.
Patient Selection
BMI Criteria
Pharmacotherapy is often considered to be a lower risk and less invasive form of therapy than surgical intervention in which a child may be exposed to risks of anesthesia (43) and potentially other complications(44, 45). Established criteria for adult obesity pharmacotherapy utilize a lower BMI threshold (BMI >27 kg/m2 with at least one obesity-related co-morbidity [such as the presence of diabetes, sleep apnea, hypertension or hyperlipidemia] or BMI >30 kg/m2 with or without comorbidity) than MBS. For MBS, the adult criteria (BMI 35–39.9 kg/m2 in the presence of serious comorbidities or when BMI is ≥40 with or without comorbidity) are already recommended for adolescent indications (>120 of the 95th percentile or BMI ≥35 with comorbidity or >140% of the 95th percentile or BMI ≥40 with or without comorbidity) (46).
We propose similarly that for pharmacotherapy, the adult criteria should be modified for pediatric indications; i.e. when BMI is in the obese range (≥ 95th percentile for age and sex or BMI >30 kg/m2 whichever is lower; Class I obesity) in the presence of at least one obesity-related co-morbidity or in the severe obesity range (≥120% of 95th percentile for age and sex or BMI >35 kg/m2 whichever is lower; Class II and Class III obesity) irrespective of the presence of co-morbidities. Of note, although adult clinical trials (20) studying obesity pharmacotherapy have recruited patients in the BMI range of 27–45 kg/m2, the efficacy of these drugs may decrease with the clinical severity of BMI given the complex progression of the disease state (47). Because no upper BMI threshold is mentioned for the practical application of obesity pharmacotherapy, one should consider the initial BMI and long-term goals to assess need for early involvement of a bariatric surgeon if MBS is likely needed, especially in adolescents where BMI ≥120% of 95th percentile with the presence of an obesity-related comorbidity or ≥140% of the 95th percentile with less severe comorbid conditions (45, 48, 49, 50)
Age Considerations
With regard to age, there are currently no data defining a lower limit for the application of pharmacotherapy or MBS in the pediatric population. The European Medicines Agency (EMA) suggests that medication could be considered for children with severe obesity as young as six years of age (51) and the 2018 adolescent MBS guidelines adopted the lower limit of 10 years (46), consistent with the World Health Organization (26) and the US Department of Health and Human Services definition of adolescence (age 10–19 years) (27). Furthermore, FDA-approved obesity pharmacotherapy is applicable to adults 18 years and older, which interestingly includes older ‘adolescents’ as per World Health Organization definition. Specifically, two obesity medications (orlistat and phentermine) are already FDA-approved in younger adolescents. Notably, there are discrepancies in the definition of “adolescence” and the American Academy of Pediatrics (AAP) strongly discourages the establishment of arbitrary age limits in pediatric care by health care providers due to the multifaceted approach encompassing a child’s overall physiological, physical, developmental, psychosocial, and mental health (52). Care delivery must take into account the needs of the child and directs the provider to meet those needs. Of note, particularly in cases involving younger patients, clinical decision-making and consultation of a multidisciplinary team on risks:benefits ratio is paramount.
Intensive Lifestyle Therapy Considerations
Intensive lifestyle therapy (without pharmacotherapy) can result in clinically meaningful weight loss in the short term in younger children (<10–12 years) but older youth tend to respond less favorably to lifestyle intervention and have higher onset of disease progression with the development of co-morbidities (15, 16, 19, 53). Combining intensive lifestyle intervention with pharmacotherapy may have enhanced synergistic or additive weight loss effects in patients with obesity as demonstrated in obesity pharmacotherapy trials(20, 54, 55). We recommend that documentation of previous (structured) attempts at lifestyle therapy upon initial encounter is sufficient as proof of non-response to lifestyle alone prior to consideration of obesity pharmacotherapy. It is understood that healthy behaviors and lifestyle modification be continued in conjunction with pharmacotherapy or MBS.
Growth and Development Considerations
Obesity affects adolescent growth and development (56, 57). In girls, higher BMI is associated with earlier menarche age (58). If the medication being prescribed is known to interfere with normal pubertal progressions, Tanner stage (sexual maturity rating) is a particularly relevant factor to consider (59), though no lower limit of adolescent age is suggested unless evidence points to developmental risks of specific agents being prescribed. Certainly there is a paucity of data with regard to obesity medications on pubertal development as well as long-term effects on developing adolescent brain; further research and long-term studies are needed.
Clinical Decision-making
Clinical decision-making is required when benefits far outweigh risks of obesity pharmacotherapy such as in an adolescent with several obesity-related medical comorbidities and severe obesity. Clinical presentation of the patient is vital in pharmacotherapeutic selection with dose titration for maximum benefit with minimal adverse effects. The patient is assessed for potential safety concerns and followed closely during the medical management visits. Obesity pharmacotherapy should not be initiated in the setting of severe psychiatric disturbance, eating disorders such as bulimia, untreated endocrinopathies, or with concomitant use of medications that have adverse interactions. Furthermore, discontinuation or substitution of medications associated with weight gain with weight-neutral alternatives before initiation of obesity pharmacotherapy is a critical consideration. Because obesity is a chronic disease, long-term treatment and follow-up care will be necessary (potentially life-long). One can expect rebound in weight and disease pathology if treatment is discontinued. Patients and family members should be counseled on the long-term use of obesity pharmacotherapy and disease chronicity along with lifelong lifestyle modification.
Decision to Continue or Discontinue Obesity Pharmacotherapy
We also recommend that the medication be continued if there is ≥5% BMI reduction from baseline at 12 weeks on the optimal dose or if arrest or slowing of weight gain is considered to be a reasonable clinical outcome, especially as linear growth occurs in adolescence. The medication should be discontinued if not tolerated by the patient or dangerous side effects occur or persist despite dose adjustment. This algorithm is consistent with adult and pediatric guidelines that recommend discontinuation of an obesity medication, at maximum appropriate dose, if less than 5% weight loss/BMI reduction from baseline is achieved within 12 weeks (60, 61, 62). When a medication or a combination is started, it can be continued longer-term based on therapeutic benefit, improvement of psycho-social comorbidities, tolerability, absence of or acceptable side effects, cardiovascular stability, and minimal effects on growth development and neurocognitive function with monitoring.
Assessment and Safety
The initial medical encounter includes a thorough clinical history and physical examination with documentation of Tanner stage, hemodynamics, and anthropometric measurements. Eating behaviors and dietary intake, family patterns, cultural cues, sleep hygiene & disorders screening, school history and psychological or neuropsychiatric metrics are assessed. In addition to usual laboratory evaluation recommended in the context of obesity(24), other laboratory assessments may be needed to monitor for side effects related to a given pharmacotherapy. Close follow-ups are recommended after the initial encounter. Pubertal stage, height, and weight, should be serially measured at all follow up visits to monitor for disruptions in pubertal development and linear growth. Because most obesity medications act through central nervous system pathways that may disrupt or affect neuropsychiatric axis, cognition and mood should be evaluated in the form of a clinical interview about school grades, concentration, memory and worsening depression/anxiety at serial follow up visits. Standardized clinical tools to track these potential side effects also need to be developed in the future. Given greater suicidality and depression risk among youth with obesity than of normal-weight youth, specific medications that may precipitate suicide or depression occurrence (e.g. such as bupropion or topiramate) also warrant uniform screening for these conditions upon the initial assessment and during subsequent follow up visits (63).
