A vaccine is urgently needed to combat HIV-1, particularly in sub-Saharan Africa, which remains disproportionately affected by the AIDS pandemic and accounts for the majority of new infections and AIDS-related deaths. In this study, two different vaccination regimens were compared. Rabbits that received two DNA primes followed by two modified vaccinia virus Ankara (MVA) and two protein inoculations developed better immune responses than those that received two MVA and three protein inoculations. In addition, DNA and MVA vaccines that expressed mosaic Gag VLPs presenting a stabilized Env antigen elicited better responses than Env alone, which supports the inclusion of Gag VLPs in an HIV-1 vaccine.
KEYWORDS: CAP256 SU, DNA, MVA, tier 2 neutralizing antibodies, human immunodeficiency virus, prime-boost, vaccine
ABSTRACT
A vaccine regimen that elicits broadly neutralizing antibodies (bNAbs) is a major goal in HIV-1 vaccine research. In this study, we assessed the immunogenicity of the CAP256 superinfecting viral envelope (CAP256 SU) protein delivered by modified vaccinia virus Ankara (MVA) and DNA vaccines in different prime-boost combinations followed by a soluble protein (P) boost. The envelope protein (Env) contained a flexible glycine linker and I559P mutation. Trimer-specific bNAbs PGT145, PG16, and CAP256 VRC26_08 efficiently bound to the membrane-bound CAP256 envelope expressed on the surface of cells transfected or infected with the DNA and MVA vaccines. The vaccines were tested in two different vaccination regimens in rabbits. Both regimens elicited autologous tier 2 neutralizing antibodies (NAbs) and high-titer binding antibodies to the matching CAP256 Env and CAP256 V1V2 loop scaffold. The immunogenicity of DNA and MVA vaccines expressing membrane-bound Env alone was compared to that of Env stabilized in a more native-like conformation on the surface of Gag virus-like particles (VLPs). The inclusion of Gag in the DNA and MVA vaccines resulted in earlier development of tier 2 NAbs for both vaccination regimens. In addition, a higher proportion of the rabbits primed with DNA and MVA vaccines that included Gag developed tier 2 NAbs than did those primed with vaccine expressing Env alone. Previously, these DNA and MVA vaccines expressing subtype C mosaic HIV-1 Gag were shown to elicit strong T cell responses in mice. Here we show that when the CAP256 SU envelope protein is included, these vaccines elicit autologous tier 2 NAbs.
IMPORTANCE A vaccine is urgently needed to combat HIV-1, particularly in sub-Saharan Africa, which remains disproportionately affected by the AIDS pandemic and accounts for the majority of new infections and AIDS-related deaths. In this study, two different vaccination regimens were compared. Rabbits that received two DNA primes followed by two modified vaccinia virus Ankara (MVA) and two protein inoculations developed better immune responses than those that received two MVA and three protein inoculations. In addition, DNA and MVA vaccines that expressed mosaic Gag VLPs presenting a stabilized Env antigen elicited better responses than Env alone, which supports the inclusion of Gag VLPs in an HIV-1 vaccine.
INTRODUCTION
Given the magnitude and duration of the human immunodeficiency virus (HIV)/AIDS pandemic, prophylactic vaccines against HIV type 1 (HIV-1) are urgently required. However, any prophylactic vaccine against HIV-1 will need to contend with the unprecedented diversity of the virus (1). While most successful antiviral vaccines confer protective immunity by the elicitation of neutralizing antibodies, this has been difficult to achieve for HIV due to several protective mechanisms inherent in the structure of the viral envelope (Env) glycoprotein. These include low spike density of the glycoprotein on the virion, poor accessibility of vulnerable epitopes, a host-derived glycan shield, and the presence of aberrantly folded glycoprotein species which misdirect the immune response against nonneutralizing epitopes, among other features (2–7).
