Abstract
The efficacy and safety of stereotactic radiosurgery (SRS) in comparison with whole brain radiotherapy (WBRT) for brain metastases (BMs) remains unclear. The present study retrospectively reviewed 44 patients who received SRS or WBRT as an initial treatment for 10–20 BMs from non-small cell lung cancer between 2009 and 2016. Of the patients, 24 (54.5%) were treated with SRS and 20 (45.5%) were treated with WBRT. Overall survival (OS), time to intracranial progression (TTIP), neurological survival (NS), and prognostic factors were examined. OS did not significantly differ between the two groups: 7.3 months in the SRS group vs. 7.2 months in the WBRT group (P=0.502). Median TTIP was significantly shorter in the SRS group than in the WBRT group (7.1 vs. 19.1 months, P=0.009). In contrast, there were no significant differences in NS between the two groups (14.5 months in the SRS group vs. 12.9 months in the WBRT group, P=0.346). Univariate and multivariate analysis revealed that the type of initial treatment for BMs (WBRT or SRS) was not a significant prognostic factor (hazard ratio=0.80, 95% confidence interval: 0.42–1.52, P=0.502). However, histology, performance status, subsequent molecular targeted drugs, subsequent chemotherapy and salvage treatment were independent prognostic factors. There were no significant differences in OS and NS between treatment with SRS and treatment with WBRT in patients with 10–20 BMs, although TTIP was improved with WBRT. As an upfront treatment for 10–20 BMs, SRS may delay WBRT and the adverse events associated with WBRT.
Keywords: Gamma Knife radiosurgery, overall survival, prognostic factor, multiple brain metastases, upfront treatment
Introduction
Up to 40% of the patients diagnosed with non-small cell lung cancer (NSCLC) develop brain metastases (BMs) over the course of their disease (1,2). Patients with untreated BMs have an extremely poor prognosis, with a median survival of 1–2 months (3), although survival can be improved to a median of 4–6 months with the introduction of whole brain radiotherapy (WBRT) (4). WBRT has long been recognized as the standard treatment for patients with multiple BMs.
Recently, owing to concern regarding the side effects and neurological dysfunction associated with WBRT, increasing numbers of studies examining the effectiveness of stereotactic radiosurgery (SRS) in patients with a low number of BMs have been performed. A multi-institutional retrospective study demonstrated that patients with fewer than four BMs from NSCLC treated with SRS as the initial therapy experienced longer survival than those treated with WBRT, even after propensity score adjustment (5). In addition, a prospective observational study showed that overall survival (OS) following initial treatment with SRS alone was similar for patients with 5–10 BMs and patients with 2–4 BMs (6). SRS has been regarded as a reasonable treatment alternative for patients with as many as 10 BMs. Additionally, some retrospective studies have shown that SRS is as safe and effective for 10 or more lesions as it is for a smaller number of lesions (7–11).
However, these previous studies included various types of primary tumors, in addition to NSCLC, and none directly compared survival outcomes for SRS and WBRT as initial radiological treatments for BMs. Therefore, we conducted this retrospective study to compare the survival benefit and prevention of neurological death for SRS and WBRT in patients with advanced NSCLC with 10–20 BMs.
Materials and methods
Patient population
The present study included all patients with 10–20 BMs from NSCLC who were treated with either SRS or WBRT as the initial treatment for brain lesions at Komaki City Hospital (Komaki, Japan) between January 2009 and December 2016. All data were retrospectively obtained from electronic medical records. All patients were diagnosed with BMs by gadolinium enhanced T1-weighed magnetic resonance imaging (MRI). Patients were included in this study if they had: i) pathologically proven NSCLC; ii) 10–20 BMs treated with SRS or WBRT; iii) a life expectancy of more than 2 months according to the attending physicians or neurologists; iv) no history of prior treatment with either SRS or WBRT or surgery for BMs; v) lesions with a maximum diameter of 4 cm or less per metastasis; and vi) no apparent leptomeningeal disease. The life expectancy was mainly predicted by diagnosis-specific graded prognostic assessment (DS-GPA) score as well as Eastern Cooperative Oncology Group Performance Status (ECOG-PS) or Karnofsky Performance Status (KPS) (12) As patients DS-GPA and Lung-molGPA scores of 0–1 showed, respectively, median OS of 3.0 and 6.9 months, some patients were included even with low performance status (12,13). Due to the lack of relevant data, pulmonologists or neurosurgeons help patients make informed decisions to select their treatment modality depending on the patients' preference. For patients with relatively larger lesion (≥10 cm3) who are not candidate for surgery, the two-session SRS would be offered as an option (14). For patients with the desire for home care, SRS would also be an option because SRS is a one-day treatment while WBRT takes over 2 weeks. Follow-up MRI to detect brain lesions or enhanced computed tomography (CT) scanning for systemic lesions was performed every 3–4 months or when clinically indicated after SRS or WBRT. Study approval was obtained from the Institutional Review Board of Komaki City Hospital (no. 171013).