Understanding Off-label drug use in Pediatrics
Off-label drug use involves the prescription of medications for indications, or using a dosage or dosage form, which have not been approved by the FDA. Off-label drug use is especially common in pediatrics, where conducting clinical trials in this population might be challenging (64, 65, 66, 67, 68, 69). More recently, in 2014, the AAP released a statement regarding off-label use of pharmaceuticals in children (64): “Therapeutic decision-making must always rely on the best available evidence and the importance of the benefit for the individual patient.” Since obesity medications are FDA-approved for ages ≥18 years (with the exception of orlistat age≥12 years and phentermine age >16 years), prescription of obesity medications in youth meeting patient selection criteria falls under the domain of off-label drug use. Because of limited efficacy and safety data in children coupled with potential unknown side effects, we caution off-label drug use in children. Though it is difficult to provide a generalized statement supporting or not supporting off-label drug use in pediatric obesity, we provide recommendations, based on our own combined clinical experience, in regards to off-label use for specific medications as described in the pharmacotherapy section of this paper. Oftentimes, off-label drug use in pediatric obesity depends on the clinical presentation, age, medical comorbidities, family history, social history, etc. among other factors influencing benefit/risk ratio and clinical decision-making.
Medical Malpractice: Informed Consent and Negligence
Previous legal claims involving physicians due to adverse reactions related to off-label drug use have primarily involved the use of a research drug (not yet FDA approved), failure to provide informed consent for an off-label drug use, and medical negligence leading to direct harm to the individual patient. To date, court systems have not mandated a formal informed consent process for off-label drug use due to concerns about impingement of direct patient care and impediments to communications along with unnecessary concerns or alarms elicited to the individual patient and/or family members involved (65). We therefore recommend standard informed consent (and assent) documentation within the electronic medical record describing that 1) the conversation about off-label drug use occurred, 2) patient/family member(s) understand risks/benefits, 3) description of potential major and minor drug side effects reviewed, 4) confirmation of the absence of contraindications to the drug, 5) appropriate follow-up care was advised, 6) the patient/family member(s) understand how to reach the medical provider should questions or concerns arise, and 7) advise urgent medical attention for emergencies including suicidal ideation and worsening depression. The clinical care team must also recognize the youth’s cognitive, emotional, and social development and take this into consideration when obtaining assent in the shared-decision making process. Supplemental Online Table 1 outlines the various medical-legal concerns with respect to individual State Medical Board rules and regulations surrounding weight management. Most States follow standard of care, with specific statutes and bylaws in some States. Obesity practitioners must understand and follow their respective State’s rules and regulations.
Available Pharmacotherapy (FDA-approved and Off-Label Drug Use for Obesity in Pediatrics)
Evidence for obesity medications in children is limited in scope due to the relatively small number of clinical trials conducted and small number of participants included in many of the trials; recent meta-analyses and systematic reviews have reported overall minimal or no benefit of obesity pharmacotherapy in children (62, 70). However, an obesity specialist is well-trained in the clinical application of obesity pharmacotherapy for successful therapeutic benefit (20). Furthermore, patient criteria, obesity phenotype, contraindications and side effect profile must be considered when selecting specific agents for a pediatric patient. Table 3 provides a summary of the efficacy, safety and clinical insights into the application of FDA-approved adult obesity medications and off-label drug use of specific highly utilized medications in the pediatric obesity population.
Table 3.
Summary of Food and Drug Administration Approved and Commonly* Prescribed Medications for Weight Loss in the Pediatric Population
| Drug Name | Mechanism of Action | Original FDA Indication | Off-label Drug Use | Side Effects | Contraindications/Warnings | Adolescent weight loss outcomes data | Ref. |
|---|---|---|---|---|---|---|---|
| FDA-APPROVED (orlistat, phentermine) | |||||||
| Orlistat | pancreatic and gastric lipase inhibitor | obesity >12 years | Not indicated | flatulence, oily spotty stools, diarrhea, vitamin/mineral deficiency Effects on Tanner Stage: None reported78 |
chronic malabsorption syndrome, cholestasis | placebo-subtracted weight loss −2.61kg at 1 yr | 54,71 |
| Phentermine | sympathomimetic amine | obesity > 16 years for “short-term” based on 1959 labeling; combination phentermine/topiramate ER approved for long term treatment of obesity in adults | <16 years or long-term; beneficial in obesity with low energy states, sleep apnea, hunger, decreased satiety | increases in heart rate, blood pressure, dry mouth, insomnia, constipation, worsening anxiety, irritability Effects on Tanner Stage: Not known |
cardiovascular disease hyperthyroidism, active drug use, glaucoma, agitated states | BMI Reduction of −4.1% at 6 months | 55 |
| NOT FDA-INDICATED FOR OBESITY (metformin, topiramate, exenatide, liraglutide, lisdexamfetamine) | |||||||
| Metformin* | activation of protein kinase pathway | ≥10 years for Type 2 diabetes mellitus | Polycystic Ovarian syndrome, insulin resistance, prediabetes, metabolic syndrome, anti-psychotic Medication induced weight gain, stress eating/emotional eating | SE: bloating, diarrhea, flatulence Effects on Tanner Stage: Not known |
Hold 48 hrs prior to contrast; lactic acidosis | BMI z-score reduction – 0.10 and BMI −0.86 | 31 |
| Topiramate* | modulation of various neurotransmitters | treatment of epilepsy >2 years and migraines >12 years; combination phentermine/topiramate ER approved for long term treatment of obesity in adults | weight loss in adult and pediatric patients; useful adjunct in binge-eating disorders and weight regain post-bariatric surgery | Cognitive dysfunction, kidney stones, metabolic acidosis, teratogenic-adolescents MUST be counseled against pregnancy or decrease in efficacy of oral contraceptives Effects on Tanner Stage: unclear |
inborn errors of metabolism with hyperammonia and encephalopathy, acute myopia and secondary angle closure glaucoma; rapid withdrawal can precipitate seizures; neuropsychiatrie dysfunction, metabolic acidosis | BMI Reduction of −4.9% on topiramate 75mg daily for at least 3 months | 86 |
| Exenatide* | GLP-1 agonist | Type 2 diabetes mellitus in adults | <18 years of age for obesity (polygenic with presence of diabetes, hypothalamic, syndromic) | bloating, nausea/vomiting, abdominal pain, elevation of pancreatic amylase and lipase Effects on Tanner Stage: not known |
post-marketing reports: pancreatitis, renal impairment, severe Gl disease | BMI Reduction of −3.42% at 3 months | 93–95 |
| Liraglutide* | GLPl-agonist | Liraglutide 3.0mg (Saxenda) dosing approved for obesity in adults; liraglutide (Victoza) approved for Type 2 diabetes in adults | <18 years of age | Gastrointestinal: abdominal pain, nausea, vomiting, diarrhea, potential hypoglycemia Effects on Tanner Stage: not known |
post-marketing reports: pancreatitis, renal impairment, severe Gl disease | Trials on-going | 101 |
| Lisdexamfetamine§ | central nervous system stimulant | age ≥6 years for Attention Deficit Hyperactivity Disorder (ADHD); short-term use of binge-eating disorder in adults | beneficial for younger children with ADHD and obesity or binge-eating disorder; | anorexia, anxiety, decreased appetite, decreased weight, diarrhea, dizziness, dry mouth, irritability, insomnia, nausea, upper abdominal pain, and vomiting Effects on Tanner Stage: Animal studies have shown neuroplasticity effect on peri-pubertal period which may negatively impact maturation of brain structures106–107. |
