Abstract
Background
Subclinical thyroid dysfunction whose typical patterns include subclinical hypothyroidism and subclinical hyperthyroidism, has been indicated to be associated with an increased risk of heart failure (HF). However, the relationship between subclinical thyroid dysfunction and the clinical outcomes of HF patients is uncertain. This meta-analysis was conducted to assess the association between subclinical thyroid dysfunction and the clinical outcomes of HF patients.
Methods
Pubmed, Embase, Web of Science and Cochrane Central Register of Clinical Trials were searched for eligible studies published up to August 1, 2018 which reported the association between subclinical thyroid dysfunction and the clinical outcomes of HF patients. The pooled hazard ratio (HR) with the corresponding 95% confidence interval (CI) was used to assess the association.
Results
Fourteen studies met the eligibility criteria and a total of 21,221 patients with heart failure were included in the meta-analysis. Compared with HF patients with euthyroidism, the pooled HR of subclinical hypothyroidism for all-cause mortality was 1.45 (95% CI 1.26–1.67) in a randomized effects model with mild heterogeneity (I2 = 40.1, P = 0.073). The pooled HR of subclinical hypothyroidism for cardiac death and/or hospitalization was 1.33 (1.17–1.50) in a randomized effects model with moderate heterogeneity (I2 = 69.4, P < 0.001). Subclinical hyperthyroid can increase the risk of all-cause mortality without heterogeneity (HR 1.31, 95% CI 1.10–1.55, I2 = 25.5%, P = 0.225) but have no influence on the risk of cardiac death and/or hospitalization (HR 1.03, 95% CI 0.87–1.23, I2 = 0.0%, P = 0.958). These significant adverse associations were also retained in subgroup analysis. Sensitivity analysis demonstrated the stability of the results of our meta-analysis.
Conclusions
Both subclinical hypothyroidism and subclinical hyperthyroidism are associated with adverse prognosis in patients with HF. Subclinical thyroid dysfunction may be a useful and promising predictor for the long-term prognosis in HF patients.
Keywords: Subclinical hypothyroidism, Subclinical hyperthyroidism, Heart failure, Prognosis
Background
Heart failure (HF) is the end stage of almost all forms of heart diseases and is one of the most common causes of hospitalization and death worldwide [1, 2]. HF patients suffer from a poor prognosis and a high mortality. The mortality of HF patients within 5 years is reported greater than 50% which is higher than in most malignancies [3]. In the past 30 years, though significant progress has been made to treat HF patients, mortality rates are still high [4]. Early risk stratification can accurately identify HF patients with higher risk for adverse clinical outcomes and thus is important for the management of patients with HF.
The poor prognosis of HF is partially due to the influence of comorbidities which include alterations of thyroid function [5–7]. Thyroid hormones have effects on all cells, tissues, and organs in human body and the homeostasis of thyroid hormones is essential to the optimal functioning of the heart [5–7]. Subclinical thyroid dysfunction is common in the adult population. A typical pattern of subclinical thyroid dysfunction include subclinical hypothyroidism and subclinical hyperthyroidism, which is defined biochemically as abnormal serum level of thyroid-stimulating hormone (TSH) with free thyroxine (FT4) and free or total triiodothyronine (FT3) within their reference range [8, 9]. The prevalence of subclinical hypothyroidism is reported to be 4–20% in the adult population [10–12], and the prevalence of subclinical hyperthyroidism has been reported to be 0.7–9% [11–13]. Increasing studies have shown that both subclinical hypothyroidism and subclinical hyperthyroidism have profoundly impact on cardiac function by modulating heart rate, cardiac contractive and diastolic function, and systemic vascular resistance [5–7]. It has also been acknowledged that both subclinical hypothyroidism and subclinical hyperthyroidism can be a cause of HF and thus the American College of Cardiology/American Heart Association guidelines for the diagnosis and management of heart failure on adults recommend measurement of thyroid function [14]. Though subclinical hypothyroidism and subclinical hyperthyroidism are associated with an increased risk of HF, the relationship between them and the clinical outcomes of HF patients is uncertain. Though several previous studies have investigated the relationship between subclinical hypothyroidism/subclinical hyperthyroidism and the prognosis of HF patients [15–28], the results are inconsistent. Some studies described an increased risk of all-cause mortality or hospitalization for HF patients with subclinical hypothyroidism or subclinical hyperthyroidism but others did not. Considering the small number of HF patients with subclinical hypothyroidism or subclinical hyperthyroidism in most studies, the results may lack statistical power.
In this studies, we performed a meta-analysis to combine the results of all available prospective studies to clarify the relationship between subclinical thyroid dysfunction and the outcomes of HF patients.
Methods
Literature search
Two reviewers (GD Yang and Y Wang) searched electronic databases of Pubmed, Embase, Web of Science, and Cochrane Central Register of Clinical Trials independently and all publications up to August 1, 2018 were considered. The search terms used to search potentially relevant studies are as follows: (‘Heart Failure’ OR ‘Cardiac Failure’ OR ‘Myocardial Failure’ OR ‘Heart Decompensation’) AND (‘Hypothyroidism’ OR ‘Hypothyroidisms’ OR ‘Thyroid-Stimulating Hormone Deficiency’ OR ‘TSH Deficiency’ OR ‘TSH Deficiencies’ OR ‘Hyperthyroidism’ OR ‘Hyperthyroid’ OR ‘Hyperthyroids’). In addition, a manual search was conducted by searching relevant bibliography including the references of the reviews on this topic and previously published meta-analysis. The search strategy was without language restriction.
Inclusion and exclusion criteria
The inclusion criteria are as follows: 1) prospective clinical studies or cohort studies; 2) involved adults (≥18 years old); 3) clear HF with reduced ejection fraction definition which is in accordance with current HF guideline; 4) investigating the relationship between subclinical hypothyroidism/subclinical hyperthyroidism and the outcomes of HF patients; 5) the outcomes of HF patients include all-cause mortality or cardiac death or hospitalization; 6) the hazard ratio (HR) with 95% confidence intervals (95% CI) for subclinical hypothyroidism/subclinical hyperthyroidism and the outcomes of HF patients were reported. Review articles, case reports, meeting abstract and editorials were excluded. We also excluded studies that only reported unadjusted HR or only reported adjusted HR without 95% CIs.
