Abstract
A basic property of endothermic thermoregulation is the ability to generate heat by increasing metabolism in response to cold ambient temperatures to maintain a stable core body temperature. This process, known as cold-induced thermogenesis (CIT), has been measured in humans as early as 1780 by Antoine Lavoisier, but has found renewed interest because of the recent 'rediscovery' of thermogenic, cold-activated brown adipose tissue (BAT) in adult humans. In this review, we summarize some of the key findings of the work involving CIT over the past two centuries and highlight some of the seminal studies focused on this topic. There has been a substantial range of variability in the reported CIT in these studies, from 0 to 280% above basal metabolism. We identify and discuss several potential sources of this variability, including both methodological (measurement device, cold exposure temperature and duration) and biological (age and body composition of subject population) discrepancies. These factors should be considered when measuring CIT going forward to better assess whether BAT or other thermogenic organs are viable targets to combat chronic positive energy balance based on their relative capacities to elevate human metabolism.
INTRODUCTION
The balance between energy expenditure (EE) and energy intake ultimately determines body weight. Resting EE is the major component (50–80%) of total daily EE in an adult human.1 Thus, even small changes in resting EE, if not compensated by changes in food intake, can have long-term effects on body weight.
Resting EE can also adapt to changes in environmental temperature. In colder temperatures, resting EE increases to help maintain a stable core body temperature, serving as a source of heat production to counterbalance heat loss. This adaptive component of resting EE is defined as cold-induced thermogenesis (CIT). In small mammals (e.g., mice), CIT-based heat production is critical to maintain consistent core body temperature because their relatively high surface-area-to-volume ratio results in increased avenues for heat loss in the cold. Consequently, these animals can have large amounts of the cold-activated, thermogenic organ known as brown adipose tissue (BAT) and can increase their metabolic rate up to 4–5 times the thermoneutral resting EE at temperatures near 4 °C.2–4 In contrast, adult humans have a much lower surface-to-volume ratio and are thought to be much less reliant on CIT to maintain body temperature. Currently, we are uncertain of the magnitude of maximal human CIT and how we can safely harness it to help us combat obesity.
The aim of this review is to briefly summarize some of the previous work on CIT measurement in humans, from the earliest measurements by Lavoisier, Voit, Swift, Hardy and Winslow to the modern-day measurements primarily targeted at understanding the contribution of adult human BAT to CIT. We attempt to identify potential sources of experimental variability, such as differences in measurement devices, subject population (sex, age and body composition), degree and duration of cold exposure, clothing level and exposure medium (air, water immersion or perfused suits and blankets). Understanding these differences may inform future experimental design to further explore the physiology of human thermoregulation and CIT.
THERMAL BIOLOGY OF CIT USING ANIMAL DATA AND MODELS
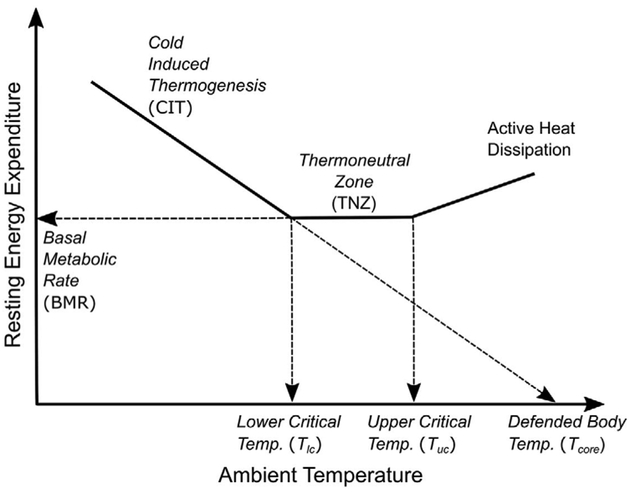
Many pioneers in the study of metabolism, including Lavoisier and Laplace (1780), Rubner (1902), Krogh (1918), Kleiber (1927), Benedict and Fox (1933), Swift and Forbes (1939) and Scholander et al. (1950), studied CIT produced by animals of various sizes and regions while gradually lowering air temperature.5 Although there have been some more recent refinements surrounding inter- and intraspecies variations in the CIT response (for instance, see Fristoe et al.6 or Fischer et al.7), the collective results of these researchers demonstrate that the framework of endothermic thermoregulation can be described by the fundamental principles of thermodynamics, that is, Newton’s law of cooling and Fourier’s law of heat flow.5 In accordance with these principles, endothermic animals are able to maintain a consistent body temperature in response to changing ambient temperatures by either altering heat conductance through physiological adaptations, such as vasoconstriction and piloerection, to reduce heat loss from the body surface or by increasing heat production (EE). The temperature range at which conductance altering mechanisms alone are sufficient to maintain a minimal and consistent level of heat production to balance heat loss is called the thermal neutral zone. This minimal EE within the thermal neutral zone is known as the basal metabolic rate (BMR). In ambient temperatures below a lower critical temperature (Tlc), these responses cannot sufficiently compensate for the increased heat loss and additional EE (i.e., CIT) is needed to prevent core body temperature from falling. As shown in Figure 1, the gradual increase in CIT with decreasing ambient temperatures reflects the effort to maintain net heat balance in the animal. Interspecies differences in insulation level (fur or hair), body size and physiology result in variations in the thermoneutral ambient temperature range, the basal metabolic rate and the rate of the CIT increase.6,8
Figure 1.
Conceptual schematic of endothermic thermoregulation.
CIT RESPONSE IN HUMANS
As early as 1780, physiologists have applied the methodologies of direct and indirect calorimetry to measure CIT in humans (Table 1).
Table 1.