Encouragingly, during natural infection approximately 30% of infected people develop broadly neutralizing antibodies (bNAbs) that can neutralize diverse viral isolates from different clades (8–16). Although these responses arise too late in infection to be of any obvious clinical benefit, their ability to protect against challenge with chimeric simian-human immunodeficiency virus (SHIV) in nonhuman primate (NHP) models suggests that they could protect against infection in humans (8, 14, 17–26). While the NAbs target a continuum of epitopes on the glycoprotein, many of these preferentially, or in some cases exclusively, recognize trimeric Env, suggesting that a vaccine immunogen should mimic this feature of the protein (27, 28).
HIV-1 isolates are ranked based on their sensitivities to neutralizing antibodies as tier 1 (sensitive), tier 2 (moderate), and tier 3 (resistant) (29). Tier 1 viruses are rarely isolated from natural infection and exhibit unusually high levels of conformational plasticity that exposes neutralization-susceptible epitopes (30, 31). Tier 2 isolates are representative of circulating viruses that a vaccine will need to confer protection against (29). The first generation of HIV-1 Env trimers was poorly representative of the native glycoprotein complex and elicited poor neutralizing antibodies against tier 2 viruses (29–31). This was partly due to the incorporation of heterologous trimerization motifs and the elimination of the native cleavage site to prevent shedding of the gp120 and gp41 subunits following proteolytic cleavage (32–39). It was subsequently reported that elimination of the Env cleavage site compromised the structure of the trimer, resulting in irregular conformations that exposed epitopes that are occluded in the native trimer (40, 41).
The importance of proteolytic cleavage in Env trimer formation was addressed by mutating the cleavage site to a hexa-arginine motif, which improved cleavage efficiency upon cotransfection with a second plasmid expressing furin (42). The sequence was then modified to introduce an artificial disulfide bond (SOS) between the gp41 ectodomain and gp120 to prevent shedding of gp120 and by the incorporation of an isoleucine-to-proline (I→P) helix-breaking mutation in gp41 (43, 44). The design was further refined to prevent aggregation by the elimination of a portion of the membrane-proximal external region (45). The incorporation of these “SOSIP” mutations into the prototype subtype A isolate, BG505, resulted in the first Env mimetic that accurately reproduced the native trimer structure (46). This approach has now been applied to a number of different Env proteins, resulting in the induction of strain-specific tier 2 virus-neutralizing antibodies in both rabbits and macaques (47–52).
A potential shortcoming of this approach is the requirement for furin coexpression, which limits its utility for delivery by genetic immunization or other vector modalities (42). In the absence of furin coexpression, the efficiency of cleavage is typically low, although this is influenced by the genetic background of the viral Env (53). To illustrate this point, in vitro expression of the prototypic BG505 Env from recombinant chimpanzee adenovirus and modified vaccinia virus (VACV) Ankara (MVA) vaccines only resulted in 66% and 33% respectively, of the recombinant Env being in the cleaved, native-like conformation (54).
A strategy employing a flexible glycine-rich linker peptide at the interface of the gp120 and gp41 subunits has been reported to enable recombinant Env to assume a native conformation in the absence of proteolytic cleavage (55). Immunization of animals with these Env trimers results in the induction of neutralizing antibodies that are comparable to those elicited by SOSIP antigens (51, 52). These Env mimetics are more suitable than the SOSIP constructs for heterologous prime-boost immunizations where endogenous furin is limited and coexpression is not possible in vivo.
A further iteration of Env trimer-based vaccination is the multivalent display of the glycoprotein trimer on the surface of liposomes or self-assembling nanoparticles (56–60). A more natural way to achieve this is to present the envelope glycoprotein on the surface of Pr55Gag virus-like particles (VLPs). Virus-like particle vaccines have been shown to be highly immunogenic and to provide protection from a number of viral diseases, such as hepatitis B virus and human papillomavirus infections (61, 62). Due to the repetitive nature of the envelope protein on the surfaces of VLPs, liposomes, and nanoparticles, cross-linking of B cell receptors specific to Env is triggered leading to improved antibody responses. These approaches are also thought to stabilize the trimeric conformation of the glycoprotein in its natural membrane context (63).