Study selection
Two independent reviewers (GD Yang and Y Wang) screened the studies using the titles or abstracts or full text to identify eligible studies. Relevant studies were assessed for compliance with the inclusion criteria. Discrepancies and uncertainties were resolved by consensus or by requiring a third author (TZ Wang) to assess it through rechecking the source data and consultation.
Data extraction
Two authors (GD Yang and Y Wang) conducted data extraction independently using the standardized data-extraction form and a third author (TZ Wang) confirmed the data for their accuracy. The data extracted from the studies include author, study population, country, mean duration of follow-up, mean age, gender percentage, clinical outcomes, adjusted cofounders and the multivariate adjusted HR with the corresponding 95% CI.
Quality assessment
The quality of the studies was evaluated by two authors (GD Yang and Y Wang) according to a modified scoring system reported previously [29]. Quality assessment was performed according to the following criteria: 1) methods of outcome adjudication and ascertainment accounted for confounders and completeness of follow-up ascertainment; 2) study populations considered a convenience or a population-based sample; 3) appropriate inclusion and exclusion criteria; 4) thyroid function measured more than once; 5) methods of outcome adjudication categorized as use of formal adjudication procedures and adjudication without knowledge of thyroid status; 6) adjustments made for age, sex, New York Heart Association (NYHA) classification, left ventricular ejection fraction (LVEF), and medication; 7) any other adjustments (such as for B-type natriuretic protein [BNP] level, thyroid drug use, and concomitant medication for HF). When a criteria was performed, a score of 1 was given. A score of 0 was given if a criteria was unclear and not achieved. The score ranges from 0 to 7 points where 7 reflects the highest quality.
Statistical analysis
HR with 95% CI were used to present the pooled effect sizes. I2 and Cochran Q statistics were used to evaluate heterogeneity among studies. I2 > 50% or P < 0.1 indicate the existence of heterogeneity and the random effects model was used. Otherwise, for I2 < 50% and P > 0.1, the fixed effects model was applied. Subgroup analysis was performed to explore the possible origin of the heterogeneity according to the study quality (≤4 and > 4), ethnicity (United States, Europe and Asia), mean age (≤65 and > 65), mean duration (month) of follow-up (≤24 and > 24), sample size (≤1000 and > 1000), and adjustment for amiodarone or thyroid treatment (Yes and No). Sensitive analysis was also performed by sequentially omitting one study to investigate the influence of a single study on the heterogeneity. Finally, publication bias was illustrated using funnel plot. Begg’s test and Egger’s test were applied to detect the significance of publication bias. Stata 15.0 (Stata Corp LP, College Station, TX, USA) was used for statistical analyses.
Results
Search results
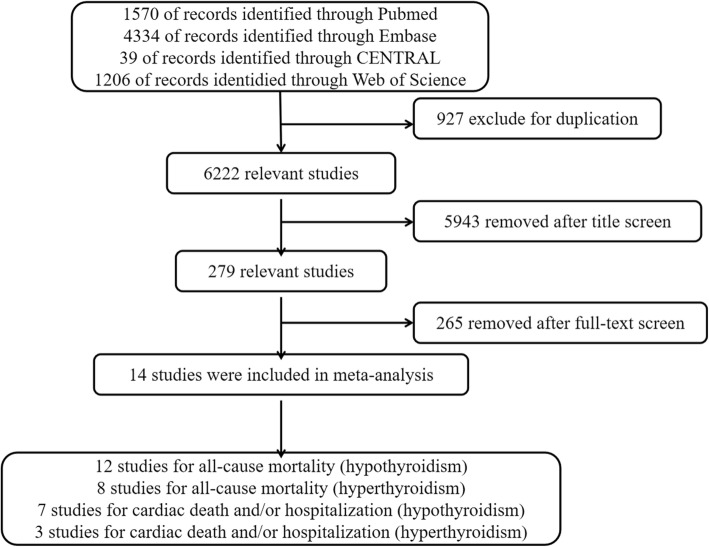
After searching the above electronic databases, a total of 7149 records were obtained. After removing 927 duplicates, 6222 records were screened using title, abstracts and full-texts. Finally, 14 relevant studies [15–28] with a total of 21,221 HF patients were obtained to do meta-analysis. A detailed flow diagram of selecting these relevant studies was presented in Fig. 1.
Fig. 1.
Flow diagram of the selection process
Summary of included studies
Table 1 listed the features of the included studies. Twelve studies reported the association between subclinical hypothyroidism and all-cause mortality of HF patients [16, 18–28] and 11 studies reported the association between subclinical hypothyroidism and cardiac death and/or hospitalization of HF patients [15–19, 21–23, 25, 27, 28]. For subclinical hyperthyroidism, 8 studies reported the association with all-cause mortality of HF patients [18, 20–25, 27] and 5 studies reported the association with cardiac death and/or hospitalization [18, 21–23, 27]. The participants in these eligible studies were primarily male and the mean age of the participants ranged from 51 to 72 years old. The follow-up duration of these studies ranged from 12.1 month to 67 month.
Table 1.