SRS and WBRT techniques and treatment
SRS was performed using Gamma Knife model C or Perfexion (Elekta AB, Stockholm, Sweden). Gamma Knife surgery was performed with the aid of the Leksell Model G stereotactic frame (Elekta AB). After administration of a mild sedative and local anesthesia, the stereotactic frame was applied. Patient treatment was planned with GammaPlan software (Elekta AB). Thin-slice axial spoiled gradient echo images with gadolinium enhancement were used for tumor delineation. Most BMs were treated at a tumor margin dose of 18–20 Gy with an isodose line of 50–95%, depending on tumor volume; treatment occurred in a single session when the tumor volume was less than 10 cm3. Some lesions were treated at a reduced margin dose of 12–16 Gy when the tumors were relatively large (≥10 cm3) or proximal to critical structures, such as the brainstem or the optic apparatus. In one patient, two lesions were treated with two-session Gamma Knife surgery within two-week interval (15), during which a margin dose of 13 Gy per session was delivered because of the multiple large BMs. All the other patients received a single session SRS as one-day treatment. We administered WBRT with 30 Gy in 10 fractions over 2 weeks using a linear accelerator. Providing that WBRT had been refused by the patient, SRS was applied for multiple BM when the patients' systemic condition was such that SRS intervention would be tolerable and fully informed consent for treatment had been obtained. In cases in which the intracranial tumor burden increased as a result of tumor growth or new metastases, repeat SRS or subsequent WBRT was recommended.
Statistical analysis
Study outcomes were comparison of OS, time to intracranial progression (TTIP), and neurological survival (NS) between patients treated with SRS and those treated with WBRT, and identification of the prognostic factors that contributed to OS. OS was defined as the time from the date of diagnosis of BMs to death from any cause. TTIP was defined as the time from the date of diagnosis of BMs to detection of any recurrence in the brain; detection of carcinomatous meningitis, which was verified by examination of cerebrospinal fluid; or the date of the last follow-up imaging. NS was defined as the time from the date of diagnosis of BMs to neurological death. In NS analysis, only deaths from neurological causes were used as endpoints, and patients who died from non-neurological causes were censored. Neurological death was defined as death from any form of progression of neurologic dysfunction caused by intracranial disease, including intracranial recurrence or carcinomatous meningitis, and other illnesses with severe neurologic dysfunction, as described by Patchell et al (16). We also conducted subgroup analysis for epidermal growth factor receptor/anaplastic lymphoma kinase (EGFR/ALK) mutation-positive patients, comparing OS between the two treatment modalities.