A brief summary of CIT measured by room calorimetry (metabolic) chambersa
| CIT (avg % increase) | Subjects | Cold exposure conditions | Exposure medium |
Temperature range (°C) |
Insulation (Clo) | Year | Authors |
|---|---|---|---|---|---|---|---|
| 36b | 1 'strong' man | 6 h sitting | Air | 4.4–30c | NS | 1878 | Voit10 |
| 11 | 21 men and women | 1.2 h supine | Air | 2, 24 | ~ 1.0 | 1932 | Swift11,12 |
| 0 (no consistent trends) | 2 men (thin man and fat man) | 2.5 h semireclined resting | Air | 16.6–33.1c | 0 (only 'atheletic supporter') | 1936 | Winslow et al.52,53 |
| 0 | 2 men | 2.5 h supine | Air | 22.5–35c | 0 | 1937 | Hardy and Dubois13 |
| 0 | 2 men | Semireclined resting | Air | 6.8–35.5c | 0 | 1937 | Winslow et al.14 |
| 7.5 for 'responders' | 13 women | 2.5 h supine | Air | 22–35c | 0 | 1952 | Dubois et al.15 |
| 7 | 9 women | 30 h daily living | Air | 22, 28 | 0.6 | 1981 | Dauncey54 |
| 5 | 4 men, 6 women; young | 24 h standardized activity | Air | 20, 28 | ~ 0.23 (28 °C, daytime); more at nighttime, more in 20 °C) | 1990 | Warwick and Busby28 |
| 8.5 | 8 normal weight females | 48 h daily living | Air | 22, 27 | NS | 2001 | van Marken Lichtenbelt et al.31 |
| 5 | 9 men | 24 h daily living | Air | 16, 22 | >0.71 | 2002 | van Marken Lichtenbelt et al.55 |
| 5.7 | 9 lean young men | 60 h daily living | Air | 16, 22 | 1.2 daytime 7.0 nighttime | 2002 | Westerterp-Plantenga et al.56 |
| 8 | 10 men | 60 h daily living | Air | 16, 22 | >0.71 | 2002 | Schrauwen et al.57 |
| 10.0 | 8 lean young women | 48 h daily living | Air | 22, 27 | 0.6 daytime, 7.0 nighttime | 2002 | Westerterp-Plantenga et al.58 |
| 5.1 | 13 lean men | 36 h at 22 °C, 84 h at 16°C, daily living | Air | 16, 22 | NS ('standardized clothing') | 2007 | Wijers et al.59 |
| 2.8 | 11 lean men | 34 h at 22 °C, 82 h at 16 °C, daily living | Air | 16, 22 | NS ('standardized clothing') | 2008 | Wijers et al.60 |
| 13.7 for lean, 17.2 for obese | 10 lean, 14 overweight/obese young men | 1 h at 22 °C, 2 h at 16 °C | Air | 16, 22 | 0.49 | 2009 | van Marken Lichtenbelt et al.38 |
| 1 for group, 2.2 for lean, 0 for obese | 9 lean, 10 obese men | 36 h at 22 °C, 48 h at 16 °C, daily living | Air | 16, 22 | 0.8 daytime 7.0 nighttime | 2010 | Wijers et al.30 |
| 5.9 | 15 men, 10 women | 12 h daytime, sitting | Air | 19, 24 | 0.55 | 2010 | Celi et al.61 |
| 3.5 | 10 lean men | 24 h daily living | Air | 16, 22 | 0.8 | 2011 | Wijers et al.62 |
| 5.3 | 14 men, 10 women | 12 h night sleeping | Air | 19, 24 | 0.65 | 2013 | Chen et al.40 |
| 6 | 5 men | 24 h daily living | Air | 19, 24 | 0.55 | 2014 | Lee et al.47 |
Abbreviations: CIT, cold-induced thermogenesis; NS, not stated.
In healthy human subjects exposed to cold vs warm room air temperatures in a laboratory setting.
Estimated from a figure.
Multiple temperature points measured over the range.
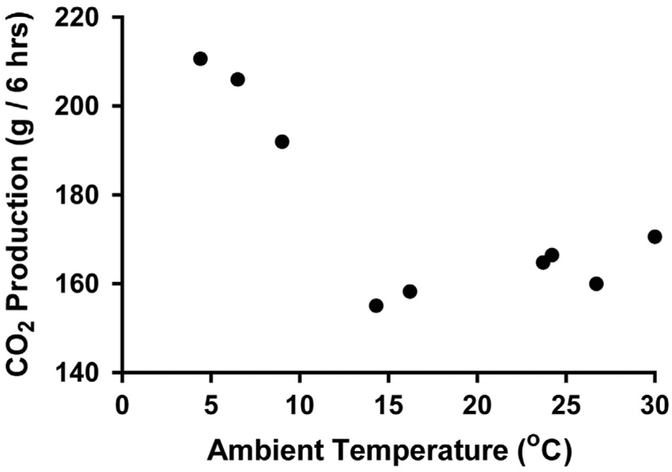
Lusk9 highlighted the earliest known work by Lavoisier who discovered that a resting man absorbed 17% more oxygen at 12 °C than at 26 °C, although the details of the study are not well documented. Nearly a century later, Voit10 carefully described a series of experiments measuring the carbon dioxide (CO2) production of a healthy male volunteer (71 kg) in temperatures ranging from warm (30 °C) to cold (4.4 °C) in the Pettenkofer respiration chamber (or as he called it the 'big Pettenkofer Respiration box'). The measurements took place on different days during a German winter (between 27 January and 24 February 1876), and the 'dedicated' subject entered at 1100 hours under fasted condition and sat in an armchair without movement (resting EE) for 6 h. The results showed that the CO2 production did not vary significantly at ambient temperatures above 16.2 °C, but increased by up to 36% in the cold, which could not be explained by movement (Figure 2).
Figure 2.
