Abstract
Adult-born neurons are continuously generated and incorporated into the circuitry of the hippocampus throughout life in mammals. Cumulative evidence supports a physiological role for adult-born neurons, yet it not clear whether this subset of dentate granule cells makes a unique contribution to hippocampal function. Perturbation or ablation of adult hippocampal neurogenesis leads to deficits in the acquisition of learned associations or memory recall, whereas an increase in adult hippocampal neurogenesis enhances some forms of learning and memory. The observed effects thus far appear to be task-dependent, species-specific, and sensitive to the timing of manipulations. Here, we review the recent evidence correlating adult-born dentate granule cells with hippocampal-dependent behavior and focus on the dynamic properties of this neuronal population that may underlie its function. We further discuss a framework for future investigations of how newly integrated neurons may contribute to hippocampal processing using advanced genetic techniques with enhanced temporal resolution.
Keywords: adult neurogenesis, dentate gyrus, cognitive function, behavior
1. Introduction
Robust adult neurogenesis, the generation of new neurons from neural progenitor cells, is observed throughout life in almost all mammals examined and there is much interest in identifying the functional significance of this phenomenon [1–5]. Two primary sites of adult neurogenesis in mammals are the dentate gyrus of the hippocampal formation and the subventricular zone/olfactory bulb system. Because the hippocampus is believed to mediate various forms of learning and memory [6–8], this region has received the bulk of attention from investigators trying to establish a causal link between adult neurogenesis and the maintenance or enhancement of cognitive abilities [9]. In the dentate gyrus of young adult mice, approximately 4,000–7,000 new cells are born each day as measured by pulsing dividing cells with nucleotide analog bromodeoxyuridine (BrdU) and in young adult rats, the rate of neurogenesis is nearly 50% higher [10–12]. In mice, less than a third of the newborn neurons survive and are ultimately integrated into neuronal circuitry in the adult brain [12–14]. Although adult neurogenesis appears to recapitulate embryonic neurodevelopment in many respects, it is unique in that adult-born neurons must incorporate into established circuitry within a functionally mature brain. A fundamental question in this field is whether these comparatively young neurons make a special contribution to information processing mediated by the local circuitry.
To address this question, there have been many attempts to correlate levels of adult neurogenesis with behavior. Suppression of adult neurogenesis in rodents has met with mixed results in that most, but not all, hippocampal-dependent tasks are negatively affected by a decrease in neurogenesis and that the effects can be species-specific and/or temporally graded [9, 15]. Although a consistent function for adult neurogenesis in all forms of hippocampal-dependent learning has not been identified, there may be confounding factors that prevent this observation. First, most manipulations used to arrest adult neurogenesis have some nonspecific effect on the system or local circuitry that could have independent effects on behavior. Second, there has been very little parametric testing to identify the critical age of adult-born neurons within the same testing conditions. Newborn neurons undergo robust changes in morphology, ion channel expression, neurotransmitter response, and other critical intracellular properties over the course of development [16, 17]. All of these factors affect signaling both within and between cells, and interactions between adult-born neurons and the local environment likely depend on the stage of cellular maturation. In this review, we discuss the contribution of adult-born dentate granule cells (DGCs) to behavior as a function of time-dependent intrinsic changes in their properties and consider optimal approaches to evaluate the role of this continually evolving population.
2. Development of newborn dentate granule cells in the adult hippocampus
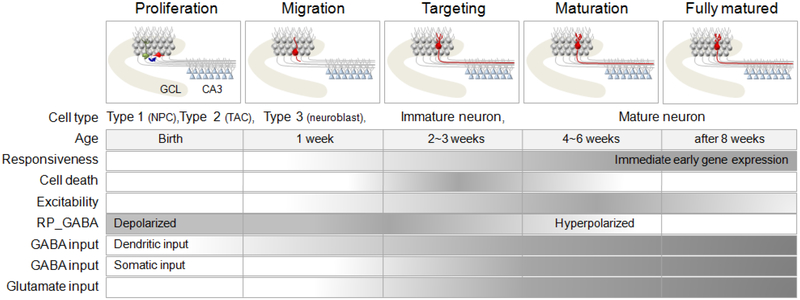
We focus on young adult mice to discuss functional stages of hippocampal neurogenesis. The progression of developmental stages is well-conserved in different species, although the timeline of neuronal development may be species-specific [18]. For example, adult neurogenesis in rats appears to occur at a faster pace and at a higher rate than in mice [12]. Quantitatively, levels of proliferation and survival are reduced in aged animals. It remains to be fully characterized whether there is a change in the pace of maturation or functional properties of the surviving adult-born neurons in aged animals [19]. We divide the development of newborn DGCs in the adult mouse hippocampus into four stages (Figure 1).
Figure 1. Development of adult-born dentate granule cells.
Top, Morphological maturation of adult-born DGC (red) after birth from neural precursors (green, left). Bottom, Newborn neurons migrate and integrate into the dentate circuit. Axons are elongated and contact the pyramidal cells of CA3 after 1–2 weeks and spines start to appear after 2 weeks. During synaptogenesis, new neurons compete to survive and many are eliminated by programmed cell death. The physiological properties of new neurons reflect a gradual change in the expression of chloride transporters during the maturation process. Immature neurons are initially depolarized by GABA and then hyperpolarized after maturation. These GABAergic inputs are initially dendritic but upon maturation and synapse refinement from afferent pathways, functional perisomatic inputs develop. Lastly, following the expression of NMDA receptors, new DGCs become activated by glutamatergic inputs. NPC: neural progenitor cell, TAC: transient amplifying cell, GCL: granule cell layer, RP-GABA: reversal potential of GABA.
2.1. Proliferation of adult neural progenitors and survival of early neuronal progeny
In the adult dentate gyrus, neural progenitors are located in the subgranular zone (SGZ) at the border between the hilus and the granule cell layer (GCL). GFAP+nestin+ radial glia-like cells [20, 21] and Sox2+ non-radial cells [22] are believed to be multipotent adult neural stem cells. These precursors give rise to rapidly dividing transient amplifying cells expressing Tbr2, which in turn generate immature neurons in the dentate gyrus [23]. Following BrdU pulsing to label proliferating cells during the S-phase, most BrdU+ cells differentiate into neurons, but some differentiate into astrocytes [11, 24]. Time course analyses using retrovirus-based lineage tracing showed that proliferating neural progenitors in young adult mice largely commit to a neuronal fate, express the immature neuronal marker DCX within 3 days, and become post-mitotic within 7 days after birth [25]. There is a significant loss of newborn progeny during the first 4 days after birth. A recent study suggests that apoptotic mechanisms trigger cell death and microglia-mediated phagocytosis rapidly clears the affected cells from the SGZ during this early critical period [14].