Characteristics of studies included in the meta-analysis
| Author (year) | Study population | Country | No. of patients Nor/Hypo/Hyper |
Defnition of Hypo/Hyper | Mean follow-up | Mean age (year) | Male % | Outcome | Adjusted variables | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Iacoviello 2008 [28] | prospective | Italy | 304/34/NA | TSH > 5.5mIU/l/ NA |
15 mo | 64 | 77 | All-cause mortality | Age, sex, BMI, DM, NYHA, HR, hypertension, LVEF, GFR, NT-proBNP, medication | 4 |
| Frey 2013 [27] | INH study | Germany | 628/34/69 | TSH > 4.0 mIU/l/ TSH < 0.3 mIU/l |
37 mo | 68 | 71 | All-cause mortality | Age | 5 |
| Rhee 2013 [26] | NHANES III | United States | 410/54/NA | TSH > 4.7 mIU/l | 14.3 mo | 52.3 | 42.6 | All-cause mortality | Age, sex, race, DM, hypertention, hypercholesterolemia, stroke, MI, BMI, GFR, medication | 4 |
| Mitchell 2013 [25] | SCD-HeFT | United States | 1930/275/23 | TSH > 5.0 mIU/l/ THS < 0.3 mIU/l |
45.5 mo | 61.3 | 65 | All-cause mortality | Age, sex, DM, renal insufficiency, hypertension, LVEF, time since HF diagnosis, 6-min walk distance, medication | 6 |
| Azemi 2013 [24] | Clinical setting | United States | 243/102/26 | TSH > 5 mIU/l/ TSH < 0.4 mIU/l |
27.2 mo | 67 | 77.9 | All-cause mortality | Age, sex, TSH, LVEF, DM, primary indication for ICD implantation, medication | 5 |
| Deursen 2014 [23] | Observational survey | Italy | 2839/290/97 | NA/NA | 12.1 mo | 66 | 70 | All-cause mortality, hospilization | Age, sex, etiology, hypertension, AF, HR, body surface area, systolic blood pressure | 4 |
| Chen 2014 [22] | HMO cohort | Israel | 4490/916/193 | TSH > 4.5 mIU/l/ TSH < 0.45 mIU/l |
14.5 mo | 75 | 49 | All-cause mortality, cardiac death and hospitalization | Age, sex, DM, ischemic heart disease, hyperlipdaemia, hypertension, AF, BMI, log transformed pulse, log transformed serum urea levels, GFR, hemoglobin, serum sodium, medication | 7 |
| Perez 2014 [21] | CORONA | Europe | 4338/237/176 | TSH > 5.0 mIU/l/ TSH < 0.3 mIU/l |
32.8 mo | 72 | 77 | All-cause mortality, cardiac death and /or hospitalization | Age, sex, NYHA, LVEF, BMI, BP, HR, MI, smoking, angina pectoris, CABG, PCI, AA, hypertension, BM, AF, ICD, stroke, CPR, medication | 6 |
| Li 2014 [20] | Clinical setting | China | 816/79/68 | TSH > 5.5 mIU/l/ TSH < 0.35 mIU/l |
42 mo | 52.1 | 73.7 | All-cause mortality | Age, sex, hypertension, AF, drinking and smoking history, QRS duration, LVEF, FT3, T3, T4, NT-Pro-BNP, medication | 6 |
| Sharma 2015 [19] | Clinical setting | United States | 427/84/NA | TSH > 5.0 mIU/l | 36 mo | 68 | 77 | All-cause mortality, hospitalization | Sex, creatinine, DM, medication | 3 |
| Wang 2015 [18] | Clinical setting | China | 353/41/35 | TSH > 4.78 mIU/l/ TSH < 0.55 mIU/l |
17 mo | 51 | 71 | All-cause mortality | Age, sex, BP, NT-Pro BNP, LVEF, smoking, AF, DM, anemia, renal dysfuntion, NYHA, medication | 5 |