Measurements of CO2 production from one subject exposed to different ambient temperatures performed by Voit using the Pettenkofer respiration chamber in 1876.10
Swift11,12 tried to quantify the role of shivering in CIT by studying 21 young subjects in a 2 °C refrigerator for 75 min. He found that in the absence of shivering, the cold condition increased heat production by ~ 11% compared with the warm basal condition (23–25 °C). However, when intense shivering occurred, the metabolism increased to ~ 400% of the BMR.
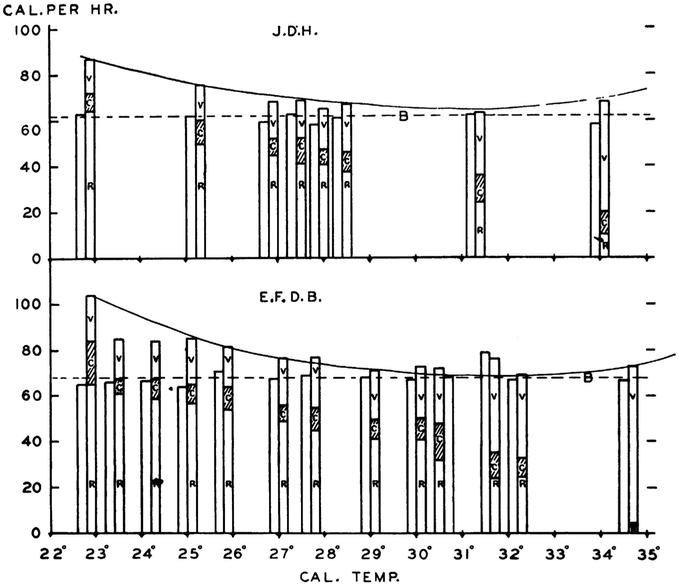
In perhaps the most classical study, Hardy and Dubois13 measured two healthy subjects (themselves) in a direct calorimetry chamber by determining the three components of heat loss: radiative, conductive/convective and evaporative under strictly controlled conditions (naked, fasted, supine, motionless and awake) for 2–3 h/day during which the air temperature was set from 22.5 to 35 °C (Figure 3). A U-shaped curve was observed in heat loss vs environmental temperature, from which they observed a minimal zone of net heat loss (similar to thermal neutral zone) of 29–33 °C. The detailed body temperature measurements collected in this study demonstrated that, while skin temperatures fell during cold exposure, decreasing much more markedly in peripheral locations compared with the central skin sites, the core (rectal) temperature was highly conserved (< 1 °C change) throughout the tested ambient temperature range. Interestingly, heat production (EE), measured using oxygen consumption and CO2 production, showed little to no change over this temperature range in these two male subjects, despite reports that shivering became uncontrollable during some of the extreme cold conditions.
Figure 3.
Measurements of heat loss and production in two subjects exposed to different ambient temperatures performed by Hardy and Dubois13 using the Russell Sage calorimeter in 1937. Figure taken from JD Hardy and EF Dubois, 1937.
Winslow et al.14 studied one 'slender' and one 'stout' healthy volunteer in a wider environmental air temperature range (6.8–35.5 °C), and found similar U-shaped curves for heat loss as Hardy and Dubois. They further observed that the slender subject experienced an increase in metabolism (CIT) only when his core temperature fell below 36 °C, but the variability appeared high. The stout subject maintained core temperature and had no CIT response.
In a follow-up to their 1937 study, Dubois et al.15 used a similar experimental design and recorded serial metabolic measurements of 13 healthy young women (age 25.9 ± 6.1 years, body mass index 21.4 ± 2.8 mg/kg2) in temperatures from 22 to 36 °C. The authors noted that 23 of the 32 measurements in the 'cold zone' (22–26.9 °C) resulted in increased metabolism compared with the 'comfort zone', presumably between 27–32 °C, which was deemed statistically significant. However, the average 'cold zone' increase was small and the 'the causes [were] still uncertain'. Great individual variability was also observed in these studies, with some subjects (60%) showing robust CIT and hot-induced thermogenesis while others exhibiting no discernible change in metabolism. According to the authors, the CIT was 'greater than the amount that could be ascribed to muscle tension or restlessness'.
A subsequent study by Buskirk et al.16 used indirect calorimetry to measure the CIT of four fasted, healthy, lean, young male volunteers for ~ 3 h in more extreme temperature conditions (26.6 °C vs 10.0 °C). They reported a much more marked increase in metabolism of 55% (32–91%), but noted that this included both body motion and shivering.
Using whole-room indirect calorimetry chambers (respiration, metabolic), more recent studies have recorded variable, but generally more modest, magnitudes of CIT (3–7%) when ambient air temperature ranges are close to normal indoor living conditions (Table 1). Other investigators used indirect calorimetry (metabolic) carts and other exposure media (e.g. water-immersion or water-perfused suits) for CIT measurements over shorter periods and have yielded relatively higher magnitudes (10–280%, Table 2).
Table 2.