Gag is also believed to be an important component of an HIV vaccine, given that Gag-specific CD4+ and CD8+ T cells are inversely correlated with viral loads during natural infection (64–66). While bNAbs against the envelope glycoprotein are expected to prevent infection, cellular immunity against Gag could play a role in controlling viremia and possibly even ameliorate pathogenesis or reduce transmission of the virus (67). In addition to the elicitation of bNAbs, several other approaches have also been suggested to contend with the global diversity of the virus, such as the elicitation of cellular immunity against conserved regions of the HIV genome and the use of mosaic immunogens (1).
These mosaic antigens are designed in silico for the maximum coverage of potential T cell epitopes from a given number of natural sequences (68). Encouragingly, HIV mosaic vaccines have been reported to elicit cell-mediated immunity against HIV with improved breadth and confer protection against stringent SHIV challenges in nonhuman primates (69, 70). We have previously reported the formation of enveloped VLPs budding from cells transfected with DNA or infected with recombinant modified vaccinia virus Ankara encoding an HIV-1 subtype C Gag mosaic antigen (71). This antigen was also reported to be considerably more immunogenic than a comparable naturally occurring Gag and has demonstrated promising immunogenicity in DNA, MVA, and Mycobacterium bovis BCG vaccine modalities in mice (71, 72). A heterologous DNA prime-MVA boost regimen generated significantly improved T cell responses compared to homologous vaccination with either the DNA or MVA vaccine. Other groups have also shown that heterologous prime-boost regimens give potent HIV-1-specific immune responses that are often better than those generated with homologous vaccination regimens (73–75).
In this study, we have combined several of the most promising approaches reported in recent years to develop an optimal vaccine regimen for the elicitation of neutralizing antibodies to HIV-1 subtype C Env in a rabbit model. These include rational selection of an HIV-1 isolate for vaccine design, priming immune responses sequentially with DNA and MVA vaccines that express mosaic Gag VLPs presenting a stabilized Env antigen, and the use of a stabilized size exclusion chromatography (SEC)-purified protein as a boost.
RESULTS
In vitro characterization of South African subtype C CAP256 Env and mosaic Gag vaccines.
The envelope sequence used in this study was based on a virus isolated from a patient in the South African CAPRISA 002 acute infection cohort, patient CAP256, who developed bNAbs following a secondary infection of HIV-1 approximately 15 weeks after the primary infection (76). The CAP256 superinfecting viral envelope (CAP256 SU) was selected, as it elicited bNAbs in this donor (77) and is sensitive to several prototype broadly neutralizing monoclonal antibodies (40). In addition, the enhanced reactivity of this Env for certain bNAb precursors makes it an appealing candidate immunogen (78, 79). For DNA and recombinant MVA (rMVA) vaccines, the Env sequence was truncated to gp150 (amino acid 730) to increase envelope protein expression and stability of the recombinant env gene (79), while retaining the transmembrane domain and part of the intracellular C-terminal domain (schematic representation in Fig. 1A). The DNA and MVA subtype C mosaic Gag (DNA-GagM and MVA-GagM) and soluble Env (gp140) protein vaccines were described previously (71, 72, 80).
FIG 1.