2.2. Migration and initial integration of immature neurons with GABAergic synaptic inputs
Newborn immature neurons migrate only a short distance into the inner granule cell layer after birth and express Prox1, a marker for DGCs [26, 27]. These new neurons lack dendritic processes and display a high membrane resistance due to a low density of somatic ion channels [25, 28, 29]. Nevertheless, within 3 days after birth, these immature neurons already exhibit functional GABAA receptors that are tonically activated by ambient GABA in the environment [25, 30]. By 7 days, newborn neurons extend dendrites toward the molecular layer and start to receive functional GABAergic synaptic inputs, before any functional glutamatergic inputs can be detected [25, 31, 32]. As in perinatal neuronal development, the classical inhibitory neurotransmitter GABA initially exerts a depolarizing influence on immature neurons [33, 34]. This GABA-mediated depolarization serves as a trophic mechanism to promote differentiation, migration and maturation of immature neurons in the dentate gyrus [25, 35–37]. Immature neurons thus respond in a diametrically opposed way to ambient GABA as compared to mature neurons. Increased GABA levels within the neurogenic region will both inhibit older neurons and promote the growth of recently born neurons. It is not yet clear how this GABA-mediated balance of excitation and inhibition in distinct neuronal subpopulations within the dentate gyrus circuitry may contribute to hippocampal function.
2.3. Activation and synaptic integration of immature neurons with glutamatergic synaptic inputs and outputs
Within 2–3 weeks after birth, newborn DGCs in young adult mice exhibit elaborated dendritic processes and project axons to the CA3 target region [25, 29, 31, 38]. These adult-born neurons also start to receive functional glutamatergic synaptic inputs [25, 31] as their efferent mossy fibers begin to make synaptic contacts with downstream hilar interneurons and CA3 pyramidal neurons [38, 39]. Because the number of efferent (CA3) and afferent (entorhinal cortex) target neurons do not change considerably in the adult brain, newly generated DGCs compete with a population of mature neurons for potential sites of synaptic contact. Dendritic filopodia of new neurons form synaptic contacts with pre-existing axonal boutons [40], whereas the axons of newborn cells initiate early synapse formation primarily on dendritic shafts in CA3 [39]. The time course of presynaptic and postsynaptic targeting appears to be synchronized and both processes are at least partially governed by activity-driven competition [16, 41]. Neuronal activity also regulates the survival of newborn neurons during this period in an NMDA receptor-dependent fashion [42]. Optogenetic and pharmacological analyses have demonstrated that adult-born DGCs release glutamate once fully mature [39], but it is unknown whether newborn DGCs may also release GABA transiently during development [43, 44]. It is also during this period of time that the polarity of GABAergic responses in newborn neurons switches from excitation to inhibition [25]. The electrophysiological properties of immature DGCs at this stage are strikingly different from their mature counterparts [25, 31, 45]. Despite similar resting potentials, immature DGCs have a higher input resistance and are capable of generating action potentials in response to weaker stimulation than that required for mature DGCs [46].
2.4. Synaptic maturation of afferent and efferent connections and critical period of synaptic plasticity
Between 4–8 weeks after birth, new neurons in young adult mice exhibit increases in dendritic arborization and dendritic spine number, as well as refinement of axon terminals and maturation of mossy fiber boutons [29, 38]. This is also the period when newborn DGCs exhibit unique properties in synaptic plasticity [16, 45, 46]. Long-term potentiation (LTP) exhibits a lower threshold for induction and larger amplitude in these adult-born neurons compared to perinatal- or adult-born neurons at more mature stages. Pharmacological analysis showed that this enhanced plasticity is mediated by NR2B-containing NMDA receptors in adult-born neurons [45, 47]. Furthermore, LTP induction in adult-born neurons during this period is insensitive to GABAergic inhibition, whereas suppression of GABAergic transmission is a requirement for LTP in mature DGCs in the acute slice preparation from adult animals [16, 45, 47]. This transient facilitation for associative plasticity in adult-born neurons could have two consequences; 1) synaptically-connected adult-born neurons make a unique contribution to information processing mediated by the dentate gyrus; and 2) adult-born neurons have an advantage in the competition with mature DGCs for stability of afferent and efferent synaptic connections [40, 42].
2.5. Maintenance of adult-born dentate granule cells
After adult-born DGCs establish stable synapses, they can survive for at least 6–11 months in rodents, and only a small fraction of cells are further eliminated by programmed cell death [26, 48]. Considering the 2–3 year life span of rodents, the evidence suggests that adult-born DGCs remain a part of the mature dentate circuitry throughout life. Whole-cell recording in acute slices prepared from adult animals showed that adult-born neurons, once they reach full maturation, appear to exhibit basic electrophysiological properties indistinguishable from those of DGCs formed in embryonic and early postnatal stages [49, 50]. However, it is possible that there may be structural differences in synaptic patterning between adult and perinatal-born neurons that could alter the input-output relationship in a more physiological context. For example, adult-born neurons may be predisposed toward establishing certain synaptic contacts due to enhanced plasticity and the need to compete with existing neurons during synapse formation. In vivo recording of adult-born DGCs at different developmental stages will be necessary to determine if adult neurogenesis produces a population of dentate granule neurons that become functionally equivalent to the pre-existing population of granule cells.
3. Timing of the functional involvement of adult-born DGCs
The dentate gyrus of the hippocampal formation has been implicated in the regulation of emotion and cognition. Lesions or manipulations of this region in rodents can alter anxiety levels and affective and cognitive-like behaviors [51–53]. Many of the behavioral tests used in functional assays of neurogenesis were designed to measure hippocampal-dependent behavior and general anxiety and depressive-like symptoms (Table 1). Computational and theoretical models of specific functions of the dentate gyrus have primarily focused on the proposed orthogonalization of inputs mediated by this region, which allows for pattern separation and the ability to discriminate between similar events [9]. For example, an expansive population of adult-born neurons could be involved in disambiguating events by providing a non-diminishing pool of dentate cells available for encoding novel experiences. In this way, different patterns could be successfully represented without overlap or distortion by distinct dentate granule cell populations. Recently, it was argued that adult-born neurons could also play a role in the temporal integration of events that occur closely in time and that the enhanced plasticity of young neurons effectively provides a timestamp for experiences [9, 54]. This temporal tagging hypothesis asserts that disambiguation of similar events can result from associating an event with a population of adult-born neurons at a distinct stage of maturation. This would occur during the period of heightened plasticity and result in a neural representation with embedded temporal reference information. The general proposal that the capacity to encode information scales with synaptic plasticity is compelling but it is unknown whether this enhanced plasticity translates into decreased stimulus selectivity and/or more robust or longer-lasting potentiation of effective synapses in the behaving animal. In vitro slice recording data appears to support both interpretations. LTP is both easier to induce and results in a higher amplitude response following high frequency stimulation when newborn cells are 4–6 weeks old [45]. However, the lasting properties of this potentiation are difficult to measure in a slice preparation. Determining how this critical period affects the acquisition and long-term expression of memory is one of the outstanding questions in the field of adult neurogenesis.