A brief summary of CIT measured by portable metabolic devices (metabolic carts or Douglas bag)a
| CIT (avg % increase) | Subjects | Cold exposure conditions | Exposure medium |
Temperature range (°C) | Insulation (Clo) | Year | Authors |
|---|---|---|---|---|---|---|---|
| 55 | 4 men | 3 h sitting | Air | 10, 26.6 | 0.08 | 1960 | Buskirk et al.16 |
| ~67% (summer), ~45% (winter) (non-acclimated)b | 10 men (summer), 6 men (winter) | 2 h supine | Air | 11.8, ? | 0 | 1961 | Davis32 |
| 13.1 (eskimos), 0 (non-eskimos) | 6 eskimos, 6 non-eskimos | 3 h seated | Air | 23–35c | ~ 0.08 | 1962 | Rennie et al.63 |
| ~ 280b | 9 men (measurements taken from 20, only measurements from 9 are reported) | 2 h supine | Air | 1, ? (thermal neutrality with blanket) | 0 | 1992 | Vallerand et al.64 |
| ~ 68b | 8 men | 4 h sitting | Air | 10.0–22 | 0.1 | 1998 | Young et al.22 |
| 11.5 winter, 7.0 summer | 10 women, 10 men, young, BMI 17–32 kg/m2 | 1 h at 22 °C, 45 min of cooling, 3 h at 15 °C, semisupine | Air | 15, 22 | 0.71 | 2004 | van Ooijen et al.65 |
| 11.8 for group, 17.2 for lean, 6.4 for overweight | 10 lean, 10 overweight young men | 1 h at 15 °C+duvet, 1 h at 15 °C – duvet | Air | 15 (+duvet = warm/− duvet = cold) | 0.71, duvet = 0.68 | 2006 | Claessens-van Ooijen et al.29 |
| 17 | 10 men | 2 h supine | Air | Individualized to just above shivering, 23–25 | 0.49 | 2012 | Vosselman et al.66 |
| 13 | 6 men | 2 h supine | Air | Individualized to just above shivering, 23–25 | 0.49 | 2013 | Vosselman et al.67 |
| 11.8 for untrained, 8.0 for trained | 12 endurance-trained and 12 untrained | 2 h supine | Air | Individualized to just above shivering, 23–25 | 0.19 | 2015 | Vosselman et al.68 |
| 10.9 | 36 lean young men | 1.5 h semisupine | Air | 15.8 ± 1.9, 25.9 ± 1.6 | 0.36–0.42 | 2016 | van der Lans et al.69 |
| 28 for BAT+, 2.9 for BAT − | 13 young men (6 BAT+/7 BAT − ) | 2 h seated | Air +intermittent ice block | 19+intermittent ice block, 27 | 0.21 | 2011 | Yoneshiro et al.70 |
| 18 for BAT+, 5.2 for BAT − | 27 young men (27 BAT+/24 BAT − ) | 2 h seated | Air +intermittent ice block | 19+intermittent ice block, 27 | 'Light clothing' | 2013 | Yoneshiro et al.49 |
| 5.5 | 4 men, 6 women | 2 h reclining | Air+water-perfused vest | 20 (room)+14 (circulated water), 23 (room) | 0.44 | 2012 | Cypess et al.71 |
| 8 | 12 men (BAT+) | 2 h reclining | Air+water-perfused vest | 20 (room)+14 (circulated water), 23 (room) | 0.44 | 2015 | Cypess et al.72 |
| 91 for lean, 26 for overweightb | 6 normal weight, 2 overweight/obese young men | ≤ 2.5 h seated, submerged to neck | Water immersion | 8–38c (individualized low temperature) | ~ 0.04 (immersed) +fleece-lined helmet | 1960 | Cannon and Keatinge21 |
| 5.7 (eskimos), − 2.6 (non-eskimos) | 5 eskimos, 5 non-eskimos | 1 h immersed except for faces | Water immersion | 33, 35 | NS | 1962 | Rennie et al.63 |
| Up to 70d | 10 men | < 1 h, water immersion | Water immersion | 24–38c | 0.08 | 1966 | Craig and Dvorak19 |
| 16–50 | 10 men | 2 h | Water-perfused blankets | 12–27c | 0.55 | 2014 | Lee et al.73 |
| 77 | 6 men | 2.5 h supine | Water-perfused suit | 18, 25 | NS | 2012 | Ouellet et al.41 |
| 11.0 for women, 11.6 for men | 9 women, 8 men, lean young | 50 min cooling+30 min non-shivering cold | Water-perfused suit | 25.4 ± 1.8 (personalized), ? ('thermoneutral') | NS | 2013 | van der Lans et al.48 |
| 67–83 | 25 men | 3 h | Water-perfused suit | −4 (skin temp), 24 (warm) | 0.08 | 2015 | Blondin et al.42 |
Abbreviations: BAT, brown adipose tissue; BMI, body mass index; CIT, cold-induced thermogenesis; NS, not stated.
In healthy human subjects exposed to cold vs warm temperatures in a laboratory setting.
Estimated from a figure.
Multiple temperature points measured over the range.
THE POTENTIAL CAUSES FOR THE WIDE VARIABILITY OF CIT RESPONSE IN HUMANS
Although individual variability in the CIT is evident and noteworthy in almost all studies on this subject, there is also considerable interstudy variation as well (e.g. Tables 1 and 2). Both methodological and biological factors may be responsible for these discrepancies. In the following section, we consider several potential sources of this variability from both of these categories.
Method of metabolic measurement
Room calorimeters have been used for more than a century to measure heat loss directly and/or heat production indirectly (through oxygen consumption and carbon dioxide production) in an environment where ambient temperature can be tightly controlled. Early experiments tended to use direct+indirect calorimeters and measured heat loss and production simultaneously.13,14 However, early calorimeters typically consisted of many custom-made components and required a team of trained operators to accomplish one experiment. This made experiments conducted with these devices quite complex, with high potential for noise, interdevice variation and operator error. Most current whole-room devices are exclusively indirect calorimeters and take advantage of modern, industrially produced gas analyzers to measure subject-generated changes in room air composition. Although these modern indirect calorimeters are incapable of directly assessing heat loss, their reported precision is 2–3% for ~ 24 h of measurement of EE at normal operating temperatures.17 By performing a series of propane combustion experiments in ambient temperatures from 16–32 °C, we have validated that the accuracy of our three indirect room calorimeters (100.0 ± 1.0% EE recovered) is not affected by ambient temperature, provided that the temperature inside and surrounding the room calorimeter is stable. Indirect calorimetry (metabolic) carts have been as an alternative to room calorimeters for shorter duration assessments of CIT because of their wider availability. They have higher sensitivity than room calorimeters because of less dead-space volume. Measurements with these devices can also be performed in close proximity to the subject, allowing the operator to better observe and potentially minimize the effects of shivering and movement. Both of these attributes may be advantageous to detect small CIT responses. However, these devices increase subject burden and potential for discomfort and thus are not recommended for longer duration measurements. The acute nature of these measurements may not allow subjects to achieve a steady-state metabolism, and transient responses may increase intra- and intersubject variability. The impact of colder ambient temperatures on the validity of metabolic carts is also unknown.