We used t-tests or Mann-Whitney U tests for continuous variables, and Chi-square tests or Fisher's exact tests for categorical variables to detect differences between the groups. Estimated survival was calculated using the Kaplan-Meier method, with 95% confidence intervals (CIs); comparisons between the groups were performed using log-rank tests. To detect the independent prognostic factors for survival, Cox proportional hazards modeling was performed for all patients. The following parameters at the time of diagnosis of BMs were included in univariate and multivariate analysis: sex, age, smoking status, ECOG performance status score (PS), initial clinical stage of lung cancer, EGFR/ALK mutation status, symptoms from the brain lesions (yes or no), extracranial metastases (yes or no), maximal diameter of the brain lesions, chemotherapy or EGFR/ALK-tyrosine kinase inhibitor (TKI) administration prior to brain radiotherapy (yes or no), chemotherapy or EGFR/ALK-TKI administration subsequent to brain radiotherapy (yes or no), DS-GPA class for NSCLC (12), Lung-molGPA score (13), and salvage treatment for recurrence of BMs such as SRS or WBRT (yes or no). All analyses were performed using the Statistical Package for the Social Sciences (SPSS v.21; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The patient characteristics are summarized in Table I. We identified 44 patients for the survival analysis. Twenty-four patients (55%) were treated with SRS and 20 patients (45%) were treated with WBRT. The median follow-up periods were 29 weeks (range: 7–233 weeks) in the SRS group and 28 weeks (range: 3–164 weeks) in the WBRT group. No significant differences were observed between the two groups in terms of patient characteristics, including age, sex, histology, smoking status. EGFR/ALK status, clinical stage, PS, systemic treatment, symptoms from BMs, DS-GPA score, Lung-molGPA score, or subsequent systemic treatment. However, patients treated with SRS had fewer lesions (median 11 vs. 15, P=0.008) and greater lesion diameters (median 17 vs. 12.5 mm, P=0.069) than patients treated with WBRT. Patients with fewer and larger lesions tended to receive SRS while patients with more and smaller lesions to receive WBRT. With regard to salvage treatment for BMs, five of 10 patients in the SRS group received repeat SRS, one patient received WBRT, and the remaining four patients received both. In contrast, one patient in the WBRT group received SRS.
Table I.
Patient characteristics (n=44).
| Characteristics | SRS group (n=24) | WBRT group (n=20) | P-value |
|---|---|---|---|
| Median age, years (range) | 67.6 (46–80) | 67.5 (49–84) | 0.519 |
| Sex (male/female) | 17/7 | 10/10 | 0.158 |
| Histology (Ad/Sq/others) | 23/1/0 | 17/1/2 | 0.278 |
| Smoking status (current/former/never) | 7/11/6 | 4/8/8 | 0.542 |
| EGFR/ALK mutation status (positive/negative/unknown) | 13/9/2 | 11/4/5 | 0.219 |
| Clinical stage at diagnosis (I–II/III/IV) | 1/6/17 | 3/1/16 | 0.118 |
| ECOG-PS (0/1/2/3/4) | 7/12/1/3/1 | 3/9/3/3/2 | 0.554 |
| Prior TKI (yes/no) | 4/20 | 5/15 | 0.710 |
| Prior chemotherapy (yes/no) | 7/17 | 8/12 | 0.450 |
| Symptoms from BMs (yes/no) | 7/17 | 8/12 | 0.450 |
| Number of BMs, median (range) | 11 (10–19) | 15 (10–20) | 0.008 |
| Maximum diameter of BMs median, mm (range) | 17.0 (8–40) | 13.5 (5–32) | 0.069 |
| Extracranial metastases (present/absent) | 24/0 | 17/3 | 0.086 |
| DS-GPA score (0–1.0/1.5–2.0/2.5-) | 17/7/0 | 14/6/0 | 0.952 |
| Lung-molGPA score (0–1.0/1.5–2.0/2.5-) | 8/9/7 | 6/9/5 | 0.879 |
| Subsequent chemotherapy (yes/no) | 14/10 | 12/8 | 0.911 |
| Subsequent TKI (yes/no) | 11/13 | 10/10 | 0.783 |
| Salvage treatment (yes/no) | 10/14 | 1/19 | 0.005 |
SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy; Ad, adenocarcinoma; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; TKI, tyrosine kinase inhibitor; BMs, brain metastases; DS-GPA, diagnosis-specific graded prognostic assessment.
Response to WBRT and SRS
Local control was achieved at 6 and 12 months in 100 and 90%, respectively, of both the SRS and WBRT groups (P=0.764). Distant brain failure was seen at 6 and 12 months in 29.9 and 69.9%, respectively, of the SRS group vs. 0 and 10.0%, respectively, of the WBRT group (P=0.005).