Table 1.
Common behavioral tests used to evaluate the function of adult neurogenesis in rodents
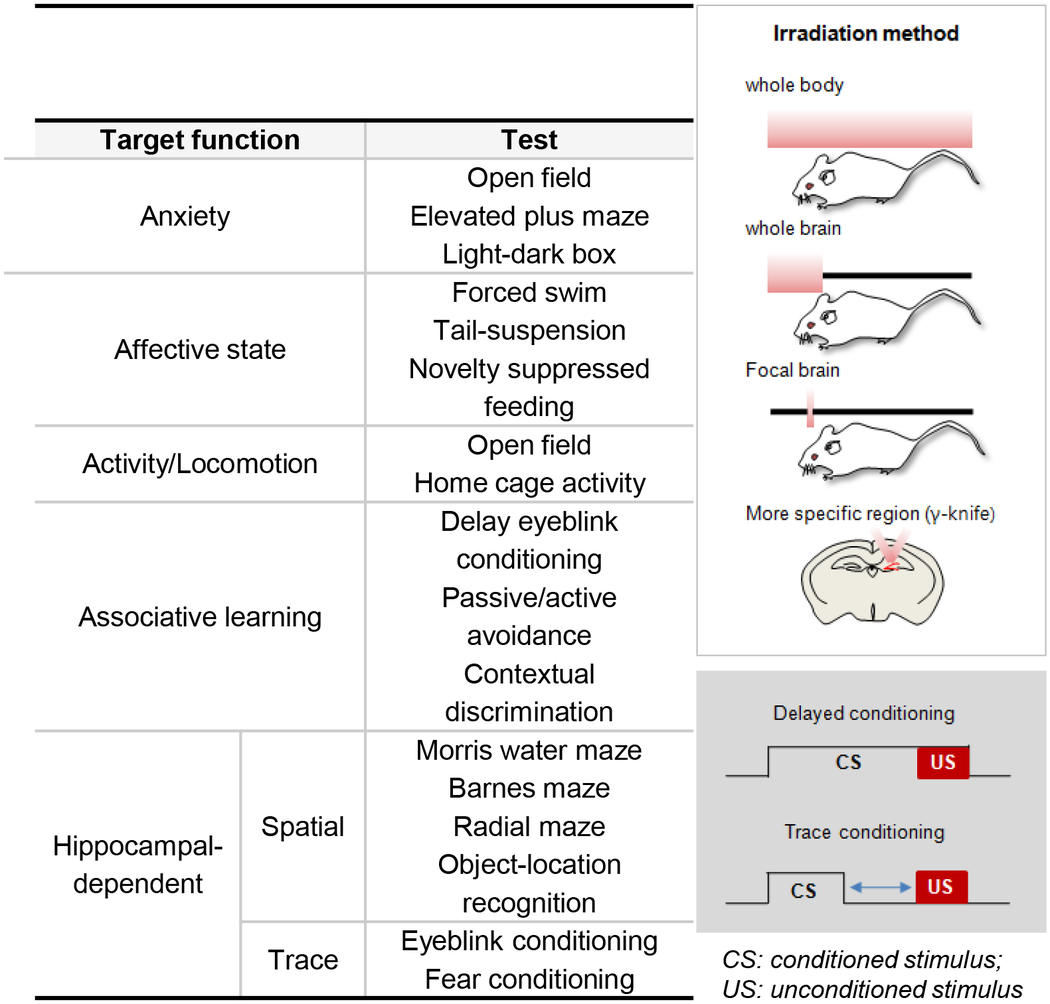
|
To date, three major experimental approaches have been used to evaluate the role of newborn neurons in hippocampal-dependent behavior. First, after birth-dating dividing cells via BrdU, EdU or GFP-tagged retrovirus injections, functional involvement of labeled DGCs can be identified based on co-labeling with markers for IEGs. IEG expression has been widely used as an index of neuronal activation following controlled exposure to environmental stimuli or direct stimulation of specific brain regions [55] and can provide information concerning the magnitude and timing of the involvement of newborn DGCs in response to a particular experience. Second, the rate of neurogenesis or the survival of newborn neurons can be increased or decreased by several factors, such as exercise, environmental enrichment, antidepressant treatment, aging and stress; and behavioral responses of animals with different levels of adult neurogenesis can then be compared. Third, elimination of adult neurogenesis has been largely achieved through irradiation and methylazoxymethanol acetate (MAM) treatment to target dividing cells or through genetic modifications to target progenitor subtypes [56]. Each of these ablation techniques has advantages and disadvantages, but the shared rationale is that effective removal of adult neurogenesis from the hippocampus will result in behavioral changes that should be indicative of the function of this population in the intact animal. We discuss the benefits and limitations of each of these approaches in identifying the contribution of newborn dentate granule neurons to behavior.
3.1. IEG expression
IEGs are transiently induced in the adult dentate gyrus for a few hours following electrical and pharmacological stimulation or exposure to a novel environment [57–59]. One of the distinct advantages of using IEG expression as a readout of the involvement of newborn neurons is that activation can be linked to an age-restricted subset of neurons based on coincident birth-dating. In mice, the initiation of a significant IEG response at the population level to behaviorally relevant stimuli does not occur until newborn granule cells are at least 3 weeks old [12, 60]. Although early studies based on IEG quantification had shown that there is a preferential activation of adult-born neurons in some tasks [61], emerging data paints a different picture. Other studies report that adult-born DGCs, once reaching the stage of heightened plasticity, are recruited at the same rate as embryonically-derived DGCs [12, 62]. Once the newborn neurons become responsive, recruitment of newborn neurons in learning a spatial task, such as the Morris water maze (MWM), gradually increases as the new neurons become mature [12]. These data thus suggest that the involvement of newborn neurons in learning and memory may reach asymptotic levels at the same time when plasticity thresholds are lowered, rather than exhibiting a transient peak in activity that correlates with enhanced plasticity.
Retrieval of remote spatial memory was shown to activate neurons that were less than two weeks old at the time of training, demonstrating the involvement of newborn neurons during memory expression, despite having been functionally immature at the time of encoding [63]. This would suggest that DGCs, regardless of developmental origin, are activated at the same rate and that involvement of new DGCs in behavioral tasks is based on functional maturation. Re-exposure to task features may occur when a completely distinct population of newborn cells is in the critical period of enhanced plasticity. If subsets of DGCs are associated with particular stimuli, it is unclear how the mature population that encoded the previously experienced environment would compete with recently born DGCs that are also primed to respond, but as though it were a novel context.