Temperature range of cold and warm exposure
The range of temperatures used to assess human CIT represents another source of variability. As CIT is defined as the change in resting EE measured at cold and thermoneutral temperatures, its magnitude can be influenced by the temperatures chosen for both cold and baseline (i.e., thermoneutral) conditions. Although the lower critical temperature (Tlc) has not been rigorously determined in humans, based on currently available data it is generally thought to occur at ambient temperatures > 24°C and could vary between individuals. A baseline EE measured below the Tlc (perhaps 22 or 24 °C) could be higher than the true BMR which would result in a falsely low CIT response. Similarly, choosing a lower fixed cold temperature (e.g., 16 °C vs 22 °C) could influence the magnitude of observed CIT, particularly when only two temperature conditions are measured. Some researchers attempt to minimize the possibility of underestimating CIT by using a personalized cooling protocol in which the subject is gradually cooled to the level of overt shivering and then the temperature is slowly increased until shivering stops. The resultant temperature is said to be the lowest non-shivering temperature and is thought to maximize individual non-shivering thermogenesis. The fixed temperature method may be more suitable for comparing CIT responses in a homogeneous subject population exposed to standardized environmental condition, whereas the personalized approach may be favorable for comparing responses in heterogeneous populations or for longitudinal intervention studies that can alter cold tolerance.18
Cold exposure medium
Researchers have applied cold using a variety of different methods to study the human CIT response, including cold air exposure, water immersion, intravenous water infusion, various water-perfused garments and intermittent localized contact with ice blocks. The physical properties of these different media may contribute to the variability of CIT observed in the literature. For example, the thermal conductivity of water is nearly 25 times that of air, thus the rate of heat loss from the body is much greater in cold water compared with that in cold air of the same temperature.19,20 One result of the difference in thermal conductivity is a shift in the reported thermoneutral zone temperatures for water immersion vs air cooling. In air, the Tlc is thought to be near 22–24 °C, but for water a Tlc of 31–33 °C is usually reported, with observable shivering commonly occurring below this temperature range.19,21 The duration of the cold exposure in each medium may also have a role in reported CIT values. Time-dependent increases in CIT have been reported in some studies using both air-based15,22 and water-based19,21 cold exposure. However, these temporal trends in CIT and heat loss appear to follow Fourier’s law of cooling, with more striking increases occurring in water,19,21 because of its high thermal conductivity, and in colder temperatures.15,19,21,22
Shivering
Increasing voluntary movement can be an effective way to keep warm in cold temperatures as a result of the additional heat created by large groups of skeletal muscles contracting. However, physical activity is typically restricted in studies of CIT. On the other hand, it is difficult to eliminate the involuntary tensing of the muscles without motion (isometric contractions) which define shivering. Shivering is a common defense mechanism against cold that can sharply increase heat production, raising the metabolism up to five times the BMR at peak intensity.23 Although voluntary movement can be observed and objectively measured, shivering and muscle tightening is much harder to quantify. Most studies of CIT attempt to measure the non-shivering component of cold thermogenesis by choosing temperatures that do not elicit visible or self-reported shivering. However, direct observation and subjective shiver reporting can be unreliable. Alternatively, electromyographic (EMG) techniques have been used to record objectively the electrical activity of muscles during shivering. In some cases, indwelling EMG sensors are placed via fine-wire needles directly into the muscle fibers of animals,24 although newer surface-based EMG methods are much more practical and less invasive for human studies. Surface EMG, however, cannot measure the activity of deeper muscles that have been found to be active in cold (e.g., scalenes, psoas, Longus colli).25 Moreover, to compare the activation level of different muscle groups within an individual or the shivering intensity of the same muscle group between individuals, EMG signals must be carefully normalized to either standardized resting, non-shivering conditions or maximal isometric voluntary contractions.26
Clothing level
Fur or fleece thickness in various animal species has been shown to have a marked influence on the CIT, with thicker fur (higher insulation) resulting in a lower Tlc and less marked CIT slope.7,27 Differing amounts of permitted clothing or bedding represent an analogous source of insulation variability seen in studies of human CIT. The level of thermal insulation provided by various garments is commonly quantified in Clo, where zero Clo corresponds to a naked person and one Clo corresponds to a person wearing a typical business suit. Most participants in studies performed before the 1970s typically were naked or wore very limited clothing. However, such conditions are rarely deemed practical or ethical by current research standards. As such, clothing conditions may not always be strictly controlled (e.g., material and fit of clothes, and blankets/duvet may be used in some but not in all subjects). For example, Warwick et al.28 allowed subjects to choose the level of daytime clothing and nighttime bedding they wore in both 28 °C and 20 °C. Not surprisingly, subjects chose to wear more clothing in the cold and while sleeping, which may have lead to a smaller and more variable CIT response (5.0 ± 5.5%). In another study, Claessens-van Ooijen et al.29 used quite different experimental conditions to study the CIT response of lean and overweight men. In that study, participants wore standardized clothing of sweat shirts and pants (Clo = 0.71) and EE measurements were conducted in a 15 °C room for 1 h under what were designated as thermoneutral conditions (covered with a 0.68 Clo duvet) and during 1 h of mild cold exposure (duvet removed). While the authors observed a large CIT response in the lean subjects (17.2%), it is possible that subjects may not have reached steady-state metabolism in both conditions because of an unusual testing conditions and the short duration of the measurements.