| Hayashi 2016 [17] | Clinical setting | Japan | 188/5/NA | TSH > 4.5 mIU/l | 26 mo | 70 | 57 | Cardiac death and hospitalization | Age, sex, LVEF, NT-Pro BNP, eGFR | 3 |
| Sato 2018 [16] | Clinical setting | Japan | 911/132/NA | TSH > 4.0 mIU/l | 36.6 mo | 68 | 57.4 | All-cause mortality, cardiac death and hospitalization | Age, sex, BMI, BP, HR, NYHA, DM, hypertension, anemia, chronic kidney disease, AF, smoking, LVEF, medication | 5 |
| Ro 2018 [15] | Clinical setting | United States | 349/25/NA | TSH > 4.7 mIU/l | 67 mo | 54.5 | 35 | hospitalization | Age, sex, BMI, race, ethnicity, DM, hypertension, hyperlipidemia, CAD, CVD | 4 |
AF atrial fibrillation, BMI body mass index, BP blood pressure, CABG coronary artery bypass grafting, eGFR chronic heart failure, HR heart rate, ICD implantable cardioverter, LVEF left ventricular ejection fraction, MI myocardial infarction, NYHA New York Heart Association, NT-Pro BNP N-terminal of the prohormone brain natriuretic peptide, CAD coronary artery disease, CVD cerebrovascular disease, DM diabetes mellitus
Subclinical thyroid dysfunction and HF outcome
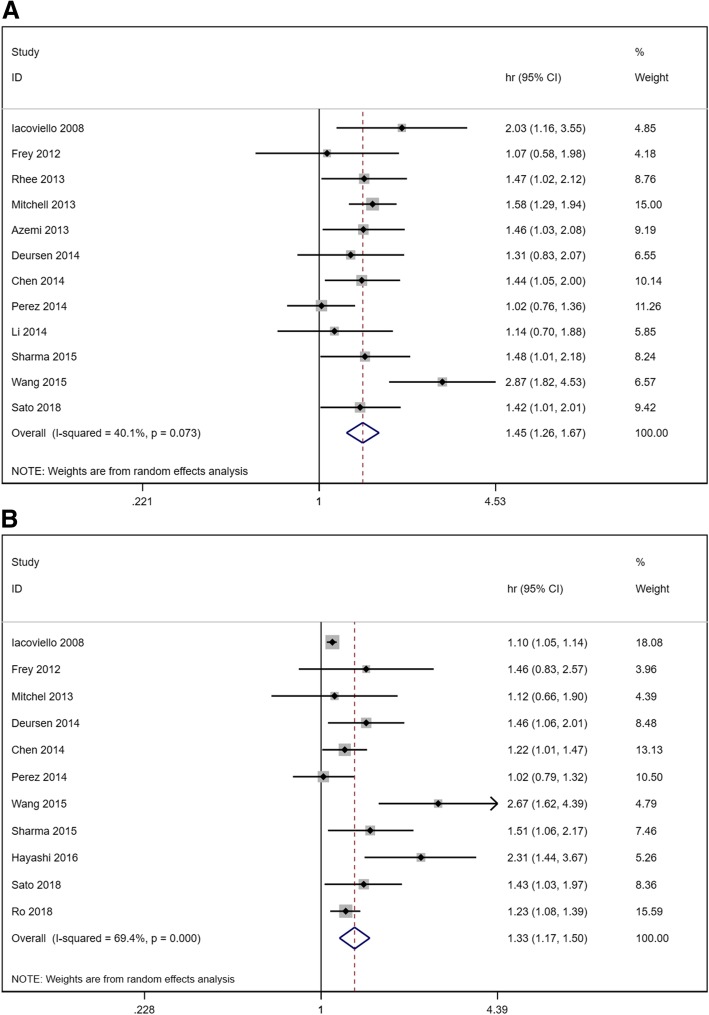
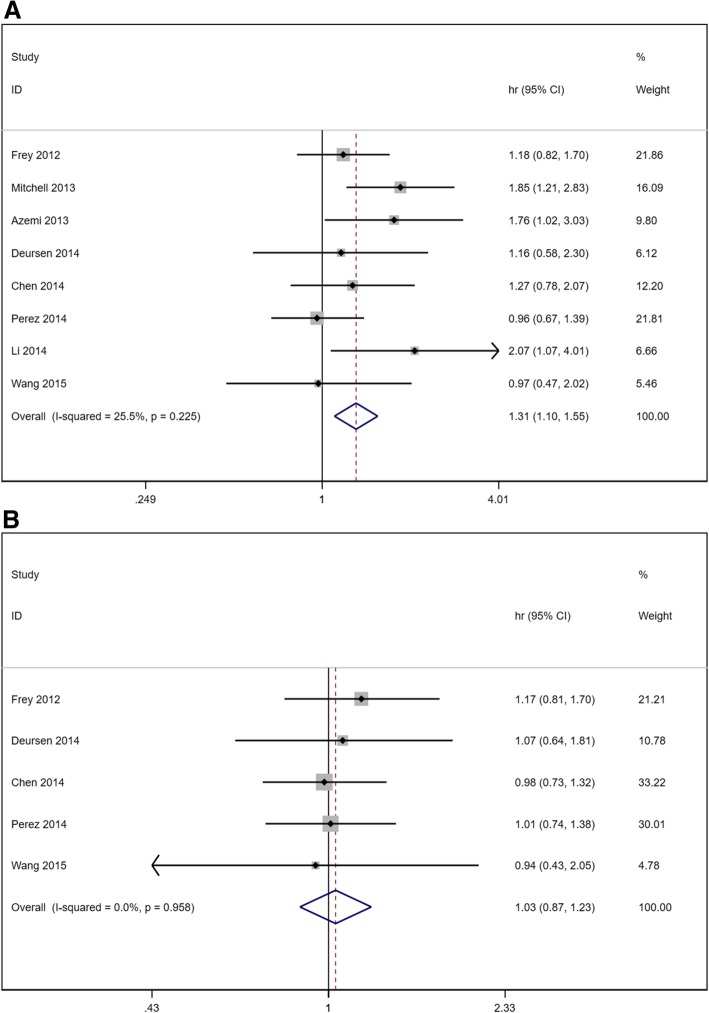
As illustrated in Fig. 2, when compared with patients with euthyroidism, the overall HR of subclinical hypothyroidism for all-cause mortality was 1.45 (1.26–1.67) in a randomized effects model with mild heterogeneity (I2 = 40.1, P = 0.073). The overall HR of subclinical hypothyroidism for cardiac death and/or hospitalization was 1.33 (1.17–1.50) in a randomized effects model with moderate heterogeneity (I2 = 69.4, P < 0.001). Figure 3 showed the overall HR of subclinical hyperthyroidism for HF outcome. We can see that subclinical hyperthyroid increases the risk of all-cause mortality without heterogeneity (HR 1.31, 95% CI 1.10–1.55, I2 = 25.5%, P = 0.225) but have no influence on the risk of cardiac death and/or hospitalization (HR 1.03, 95% CI 0.87–1.23, I2 = 0.0%, P = 0.958).
Fig. 2.
Forest plot of hazard ratio (HR) for hypothyroidism. a all-cause mortality. b cardiac death and/or hospitalization
Fig. 3.
Forest plot of hazard ratio (HR) for hyperthyroidism. a all-cause mortality. b cardiac death and/or hospitalization
Subgroup analysis and sensitive analysis
A subgroup analysis according to age, ethnicity, mean age, mean duration of follow-up, sample size, score and adjustment of amiodarone or thyroid treatment was performed to investigate the possible origin of the heterogeneity among studies which reported the association between subclinical hypothyroidism and HF. As shown in Table 2, sample size and ethnicity may be the mainly origin of heterogeneity. Besides, our subgroup analysis showed that all-cause mortality had an even stronger relationship with Asian patients (HR 1.67, 95% CI 1.01–2.78) and patients less than 65 years old (HR 1.70, 95% CI 1.31–2.20). In addition, Asian patients also had a stronger association with cardiac death and/or hospitalization (HR 1.76, 95% CI 1.11–2.81).
Table 2.