Survival data in WBRT and SRS groups
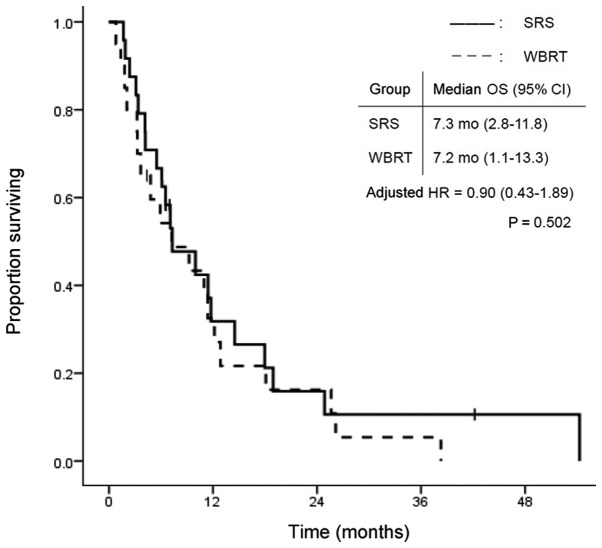
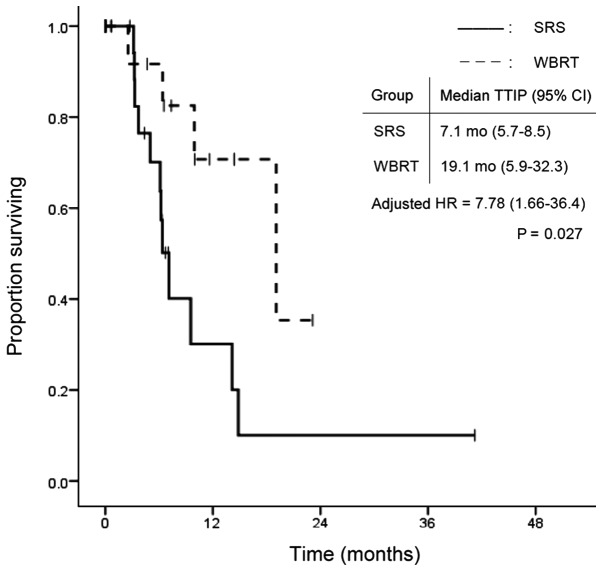
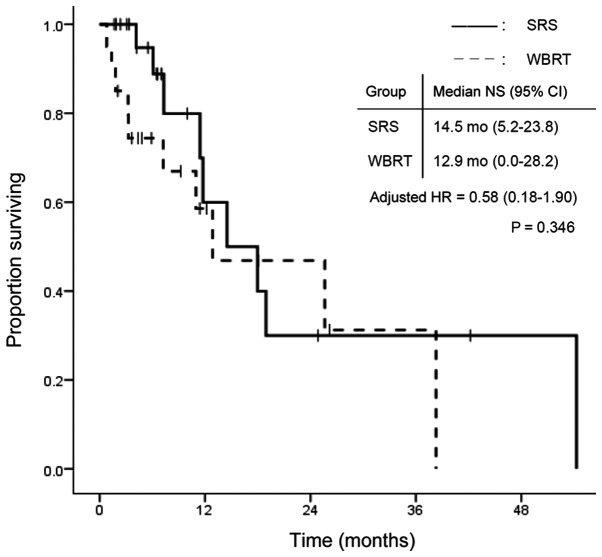
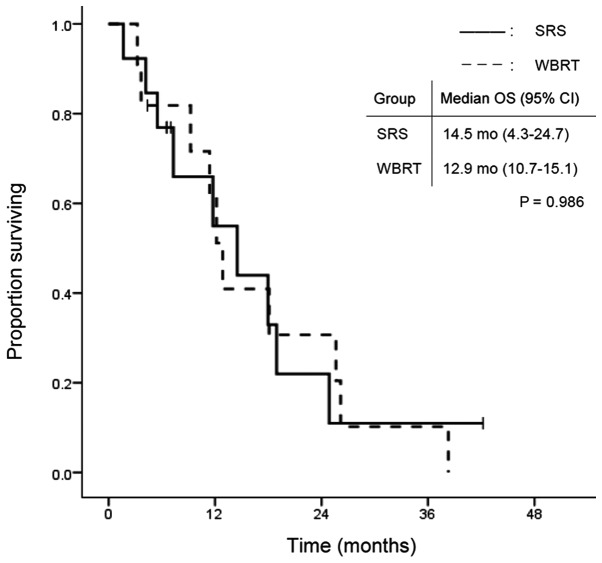
The overall group survival rates were 84.1, 59.1 and 27.3% at 3 and 6 months, and 1 year, respectively, after WBRT or SRS treatment. Fig. 1 shows the survival curves in the SRS and WBRT groups. The median survival time was 7.3 months (95% CI: 2.8–11.8) in the SRS group and 7.2 months (95% CI: 1.1–13.3) in the WBRT group. There was no statistically significant difference in OS between the two groups (P=0.502). In contrast, TTIP was significantly longer in the WBRT group. Median TTIP was 7.1 months (95% CI: 5.7–8.5) in the SRS group and 19.1 months (95% CI: 5.9–32.3) in the WBRT group (P=0.009; Fig. 2). Fig. 3 shows the time to death from neurological causes for the two treatment modalities. There was no significant difference between the two groups: 14.5 months (95% CI: 5.2–23.8) in the SRS group and 12.9 months (95% CI: 0.0–28.2) in the WBRT group (P=0.346). In the subgroup analysis for EGFR/ALK mutation-positive patients, OS did not differ significantly between the two treatment modalities (Fig. 4).