Adiposity
Based largely on earlier studies of CIT in humans, higher body fat content is thought to provide increased insulation and have a blunting effect on CIT, similar to external insulators. The work Hardy and Dubious,13 Winslow et al.14 and Cannon and Keatinge21 seem to lend support to this premise, although the number of overweight or obese participants was rather low in all cases (≤2 subjects). More recent studies comparing lean and overweight/obese CIT response have reported mixed results. In the previously mentioned study by Claessens-van Ooijen et al.,29 overweight subjects showed a blunted CIT response to a 'mild cold' (15 °C air) for 60 min as compared with lean subjects (6.4% vs 17.2%, P = 0.04). However, longer duration studies have shown less striking differences (2.2% for lean vs 0% for obese subjects for >24 h at 22 °C and 16 °C)30 and even opposite trends (13.7% for lean vs 17.2% for obese subjects for 1 h at 22 °C and 2 h at 16 °C)31 in CIT. Recent data in mice demonstrated that the insulation level of obese animals, quantified as the inverse of the CIT slope, is similar to lean counterparts, suggesting that greater adiposity and more abundant external insulation (fur) may have differential influences on CIT.7
Cold tolerance and brown adipose tissue
Acclimation to cold via chronic cold exposure may also impact the CIT response. In an early landmark study, Davis32 conducted weekly measurements of the CIT of 10 young men over 31 days in the summer months while they were being cold acclimatized by remaining sedentary in 12 °C room wearing only shorts for 8 h/ day. He found that the subjects’ CIT response decreased monotonically for the first 21 days, from ~ 55 to ~ 25% above BMR, where it plateaued. At the same time, EMG-measured shivering also decreased substantially, decreasing ~ 80% from day 0 levels, but was never completely abolished. When the study was repeated with a separate group of six subjects in the winter months, the initial (day 0) CIT response was lower but plateaued at nearly the same level, which suggested to the authors that natural seasonal acclimatization can occur. This standardized cold acclimation protocol supported previous reports from less standardized field studies conducted in different populations during which metabolic adaptation and shiver reduction occurred following repeated exposure to cold outdoor temperatures.33–35 These early studies demonstrated that cold metabolic acclimation was possible but, as noted by Davis,32 the mechanism for these changes remained unclear.
With the recent 'rediscovery' of BAT in adult humans via positron emission tomography-computed tomography scanning,36–39 interest has been building about its metabolic potential, its role in CIT and its contribution to cold acclimatization in humans. Initial cros-ssectional association studies between CIT and human BAT demonstrated mixed results. Several investigators have identified a correlation between CIT and BAT volume or activity,40,41 while others provided evidence that skeletal muscle is more predominant in CIT.42,43 Currently, we cannot definitively delineate the relative contributions of BAT and skeletal muscle to whole-body human CIT because of the challenges of measuring their separate metabolic activity in vivo.18,44 However, several more recent studies with longitudinal designs have shown that BAT accumulates, and CIT measured during non-shivering cold challenges tends to increase following cold acclimatization.25,45–49 Lee et al.47 showed a small increase of BAT abundance in young men following 1 month of mild cold sleeping conditions (19 °C, hospital scrubs and sheet, ~ 8 h/day), but this was not accompanied by a change in CIT during a 24 h stay in a warm and cold respiration chamber. Using a lower temperature (17 °C wearing light clothing) but shorter daily exposure (2 h/day), Yoneshiro et al.49 saw a positively correlated increase in BAT activity (58% increase from week 0) and CIT of a mild cold challenge (19 °C, 268% increase from week 0) after 6 weeks of acclimation. Similarly, van der Lans et al.48 also showed a related, but less substantial increase in BAT(17% from week 0) and CIT (7% from week 0) using a shorter duration acclimation (10 days) with longer daily bouts of cold exposure (15–16 °C, 6 h/day wearing T-shirt and shorts) and a cold challenge involving the previously described personalized, maximal non-shivering cooling protocol. Using this cold acclimation protocol and CIT-testing procedure, the authors observed similar trends in BAT and CIT in obese participants46 and subject with type 2 diabetes.45 These studies suggest that both BAT activity and CIT are modifiable and may both be influenced by the degree and duration of repeated cold exposure. It is worth noting that while the daily exposures of these studies were less severe than those of the Davis study, the cold-challenge temperatures were warmer and not meant to elicit shivering, which may have contributed to their differential results in the CIT pattern before and after cold acclimation.
Age
Although older subjects have been shown to have reduced BAT volume in cross-sectional analyses,36,50 to our knowledge, there is limited CIT data comparing young vs old subjects using air cooling. Using a 30- min 4 °C saline intravenous infusion method of cooling, Frank et al.51 showed that older men (mean of 63 years) had a severely blunted thermoregulatory responses as compared with younger men (mean age of 21 years), including lower oxygen consumption rates (5.9 ± 0.6 vs 8.1 ± 0.5 ml/kg lean body mass/min, P = 0.05), reduced vasoconstriction and plasma norepinephrine responses. Core temperature also decreased more significantly in older vs younger subjects (P = 0.001).
SUMMARY
The recent interests in the area of CIT stem from its potential as a target for obesity prevention or treatment. We conducted a non-exhaustive review of 40+ clinical studies that primarily focused on the metabolic response to mild cold, to minimize the stress of overt shivering, CIT responses ranged from 0% to up to 90% above the 'warm' baseline levels. In general, being exposed to colder temperatures, over longer durations, in water (as opposed to air), wearing less clothing, and being younger, leaner and more cold acclimated can result in a higher relative CIT response. Studies using metabolic carts to measure cold-induced changes in metabolism over shorter durations tend to identify higher CIT responses compared with those using room calorimeters to measure more than 12 h. This trend may be the result of the metabolic cart capturing larger, transient increases in CIT that would remit if given more time or from confounding factors of the near-free-living conditions of the metabolic chamber such as physical activity, sleep and thermic effect of food. Less stringent thermal neutral conditions at baseline and individualized cooling protocols can also impact the variability in CIT responses across subjects. Shivering, although difficult to quantify, can also have a critical role in CIT. Moreover, most clinical research in this area has been performed on young, healthy, lean subjects, and on men more often than on women. Typically, very few subjects and very few temperature points (generally one warm and one cold) are measured per study. Thus, despite more than two centuries of effort to understand the mechanisms of CIT, we are still somewhat 'left out in the cold'.