serious cardiovascular reactions such as sudden death; blood pressure and heart rate increases; psychiatric adverse reactions; suppression of growth; peripheral vasculopathy such as Raynaud’s, serotonin syndrome with use of serotonergic agents | In 6–12 years age group, mean weight loss was −2.5 lbs. with 70mg dose over 4 weeks; In 13–17 years age group, mean weight loss from baseline was −4.8 lbs. with 70mg dose over 4 weeks | 105 |
| NO PEDIATRIC DATA (lorcaserin, naltrexone/bupropion SR) | |||||||
| Lorcaserin | 5-Hydroxytriptamine receptor 2C agonist | long term treatment of obesity in adults | <18 years of age with obesity | headache, dizziness, fatigue, dry mouth, constipation; headache, back pain, cough in patients with diabetes Effects on Tanner Stage: Not known |
serotonin syndrome or neuroleptic malignant like syndrome when co-administered with other serotonergic or antidopaminergic agents; discontinue with signs of valvular heart disease | safety and outcomes data not available for <18 years | 20 |
| naltrexone/bupropion SR | blockage of opioid-receptor-mediated POMC auto-inhibition (naltrexone) and Selective inhibition of reuptake of dopamine and noradrenaline (bupropion) | long term treatment of obesity in adults | Children and adolescents: WARNING for Increased suicidal ideation | nausea, constipation, headache, dizziness, insomnia, dry mouth, diarrhea Effects on Tanner Stage: Not known |
uncontrolled hypertension, seizures, anorexia nervosa or bulimia, active alcohol or chronic opioid use, angle closure glaucoma, increase in suicidal thought and ideation | safety and outcomes data not available for <18 years | 20 |
| PENDING NEW FDA-APPROVAL (setmelanotide) | |||||||
| Setmelanotide | melanocortin-4-receptor agonist | Phase 3 trials for monogenic obesity; FDA approval pending for monogenic obesity in adults and children | None; drug will be approved for pediatric patients as well | drymouth, mild induration at injection site, darkening of skin nevi Effects on Tanner Stage: Not known |
caution use in structural heart disease and arrhythmias due to potential to increase heart rate and blood pressure | Sustainable Reduction in hunger and substantial weight loss −20.5kg after 12 weeks in one patient and −51.0kg in another after 42 weeks | 118 |
Not FDA indicated for the treatment of obesity, but commonly prescribed by trained providers for adolescents with obesity
Not FDA indicated for the treatment of obesity, but prescribed for eating-disorder
FDA-Approved Obesity Medications in Pediatrics
Orlistat (FDA-approved for treatment of obesity ≥12 years of age); mechanism of action: pancreatic and gastric lipase inhibitor
Efficacy:
In a recent pediatric meta-analysis (31) involving 779 adolescents ages 12–18 years with baseline average BMI 37.4 kg/m2, there were small BMI differences between orlistat and placebo groups: −0.94 (95% CI, −1.58 to −0.30) to −0.50 (95% CI, −7.62 to 6.62), with absolute weight changes ranging from +1 to −12 lbs. with orlistat. The largest randomized-controlled trial evaluating orlistat combined with a hypocaloric (30% fat calories) diet, exercise, and behavioral therapy in adolescents (n=352; also included in the above meta-analyses) showed a −2.61 kg placebo-subtracted weight loss at one year after treatment (p<0.001)(54).
Safety:
Gastrointestinal adverse effects were quite common with orlistat use: abdominal pain or cramps (16–65% of participants vs. 11–26% placebo), flatus with discharge (20–43% vs. 3–11% placebo), fecal incontinence (9% vs. 0–1% placebo); changes in glucose, insulin, and lipid profile were not statistically significant in adolescents with obesity (54, 71). Rare but serious associations of hepatic and renal illness with orlistat use have been described in the product brochure (72).
Additional Insight:
Orlistat is also available over-the-counter in a lower dosage formulation. A multivitamin is recommended with orlistat use due to increased risks of fat-soluble vitamin and mineral deficiencies. Due to little cardiometabolic benefit, expense and adverse tolerability in adolescents attending school where bathroom privileges may be limited, orlistat is not considered a first-line drug for the treatment of pediatric obesity as monotherapy.
Effects on pubertal development or puberty:
Based on data from several human studies, treatment with orlistat for one year does not appear to affect pubertal development or the expected increase in lean body mass during puberty (73). Longer-term studies are needed to understand the effects of orlistat on pubertal development.
Phentermine (FDA approved for age >16 years for short-term treatment (often interpreted as 12 weeks but unspecified on the label) based on 1959 labeling which has not been updated, off-label drug use for obesity when used in age <16 years or long-term as monotherapy; combination therapy with phentermine/topiramate extended-release is FDA-approved for chronic weight management in adults); mechanism of action: sympathomimetic amine, releases catecholamines from hypothalamus, insignificant dopamine release
Efficacy:
A small retrospective chart review compared adolescents (mean age 16.1±1.3 years) treated with phentermine 15mg once daily plus lifestyle modification therapy (n =25) to lifestyle modification therapy alone (n= 274)(55). The study found a −4.1% BMI reduction at 6 months (95% CI: −7.1, −1.0%; p=0.009) with phentermine plus lifestyle modification therapy compared to lifestyle therapy alone with no changes in baseline systolic or diastolic blood pressure readings (55). This is comparable to an adult study that found −5.1% weight loss at 28 weeks with phentermine 15mg once daily monotherapy (74).
Safety:
In the same retrospective study, though no changes were elicited in baseline blood pressure measurements in the treatment arm, heart-rate was higher at all time points in the phentermine group compared to lifestyle modification therapy alone for adolescents (55). Phentermine is a sympathomimetic which can cause increases in heart rate, blood pressure, nervousness and/or insomnia. Adverse effects can also include dizziness, dry mouth, difficulty sleeping, irritability, nausea/vomiting, diarrhea and constipation. Phentermine is contraindicated in patients with cardiovascular disease, hyperthyroidism, glaucoma, history of drug abuse, or women who are pregnant. Phentermine should not be used while taking, and for 14 days after stopping, a monoamine oxidase (MAO) inhibitor because of the risk of hypertensive crisis (20).
Additional Insight:
Phentermine is a Class IV controlled substance and state bylaws and statues should be followed. Baseline electrolytes, kidney function, blood pressure measurements, and heart rate need to be assessed in adolescents along with screening for congenital heart disease or other cardiac pathology such as family history of sudden death, Wolff-Parkinson-White syndrome, and newly auscultated murmurs; obtain cardiology clearance if concerns arise prior to phentermine prescribing as there have been case reports of myocardial infarction when taking phentermine (75, 76).
Off-label drug use:
Off-label drug use documentation along with consent for treatment from the patient’s parent or guardian is recommended.
Effects on pubertal development or puberty:
The effects of phentermine on pubertal development in humans are not known.
Non-FDA Approved Medications for Obesity but with Pediatric Evidence
Metformin (FDA approved for ≥10 years of age for Type 2 diabetes, off-label drug use for other indications); mechanism of action: mainly activation of activated protein kinase
Efficacy:
In a meta-analysis (n=616) comparing the use of metformin versus placebo for weight loss as part of pediatric obesity or endocrine clinics (baseline BMI of 36.0 with metformin dose ranging from 1–2 g per day), metformin treatment reduced BMI z-score (−0.10 [95% CI, −0.17 to −0.03] and BMI (−0.86 [95% CI, −1.44 to −0.29])(31).
Safety:
The medication in the same meta-analysis was well tolerated with minimal discontinuation rates (<5%), with no reported cases of lactic acidosis and hypoglycemia in children (31). Commonly reported side effects are usually gastrointestinal including bloating, diarrhea, and flatus. Metformin-associated lactic acidosis is quite rare with incidence estimated to be 3–10 per 100,000 person years (77) and there are a few cases reports of rhabdomyolysis in the literature although quite rare (78).