Subgroup analysis of the association between hypothyroidism and all-cause mortality or cardiac death and/or hospitalization in heart failure patients
| All-cause mortality | Cardiac death and/or hospitalization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity | Meta-analysis | Heterogeneity | Meta-analysis | |||||||
| Subgroup | Number of studies | I2% | P value | HR | 95% CI | Number of studies | I2% | P value | HR | 95% CI |
| Age | ||||||||||
| ≤ 65 | 5 | 55.0 | 0.064 | 1.70 | 1.31–2.20 | 1 | 1.23 | 1.08–1.40 | ||
| > 65 | 7 | 0.0 | 0.603 | 1.31 | 1.14–1.50 | 6 | 55.3 | 0.048 | 1.37 | 1.14–1.65 |
| Ethnicity | ||||||||||
| Europe | 6 | 20.9 | 0.276 | 1.31 | 1.09–1.58 | 4 | 32.0 | 0.220 | 1.25 | 1.06–1.47 |
| United States | 3 | 0.0 | 0.901 | 1.53 | 1.31–1.80 | 1 | 1.23 | 1.08–1.40 | ||
| Asian | 3 | 76.6 | 0.014 | 1.67 | 1.00–2.78 | 2 | 63.3 | 0.099 | 1.76 | 1.11–2.81 |
| Follow-up | ||||||||||
| ≤ 24 | 5 | 51.4 | 0.083 | 1.70 | 1.30–2.23 | 2 | 0.0 | 0.343 | 1.28 | 1.09–1.50 |
| > 24 | 7 | 17.8 | 0.294 | 1.35 | 1.17–1.56 | 5 | 62.8 | 0.030 | 1.36 | 1.11–1.66 |
| Sample size | ||||||||||
| ≤ 1000 | 7 | 45.6 | 0.088 | 1.57 | 1.25–1.97 | 3 | 72.3 | 0.027 | 1.53 | 1.09–2.15 |
| > 1000 | 5 | 33.1 | 0.201 | 1.36 | 1.15–1.61 | 4 | 24.7 | 0.263 | 1.24 | 1.07–1.44 |
| Score | ||||||||||
| ≤4 | 4 | 0.0 | 0.688 | 1.51 | 1.22–1.86 | 4 | 60.6 | 0.054 | 1.48 | 1.17–1.87 |
| > 4 | 8 | 58.2 | 0.019 | 1.43 | 1.18–1.73 | 3 | 25.5 | 0.261 | 1.32 | 1.15–1.51 |
| Thyroid drug use | ||||||||||
| Yes | 5 | 0.0 | 0.826 | 1.48 | 1.29–1.70 | 4 | 56.5 | 0.075 | 1.32 | 1.08–1.60 |
| No | 7 | 64.0 | 0.011 | 1.48 | 1.14–1.94 | 7 | 71.8 | 0.002 | 1.36 | 1.12–1.66 |
| Amidarone use | ||||||||||
| Yes | 6 | 43.8 | 0.113 | 1.31 | 1.08–1.57 | 5 | 46.2 | 0.115 | 1.33 | 1.13–1.56 |
| No | 6 | 19.1 | 0.289 | 1.57 | 1.30–1.90 | 6 | 73.7 | 0.002 | 1.36 | 1.09–1.70 |
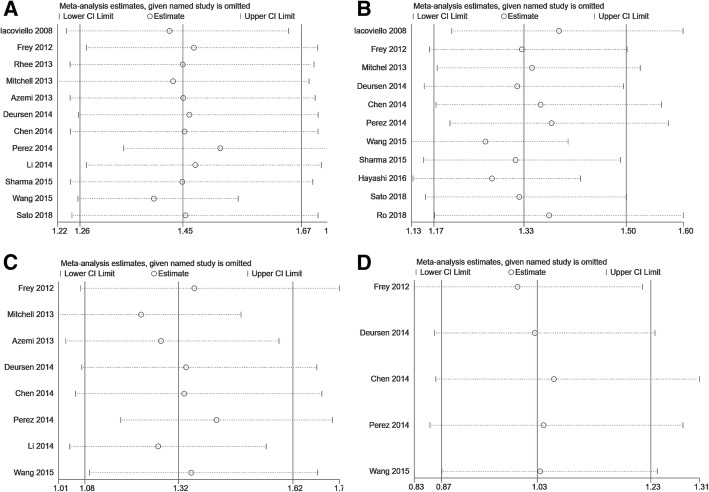
The sensitive analysis was performed by removing one study at a time. Figure 4 illustrated the sensitive analysis. The results didn’t find any study changing the magnitude and direction of the results.
Fig. 4.
Sensitive analysis. a hypothyroidism and all-cause mortality. b hypothyroidism and cardiac death and/or hospitalization. c hyperthyroidism and all-cause mortality. d hyperthyroidism and cardiac death and/or hospitalization
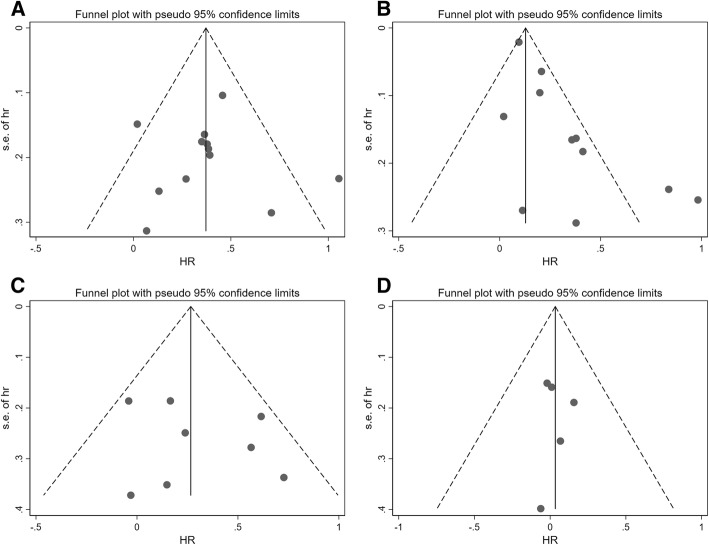
Publication bias
We performed funnel plot, Begg’s test and Egger’s test to evaluate the publication bias. The results showed in Fig. 5 and Table 3 indicated there was no publication bias existed among the included studies.
Fig. 5.
Funnel plot assessing publication bias. a hypothyroidism and all-cause mortality. b hypothyroidism and cardiac death and/or hospitalization. c hyperthyroidism and all-cause mortality. d hyperthyroidism and cardiac death and/or hospitalization
Table 3.