ACKNOWLEDGEMENTS
This work was funded by NIH Intramural research funding resources (NIDDK and Clinical Center).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Snitker S, Macdonald I, Ravussin E, Astrup A. The sympathetic nervous system and obesity: role in aetiology and treatment. Obes Rev 2000; 1: 5–15. [DOI] [PubMed] [Google Scholar]
- 2.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab 2015; 4: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 2011; 214: 242–253. [DOI] [PubMed] [Google Scholar]
- 4.Gordon CJ. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 2012; 37: 654–685. [Google Scholar]
- 5.Kleiber M The Fire of Life: An Introduction to Animal Energetics. Wiley: New York, NY, USA, 1961, pp 105–171. [Google Scholar]
- 6.Fristoe TS, Burger JR, Balk MA, Khaliq I, Hof C, Brown JH. Metabolic heat production and thermal conductance are mass-independent adaptations to thermal environment in birds and mammals. Proc Natl Acad Sci USA 2015; 112: 15934–15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer AW, Csikasz RI, von Essen G, Cannon B, Nedergaard J. No insulating effect of obesity. Am J Physiol Endocrinol Metab 2016; 311: E202–E213. [DOI] [PubMed] [Google Scholar]
- 8.Scholander PF, Hock R, Walters V, Johnson F, Irving L. Heat regulation in some arctic and tropical mammals and birds. Biol Bull 1950; 99: 237–258. [DOI] [PubMed] [Google Scholar]
- 9.Lusk G The Elements of the Science of Nutrition, 4th edn Academic Press: New York, NY, USA, 1976, pp 18–19. [Google Scholar]
- 10.Voit C Ueber die Wirkung der Temperatur der umgebenden Luft auf die Zersetsung im Organismus der Warmblüter. Z Biol 1878; 14: 57–160. [Google Scholar]
- 11.Swift RW. The effects of low environmental temperature upon metabolism II. The influence of shivering, subcutaneous fat, and skin temperature on heat production. J Nutr 1932; 5: 227–249. [Google Scholar]
- 12.Swift RW. The effects of low environmental temperature upon metabolism I. Technic and respiratory quotient. J Nutr 1932; 5: 213–225. [Google Scholar]
- 13.Hardy JD, Dubois EF. Regulation of heat loss from the human body. Proc Natl Acad Sci USA 1937; 23: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winslow CEA, Herrington LP, Gagge AP. Physiological reactions of the human body to varying environmental temperatures. Am J Physiol 1937; 120: 1–22. [Google Scholar]
- 15.Dubois EF, Ebaugh FG Jr, Hardy JD. Basal heat production and elimination of thirteen normal women at temperatures from 22 degrees C. to 35 degrees C. J Nutr 1952; 48: 257–293. [DOI] [PubMed] [Google Scholar]
- 16.Buskirk ER, Thompson RH, Moore R, Whedon GD. Human energy expenditure studies in the National Institute of Arthritis and Metabolic Diseases metabolic chamber.1. Interaction of cold environment and spcific dynamic effect. 2. Sleep. Am J Clin Nutr 1960; 8: 602–613. [Google Scholar]
- 17.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr Opin Clin Nutr Metab Care 2004; 7: 599–605. [DOI] [PubMed] [Google Scholar]
- 18.Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab 2016; 24: 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AB Jr, Dvorak M. Thermal regulation during water immersion. J Appl Physiol 1966; 21: 1577–1585. [DOI] [PubMed] [Google Scholar]
- 20.Young A, Sawka M, Pandolf K. The Physiology of Cold Exposure In: Marriot BM, Carlson SJ (eds). Nutritional Needs in Cold and High-Altitude Environments: Applications for Military Personnel in Field Operations, Chapter 7. National Academy Press: Washington, DC, USA, 1996, pp 127–148. [PubMed] [Google Scholar]
- 21.Cannon P, Keatinge WR. The metabolic rate and heat loss of fat and thin men in heat balance in cold and warm water. J Physiol 1960; 154: 329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young AJ, Castellani JW, O'Brien C, Shippee RL, Tikuisis P, Meyer LG et al. Exertional fatigue, sleep loss, and negative energy balance increase susceptibility to hypothermia. J Appl Physiol (1985) 1998; 85: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 23.Haman F Shivering in the cold: from mechanisms of fuel selection to survival. J Appl Physiol (1985) 2006; 100: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 24.Kilgour RD, Williams PA. Effects of diabetes and food deprivation on shivering activity during progressive hypothermia in the rat. Comp Biochem Physiol A 1996; 114: 159–165. [DOI] [PubMed] [Google Scholar]
- 25.Blondin DP, Labbé SM, Tingelstad HC, Noll C, Kunach M, Phoenix S et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab 2014; 99: E438–E446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tikuisis P, Bell DG, Jacobs I. Shivering onset, metabolic response, and convective heat transfer during cold air exposure. J Appl Physiol (1985) 1991; 70: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 27.Blaxter K Energy Metabolism in Animals and Man, vol. 110 Cambridge University Press: Cambridge, MA, USA, 1989. [Google Scholar]
- 28.Warwick PM, Busby R. Influence of mild cold on 24 h energy expenditure in 'normally' clothed adults. Br J Nutr 1990; 63: 481–488. [DOI] [PubMed] [Google Scholar]
- 29.Claessens-van Ooijen AM, Westerterp KR, Wouters L, Schoffelen PF, van Steenhoven AA, van Marken Lichtenbelt WD et al. Heat production and body temperature during cooling and rewarming in overweight and lean men. Obesity (Silver Spring) 2006; 14: 1914–1920. [DOI] [PubMed] [Google Scholar]
- 30.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Cold-induced adaptive thermogenesis in lean and obese. Obesity (Silver Spring) 2010; 18: 1092–1099. [DOI] [PubMed] [Google Scholar]
- 31.van Marken Lichtenbelt WD, Westerterp-Plantenga MS, van Hoydonck P. Individual variation in the relation between body temperature and energy expenditure in response to elevated ambient temperature. Physiol Behav 2001; 73: 235–242. [DOI] [PubMed] [Google Scholar]