Figure 1.
OS according to treatment modality. OS, overall survival; SRS, safety of stereotactic radiosurgery; WBRT, whole brain radiotherapy; CI, confidence interval; HR, hazard ratio.
Figure 2.
TTIP according to treatment modality. TTIP, time to intracranial progression; SRS, safety of stereotactic radiosurgery; WBRT, whole brain radiotherapy; CI, confidence interval; HR, hazard ratio.
Figure 3.
NS according to treatment modality. NS, neurological survival; SRS, safety of stereotactic radiosurgery; WBRT, whole brain radiotherapy; CI, confidence interval; HR, hazard ratio.
Figure 4.
OS among the patients with EGFR or ALK mutations according to treatment modality. OS, overall survival; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; SRS, safety of stereotactic radiosurgery; WBRT, whole brain radiotherapy; CI, confidence interval.
Prognostic factors for survival in univariate and multivariate analyses
The prognostic factors for survival in univariate and multivariate analyses are shown in Table II. Multivariate analysis confirmed that non-adenocarcinomatous histology [hazard ratio (HR)=7.39; 95% CI, 1.88–28.99; P=0.004], lower PS (HR=1.38; 95% CI, 1.01–1.90; P=0.045), subsequent EGFR/ALK-TKI administration (HR=0.21; 95% CI, 0.09–0.47; P<0.001), subsequent chemotherapy (HR=0.36; 95% CI, 0.15–0.83; P=0.017) and salvage treatment (HR=0.33; 95% CI, 0.12–0.91; P=0.032) were independent prognostic factors, but there were no significant differences between the SRS group and WBRT group with respect to these factors.
Table II.
Univariate and multivariate analyses of covariates associated with overall survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Treatment (SRS/WBRT) | 0.80 | 0.42–1.52 | 0.502 | |||
| Age | 1.00 | 0.97–1.04 | 0.900 | |||
| Sex (male/female) | 1.21 | 0.62–2.37 | 0.580 | |||
| Histology (non-Ad/Ad) | 6.38 | 2.11–19.35 | 0.001 | 7.39 | 1.88–28.99 | 0.004 |
| EGFR/ALK mutation status (positive/negative/unknown) | 0.34 | 0.18–0.67 | 0.002 | |||
| Clinical stage (IV/I–III) | 1.43 | 0.67–3.06 | 0.358 | |||
| Prior TKI (yes/no) | 0.88 | 0.40–1.95 | 0.754 | |||
| Prior chemotherapy (yes/no) | 1.39 | 0.71–2.75 | 0.340 | |||
| Smoking status (current/former/never) | 1.21 | 0.58–2.50 | 0.613 | |||
| Symptoms from BMs (yes/no) | 1.17 | 0.59–2.32 | 0.660 | |||
| Number of BMs | 0.98 | 0.89–1.08 | 0.618 | |||
| Maximal diameter | 1.04 | 1.00–1.08 | 0.055 | |||
| ECOG-PS | 1.48 | 1.12–1.97 | 0.006 | 1.38 | 1.01–1.90 | 0.045 |
| Extracranial metastases (present/absent) | 0.89 | 0.21–3.75 | 0.878 | |||
| DS-GPA score | 0.62 | 0.28–1.40 | 0.248 | |||
| Lung-molGPA score | 0.53 | 0.34–0.83 | 0.005 | 1.11 | 0.61–2.03 | 0.728 |
| Subsequent TKI (yes/no) | 0.37 | 0.19–0.71 | 0.003 | 0.21 | 0.09–0.47 | <0.001 |
| Subsequent chemotherapy (yes/no) | 0.48 | 0.24–0.95 | 0.035 | 0.36 | 0.15–0.83 | 0.017 |
| CNS-PD (yes/no) | 0.59 | 0.30–1.15 | 0.123 | |||
| Salvage treatment for BMs (yes/no) | 0.46 | 0.22–0.99 | 0.046 | 0.33 | 0.12–0.91 | 0.032 |
HR, hazard ratio; CI, confidence interval; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy; Ad, adenocarcinoma; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; BMs, brain metastases; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; DS-GPA, diagnosis-specific graded prognostic assessment; CNS-PD, central nervous system progression disease.