P values of Begg’s and Egger’s test for investigating the publication bias
| Begg’s test | Egger’s test | |
|---|---|---|
| All cause mortality | ||
| Hypothyroidism | 1.00 | 0.870 |
| Hyperthyroidism | 1.00 | 0.504 |
| Cardiac death and/or hospitalization | ||
| Hypothyroidism | 0.119 | 0.005 |
| Hyperthyroidism | 0.806 | 0.932 |
Discussion
The present study demonstrated that both subclinical hypothyroidism and subclinical hyperthyroidism are associated with adverse prognosis in HF patients. Subclinical hypothyroidism can increase the risk of both all-cause mortality and cardiac death and/or hospitality in HF patients. Subclinical hyperthyroidism can also increase the risk of all-cause mortality but appeared to have no distinguishing association with cardiac death and/or hospitality in patients with HF. In addition, these significant adverse associations were also retained in subgroup analysis when adjusting for study quality, ethnicity, mean age, mean duration of follow-up, sample size, amiodarone and thyroid treatment. Besides, sensitivity analysis indicated that no individual study had a remarkable effect on the overall results of the present meta-analysis, demonstrating the results of the current meta-analysis were stable. Considering both subclinical hypothyroidism and subclinical hyperthyroidism are associated with adverse prognosis in HF patients and the test of thyroid function is inexpensive and simple to determine, subclinical thyroid dysfunction may potentially be a useful and promising predictor for the long-term prognosis in HF patients.
In our present meta-analysis, we investigated the association between both subclinical hypothyroidism and subclinical hyperthyroidism and the clinical prognosis in HF patients. A previous meta-analysis published in 2015 [29] has investigated the association between subclinical hypothyroidism and the clinical prognosis in HF patients. Though the previous meta-analysis got the same results as ours, our meta-analysis has some advantages over the previous one. First, this is an update of the previous one. In our meta-analysis, we included four new studies [15–18] which were not contained in the previous meta-analysis and excluded two studies contained in the previous meta-analysis [30, 31] which didn’t report multivariate adjusted HR with 95% CI. Second, our meta-analysis also investigated the association between subclinical hyperthyroidism and the clinical prognosis in HF patients which did not contained in the previous meta-analysis. Third, to our knowledge, this is the first meta-analysis to clarify the relationship between subclinical hyperthyroidism and the outcomes of HF patients. Our results showed that hyperthyroidism can only increase the risk of all-cause mortality but have no influence on cardiac death and/or hospitalization. The number of studies reporting the association between hyperthyroidism and cardiac death and/or hospitalization is relatively small which may lead to a lack of statistical power. Besides, despite the negative effects of hyperthyroidism, there are also potentially positive effects of hyperthyroidism, such as increased contractility [32, 33], reduced peripheral resistance [32, 33], and increased production of natriuretic peptides [34], which may to some extent have compensatory effect.
There are several possible mechanisms accounting for the adverse prognosis of hypothyroidism on HF patients. First, previous studies have reported that hypothyroidism has influence on the structure and function of heart and these alterations can be reversed by thyroid hormone substitutive therapy [35–37]. Second, several studies have reported the link between hypothyroidism and pulmonary hypertension [38–40] which is associated with the mortality with HF patients [41, 42]. Thyroid hormone substitutive therapy can lead to the modification of pulmonary hypertension [38–40]. Third, hypothyroidism can significantly reduce cardiac preload, whereas increasing cardiac afterload results in a consequent reduction in stroke volume and cardiac output [8]. Replacement treatment of thyroid hormone can fully normalized the alterations of hemodynamics [8]. Fourth, hypothyroidism is reported to be associated with anemia which might be one of the causes leading to reduced exercise capacity [43, 44]. Besides the potential mechanisms above, hypothyroidism can also lead to altered lipid metabolism [45], elevated C-reactive protein [46], and increased prevalence of aortic atherosclerosis [47], which can increase the prevalence of myocardial infarction and mortality in HF patients [47, 48].
The potential reasons of why hyperthyroidism is associated with an increased mortality may be as follows. First, hyperthyroidism can cause a high cardiac output state with the increase in heart rate and cardiac preload and the reduced resistance of peripheral vascular [49]. Second, hyperthyroidism is associated with increased heart rate and increased risk of atrial fibrillation [50] which are attributable to the effects of thyroid hormone T3 on systolic depolarization and diastolic repolarization with decreased action potential and refractory period duration in atrial and ventricular myocardium [51]. Development of atrial fibrillation may account for increased vascular mortality [51]. In addition, hyperthyroidism is also related to an increased mass of left ventricle [52] which can lead to late diastolic dysfunction [53] and decreased exercise tolerance [54].
There are several strengths of the present study. First, all articles of the eligible cohort studies are published without conference abstract. Moreover, the analysis of included studies is depended on definite inclusion and exclusion criteria. Besides, the present study is an update of the previous meta-analysis investigating the association between subclinical hypothyroidism and the prognosis of HF patients. In the present study, we included four new studies which are not involved in the previous meta-analysis. In addition, to our knowledge, this is the first meta-analysis to investigate the association between subclinical hyperthyroidism and the prognosis of HF patients. The present study also has several limitations. One possible limitation of the present studies is that there is heterogeneity in the included hypothyroidism-related studies. Second, the confounding factors adjusted in different studies are varied. Some well-established variables, such as the history of cardiovascular disease, renal function, natriuretic peptides, and troponins are not adjusted in several included studies. Third, sample size in some included studies is not large enough. Fourth, the number of studies for performing meta-analysis investigating the association of hyperthyroidism and cardiac death and/or hospitalization is relatively small. Because of these general limitations, the results should be interpreted with caution and further studies with larger sample size should be taken to confirm the results.
Conclusion
The present study demonstrated that both subclinical hypothyroidism and subclinical hyperthyroidism are associated with adverse prognosis in patients with HF. Subclinical thyroid dysfunction may be potentially a useful and promising predictor for the long-term prognosis in HF patients.
Acknowledgements
None.
Funding
This work was supported by grant from Science and Technology Program for Public Wellbeing of China (2012GS610101) for data collection, analysis, and English language proofreading service.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AF
Atrial fibrillation
- BMI
Body mass index
- BNP
B-type natriuretic protein
- BP
Blood pressure
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CI
Confidence interval
- CVD
Cerebrovascular disease
- DM
Diabetes mellitus
- eGFR
Chronic heart failure
- FT3
Triiodothyronine
- FT4
Thyroxine
- HF
Heart failure
- HR
Hazard ratio
- HR
Heart rate
- ICD
Implantable cardioverter
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- NT-Pro BNP
N-terminal of the prohormone brain natriuretic peptide
- NYHA
New York Heart Association
- TSH
Thyroid-stimulating hormone
Authors’ contributions
GDY and AQM contributed to the conception and design of the study. GDY, YW, and TZW contributed to the collection and analysis of the data. GDY and TZW contributed to the drafting of the article. All authors approved the final version of the manuscript for publication.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guodong Yang, Email: ygddragon@qq.com.