Additional Insight:
Metformin is usually a first-line medication in a patient with insulin resistance, prediabetes or metabolic syndrome given the minimal safety concerns and tolerability. Though the meta-analyses showed only a small reduction in excess weight in youth, metformin has been effective for weight regain related to anti-psychotic medications (79) in non-diabetic children (−4.1% weight reduction, 95% CI 2.2–6.0) (80), and in adults, for weight gain related to mood disorders, steroid-exposure, stress-eating, and emotional eating related to cognitive dysfunction, possibly related to aberrant insulin signaling, inflammation, and glucocorticoid activity which may be emanated by iatrogenic causes (81). Metformin is extensively utilized for polycystic ovarian syndrome treatment with or without obesity diagnosis in children and adolescents with improvement seen on lipid profile, hirsutism, and weight loss (82, 83, 84).
Off-label drug use:
Off-label drug use documentation along with consent for treatment from the patient’s parent or guardian is recommended.
Effects on pubertal development or puberty:
Effect of metformin on pubertal development is unknown.
Topiramate (FDA approved for the treatment of epilepsy ≥2 years of age and migraine prophylaxis in ≥12 years old; causes weight loss in adult obesity trials; combination obesity medication phentermine/topiramate extended-release is FDA approved for chronic weight management in adults. Mechanism of action is possibly through modulation of various neurotransmitters, including the inhibition of voltage-dependent sodium channels, glutamate receptors and carbonic anhydrase and the potentiation of γ-aminobutyrate activity; off-label drug use for obesity treatment and also binge-eating disorder in adults)
Efficacy:
A small randomized, placebo-controlled pilot clinical trial evaluating topiramate in 30 adolescents ages 12–17 years with severe obesity (BMI ≥120% of the 95th percentile or BMI ≥ 35 kg/m2) showed a 2% BMI reduction on 75mg topiramate at 6 months which did not reach statistical significance compared to placebo following a short-term (1 month) meal replacement phase (−1.9%; 95% CI: −5.2% to +1.5%; P = 0.291). Significant improvements in visceral fat and very-low-density lipoprotein cholesterol were observed in the topiramate compared with the placebo group (85). Another study, a retrospective chart review examining the effect of topiramate 75mg once daily dosing for at least 3 months plus lifestyle intervention on BMI reduction in adolescents with severe obesity (n= 28; mean age 15.2 +/− 2.5 years, mean baseline BMI 46.2 +/− 10.3 kg/m2) found clinically meaningful −4.9% BMI reduction (95% CI:−7.1, −2.8; P< .001) with no significant adverse effects (86).
Safety:
In both trials, there were no concerning changes in neurocognitive function with low dose topiramate in adolescents (85, 86). Of the 28 patients in one of the trials, only 2 experienced paresthesia (86). Adult clinical trials using combination therapy with phentermine/topiramate ER reported paresthesia, depression and anxiety as common side effects most likely related to the topiramate component of the combination (20). The drug is a teratogen with potential to cause cleft palate and/or lip during first trimester of pregnancy. It might also decrease the efficacy of oral contraceptives though less likely at dosages <200mg/day (20). Adolescents must be strongly counseled against pregnancy with effective contraception in place; a monthly urine pregnancy test in all adolescents is recommended.
Additional Insight:
Topiramate has documented efficacy in eating disorders, including binge-eating (87, 88, 89, 90), and weight regain post-bariatric surgery (91). Topiramate can cause reversible cognitive and psychiatric dysfunction as well as metabolic acidosis. Adolescents with fatigue, sleep disturbances, worsening school performance need to be evaluated for possible cognitive side effects related to topiramate. Quick withdrawal may prompt seizures in children and thus gradual titration downwards for discontinuation is recommended.
Off-label drug use:
Off-label drug use documentation along with consent for treatment from the patient’s parent or guardian is recommended.
Effects on pubertal development or puberty:
Topiramate affects BMI, weight, insulin, leptin, and adipocytokine levels in pre-pubertal children (92). The effect on puberty is unknown.
GLP-1 Receptor Agonists (exenatide, liraglutide):
Exenatide (FDA approved for Type 2 diabetes mellitus in adults; off-label drug use in <18 years of age; mechanism of action: glucagon-like-1 receptor (GLP-1) agonist)
Liraglutide (3.0mg dosing FDA approved for obesity in adults; off-label drug use <18 years of age for patients; mechanism of action: glucagon-like-1 receptor (GLP-1) agonist)
Efficacy (Exenatide):
In a small randomized, controlled, crossover trial of 12 adolescents and children (9–16 years of age) with severe obesity, 3 months of treatment with exenatide plus lifestyle modification therapy significantly reduced BMI (−1.7 kg/m2, [95% CI −3.0, −0.4], P = 0.01), body weight (−3.9 kg, [95% CI −7.11, −0.69], p = 0.02), and fasting insulin (−7.5 mU/l, [95% CI −13.71, −1.37], p = 0.02)(93). In another randomized, placebo-controlled clinical trial of 26 adolescents (ages 12–19 years) with severe obesity followed by a 3 month open-label extension, treatment with exenatide elicited a greater reduction in percent change in BMI compared with placebo (−2.70% [95% CI, −5.02% t −0.37%]; p = 0.03) and body weight (−3.26 kg [95% CI, −5.87 to −0.66 kg]; p = 0.02); during the open-label extension, BMI was further reduced in those initially randomized to exenatide (cumulative BMI reduction of 4%)(94). Pooled-data (n = 32 [mean age 14.3 ± 2.2 years; 69% female; mean BMI 39.8 ± 5.8 kg/m2) from these near identical trials showed an absolute BMI reduction of −3.42% [95% CI −5.41, −1.42] compared to placebo at 3 months (95).
GLP-1 agonists may have some role in syndromic obesity such as Prader-Willi syndrome (PWS) though more robust studies are needed. A 6 month open-label, non-randomized, longitudinal study recruited 10 patients (13–25 years) with PWS and placed them on diabetes dosing exenatide without dietary modification. Though appetite scores and hemoglobin A1c decreased significantly after treatment, weight and adiposity were unaffected with no significant changes in ghrelin (96).
Safety (Exenatide, liraglutide):
GLP-1 agonists such as exenatide and liraglutide have been associated with pancreatitis on post-marketing surveillance reports though this association has not been statistically significant or clearly evident (20). GLP-1 agonists have also been associated with acute renal failure and worsening of chronic renal failure, sometimes requiring hemodialysis and thus are not recommended in severe renal impairment, or end-stage- renal disease and should be used with caution in renal transplant patients (97, 98). Newer extended-release formulations such as dulaglutide have a better safety profile in renal impairment (99). GLP-1 agonists are not recommended in patients with severe gastrointestinal disease such as gastroparesis (97, 98, 99, 100). GLP-1 agonists are contraindicated in patients with a family or personal history of medullary thyroid carcinoma or patients with multiple endocrine neoplasia Type 2 (MEN2) (20) syndrome (100). In adolescent trials, compliance with the injection regimen was excellent (≥94%) and exenatide was generally well-tolerated (the most common adverse event was mild nausea in 36% followed by vomiting, headache, abdominal pain and diarrhea)(93, 94, 95). Though the safety and tolerability of newer GLP-1 agonists such as dulaglutide and semaglutide have not been assessed in adolescents, these preliminary adolescent studies provide support for safety, tolerability, and feasibility of GLP-1 agonists in this patient population.