Contrary to these data which minimize the “uniqueness” of the newborn neurons, a recent study made the provocative claim that the most, and perhaps only, functional and behaviorally relevant population of neurons in the dentate gyrus consists of recently born DGCs, as mature cells are phased out and become unresponsive [64]. If most mature DGCs no longer contribute to neural representations mediated by the dentate gyrus, it is unlikely that a particular population of newborn neurons is linked to a specific memory trace for an unbounded time. In this study, IEG expression was quantified following either re-exposure to the same environment at several time points or a series of novel environments, and the analysis showed that only a small number of granule cells are active in any environment, suggestive of a restricted pool of readily available neurons. If young neurons remain associated with particular events as they mature, as predicted by the temporal tagging hypothesis, then re-exposure would result in more cumulative activation due to the inclusion of additional populations entering the critical period at each time point. The authors instead observed that the total number of active cells was independent of previous exposure to the experimental contexts. The interpretation of these data was that the critical determinant of DGCs involvement in hippocampal-dependent memory is the age of the cell, with new populations being recruited almost exclusively.
3.2. Physiological regulation of neurogenesis
The rate of adult neurogenesis is modulated by various factors: activities such as voluntary wheel running and learning, or prolonged exposure to enriched environment, stress, hormones, antidepressants and neurotransmitters [65, 66]. Up- or down-regulation of adult neurogenesis can, in turn, affect behavioral performance in some tasks. When animals are exposed to an enriched environment or exercise for 3–4 weeks, the numbers of newborn DGCs can increase up to 50% [67–70]. Under these conditions, spatial learning and memory are enhanced in the MWM and radial arm maze. When mice are given accelerating rotarod training for 5 days, adult neurogenesis is increased approximately 40% [71]. Subsequently, instrumental conditioning is enhanced in the pre-trained group, whereas trace eye-blink conditioning, a hippocampal-dependent task, is unaffected. Therefore, not all types of hippocampal-dependent learning are enhanced following exposure to pro-neurogenic stimuli. Although the impact of increased neurogenesis on behavior appears to be task-dependent, there is a consistently positive effect on the survival of newborn neurons born during a restricted time period before exposure to explicit learning protocols, exercise or environmental enrichment. Converging evidence suggests that activity-driven reduction in programmed cell death is most prominent between one and three weeks after the birth of the neurons [60].
A decline in neurogenesis of 32–70% can be induced by physiological insults, such as restraint stress for 21 days or sleep disruption [72, 73]. Functional consequences include impairments in spatial reference memory in the Barnes maze and a radial arm maze. Although sleep deprivation appears to have a consistently negative effect on neurogenesis and behavior, the behavioral effects of stress depend on the species, induction protocol, and duration of exposure [60, 74]. The data thus far suggests that stress and sleep disruption are most detrimental to the proliferation and survival of recently born neurons. Whether these conditions also alter the likelihood of newly matured neurons to be recruited during the formation of hippocampal-dependent memory remains to be determined.
One challenge in identifying the functional role of these exogenous regulators is that many of these treatments affect many physiological processes that have independent effects on learning and memory. In addition, these treatments have the most pronounced effect when administered chronically and thus it is difficult to target an age-constrained population of adult-born cells. It is therefore difficult to argue for a causal link between a specific effect on neurogenesis and changes in behavior without dissociating the neurogenic and systemic effects of the manipulation. Recent studies to explicitly test this relationship have shown that environmental enrichment can enhance learning and decrease anxiety-related behavior in the absence of adult neurogenesis [75], and conversely, that enhanced neurogenesis is not sufficient to induce anxiolytic-like behavior [76]. However, it has also been reported that suppressing neurogenesis during exposure to an enriched environment blocked long term memory enhancement [77]. More data is needed to resolve whether the impact on neurogenesis mediates the neural changes that underlie exogenous modulation of cognitive and affective behaviors.
3.3. Targeted ablation and increase of adult-born dentate granule cells
Because the population of newborn granule neurons is distributed throughout the dentate gyrus, it is impossible to selectively target these cells using traditional lesion and inactivation methods (Table 1). Instead, ablation techniques have been employed that take advantage of one of the unique properties of this population, i.e. cell division. MAM, an anti-mitotic agent, was first used to induce a targeted ablation of adult-born neurons [78–80]. After 2 weeks of treatment, the population of newly born DGCs is reduced over 75%. In rats treated with these drugs, hippocampal-dependent trace eye-blink and fear conditioning are impaired. Other approaches include injection of the anti-neoplastic agent cyclophosphamide, or neurotoxin 192 IgG-saporinin, which also leads to a reduction in the number of proliferating cells by 50–80% [81–83]. Following these treatments, spatial memory in the water maze, fear conditioning in the passive avoidance test, and object memory are severely impaired.
Because of the detrimental systemic effects of all of these drug treatments, cranial irradiation has been recently become the most prevalent means of neurogenic ablation. Actively dividing cells are sensitive to irradiation and undergo apoptosis, so it is possible to selectively ablate proliferating neural progenitors and neuroblasts with minimal damage to nearby mature neurons, glia or endothelial cells [84–88]. After irradiation, proliferating cells are reduced 70–95% and the number decreases further for another 2–3 months [78, 89]. Thus, this manipulation allows investigation of the cumulative contribution of adult-born DGCs at various time intervals from the onset of ablation. Interestingly, 2–4 weeks following either whole or focal brain irradiation in rats, contextual fear conditioning and place memory are selectively impaired, but spatial and object memory are virtually intact [90–94]. In mice, however, the most severe impairments of learning and memory have been reported to occur 2–3 months after irradiation. In the absence of adult neurogenesis for 2 months, spatial pattern separation is selectively impaired in a delayed non-matching to place task [95].
Recent evidence suggests that newly generated DGCs may play a role in the gradual decay of hippocampal-dependence of recently formed memory traces [89]. In the absence of adult neurogenesis for 5 weeks following irradiation, at a time-point when the memory trace is thought to rely on extra-hippocampal cortical structures, recall of the remote memory was still dependent on the hippocampus [89]. This result suggests that new neurons can regulate the transfer of memory to a reliance on extra-hippocampal structures, presumably to maintain the online storage capacity of hippocampus. Consistent with this hypothesis, enhanced neurogenesis speeds up the decay (clearance) rate of memory from hippocampus. Although 5 week-old new neurons express mature neuronal markers, synaptic plasticity is still higher than in the pre-existing mature neurons [45]. Taken together, we could hypothesize that if new neurons are selectively involved in the encoding of individual events and also the effective reorganization of memory traces, then both processes may due to enhanced synaptic plasticity of newborn neurons. Because new DGCs are more excitable, they could be primed to respond to new information. Furthermore, because they actively invade and incorporate into pre-existing circuits during competitive synapse formation, new neurons could also interfere with the efficacy of previously formed synapses. Thus, this population of developing neurons may be involved in both memory formation and decay. Because the memory traces are maintained by other neural systems, the decay of memory in the hippocampus should be viewed in terms of a homeostatic process that allows for the acquisition of new information through reorganization of more remotely acquired memories, rather than complete elimination of the memory trace. It is possible that the newborn neurons are promoting hippocampal independence through an active mechanism that also ensures the fidelity of the original memory. What does seem to be clear from the irradiation studies in mice is that there may be a cumulative effect such that prolonged training intervals from the time of irradiation may reveal additional deficits. By more carefully teasing apart the time-dependence of irradiation effects, we can get a better picture of how neuronal age determines the extent of behavioral impairments.