Ya Wang, Email: 244908542@qq.com.
Aiqun Ma, Phone: 86-29-85323524, Email: maaiqun@medmail.com.cn.
Tingzhong Wang, Phone: 86-29-85323524, Email: tingzhong.wang@mail.xjtu.edu.cn.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, F. American College of Cardiology, G. American Heart Association Task Force on Practice 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, Katus HA, Debus J, Bischof M. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer. 2010;10:105. doi: 10.1186/1471-2407-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis KS, Butler J, Bauersachs J, Sandner P. The three-decade long journey in heart failure drug development. Handb Exp Pharmacol. 2017;243:1–14. doi: 10.1007/164_2016_101. [DOI] [PubMed] [Google Scholar]
- 5.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 6.Dillmann WH. Cellular action of thyroid hormone on the heart. Thyroid. 2002;12(6):447–452. doi: 10.1089/105072502760143809. [DOI] [PubMed] [Google Scholar]
- 7.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 8.Ripoli A, Pingitore A, Favilli B, Bottoni A, Turchi S, Osman NF, De Marchi D, Lombardi M, L’Abbate A, Iervasi G. Does subclinical hypothyroidism affect cardiac pump performance? Evidence from a magnetic resonance imaging study. J Am Coll Cardiol. 2005;45(3):439–445. doi: 10.1016/j.jacc.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Biondi B, Palmieri EA, Lombardi G, Fazio S. Subclinical hypothyroidism and cardiac function. Thyroid. 2002;12(6):505–510. doi: 10.1089/105072502760143890. [DOI] [PubMed] [Google Scholar]
- 10.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 12.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 13.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95(2):496–502. doi: 10.1210/jc.2009-1845. [DOI] [PubMed] [Google Scholar]
- 14.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 15.Ro K, Yuen AD, Du L, Ro CC, Seger C, Yeh MW, Leung AM, Rhee CM. Impact of hypothyroidism and heart failure on hospitalization risk. Thyroid. 2018; 28(9):1094–1100. [DOI] [PMC free article] [PubMed]
- 16.Sato Y, Yoshihisa A, Kimishima Y, Kiko T, Watanabe S, Kanno Y, Abe S, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Ishida T, Takeishi Y. Subclinical hypothyroidism is associated with adverse prognosis in heart failure patients. Can J Cardiol. 2018;34(1):80–87. doi: 10.1016/j.cjca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Hasegawa T, Kanzaki H, Funada A, Amaki M, Takahama H, Ohara T, Sugano Y, Yasuda S, Ogawa H, Anzai T. Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC Heart Fail. 2016;3(3):168–176. doi: 10.1002/ehf2.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Guan H, Gerdes AM, Iervasi G, Yang Y, Tang YD. Thyroid status, cardiac function, and mortality in patients with idiopathic dilated cardiomyopathy. J Clin Endocrinol Metab. 2015;100(8):3210–8. doi: 10.1210/jc.2014-4159. [DOI] [PubMed] [Google Scholar]
- 19.Sharma AK, Vegh E, Orencole M, Miller A, Blendea D, Moore S, Lewis GD, Singh JP, Parks KA, Heist EK. Association of hypothyroidism with adverse events in patients with heart failure receiving cardiac resynchronization therapy. Am J Cardiol. 2015;115(9):1249–1253. doi: 10.1016/j.amjcard.2015.01.559. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Yang X, Wang Y, Ding L, Wang J, Hua W. The prevalence and prognostic effects of subclinical thyroid dysfunction in dilated cardiomyopathy patients: a single-center cohort study. J Card Fail. 2014;20(7):506–512. doi: 10.1016/j.cardfail.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Perez AC, Jhund PS, Stott DJ, Gullestad L, Cleland JG, van Veldhuisen DJ, Wikstrand J, Kjekshus J, McMurray JJ. Thyroid-stimulating hormone and clinical outcomes: the CORONA trial (controlled rosuvastatin multinational study in heart failure) JACC Heart Fail. 2014;2(1):35–40. doi: 10.1016/j.jchf.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Shauer A, Zwas DR, Lotan C, Keren A, Gotsman I. The effect of thyroid function on clinical outcome in patients with heart failure. Eur J Heart Fail. 2014;16(2):217–226. doi: 10.1002/ejhf.42. [DOI] [PubMed] [Google Scholar]
- 23.van Deursen VM, Urso R, Laroche C, Damman K, Dahlstrom U, Tavazzi L, Maggioni AP, Voors AA. Co-morbidities in patients with heart failure: an analysis of the European heart failure pilot survey. Eur J Heart Fail. 2014;16(1):103–111. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 24.Azemi T, Bhavnani S, Kazi F, Coleman CI, Guertin D, Kluger J, Clyne CA. Prognostic impact of thyroid stimulating hormone levels in patients with cardiomyopathy. Conn Med. 2013;77(7):409–415. [PubMed] [Google Scholar]
- 25.Mitchell JE, Hellkamp AS, Mark DB, Anderson J, Johnson GW, Poole JE, Lee KL, Bardy GH. Thyroid function in heart failure and impact on mortality. JACC Heart Fail. 2013;1(1):48–55. doi: 10.1016/j.jchf.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM. Subclinical hypothyroidism and survival: the effects of heart failure and race. J Clin Endocrinol Metab. 2013;98(6):2326–2336. doi: 10.1210/jc.2013-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey A, Kroiss M, Berliner D, Seifert M, Allolio B, Guder G, Ertl G, Angermann CE, Stork S, Fassnacht M. Prognostic impact of subclinical thyroid dysfunction in heart failure. Int J Cardiol. 2013;168(1):300–305. doi: 10.1016/j.ijcard.2012.09.064. [DOI] [PubMed] [Google Scholar]
- 28.Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R, Cicala M, Basile M, Sorrentino S, Favale S. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des. 2008;14(26):2686–2692. doi: 10.2174/138161208786264142. [DOI] [PubMed] [Google Scholar]