Safety and Tolerability (Liraglutide):
A randomized, placebo-controlled, double-blind study to assess safety, tolerability, and pharmacokinetics of liraglutide 3.0mg in adolescents (12–17 years of age, Tanner staging 2–5; n=21; BMI ≥95th percentile for age-and-sex [BMI ≥30 kg/m2 to ≤45 kg/m2) was recently completed (101). Adolescents were randomized (2:1) to receive 5 weeks of treatment with liraglutide (0.6 mg with weekly dose increase to a maximum of 3.0 mg for the last week; [n = 14] or placebo [n = 7]). The most common adverse events associated with liraglutide 3.0mg dose were gastrointestinal (abdominal pain, nausea, vomiting, and diarrhea). Twelve hypoglycemic episodes occurred in 8 patients while 2 similar events occurred in a patient in the placebo group. Participants did not have symptoms and need assistance from another person; the hypoglycemia was found only during routine glucose monitoring(102, 103). Liraglutide had a similar safety and tolerability profile compared with adults when administered to adolescents with obesity, with no unexpected safety/tolerability issues. Results suggest that the dosing regimen approved for weight management in adults may be appropriate for use in adolescents(101). Further studies evaluating the efficacy of liraglutide in the adolescent population are currently on-going.
Additional Insight:
GLP-1 agonist therapy has potential for weight loss and weight stabilization in patients with syndromic and hypothalamic obesity with hyperphagia. In patients with hypothalamic obesity as a result of tumor or trauma injury, exenatide therapy may help stabilize weight and increase satiety (104). GLP-1 agonist therapy is frequently considered next in line for patients with poorly controlled Type 2 diabetes mellitus and obesity in conjunction with metformin and intensive lifestyle modification (60). Because insurance coverage for GLP-1 agonists may be limited in adolescents, especially for newer GLP-1 agonists, older generic analogs such as exenatide are more likely to be covered in some states for adolescents with Type 2 diabetes mellitus.
Off-label drug use:
Off-label drug use documentation along with consent for treatment from the patient’s parent or guardian is recommended.
Effects on pubertal development or puberty:
The effects of GLP-1 agonists on puberty are unknown.
FDA Approved Medication for Eating Disorder (Non-FDA Approved for Obesity) but with Pediatric Evidence
Lisdexamfetamine (FDA-approved for attention deficit hyperactivity disorder [ADHD] in children ≥6 years and adults, and binge-eating disorder in adults. Not FDA-indicated for weight loss treatment in either children or adults; mechanism of action: dopamine agonist)
Efficacy (children, likely normal weight, with ADHD in the study):
In a controlled trial of children ages 6 to 12 years being prescribed lisdexamfetamine for ADHD, mean weight loss from baseline after 4 weeks of therapy was −0.9, −1.9, and −2.5 pounds, respectively, for patients receiving 30 mg, 50 mg, and 70 mg (vs. +1 lb. weight gain placebo)(105). In adolescents ages 13 to 17 years, mean weight loss from baseline to endpoint was −2.7, −4.3, and −4.8 lbs., respectively, for patients receiving 30 mg, 50 mg, and 70 mg of lisdexamfetamine over 4 weeks (vs. +2.0 lb. weight gain placebo)(105).
Safety:
Careful follow up of children who received lisdexamfetamine for over 12 months had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development (105). Sudden death has been reported in children and adolescents with structural cardiac abnormalities and other serious heart problems taking amphetamines at recommended doses for ADHD. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmia, coronary artery disease, and other serious heart problems. Central nervous system (CNS) stimulants cause an increase in blood pressure (mean increase about 2–4 mm Hg) and heart rate (mean increase about 3–6 bpm). CNS stimulants can also provoke pre-existing psychiatric disorders and psychosis, delusions or hallucinations in children and adolescents with no prior mental illness. Post-marketing reports have also associated lisdexamfetamine with Raynaud’s phenomenon in adults (105).
Additional Insight:
Because lisdexamfetamine is FDA-approved lower than age 10 years, it may be a beneficial option in younger children ≥6 years of age with ADHD and binge-eating disorder. Though it is approved for binge-eating disorder in adults, it is not approved for the same indication in children in the absence of an ADHD diagnosis. Long-term safety of amphetamines has been studied in the pediatric population with concerns for developmental growth retardation and careful monitoring for cardiac arrhythmias. Treatment of children and adolescents who have impulsive behaviors such as impulsive excessive food intake as a manifestation of their ADHD may find benefit from lisdexamfetamine or other ADHD medications in achieving healthier eating behaviors. Given the history of abuse potential of amphetamines in the past coupled with potential adverse psychiatric side effects, caution is advised if used for the long-term treatment of obesity. In youth with obesity or binge eating disorder, regardless of an ADHD diagnosis, there is limited data, if at all, reporting potential benefits with lisdexamfetamine use.
Off-label drug use:
Off-label drug use is not recommended at this time.
Effects on pubertal development or puberty:
Animal studies have demonstrated that amphetamine drug exposure during different development stages such as peri-pubertal vs. pre-pubertal result in distinct neurobehavioral abnormalities (106, 107).
FDA-approved Obesity Medications With No Pediatric Evidence
Lorcaserin
This medication is a 5-hydroxytryptamine receptor 2C agonist that acts on anorexigenic proopiomelanocortin (POMC) neurons in the hypothalamus.
Efficacy:
Lorcaserin, is FDA-approved for the chronic treatment of obesity in adults (20). However, there are no pediatric outcome data available with regards to lorcaserin. Adult clinical trials have demonstrated 3% placebo-subtracted weight loss from baseline (20).
Safety:
No adverse cardiovascular safety signals have thus far emerged with lorcaserin, from the large cardiovascular safety trial(108). Common adverse effects of lorcaserin reported in clinical trials are headache, dizziness, fatigue, nausea, dry mouth and constipation. Lorcaserin should not be prescribed concurrently with a serotonergic medication due to the risk of serotonin syndrome (20).
Off-label drug use:
Off-label drug use documentation along with consent for treatment from the patient’s parent or guardian is recommended.
Effects on pubertal development or puberty:
The effects of lorcaserin on pubertal development in humans are not known.
Naltrexone SR/bupropion SR (NB)
This medication blocks opioid receptor-mediated POMC autoinhibition (naltrexone) and selectively inhibits reuptake of dopamine and noradrenaline (bupropion, an antidepressant), and it is FDA approved for the chronic treatment of obesity in adults (20). NB has shown benefit in patients with obesity with addiction behaviors, reward pathways, and hedonic drive. Though there are no pediatric outcomes data available with regards to obesity for NB, naltrexone and bupropion monotherapy have been used for other indications in children. Adult clinical trials have demonstrated 4.8% placebo-subtracted weight loss from baseline for NB on the highest optimal dose (20).
Safety:
Common adverse effects of NB reported in clinical trials are transient nausea during the dose escalation period, constipation, headaches, vomiting, dizziness, and dry mouth (20). NB did not increase rates of depression and suicidal ideation more than placebo in the clinical trials (20). Though monotherapy with bupropion has been utilized in adolescents (ages 12–17 years) for depression, with weight loss noted as a side effect in a majority of patients (109), caution is needed as bupropion, as with other antidepressants, may increase risk of suicidal ideation in children, adolescents and young adults (110). Therefore, NB carries a black-box warning in regards to increased suicidal risk and ideation in young adults and is not approved for pediatric patients (110). Of note, bupropion monotherapy has not been FDA-approved for the treatment of depression or other condition in youth.
Additional Insight:
Limited pediatric data are available for naltrexone monotherapy. Naltrexone monotherapy has been studied for opioid drug use in adolescents (ages 13 years and older)(111) and PWS for appetite reduction (112) since the 1980s, although long-term safety has not been established in the pediatric population. There is one case-report in the literature of using NB combination therapy for PWS in a 13-year old girl with small reduction in BMI and improvements in hyperphagia (113).
Off-label drug use:
Off-label drug use documentation along with consent for treatment from the patient’s parent or guardian is recommended.
Effects on pubertal development or puberty:
The effects of naltrexone and bupropion on pubertal development in humans are not known. In an animal study, bupropion did appear to alter pubertal onset (114). On the other hand, naltrexone effects on puberty have been studied. In female rat models, naltrexone has been shown to advance first ovulation through changes in pituitary responsiveness to luteinizing-releasing-hormone (115). One human study demonstrated a more sensitive luteinizing hormone surge in pubertal boys than prepubertal sexually immature boys after chronic one month exposure of naltrexone (116). Another study found no effect on puberty in boys with confirmed bone age 10–15 years when given naltrexone for one month (117).