- 32.Davis TR. Chamber cold acclimatization in man. J Appl Physiol 1961; 16: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 33.Irving L Human adaptation to cold. Nature 1960; 185: 572–574. [DOI] [PubMed] [Google Scholar]
- 34.Scholander PF, Hammel HT, Andersen KL, Loyning Y. Metabolic acclimation to cold in man. J Appl Physiol 1958; 12: 1–8. [DOI] [PubMed] [Google Scholar]
- 35.Scholander PF, Hammel HT, Hart JS, Lemessurier DH, Steen J. Cold adaptation in Australian aborigines. J Appl Physiol 1958; 13: 211–218. [DOI] [PubMed] [Google Scholar]
- 36.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 39.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 40.Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab 2013; 98: E1218–E1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012; 122: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blondin DP, Labbé SM, Noll C, Kunach M, Phoenix S, Guérin B et al. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 2015; 64: 2388–2397. [DOI] [PubMed] [Google Scholar]
- 43.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG et al. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 2013; 54: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulloo AG. Translational issues in targeting brown adipose tissue thermogenesis for human obesity management. Ann NY Acad Sci 2013; 1302: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015; 21: 863–865. [DOI] [PubMed] [Google Scholar]
- 46.Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G et al. Shortterm cold acclimation recruits brown adipose tissue in obese humans. Diabetes 2016; 65: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 47.Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 2014; 63: 3686–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013; 123: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013; 123: 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011; 19: 1755–1760. [DOI] [PubMed] [Google Scholar]
- 51.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol 2000; 279: R349–R354. [DOI] [PubMed] [Google Scholar]
- 52.Winslow CEA, Herrington LP, Gagge AP. The determination of radiation and convection exchanges by partitional calorimetry. Am J Physiol 1936; 116: 669–684. [Google Scholar]
- 53.Winslow CEA, Herrington LP, Gagge AP. A new method of partitional calorimetry. Am J Physiol 1936; 116: 641–655. [Google Scholar]
- 54.Dauncey MJ. Influence of mild cold on 24 h energy expenditure, resting metabolism and diet-induced thermogenesis. Br J Nutr 1981; 45: 257–267. [DOI] [PubMed] [Google Scholar]
- 55.van Marken Lichtenbelt WD, Schrauwen P, van De Kerckhove S, Westerterp-Plantenga MS. Individual variation in body temperature and energy expenditure in response to mild cold. Am J Physiol Endocrinol Metab 2002; 282: E1077–E1083. [DOI] [PubMed] [Google Scholar]
- 56.Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Strobbe H, Schrauwen P. Energy metabolism in humans at a lowered ambient temperature. Eur J Clin Nutr 2002; 56: 288–296. [DOI] [PubMed] [Google Scholar]
- 57.Schrauwen P, Westerterp-Plantenga MS, Kornips E, Schaart G, van Marken Lichtenbelt WD The effect of mild cold exposure on UCP3 mRNA expression and UCP3 protein content in humans. Int J Obes Relat Metab Disord 2002; 26: 450–457. [DOI] [PubMed] [Google Scholar]
- 58.Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Cilissen C, Top S. Energy metabolism in women during short exposure to the thermoneutral zone. Physiol Behav 2002; 75: 227–235. [DOI] [PubMed] [Google Scholar]
- 59.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab 2007; 92: 4299–4305. [DOI] [PubMed] [Google Scholar]
- 60.Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One 2008; 3: e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Celi FS, Brychta RJ, Linderman JD, Butler PW, Alberobello AT, Smith S et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol 2010; 163: 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab 2011; 96: E598–E605. [DOI] [PubMed] [Google Scholar]
- 63.Rennie DW, Covino BG, Blair MR, Rodahl K. Physical regulation of temperature in Eskimos. J Appl Physiol 1962; 17: 326–332. [DOI] [PubMed] [Google Scholar]
- 64.Vallerand AL, Savourey G, Bittel JH. Determination of heat debt in the cold: partitional calorimetry vs. conventional methods. J Appl Physiol (1985) 1992; 72: 1380–1385. [DOI] [PubMed] [Google Scholar]
- 65.van Ooijen AM, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav 2004; 82: 545–553. [DOI] [PubMed] [Google Scholar]
- 66.Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P et al. Systemic beta-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 2012; 61: 3106–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM et al. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr 2013; 98: 57–64. [DOI] [PubMed] [Google Scholar]
- 68.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes (Lond) 2015; 39: 1696–1702. [DOI] [PubMed] [Google Scholar]
- 69.van der Lans AA, Vosselman MJ, Hanssen MJ, Brans B, van Marken Lichtenbelt WD. Supraclavicular skin temperature and BAT activity in lean healthy adults. J Physiol Sci 2016; 66: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011; 19: 13–16. [DOI] [PubMed] [Google Scholar]
- 71.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA 2012; 109: 10001–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cypess AM, Weiner LS, Roberts-Toler C, Franquet-Elia E, Kessler SH, Kahn PA et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 2015; 21: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 2014; 19: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]