- 29.Ning N, Gao D, Triggiani V, Iacoviello M, Mitchell JE, Ma R, Zhang Y, Kou H. Prognostic role of hypothyroidism in heart failure: a meta-analysis. Medicine (Baltimore) 2015;94(30):e1159. doi: 10.1097/MD.0000000000001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triggiani V, Iacoviello M. Thyroid disorders in chronic heart failure: from prognostic set-up to therapeutic management. Endocr Metab Immune Disord Drug Targets. 2013;13(1):22–37. doi: 10.2174/1871530311313010005. [DOI] [PubMed] [Google Scholar]
- 31.Triggiani V, Iacoviello M, Monzani F, Puzzovivo A, Guida P, Forleo C, Ciccone MM, Catanzaro R, Tafaro E, Licchelli B, Giagulli VA, Guastamacchia E, Favale S. Incidence and prevalence of hypothyroidism in patients affected by chronic heart failure: role of amiodarone. Endocr Metab Immune Disord Drug Targets. 2012;12(1):86–94. doi: 10.2174/187153012799278947. [DOI] [PubMed] [Google Scholar]
- 32.Marcisz C, Jonderko G, Wroblewski T, Kurzawska G, Mazur F. Left ventricular mass in patients with hyperthyroidism. Med Sci Monit. 2006;12(11):CR481–CR486. [PubMed] [Google Scholar]
- 33.Marcisz C, Kucharz EJ, Jonderko G, Wojewodka J. The systolic function of the left ventricle of the heart in patients with hyperthyroidism during therapy. Pol Arch Med Wewn. 2001;105(2):131–138. [PubMed] [Google Scholar]
- 34.Ozmen B, Ozmen D, Parildar Z, Mutaf I, Bayindir O. Serum N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) levels in hyperthyroidism and hypothyroidism. Endocr Res. 2007;32(1–2):1–8. doi: 10.1080/07435800701670047. [DOI] [PubMed] [Google Scholar]
- 35.Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, Ferrannini E. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86(3):1110–1115. doi: 10.1210/jcem.86.3.7291. [DOI] [PubMed] [Google Scholar]
- 36.Di Bello V, Monzani F, Giorgi D, Bertini A, Caraccio N, Valenti G, Talini E, Paterni M, Ferrannini E, Giusti C. Ultrasonic myocardial textural analysis in subclinical hypothyroidism. J Am Soc Echocardiogr. 2000;13(9):832–840. doi: 10.1067/mje.2000.106397. [DOI] [PubMed] [Google Scholar]
- 37.Biondi B, Fazio S, Palmieri EA, Carella C, Panza N, Cittadini A, Bone F, Lombardi G, Sacca L. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J Clin Endocrinol Metab. 1999;84(6):2064–2067. doi: 10.1210/jcem.84.6.5733. [DOI] [PubMed] [Google Scholar]
- 38.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, E.S.C.S.D. Group 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 39.Li JH, Safford RE, Aduen JF, Heckman MG, Crook JE, Burger CD. Pulmonary hypertension and thyroid disease. Chest. 2007;132(3):793–797. doi: 10.1378/chest.07-0366. [DOI] [PubMed] [Google Scholar]
- 40.Vakilian F, Attaran D, Shegofte M, Lari S, Ghare S. Assessment of thyroid function in idiopathic pulmonary hypertension. Res Cardiovasc Med. 2016;5(2):e29361. doi: 10.5812/cardiovascmed.29361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183–188. doi: 10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 42.Fabregat-Andres O, Estornell-Erill J, Ridocci-Soriano F, Garcia-Gonzalez P, Bochard-Villanueva B, Cubillos-Arango A, Espriella-Juan Rde L, Facila L, Morell S, Cortijo J. Prognostic value of pulmonary vascular resistance estimated by cardiac magnetic resonance in patients with chronic heart failure. Eur Heart J Cardiovasc Imaging. 2014;15(12):1391–1399. doi: 10.1093/ehjci/jeu147. [DOI] [PubMed] [Google Scholar]
- 43.Duntas LH, Papanastasiou L, Mantzou E, Koutras DA. Incidence of sideropenia and effects of iron repletion treatment in women with subclinical hypothyroidism. Exp Clin Endocrinol Diabetes. 1999;107(6):356–360. doi: 10.1055/s-0029-1212126. [DOI] [PubMed] [Google Scholar]
- 44.Horton L, Coburn RJ, England JM, Himsworth RL. The haematology of hypothyroidism. Q J Med. 1976;45(177):101–123. [PubMed] [Google Scholar]
- 45.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 46.Christ-Crain M, Meier C, Guglielmetti M, Huber PR, Riesen W, Staub JJ, Muller B. Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis. 2003;166(2):379–386. doi: 10.1016/S0021-9150(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 47.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med. 2000;132(4):270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 48.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J, Thyroid Studies C. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31–50. doi: 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 50.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D’Agostino RB. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 51.Osman F, Gammage MD, Sheppard MC, Franklyn JA. Clinical review 142: cardiac dysrhythmias and thyroid dysfunction: the hidden menace? J Clin Endocrinol Metab. 2002;87(3):963–967. doi: 10.1210/jcem.87.3.8217. [DOI] [PubMed] [Google Scholar]
- 52.Biondi B, Palmieri EA, Fazio S, Cosco C, Nocera M, Sacca L, Filetti S, Lombardi G, Perticone F. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab. 2000;85(12):4701–4705. doi: 10.1210/jcem.85.12.7085. [DOI] [PubMed] [Google Scholar]
- 53.Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137(11):904–914. doi: 10.7326/0003-4819-137-11-200212030-00011. [DOI] [PubMed] [Google Scholar]
- 54.Mercuro G, Panzuto MG, Bina A, Leo M, Cabula R, Petrini L, Pigliaru F, Mariotti S. Cardiac function, physical exercise capacity, and quality of life during long-term thyrotropin-suppressive therapy with levothyroxine: effect of individual dose tailoring. J Clin Endocrinol Metab. 2000;85(1):159–164. doi: 10.1210/jcem.85.1.6298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.