Genetic targeting, including the expression of toxins or pro-apoptotic genes under control of neural progenitor-specific promoters, allows for the specific ablation of adult-born DGCs with minimal confounds. In addition, inducible genetic manipulations increase temporal precision, which is critical for understanding the role of adult-born DGCs in hippocampal-dependent behavior. Four lines of transgenic (Tg) mice have been developed to explore the functional involvement of adult-born DGCs. One line expresses herpes virus thymidine kinase (TK) under the regulation of the mouse GFAP promoter. Proliferating cells are reduced by 75% following chronic delivery of the antiviral pro-drug ganciclovir (GCV) for 6–10 weeks. In these mice, working memory and contextual fear conditioning are impaired, but after 10 weeks recovery in the absence of GCV, working memory has fully recovered [96, 97]. This result suggests that new neurons, under 10 weeks of age, are involved in working memory processes. When TK is expressed under the nestin promoter and enhancer, only 2 weeks of treatment with GCV results in a 50% reduction in neurogenesis and deficits in both spatial memory and contextual fear memory extinction. However, after 4–9 weeks of recovery in the absence of the drug, the behavioral impairments fully recovered [98]. Similarly, using a Tet-On inducible system, the pro-apoptotic gene, Bax, is expressed under the regulation of the nestin promoter to ablate new born neurons. Following 6 weeks of treatment with doxycycline (Dox), there is a 60% reduction in proliferating cells and spatial learning is impaired, although contextual fear conditioning remains intact [99]. Much of the data suggests that 6–10 week old new neurons are critically involved in the functional deficits following transient reduction of neurogenesis, similar to the time course of involvement shown by IEG expression. Importantly, these studies using inducible techniques also demonstrate that impaired functions can be recovered when neurogenesis is restored, thus providing a link between adult neurogenesis and specific functions. A recent study employed a targeted genetic approach to suppress endogenous expression of Bax and increase adult neurogenesis in a gain-of-function experiment. Although novel object recognition and spatial memory were unaffected, the manipulation did result in a significant improvement in the ability of the mice to discriminate between similar contexts, suggesting that adult neurogenesis plays a role in pattern separation [76]. Despite the preponderance of evidence indicating that adult-born neurons are active between 1 and 3 months after birth, it is still difficult to identify a critical period during which these neurons play a distinctive role in hippocampal-dependent behavior from these studies. Even targeted treatments create a transient inflammatory response with potential functional consequences. Moreover, many of the ablation methods are irreversible and temporal control is limited. To cope with these limitations, new approaches are necessary to clarify the optimal timing for functional involvement and the specific role of adult born DGCs.
4. Newly advanced approach: optogenetics
Optogenetic techniques have emerged as an extremely effective and specific tool to answer some of the fundamental questions regarding the temporal involvement of adult-born neurons in behavior [100]. Through genetically controlled introduction of an opsin gene, we can control the activity of specific populations in the neurogenic regions through light-driven activation or suppression of targeted cells. Briefly, channelrhodopsin-2 (ChR2) is a light-gated, cation-permeable channel derived from Chlamydomonas reinhardtii, which can be activated by blue light at 470 nm. Halorhodopsin (NpHR), derived from Natronomonas pharaonis, is a chloride pump that responds to yellow light at 589 nm, which effectively silences NpHR expressing neurons. If both ChR2 and NpHR are simultaneously expressed in the same neurons, we can bidirectionally control the activity though two different wavelengths of light (Figure 2). This technique holds significant promise for experimental tractability in understanding how newborn neurons contribute to behavior. It is now theoretically possible to disrupt the activity of this population acutely during episodes of encoding and recall to determine how this dynamic granule cell population contributes to hippocampus-dependent information processing. In addition to examining behavioral effects of disrupted signaling in adult-born cells, we can also begin to address the downstream effects on neural processing in an intact system. By recording from adjacent areas and efferent targets of the hippocampal formation, we can monitor whether activation or suppression of this group of cells may impact population activity, long-range synchronous responses and oscillatory phase-dependent firing within the hippocampus. We anticipate much progress in the effort to understand why new neurons are necessary, when they become involved in hippocampal function, and how they contribute to specific forms of learning and memory. One potential drawback of this technique is that chronic disruption of newborn granule cell activity will be more technically challenging to achieve. Although the temporal resolution is sufficient to perturb activity on a millisecond scale and therefore ideal for acute investigations during task performance, it does not easily allow for ongoing manipulation outside the experimental setting. This will be critical to evaluate the involvement of adult-born neurons in systems-level consolidation or offline memory reorganization. Another serious challenge will be to ensure that a sufficient portion of the population of newborn neurons is targeted through acute viral injections. The appeal of in vivo studies of function is largely derived from the fact that neuronal networks remain intact, which makes it possible to investigate mechanisms in a physiological context. But if a virally-mediated manipulation affects only a subset of targeted cells, then there could be consequences owing to a perturbation of the network properties that do not reflect the endogenous function of the population as a whole. The most informative aspect of optogenetic manipulation of newborn neurons may be to identify how single cells respond to activity and environmental demands. Understanding the most basic properties of adult-born neurons, even in a cell-autonomous manner, could lead to new hypotheses of how this dynamic population could impact hippocampal-dependent behavior.
Figure 2. Optogenetic approach to clarify the timing for functional involvement of adult-born DGCs.
Top, Optical manipulation of neuronal activity with light-sensitive rhodopsins: Blue light (470 nm wavelength)-induced neuronal activation by channelrhodopsin-2 (ChR2), a cation channel (left). Yellow light (589 nm)-induced neuronal suppression by halorhodopsin (NpHR), a chloride pump, derived from Natronomonas pharaonis (right). GCL: granule cell layer. Bottom, Hippocampal circuitry and afferent connections from the entorhinal cortex (EC). Granule cells of the dentate gyrus (DG) project their axons, mossy fibers (MF), to the pyramidal cells of CA3. CA3 neurons target CA1 pyramidal neurons via the Schaffer collateral pathway (S/C). CA1 neurons also project back to the EC. The EC sends cortical information and hippocampal feedback to the DG through the medial perforant pathway (mPP), to CA3 through lateral the perforant pathway (LPP) and directly to CA1 through the temporoammonic pathway (TA). Some memories appear to have a temporary dependence on the hippocampus before cortical structures are capable of mediating the long-term maintenance of the memory trace.