Adverse events
Based on Common Terminology Criteria for Adverse Events (CTCAE) v4.0, the rate of radiation-induced leukoencephalopathy on follow-up MRI were as follows: grade 1/2/3/4/not evaluated = 14/4/1/0/5 in the SRS group and 5/3/4/8 in the WBRT group, respectively. Radiation induced changes in the SRS group were relatively lower than in the WBRT group. The other radiation-induced adverse events such as neuropathy, seizure, symptomatic radiation necrosis, cerebral hemorrhage were not detected in any patients included in our analysis.
Discussion
In this single-center retrospective study, we compared SRS and WBRT as the initial treatment for patients with 10–20 BMs from NSCLC. We found that there were no significant differences in OS between the treatment groups, although TTIP and the local control rate were significantly better in the WBRT group.
Conventionally, WBRT has been considered the standard treatment for multiple BMs. However, there have been numerous reports of survival time comparable to that of WBRT in patients treated with SRS for 5–10 BMs, with similar side effects, and the adoption of SRS has therefore been gradually increasing (6,17–19). Recently, some retrospective studies have evaluated the use of SRS for 10 or more BMs (7–11). The findings of those studies have suggested that treatment with SRS for 10 or more lesions is not inferior to that in patients with smaller numbers of BMs in terms of safety and efficacy. However, the studies included various primary sites besides NSCLC, and none directly compared SRS with WBRT with respect to survival. Therefore, the present study focused on initial radiological treatment for more than 10 BMs from NSCLC and compared the two treatment options.
SRS generally has advantages when compared with WBRT in terms of safety, such as the late development of cognitive dysfunction and alopecia, which could affect patients' quality of life (20–25). Yamamoto et al reported the safety and feasibility of SRS for ten or more BMs when compared to the patients with less lesions (11) In addition SRS can be applied repeatedly for BMs and can be performed in a shorter time, resulting in a reduced burden on patients. In fact, 10 patients in the SRS group in the present study required re-irradiation for recurrent BMs; five of these underwent SRS alone without WBRT, while the remainder needed WBRT. Thus, if patients would be tolerable for the adverse events of SRS such as stereotactic frame application for immobilization, SRS for 10 or more BMs may delay the administration of WBRT and the adverse events associated with this treatment modality.
Patients treated with WBRT compared to SRS in the present study showed superior intracranial control and TTIP, but these outcomes did not lead to either fewer neurological deaths or OS prolongation. While the risk of intracranial recurrence in the SRS group was higher than WBRT group, the adverse events such as leukoencephalopathy were fewer in the SRS group. Besides severe neuropathy, seizure, symptomatic radiation necrosis, cerebral hemorrhage were not observed in either group. These results were similar to the findings of previous prospective studies conducted for fewer than 10 BMs (21,22,26,27). On the basis of those findings, upfront SRS is a good option for patients with 10 or more BMs from NSCLC to shorten the duration of the treatment and avoid the long-term adverse events from WBRT if patients are appropriate for local anesthesia and mild sedation.