New FDA-Approval Pending for Pediatric Obesity
Setmelanotide (FDA approval pending for monogenic obesity; mechanism of action: melanocortin-4-receptor [MC4R] agonist)
Efficacy:
Setmelanotide is currently being evaluated for the treatment of the following genetic disorders of obesity: POMC deficiency obesity, LepR deficiency obesity, Prader-Willi syndrome, Bardet-Biedl syndrome, Alström syndrome, POMC heterozygous deficiency obesity, and POMC epigenetic disorders. In an investigator-initiated, open-label study, two patients with POMC deficiency were treated with setmelanotide with a sustainable reduction in hunger and substantial weight loss (51.0 kg after 42 weeks in Patient 1 and 20.5 kg after 12 weeks in Patient 2)(118).
Safety:
Dry mouth, mild induration at injection site, and darkening of skin nevi were notable adverse affects. There were no increases in blood pressure, with improvements in both heart rate and blood pressure in one of the patients (118).
Additional Insight:
Setmelanotide provides promise for rare genetic obesity disorders which should be considered in children with hyperphagia, early adiposity rebound, and severe obesity at a young age.
Effects on pubertal development or puberty:
The effects of setmelanotide on pubertal development in humans are not known.
Off-label drug use:
Off-label drug use is not recommended at this time.
CONCLUSION
Childhood and adolescent obesity is already a global epidemic and poses significant health risks. As adolescent obesity often leads to obesity in adulthood and is already accompanied by a multitude of weight-related comorbidities including an increased risk for cardiovascular disease and certain types of cancers, treating obesity in children/adolescents should not be delayed. As an adjunct to intensive lifestyle therapy and MBS, the potential role of pharmacotherapy in the treatment of pediatric obesity should not be ignored and may represent a useful additional option for some patients who suffer from obesity. Additionally, in clinically severe obesity, pharmacotherapy may play a larger role as adjunct to MBS (91). This therapeutic need highlights the value of experienced pediatric obesity medicine specialists at tertiary care centers and yet presents challenges as clinical trials evaluating long-term safety and efficacy of obesity medications remain scant in the pediatric population. Emphasis needs to be placed on the concurrent development of appropriate, high-quality, well-designed pediatric obesity clinical trials to validate the use of medications for obesity in adolescents. Moreover, there exists a great need to develop specialized pediatric obesity medicine training programs, applicable protocols, screening tools, and guidelines to further advance the burgeoning field of pediatric obesity medicine.
Supplementary Material
What is known about this subject?
There is a rise in severe obesity in children and adolescents.
There is an emerging population of adolescents “stuck” in between lifestyle modification therapy and bariatric surgery for which obesity pharmacotherapy may be helpful.
There is limited evidence for the safety and efficacy of pediatric obesity pharmacotherapy. Practitioner guidance is needed regarding how adult safety and clinical considerations might apply to youth with severe obesity.
What does this study add?
A group of pediatric obesity medicine and surgery specialists provide guidance on current best practices for the use of obesity medications in the pediatric population.
It is hoped that this opinion piece on pediatric obesity pharmacotherapy will be followed by accumulating clinical trials data and ultimately formal recommendations on the clinical use of medications to treat pediatric obesity.
ACKNOWLEDGEMENTS
The authors would like to especially thank co-author Nancy Browne, MS, PPCNP-BC, FAANP in addition for administrative support, networking, and coordination of communication from the State Medical Boards.
This work was funded with core support from P30DK046200 and P30DK040561. GS serves as consultant for Johnson and Johnson. CF receives research support from Novo Nordisk. AK receives research support (drug/placebo) from Astra Zeneca Pharmaceuticals and serves as a consultant for Novo Nordisk, Orexigen, and Vivus Pharmaceuticals but does not accept personal or professional income for these activities. AMJ has served as a consultant for Novo Nordisk and Medtronic. CML receives support from New Balance Foundation. CMA reports grants from Aspire Bariatrics, Myos, the Vela Foundation, the Dr. Robert C. and Veronica Atkins Foundation, Coherence Lab, Energesis, NIH, and PCORI, grants and personal fees from Orexigen, GI Dynamics, Takeda, personal fees from Nutrisystem, Zafgen, Sanofi-Aventis, NovoNordisk, Scientific Intake, Xeno Biosciences, Rhythm Pharmaceuticals, Eisai, EnteroMedics, Bariatrix Nutrition, and other from Science-Smart LLC, outside the submitted work.
Abbreviations:
- MBS
metabolic and bariatric surgery
Footnotes
Disclosures: AFB, NTB, JSAP, CB, MPM, and SC report no competing interests.
REFERENCES
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017: 1–8. [PubMed] [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018. [DOI] [PMC free article] [PubMed]
- 3.Finegood DT, Merth TD, Rutter H. Implications of the foresight obesity system map for solutions to childhood obesity. Obesity (Silver Spring) 2010;18 Suppl 1: S13–16. [DOI] [PubMed] [Google Scholar]
- 4.Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N Engl J Med 2016;374: 2430–2440. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007;150: 12–17 e12. [DOI] [PubMed] [Google Scholar]
- 6.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics 2012;129: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Accelerating progress in obesity prevention: solving the weight of the nation Washington, DC: National Academies Press; 2012. [Google Scholar]
- 8.Twig G, Tirosh A, Leiba A, Levine H, Ben-Ami Shor D, Derazne E, et al. BMI at Age 17 Years and Diabetes Mortality in Midlife: A Nationwide Cohort of 2.3 Million Adolescents. Diabetes Care 2016;39: 1996–2003. [DOI] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA 2003;289: 1813–1819. [DOI] [PubMed] [Google Scholar]
- 10.van Geel M, Vedder P, Tanilon J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the correlation between weight status and bullying. Int J Obes (Lond) 2014;38: 1263–1267. [DOI] [PubMed] [Google Scholar]
- 11.Fox CL, Farrow CV. Global and physical self-esteem and body dissatisfaction as mediators of the relationship between weight status and being a victim of bullying. J Adolesc 2009;32: 1287–1301. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes 2010;5: 282–304. [DOI] [PubMed] [Google Scholar]
- 13.Lo JC, Chandra M, Sinaiko A, Daniels SR, Prineas RJ, Maring B, et al. Severe obesity in children: prevalence, persistence and relation to hypertension. Int J Pediatr Endocrinol 2014;2014: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr 2010;91: 1499S–1505S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med 2012;166: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 16.Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatr 2011;158: 624–627. [DOI] [PubMed] [Google Scholar]
- 17.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord 2001;30: 318–328. [DOI] [PubMed] [Google Scholar]
- 18.Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics 2009;124: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knop C, Singer V, Uysal Y, Schaefer A, Wolters B, Reinehr T. Extremely obese children respond better than extremely obese adolescents to lifestyle interventions. Pediatr Obes 2015;10: 7–14. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol 2017. [DOI] [PubMed]
- 21.Grummer-Strawn LM, Reinold C, Krebs NF, Centers for Disease C, Prevention. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep 2010;59: 1–15. [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data 2000: 1–27. [PubMed]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002: 1–190. [PubMed] [Google Scholar]
- 24.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4: S164–192. [DOI] [PubMed] [Google Scholar]
- 25.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr 2009;90: 1314–1320. [DOI] [PubMed] [Google Scholar]
- 26.Age limits and adolescents. Paediatr Child Health 2003;8: 577–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services: The Changing Face of America’s Adolescence. https://www.hhs.gov/ash/oah/facts-and-stats/changing-face-of-americas-adolescents/index.html;Accessed February 20, 2018.