5. Conclusion and perspective
Adult neurogenesis recapitulates embryonic and early postnatal neurodevelopment and shares many underlying mechanisms, but the functional significance of this phenomenon in the mature brain is not well understood. In terms of hippocampal neurogenesis, in particular, the challenge to identify its functional role is further complicated by our limited understanding of how the dentate gyrus itself contributes to cognitive and affective-like behaviors. Numerous studies support the notion that adult neurogenesis positively correlates with many aspects of learning and memory and that disrupting this phenomenon can lead to selective deficits in some forms of hippocampal-dependent memory. Beyond a desire to understand how adult neurogenesis contributes to the processing capacity of the dentate granule cell network, a mechanistic description of how newly introduced neurons are incorporated into the local circuitry may be generalizable to stem cell-mediated therapeutic strategies for neuronal replacement. But one of the most critical questions that remains to be addressed is how these two populations – the dynamic, regenerative, adult-born neurons and the fully integrated, mature, perinatal-born neurons – interact to enhance, or regulate, hippocampal function. The continuous birth of new neurons in the dentate gyrus results in a strikingly plastic structure that is rare in the adult mammalian brain in the absence of pathology. At any given moment, this region is comprised of DGCs that cover an entire spectrum of ages as old as the organism itself and as young as a few hours old. It seems that the unique intrinsic features of newborn DGCs such as an initial phase of atypical GABAergic depolarization compared to surrounding cells, competition for synaptic integration with mature neurons, and finally, a significant period of enhanced plasticity, are designed to maximize the likelihood of survival of newborn neurons in a potentially less hospitable developmental environment in the adult brain. What we still need to understand is why this particular region is so highly neurogenic and how these features of young neurons can be co-opted to enhance memory formation and behavioral modification. It is precisely this juxtaposition of continuously evolving neuronal populations against a background of a structurally stable dentate gyrus that suggests discrete time-limited and age-dependent roles of DGCs. Only by transiently, and reversibly, perturbing the intercellular communication between these populations in a systematic way, can we isolate the impact of one group of cells on the rest of the circuitry in real-time. At that point, we can begin to unravel the fine-grained interactions between mature and newborn neurons and begin to build a comprehensive picture of how these populations may interact to optimize the hippocampal function.
Table 2.
Timing of functional involvement of adult-born neurons in behavior
| State | % | SP | Treatment | Time interval | Impaired | Enhanced | No effect | Ref |
|---|---|---|---|---|---|---|---|---|
| Increased NG | 40 | mice | Physical enrichment (36rpm/5days) | 5 d | Instrumental | Trace eyeblink | (72) | |
| 42 | mice | NR2B antagonist (Ro25–6981) ip. | 1 mo | WM | (100) | |||
| NM | mice | Enriched environment (60 days) | 4 mos | WM | (71) | |||
| 30 | mice | Enriched environment (3 wks) | 3 wks EE | WM, RAM, PA | (68) | |||
| 60 | mice | Voluntary wheel running | 42 d | WM | (101) | |||
| 47 | rats | Enriched environment (4 wks) | 4 wks EE | WM | (69) | |||
| Decreased NG | 50 | mice | Cyclophosphamide | 12 hrs | PA, Object | (82) | ||
| 50 | mice | Cyclophosphamide | 10 d | PA, Object | (82) | |||
| 27 | mice | NR2A-containing NMDA receptors inhibitor (NVP-AAM077), (34 days) | 34 d | WM | (80) | |||
| 32 | rats | Sleep fragmentation (12 days) | 1 mo | Barnes | (74) | |||
| 70 | rats | Ozone exposure (4 hr/90 days) | 3 mos | PA, Context fear | (102) | |||
| 30 | rats | Olfactory bulbectomy | 1.5 mos | PA | (103) | |||
| 70 | rats | Restraint stress (6 hr/day, 21 days) | 21 d | RAM | (75) | |||
| 80 | rats | Neurotoxin (192 IgG-saporin) | 1 mo | WM | (81) | |||
| 74 | rats | Dominant-negative WNT Lentivirus | 2 mos | WM, Object | (104) | |||
| Ablation by irradiation | 74 | mice | Whole-body (2 Gy), single | 1 d | PA, Object | Open | (105) | |
| 74 | mice | Whole-body (2 Gy), single | 3 d | Object | PA | (105) | ||
| 74 | mice | Whole-body (2 Gy), single | 5 d | PA | (105) | |||
| 77 | mice | Whole brain (5 Gy), single | 3 mos | WM | Barnes, Object | (106) | ||
| 68 | rats | Whole brain (7.5 Gy), 2 / 2 days | 2 wks | T-maze | (89) | |||
| 90 | rats | Whole brain (7.5 Gy), 10 min/2 days | 4 wks | Context fear | (90) | |||
| 50 | rats | Whole brain (8Gy), then running | 5 wks | Context fear | WM | (91) | ||
| 95 | rats | Whole brain (10 Gy), 10 min/2 days | 4 wks | WM | (92) | |||
| 75 | gerbils | Focal brain(10 Gy,1 M), EE (2 M) | 3 mos | WM, then recover | (107) | |||
| 95 | mice | Focal brain, (5 Gy), 3 times | 2 mos | RAM: spatial separation | (94) | |||
| 60 | mice | Focal brain (5 Gy), 3 times | 3 mos | RAM: working | (95) | |||
| 78 | mice | Focal brain (5 Gy), 3 times, (after 2 months) 54 days running | 2 mos +54d | WM | Context fear | (108) | ||
| 85 | mice | Focal brain (5 Gy), 3 times | 3 mos | Context fear | WM,Y-maze | (96) | ||
| 80 | mice | Focal brain (10Gy), single | 3 mos | Fear extinct. | (77) | |||
| 88 | mice | Focal brain (20Gy), single | 3 mos | Fear retention | Fear extinct. | (77) | ||
| 43 | rats | Focal brain (7.5 Gy), 2 times/2 days | 9 wks | Context fear | (89) | |||
| 95 | rats | Focal brain (4.58 Gy), 8 days | 3 wks | Place | (93) | |||
| 95 | rats | Focal brain (4.58 Gy), 8 days | 2 wks | WM, Object | (93) | |||
| 95 | rats | Focal brain (4.58 Gy), 8 days | 7 wks | Place | (93) | |||
| Ablation by MAM | 75 | rats | MAM (2 wks) | 2 wks | Trace fear (30 s) | Delayed fear | (79) | |
| 75 | rats | MAM (2 wks) | 2 wks | WM | (79) | |||
| 75 | mice | MAM (2 wks) | 2 wks | Fear | (77) | |||
| Ablation by transgenic expression of toxins | 75 | mice | GFAP-TK | GCV: 6 wks | Context fear | WM,Y-maze | (96) | |
| 75 | mice | GFAP-TK | GCV: 10 wks | RAM: working | (95) | |||
| 75 | mice | GFAP-TK (GCV:10 wks) | 10 wks recover | RAM: working | (95) | |||
| 50 | mice | Nestin-TK (GCV:2 wks) | 1 wk | WM, Fear extinct. | (97) | |||
| 50 | mice | Nestin-TK (GCV:2 wks) | 3.5, 9 wks | WM (1 wk) | (97) | |||
| 50 | mice | Nestin-TK (GCV:2 wks) | 5 wks | Fear extinct. | (97) | |||
| 60 | mice | Nestin-rtTA/TRE-BAX | Dox: 6wks | WM | Context fear | (98) | ||
| 90 | mice | Nestin-CreER/NSE-DTA (TM:4 days) | 1.5 mos | Fear, Barnes | (109) |
NG: neurogenesis, SP: species, EE: enriched environment, WM: water maze, PA: passive avoidance, RAM: radial arm maze, Fear extinct: fear extinction, Gy: gray, MAM: methylazoxymethanol acetate, GCV: Ganciclovir, Dox: Doxycycline, TM: Tamoxifen, NM: not measured
Adult neurogenesis continues throughout life but its physiological role is still uncertain
Contribution of newborn neurons to behavior is regulated by dynamic intracellular environment
Increased temporal resolution is required to assess role of adult-born cells in learned behavior
Determining process of functional integration can inform research of stem cell therapeutics
Acknowledgements:
Supported by NIH (HD069184, NS048271) to G.L.M., by NIH (NS047344, AG024984, MH087874) to H.J.S., by postdoctoral fellowship from Korea Research Foundation and Maryland Stem Cell Research Fund to W.R.K., and by postdoctoral fellowship from Maryland Stem Cell Research Foundation to K.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ming G. l. and Song H.-j.. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron, 2011: p. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sahay A, Wilson DA, and Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron, 2011; 70(4): p. 582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aimone JB, Deng W, and Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron, 2011; 70(4): p. 589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lledo PM, Alonso M, and Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci, 2006; 7(3): p. 179–93. [DOI] [PubMed] [Google Scholar]
- [5].Abrous DN, Koehl M, and Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev, 2005; 85(2): p. 523–69. [DOI] [PubMed] [Google Scholar]
- [6].Burgess N, Maguire EA, and O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron, 2002; 35(4): p. 625–41. [DOI] [PubMed] [Google Scholar]
- [7].Scoville WB and Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 1957; 20(1): p. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Squire LR The organization and neural substrates of human memory. Int J Neurol, 1987; 21–22: p. 218–22. [PubMed] [Google Scholar]
- [9].Deng W, Aimone JB, and Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci, 2010; 11(5): p. 339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cameron HA and McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol, 2001; 435(4): p. 406–17. [DOI] [PubMed] [Google Scholar]
- [11].Kempermann G, Kuhn HG, and Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A, 1997; 94(19): p. 10409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Snyder JS, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci, 2009; 29(46): p. 14484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mandyam CD, Harburg GC, and Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience, 2007; 146(1): p. 108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell, 2010; 7(4): p. 483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kitabatake Y, Sailor KA, Ming GL, and Song H. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg Clin N Am, 2007; 18(1): p. 105–13, x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ge S, Sailor KA, Ming GL, and Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol, 2008; 586(16): p. 3759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mongiat LA and Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci, 2011; 33(6): p. 1055–61. [DOI] [PubMed] [Google Scholar]
- [18].Duan X, Kang E, Liu CY, Ming GL, and Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol, 2008; 18(1): p. 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Morgenstern NA, Lombardi G, and Schinder AF. Newborn granule cells in the ageing dentate gyrus. J Physiol, 2008; 586(16): p. 3751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seri B, Garcia-Verdugo JM, McEwen BS, and Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci, 2001; 21(18): p. 7153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell, 2011; 145(7): p. 1142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, and Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell, 2007; 1(5): p. 515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hodge RD, et al. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci, 2008; 28(14): p. 3707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cameron HA, Woolley CS, McEwen BS, and Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience, 1993; 56(2): p. 337–44. [DOI] [PubMed] [Google Scholar]
- [25].Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, and Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature, 2006; 439(7076): p. 589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kempermann G, Gast D, Kronenberg G, Yamaguchi M, and Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development, 2003; 130(2): p. 391–9. [DOI] [PubMed] [Google Scholar]
- [27].Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell, 2007; 130(6): p. 1146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ambrogini P, et al. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res, 2004; 1017(1–2): p. 21–31. [DOI] [PubMed] [Google Scholar]
- [29].Zhao C, Teng EM, Summers RG Jr., Ming GL, and Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci, 2006; 26(1): p. 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bhattacharyya BJ, et al. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci, 2008; 28(26): p. 6720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Esposito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci, 2005; 25(44): p. 10074–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Overstreet-Wadiche LS, Bensen AL, and Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci, 2006; 26(8): p. 2326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Owens DF and Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci, 2002; 3(9): p. 715–27. [DOI] [PubMed] [Google Scholar]
- [34].Ben-Ari Y and Spitzer NC. Nature and nurture in brain development. Trends Neurosci, 2004; 27(7): p. 361. [DOI] [PubMed] [Google Scholar]
- [35].Tozuka Y, Fukuda S, Namba T, Seki T, and Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron, 2005; 47(6): p. 803–15. [DOI] [PubMed] [Google Scholar]
- [36].Ge S, Pradhan DA, Ming GL, and Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci, 2007; 30(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- [37].Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci, 2009; 29(25): p. 7966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Faulkner RL, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A, 2008; 105(37): p. 14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci, 2008; 11(8): p. 901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci, 2007; 10(6): p. 727–34. [DOI] [PubMed] [Google Scholar]
- [41].Jessberger S and Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci, 2003; 18(10): p. 2707–12. [DOI] [PubMed] [Google Scholar]
- [42].Tashiro A, Sandler VM, Toni N, Zhao C, and Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature, 2006; 442(7105): p. 929–33. [DOI] [PubMed] [Google Scholar]
- [43].Uchigashima M, Fukaya M, Watanabe M, and Kamiya H. Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J Neurosci, 2007; 27(30): p. 8088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gutierrez R The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci, 2005; 28(6): p. 297–303. [DOI] [PubMed] [Google Scholar]
- [45].Ge S, Yang CH, Hsu KS, Ming GL, and Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron, 2007; 54(4): p. 559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schmidt-Hieber C, Jonas P, and Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature, 2004; 429(6988): p. 184–7. [DOI] [PubMed] [Google Scholar]
- [47].Snyder JS, Kee N, and Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol, 2001; 85(6): p. 2423–31. [DOI] [PubMed] [Google Scholar]
- [48].Dayer AG, Ford AA, Cleaver KM, Yassaee M, and Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol, 2003; 460(4): p. 563–72. [DOI] [PubMed] [Google Scholar]
- [49].Laplagne DA, et al. Functional Convergence of Neurons Generated in the Developing and Adult Hippocampus. PLoS Biol, 2006; 4(12): p. e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Laplagne DA, et al. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci, 2007; 25(10): p. 2973–81. [DOI] [PubMed] [Google Scholar]