With respect to EGFR and ALK mutational status, patients with those mutations accounted for half of the patients in this study. No statistical difference in survival was seen between the WBRT group and the SRS group with regard to patients with these mutations. In general, EGFR and ALK-TKIs are effective treatment for BMs, leading to better prognosis for patients with such driver mutations. As reported by Mangnuson et al, upfront SRS followed by TKI administration is a reasonable treatment to avoid late adverse events derived from WBRT, especially for patients with these mutations (28).
The present study has several limitations. First, this was a retrospective study with small sample size and lacked evaluation of neurocognitive functions and detailed complications. In a retrospective cohort study that compared 2–9 BMs with 10 or more BMs treated with SRS, neurological deterioration and SRS-related complications did not differ between the groups (11). In addition, another prospective observational study showed that patients with 5–10 BMs did not experience more neurocognitive deterioration or post-SRS complications compared to those with 2–4 BMs, whereas the addition of WBRT generally resulted in worse effects on neurocognitive function (21,22). These findings suggest that, even in patients with 10 or more BMs, SRS may be safer and may better maintain neurocognitive function than WBRT. In fact, in the SRS group there were fewer leukoencephalopathy and may be fewer adverse events related to WBRT described in other studies. We could not show the detailed adverse events data because this was a retrospective study and many of patients were outpatients. Second, this study did not include details on control of primary tumor which could affect the prognosis (29), because there were some patients without any radiologic findings for primary lesions outside central nervous system at the time of progression of BMs. Third, there were differences in patient backgrounds between the two groups, such as the number of BMs, diameter of BMs, PS, histology, and systemic therapy. In fact, PS, histology, EGFR/ALK-TKI administration or chemotherapy after diagnosis of BMs, and salvage treatment can influence OS (30–32). Therefore, we conducted a multivariate analysis that included those factors to adjust for bias with respect to patient characteristics. Additionally, the absence of the evaluation of the volume of BMs is also a limitation. Fourth, we do not have the data of which sum of the volumes of BMs is smaller or bigger between the two modalities because this study was a retrospective study based on the electronic clinical records, which did not include the information about the volume of BMs. We also do not have the data or evidence if the high dose provided by SRS alone would bring more benefit than WBRT alone especially in patients with multiple BMs. Fifth, the assignment of patients to treatment was based solely on the judgment of physicians. Thus, there is a possibility that some bias not included in the analysis was present. In general, patients with multiple small metastases would generally be selected for WBRT, whereas those with fewer and larger (≥10 cm3) lesions would be offered SRS in our institution. Patients with long-term prognosis might be treated with SRS repeatedly to prevent complications from WBRT. Further study evaluating neurocognitive function, detailed complications, control of the primary tumor, and the actual tumor volume or planning tumor volume are warranted.
In conclusion, we compared SRS to WBRT as the initial treatment for 10–20 BMs from NSCLC. The present study demonstrated that there were no significant differences in OS and NS between treatment with SRS and WBRT for BMs. Therefore, SRS may be a useful alternative treatment for 10–20 BMs from NSCLC. Further prospective randomized studies that evaluate neurocognitive functions and complications are needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TM drafted the manuscript and analyzed the data. TH, HK, TS, YY, NA, EK contributed to study design and data collection. TY helped to interpret the data and draft the manuscript. KM helped to analyze the data in this study. KT conceived the study and participated in its design and coordination. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Study approval was obtained from the Institutional Review Board of Komaki City Hospital (approval no. 171013; Komaki, Japan). Due to the anonymous and retrospective nature of the study, the requirement for individual informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