- 28.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics 2007;120 Suppl 4: S193–228. [DOI] [PubMed] [Google Scholar]
- 29.Baker JL, Farpour-Lambert NJ, Nowicka P, Pietrobelli A, Weiss R, Childhood Obesity Task Force of the European Association for the Study of O. Evaluation of the overweight/obese child--practical tips for the primary health care provider: recommendations from the Childhood Obesity Task Force of the European Association for the Study of Obesity. Obes Facts 2010;3: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007;120 Suppl 4: S254–288. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017;317: 2427–2444. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand S, Keller KM, Lob-Corzilius T, Pott W, Reinehr T, Robl M, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr 2014;82: 380–387. [DOI] [PubMed] [Google Scholar]
- 33.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes (Lond) 2006;30: 590–594. [DOI] [PubMed] [Google Scholar]
- 34.Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which Amount of BMI-SDS Reduction Is Necessary to Improve Cardiovascular Risk Factors in Overweight Children? J Clin Endocrinol Metab 2016;101: 3171–3179. [DOI] [PubMed] [Google Scholar]
- 35.Kelly AS, Daniels SR. Rethinking the Use of Body Mass Index z-Score in Children and Adolescents with Severe Obesity: Time to Kick It to the Curb? J Pediatr 2017;188: 7–8. [DOI] [PubMed] [Google Scholar]
- 36.Freedman DS, Butte NF, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, et al. BMI z-Scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring) 2017;25: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr 2010;91: 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalsky M, Reichard K, Inge T, Pratt J, Lenders C, American Society for M, et al. ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis 2012;8: 1–7. [DOI] [PubMed] [Google Scholar]
- 39.Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: Conceptual issues and evidence review. J Am Diet Assoc 2006;106: 2024–2033. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz RP, Hamre R, Dietz WH, Wasserman RC, Slora EJ, Myers EF, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med 2007;161: 495–501. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz RP. Motivational interviewing (patient-centered counseling) to address childhood obesity. Pediatr Ann 2010;39: 154–158. [DOI] [PubMed] [Google Scholar]
- 42.Hoelscher DM, Kirk S, Ritchie L, Cunningham-Sabo L, Academy Positions C. Position of the Academy of Nutrition and Dietetics: interventions for the prevention and treatment of pediatric overweight and obesity. J Acad Nutr Diet 2013;113: 1375–1394. [DOI] [PubMed] [Google Scholar]
- 43.Smith HL, Meldrum DJ, Brennan LJ. Childhood obesity: a challenge for the anaesthetist? Paediatr Anaesth 2002;12: 750–761. [DOI] [PubMed] [Google Scholar]
- 44.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr 2014;168: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med 2016;374: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratt JSA, Browne A, Browne N, Bruzoni M, Cohen M, Desai A, et al. ASMBS Pediatric Metabolic and Bariatric Surgery Guidelines. Surgery for Obesity and Related Disease 2018;In Press. [DOI] [PMC free article] [PubMed]
- 47.Srivastava G, Buffington C. A Specialized Medical Management Program to Address Post-operative Weight Regain in Bariatric Patients. Obes Surg 2018. [DOI] [PubMed]
- 48.Black JA, White B, Viner RM, Simmons RK. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev 2013;14: 634–644. [DOI] [PubMed] [Google Scholar]
- 49.Paulus GF, de Vaan LE, Verdam FJ, Bouvy ND, Ambergen TA, van Heurn LW. Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes Surg 2015;25: 860–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol 2017;5: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karres J, Tomasi P, Saint Raymond A. The development of pharmacological treatment of obesity in children. A European regulatory perspective. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2011;54: 570–576. [DOI] [PubMed] [Google Scholar]
- 52.Hardin AP, Hackell JM, Committee On P, Ambulatory M. Age Limit of Pediatrics. Pediatrics 2017;140. [DOI] [PubMed]
- 53.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128: 1689–1712. [DOI] [PubMed] [Google Scholar]
- 54.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 2005;293: 2873–2883. [DOI] [PubMed] [Google Scholar]
- 55.Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes (Lond) 2017;41: 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Pubertal timing and body mass index gain from birth to maturity in relation with femoral neck BMD and distal tibia microstructure in healthy female subjects. Osteoporos Int 2011;22: 2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohamad K, Jamshidi L, Nouri Jelyani K. Is Age of Menarche Related with Body Mass Index? Iran J Public Health 2013;42: 1043–1048. [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. Int J Obes (Lond) 2016;40: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100: 342–362. [DOI] [PubMed] [Google Scholar]
- 61.Lenders CM. Paediatric obesity: can medications help. Curr Opin Endocrinol Diabetes Obes 2015;22: 331–339. [DOI] [PubMed] [Google Scholar]
- 62.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017;102: 709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siu AL, Force USPST. Screening for Depression in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. Pediatrics 2016;137: e20154467. [DOI] [PubMed] [Google Scholar]
- 64.Frattarelli DA, Galinkin JL, Green TP, Johnson TD, Neville KA, Paul IM, et al. Off-label use of drugs in children. Pediatrics 2014;133: 563–567. [DOI] [PubMed] [Google Scholar]
- 65.Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc 2012;87: 982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 67.Bazzano AT, Mangione-Smith R, Schonlau M, Suttorp MJ, Brook RH. Off-label prescribing to children in the United States outpatient setting. Acad Pediatr 2009;9: 81–88. [DOI] [PubMed] [Google Scholar]
- 68.Shah SS, Hall M, Goodman DM, Feuer P, Sharma V, Fargason C Jr., et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med 2007;161: 282–290. [DOI] [PubMed] [Google Scholar]
- 69.Phan H, Leder M, Fishley M, Moeller M, Nahata M. Off-label and unlicensed medication use and associated adverse drug events in a pediatric emergency department. Pediatr Emerg Care 2010;26: 424–430. [DOI] [PubMed] [Google Scholar]
- 70.Force USPST Grossman DC, Bibbins-Domingo K Curry SJ, Barry MJ Davidson KW, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317: 2417–2426. [DOI] [PubMed] [Google Scholar]
- 71.Maahs D, de Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract 2006;12: 18–28. [DOI] [PubMed] [Google Scholar]
- 72.Orlistat [Xenical] package insert. Roche Laboratories; Nutley, NJ: 2009. [Google Scholar]
- 73.Bogarin Ra J-P, Chanoine. Efficacy, safety and tolerability of orlistat, a lipase inhibitor, in the treatment of adolescent weight excess. Therapy 2009;6: 23–30. [Google Scholar]
- 74.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21: 2163–2171. [DOI] [PubMed] [Google Scholar]
- 75.Azarisman SM, Magdi YA, Noorfaizan S, Oteh M. Myocardial infarction induced by appetite suppressants in Malaysia. N Engl J Med 2007;357: 1873–1874. [DOI] [PubMed] [Google Scholar]
- 76.Anwar MO, Bodagh N, Iqbal MH, Timmis A. Vasospastic myocardial infarction caused by a slimming agent-do not forget non-prescription drugs. Oxf Med Case Reports 2017;2017: omx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312: 2668–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2006: CD002967. [DOI] [PubMed]
- 79.Anagnostou E, Aman MG, Handen BL, Sanders KB, Shui A, Hollway JA, et al. Metformin for Treatment of Overweight Induced by Atypical Antipsychotic Medication in Young People With Autism Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016;73: 928–937. [DOI] [PubMed] [Google Scholar]