- [51].McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science, 2007; 317(5834): p. 94–9. [DOI] [PubMed] [Google Scholar]
- [52].Oomen CA, et al. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, and Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci, 2007; 10(7): p. 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Aimone JB, Deng W, and Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci, 2010; 14(7): p. 325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, and Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol, 2005; 15(5): p. 599–606. [DOI] [PubMed] [Google Scholar]
- [56].Breunig JJ, Arellano JI, Macklis JD, and Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell, 2007; 1(6): p. 612–27. [DOI] [PubMed] [Google Scholar]
- [57].French PJ, et al. Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo. Eur J Neurosci, 2001; 13(5): p. 968–76. [DOI] [PubMed] [Google Scholar]
- [58].Montag-Sallaz M, Welzl H, Kuhl D, Montag D, and Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol, 1999; 38(2): p. 234–46. [PubMed] [Google Scholar]
- [59].Ma DK, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science, 2009; 323(5917): p. 1074–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Snyder JS, Glover LR, Sanzone KM, Kamhi JF, and Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus, 2009; 19(10): p. 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kee N, Teixeira CM, Wang AH, and Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci, 2007; 10(3): p. 355–62. [DOI] [PubMed] [Google Scholar]
- [62].Stone SS, et al. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus, 2010. [DOI] [PubMed] [Google Scholar]
- [63].Trouche S, Bontempi B, Roullet P, and Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A, 2009; 106(14): p. 5919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Alme CB, et al. Hippocampal granule cells opt for early retirement. Hippocampus, 2010; 20(10): p. 1109–23. [DOI] [PubMed] [Google Scholar]
- [65].Ming GL and Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci, 2005; 28: p. 223–50. [DOI] [PubMed] [Google Scholar]
- [66].Zhao C, Deng W, and Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell, 2008; 132(4): p. 645–60. [DOI] [PubMed] [Google Scholar]
- [67].Huang FL, Huang KP, Wu J, and Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci, 2006; 26(23): p. 6230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nilsson M, Perfilieva E, Johansson U, Orwar O, and Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol, 1999; 39(4): p. 569–78. [DOI] [PubMed] [Google Scholar]
- [69].van Praag H, Kempermann G, and Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci, 1999; 2(3): p. 266–70. [DOI] [PubMed] [Google Scholar]
- [70].Williams BM, et al. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav, 2001; 73(4): p. 649–58. [DOI] [PubMed] [Google Scholar]
- [71].Madronal N, Lopez-Aracil C, Rangel A, del Rio JA, Delgado-Garcia JM, and Gruart A. Effects of enriched physical and social environments on motor performance, associative learning, and hippocampal neurogenesis in mice. PLoS One; 5(6): p. e11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sportiche N, et al. Sustained sleep fragmentation results in delayed changes in hippocampal-dependent cognitive function associated with reduced dentate gyrus neurogenesis. Neuroscience; 170(1): p. 247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, and Shankaranarayana Rao BS. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res, 2009; 87(4): p. 831–43. [DOI] [PubMed] [Google Scholar]
- [74].Bain MJ, Dwyer SM, and Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett, 2004; 368(1): p. 7–10. [DOI] [PubMed] [Google Scholar]
- [75].Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci, 2006; 9(6): p. 729–31. [DOI] [PubMed] [Google Scholar]
- [76].Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature, 2011; 472(7344): p. 466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bruel-Jungerman E, Laroche S, and Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci, 2005; 21(2): p. 513–21. [DOI] [PubMed] [Google Scholar]
- [78].Ko HG, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain, 2009; 2(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, and Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature, 2001; 410(6826): p. 372–6. [DOI] [PubMed] [Google Scholar]
- [80].Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, and Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus, 2002; 12(5): p. 578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hu M, et al. Reduced spatial learning in mice treated with NVP-AAM077 through down-regulating neurogenesis. Eur J Pharmacol, 2009; 622(1–3): p. 37–44. [DOI] [PubMed] [Google Scholar]
- [82].Mohapel P, Leanza G, Kokaia M, and Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging, 2005; 26(6): p. 939–46. [DOI] [PubMed] [Google Scholar]
- [83].Yang M, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem; 93(4): p. 487–94. [DOI] [PubMed] [Google Scholar]
- [84].Nagai R, Tsunoda S, Hori Y, and Asada H. Selective vulnerability to radiation in the hippocampal dentate granule cells. Surg Neurol, 2000; 53(5): p. 503–6; discussion 506–7. [DOI] [PubMed] [Google Scholar]
- [85].Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science, 2003; 301(5634): p. 805–9. [DOI] [PubMed] [Google Scholar]
- [86].Tada E, Yang C, Gobbel GT, Lamborn KR, and Fike JR. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol, 1999; 160(1): p. 66–77. [DOI] [PubMed] [Google Scholar]
- [87].Uberti D, Piccioni L, Cadei M, Grigolato P, Rotter V, and Memo M. p53 is dispensable for apoptosis but controls neurogenesis of mouse dentate gyrus cells following gamma-irradiation. Brain Res Mol Brain Res, 2001; 93(1): p. 81–9. [DOI] [PubMed] [Google Scholar]
- [88].Wojtowicz JM Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus, 2006; 16(3): p. 261–6. [DOI] [PubMed] [Google Scholar]
- [89].Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 2009; 139(4): p. 814–27. [DOI] [PubMed] [Google Scholar]
- [90].Hernandez-Rabaza V, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience, 2009; 159(1): p. 59–68. [DOI] [PubMed] [Google Scholar]
- [91].Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, and Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus, 2006; 16(3): p. 296–304. [DOI] [PubMed] [Google Scholar]
- [92].Wojtowicz JM, Askew ML, and Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci, 2008; 27(6): p. 1494–502. [DOI] [PubMed] [Google Scholar]
- [93].Snyder JS, Hong NS, McDonald RJ, and Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience, 2005; 130(4): p. 843–52. [DOI] [PubMed] [Google Scholar]
- [94].Madsen TM, Kristjansen PE, Bolwig TG, and Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience, 2003; 119(3): p. 635–42. [DOI] [PubMed] [Google Scholar]
- [95].Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 2009; 325(5937): p. 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Saxe MD, et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A, 2007; 104(11): p. 4642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A, 2006; 103(46): p. 17501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Deng W, Saxe MD, Gallina IS, and Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci, 2009; 29(43): p. 13532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One, 2008; 3(4): p. e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhang F, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc; 5(3): p. 439–56. [DOI] [PMC free article] [PubMed] [Google Scholar]