TY has previously obtained research grants from Nippon Boehringer Ingelheim; however, this had no bearing on the design of the present study.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thoracic Oncol. 2014;9:195–199. doi: 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 3.Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384–394. doi: 10.1002/1097-0142(19810715)48:2<384::AID-CNCR2820480227>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, Perez CA, Hendrickson FR. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 5.Halasz LM, Uno H, Hughes M, D'Amico T, Dexter EU, Edge SB, Hayman JA, Niland JC, Otterson GA, Pisters KM, et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer. Cancer. 2016;122:2091–2100. doi: 10.1002/cncr.30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 7.Chang WS, Kim HY, Chang JW, Park YG, Chang JH. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113(Suppl):73–78. doi: 10.3171/2010.8.GKS10994. [DOI] [PubMed] [Google Scholar]
- 8.Grandhi R, Kondziolka D, Panczykowski D, Monaco EA, III, Kano H, Niranjan A, Flickinger JC, Lunsford LD. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg. 2012;117:237–245. doi: 10.3171/2012.4.JNS11870. [DOI] [PubMed] [Google Scholar]
- 9.Kim CH, Im YS, Nam DH, Park K, Kim JH, Lee JI. Gamma knife radiosurgery for ten or more brain metastases. J Korean Neurosurg Soc. 2008;44:358–363. doi: 10.3340/jkns.2008.44.6.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rava P, Leonard K, Sioshansi S, Curran B, Wazer DE, Cosgrove GR, Norén G, Hepel JT. Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2013;119:457–462. doi: 10.3171/2013.4.JNS121751. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Watanabe S, Kasuya H. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2–9 versus 10 or more tumors. J Neurosurg. 2014;121(Suppl):16–25. doi: 10.3171/2014.8.GKS141421. [DOI] [PubMed] [Google Scholar]
- 12.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the Graded Prognostic Assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serizawa T, Higuchi Y, Yamamoto M, Matsunaga S, Nagano O, Sato Y, Aoyagi K, Yomo S, Koiso T, Hasegawa T, et al. Comparison of treatment results between 3- and 2-stage Gamma Knife radiosurgery for large brain metastases: a retrospective multi-institutional study. J Neurosurg. 2018 Sep 1; doi: 10.3171/2018.4.JNS172596. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa T, Kato T, Yamamoto T, Iizuka H, Nishikawa T, Ito H, Kato N. Multisession gamma knife surgery for large brain metastases. J Neurooncol. 2017;131:517–524. doi: 10.1007/s11060-016-2317-4. [DOI] [PubMed] [Google Scholar]
- 16.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 17.Hunter GK, Suh JH, Reuther AM, Vogelbaum MA, Barnett GH, Angelov L, Weil RJ, Neyman G, Chao ST. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:1394–1398. doi: 10.1016/j.ijrobp.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Raldow AC, Chiang VL, Knisely JP, Yu JB. Survival and intracranial control of patients with 5 or more brain metastases treated with gamma knife stereotactic radiosurgery. Am J Clin Oncol. 2013;36:486–490. doi: 10.1097/COC.0b013e31825494ef. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Barfod BE, Kasuya H, Urakawa Y. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1–4 vs ≥ 5 tumors: clinical article. J Neurosurg. 2013;118:1258–1268. doi: 10.3171/2013.3.JNS121900. [DOI] [PubMed] [Google Scholar]
- 20.Sahgal A, Ruschin M, Ma L, Verbakel W, Larson D, Brown PD. Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neuro Oncol. 2017;19(Suppl 2):ii2–ii15. doi: 10.1093/neuonc/nox001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, II, Deming R, Burri SH, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 23.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 24.Habets EJ, Dirven L, Wiggenraad RG, Verbeek-de Kanter A, Lycklama À, Nijeholt GJ, Zwinkels H, Klein M, Taphoorn MJ. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol. 2016;18:435–444. doi: 10.1093/neuonc/nov186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 26.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 27.Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, Beal K, Amini A, Patil T, Kavanagh BD, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzoni J, Devriendt D, Massager N, David P, Ruíz S, Vanderlinden B, Van Houtte P, Brotchi J, Levivier M. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–224. doi: 10.1016/j.ijrobp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Jiang T, Min W, Li Y, Yue Z, Wu C, Zhou C. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: An update meta-analysis. Cancer Med. 2016;5:1055–1065. doi: 10.1002/cam4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo S, Chen L, Chen X, Xie X. Evaluation on efficacy and safety of tyrosine kinase inhibitors plus radiotherapy in NSCLC patients with brain metastases. Oncotarget. 2015;6:16725–16734. doi: 10.18632/oncotarget.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DY, Lee KW, Yun T, Kim DW, Kim TY, Heo DS, Bang YJ, Kim NK. Efficacy of platinum-based chemotherapy after cranial radiation in patients with brain metastasis from non-small cell lung cancer. Oncol Rep. 2005;14:207–211. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.