- 80.Bjorkhem-Bergman L, Asplund AB, Lindh JD. Metformin for weight reduction in non-diabetic patients on antipsychotic drugs: a systematic review and meta-analysis. J Psychopharmacol 2011;25: 299–305. [DOI] [PubMed] [Google Scholar]
- 81.Cha DS, Vahtra M, Ahmed J, Kudlow PA, Mansur RB, Carvalho AF, et al. Repurposing of Anti-Diabetic Agents for the Treatment of Cognitive Impairment and Mood Disorders. Curr Mol Med 2016;16: 465–473. [DOI] [PubMed] [Google Scholar]
- 82.Hsia Y, Dawoud D, Sutcliffe AG, Viner RM, Kinra S, Wong IC. Unlicensed use of metformin in children and adolescents in the UK. Br J Clin Pharmacol 2012;73: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al Khalifah RA, Florez ID, Dennis B, Thabane L, Bassilious E. Metformin or Oral Contraceptives for Adolescents With Polycystic Ovarian Syndrome: A Meta-analysis. Pediatrics 2016;137. [DOI] [PubMed]
- 84.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98: 4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fox CK, Kaizer AM, Rudser KD, Nathan BM, Gross AC, Sunni M, et al. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: A pilot randomized controlled trial. Obesity (Silver Spring) 2016;24: 2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox CK, Marlatt KL, Rudser KD, Kelly AS. Topiramate for weight reduction in adolescents with severe obesity. Clin Pediatr (Phila) 2015;54: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalai SS, Adler S, Najarian T, Safer DL. Study protocol and rationale for a randomized double-blinded crossover trial of phentermine-topiramate ER versus placebo to treat binge eating disorder and bulimia nervosa. Contemp Clin Trials 2017. [DOI] [PubMed]
- 88.Guardia D, Rolland B, Deheul S, Danel T, Bordet R, Cottencin O. [Supervised off-label prescribing of topiramate for binge eating disorder within the system CAMTEA]. Therapie 2012;67: 480–481. [DOI] [PubMed] [Google Scholar]
- 89.Guerdjikova AI, Fitch A, McElroy SL. Successful Treatment of Binge Eating Disorder With Combination Phentermine/Topiramate Extended Release. Prim Care Companion CNS Disord 2015;17. [DOI] [PMC free article] [PubMed]
- 90.Leombruni P, Lavagnino L, Fassino S. Treatment of obese patients with binge eating disorder using topiramate: a review. Neuropsychiatr Dis Treat 2009;5: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stanford FC, Alfaris N, Gomez G, Ricks ET, Shukla AP, Corey KE, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surg Obes Relat Dis 2017;13: 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonmez FM, Zaman D, Aksoy A, Deger O, Aliyazicioglu R, Karaguzel G, et al. The effects of topiramate and valproate therapy on insulin, c-peptide, leptin, neuropeptide Y, adiponectin, visfatin, and resistin levels in children with epilepsy. Seizure 2013;22: 856–861. [DOI] [PubMed] [Google Scholar]
- 93.Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring) 2012;20: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr 2013;167: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nathan BM, Rudser KD, Abuzzahab MJ, Fox CK, Coombes BJ, Bomberg EM, et al. Predictors of weight-loss response with glucagon-like peptide-1 receptor agonist treatment among adolescents with severe obesity. Clin Obes 2016;6: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr Obes 2017;12: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bydureon [exenatide] package insert Amylin Pharmaceuticals, Inc; San Diego, CA: 2012;ccessed March 9, 2018. [Google Scholar]
- 98.Saxenda (liraglutide [rDNA origin] injection) package insert Novo Nordisk; Plainsboro, NJ: 2014;accessed March 8, 2018. [Google Scholar]
- 99.Trulicty [dulaglutide] package insert Eli Lilly and Company; Indianapolis, IN: 2014;accessed March 9, 2018. [Google Scholar]
- 100.Exenatide [Byetta] package insert Amylin Pharmaceuticals; San Diego, CA: 2009. [Google Scholar]
- 101.Danne T, Biester T, Kapitzke K, Jacobsen SH, Jacobsen LV, Petri KCC, et al. Liraglutide in an Adolescent Population with Obesity: A Randomized, Double-Blind, Placebo-Controlled 5-Week Trial to Assess Safety, Tolerability, and Pharmacokinetics of Liraglutide in Adolescents Aged 12–17 Years. J Pediatr 2017;181: 146–153 e143. [DOI] [PubMed] [Google Scholar]
- 102.Petri KC, Jacobsen LV, Klein DJ. Comparable liraglutide pharmacokinetics in pediatric and adult populations with type 2 diabetes: a population pharmacokinetic analysis. Clin Pharmacokinet 2015;54: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klein DJ, Battelino T, Chatterjee DJ, Jacobsen LV, Hale PM, Arslanian S, et al. Liraglutide’s safety, tolerability, pharmacokinetics, and pharmacodynamics in pediatric type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Technol Ther 2014;16: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lomenick JP, Buchowski MS, Shoemaker AH. A 52-week pilot study of the effects of exenatide on body weight in patients with hypothalamic obesity. Obesity (Silver Spring) 2016;24: 1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vyvanse (lisdexamfetamine dimesylate) [package insert] Shire US, Lexington, MA: 2017. [Google Scholar]
- 106.Calabrese F, Richetto J, Racagni G, Feldon J, Meyer U, Riva MA. Effects of withdrawal from repeated amphetamine exposure in peri-puberty on neuroplasticity-related genes in mice. Neuroscience 2013;250: 222–231. [DOI] [PubMed] [Google Scholar]
- 107.Kang S, Wu MM, Galvez R, Gulley JM. Timing of amphetamine exposure in relation to puberty onset determines its effects on anhedonia, exploratory behavior, and dopamine D1 receptor expression in young adulthood. Neuroscience 2016;339: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, et al. Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N Engl J Med 2018;379: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 109.Glod CA, Lynch A, Flynn E, Berkowitz C, Baldessarini RJ. Open trial of bupropion SR in adolescent major depression. J Child Adolesc Psychiatr Nurs 2003;16: 123–130. [DOI] [PubMed] [Google Scholar]
- 110.Contrave (naltrexone HCl and buproprion HCl extended release) [package insert] Takeda Pharmaceuticals, Deerfield, IL: 2014. [Google Scholar]
- 111.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in Receipt of Buprenorphine and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001–2014. JAMA Pediatr 2017;171: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benjamin E, Buot-Smith T. Naltrexone and fluoxetine in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 1993;32: 870–873. [DOI] [PubMed] [Google Scholar]
- 113.Puri MR, Sahl R, Ogden S, Malik S. Prader-Willi Syndrome, Management of Impulsivity, and Hyperphagia in an Adolescent. J Child Adolesc Psychopharmacol 2016;26: 403–404. [DOI] [PubMed] [Google Scholar]
- 114.De Long N, Hyslop JR, Nicholson CJ, Morrison KM, Gerstein HC, Holloway AC. Postnatal metabolic and reproductive consequences of fetal and neonatal exposure to the smoking cessation drug bupropion. Reprod Sci 2013;20: 1156–1161. [DOI] [PubMed] [Google Scholar]
- 115.Meijs-Roelofs HM, Kramer P. Advancement of first ovulation by the opioid antagonist naltrexone. Biol Reprod 1989;41: 842–847. [DOI] [PubMed] [Google Scholar]
- 116.Mauras N, Veldhuis JD, Rogol AD. Role of endogenous opiates in pubertal maturation: opposing actions of naltrexone in prepubertal and late pubertal boys. J Clin Endocrinol Metab 1986;62: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 117.Kulin HE, Demers LM, Rogol AD, Veldhuis JD. The effect of long-term opiate antagonist administration to pubertal boys. J Androl 1987;8: 374–377. [DOI] [PubMed] [Google Scholar]
- 118.Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, et al. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N Engl J Med 2016;375: 240–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