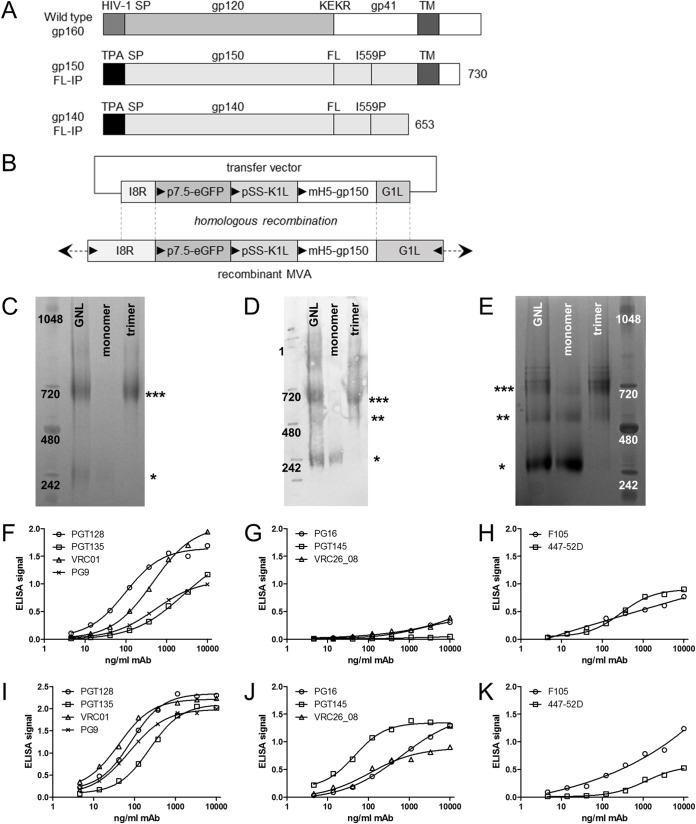
CAP256 DNA, rMVA and protein vaccine design and characterization of protein vaccine. (A) Schematic representation of wild-type Env and the truncated CAP256 gp150-FL-IP (gp150) used in DNA and MVA vaccines. The native signal peptide (HIV-1 SP) was replaced with the human tissue plasminogen activator (TPA SP) sequence, the furin cleavage site (KEKR) was replaced with a flexible linker (FL) sequence, and an I559P mutation was introduced. This sequence was further truncated for soluble gp140-FL-IP (soluble Env) protein vaccines. (B) Schematic representation of transfer vector for targeting gp150, expressed by the mH5 promoter, into the I8R-G1L locus of wild-type MVA or rMVA GagM. Triangles indicate direction of open reading frames. (C and D) Soluble Env was purified from a stable HEK293 cell line expressing CAP256 gp140-FL-IP by Galanthus nivalis lectin affinity chromatography followed by size exclusion chromatography. Coomassie staining (C) and anti-Env Western blotting (D) show purification of soluble, trimeric Env (***, trimer; **, dimer; *, monomer). (E) Coomassie staining of soluble Env-His purified from a stable HEK293 cell line expressing CAP256 gp140-FL-IP-6×His by Galanthus nivalis lectin affinity chromatography followed by size exclusion chromatography. (F to H) ELISA for binding of MAbs to soluble, trimeric Env-His (representative traces). (I to K) ELISA for binding of MAbs to His-tagged BG505 SOSIP.664 (representative traces). No ELISA signal was observed for ELISA control without protein (data not shown).
Our expression vector pTHCapR, which contains a porcine circovirus enhancer sequence that gives increased antigen expression, was used as the vector backbone for the DNA vaccines (71, 81). To generate the recombinant MVA vaccines, gp150 was targeted between the transcriptionally convergent open reading frames of the essential I8R and G1L genes of either wild-type (WT) MVA or MVA-GagM (Fig. 1B) to generate MVA Env and MVA Env+GagM, respectively. Selection of recombinant MVA, which expressed both the enhanced green fluorescent protein (eGFP) marker gene and the vaccinia virus K1L host range gene, was performed in RK13 cells (82, 83). High-titer rMVA stocks (>0.5 × 109 PFU) were generated in RK13 cells, and correct gp150 insertion was confirmed by PCR and sequencing.
As reported previously (80), soluble Env isolated via Galanthus nivalis lectin (GNL) affinity chromatography was mainly in a trimeric conformation as measured by molecular weight on NativePAGE protein gels. This trimeric fraction was further purified by size exclusion chromatography (Coomassie blue [Fig. 1C] and anti-Env Western blotting [Fig. 1D]). More monomeric and dimeric Env was seen following lectin affinity purification of the His-tagged Env (Fig. 1E, GNL) than the untagged Env (Fig. 1C, GNL).
The quaternary structure of this soluble presumptively native trimeric Env was assessed by capturing His-tagged Env on nickel-coated enzyme-linked immunosorbent assay (ELISA) plates and assaying binding of human monoclonal antibodies (MAbs) to native/trimeric Env. For soluble trimeric Env, both the CD4 binding site (MAb VRC01) and the Env V3-glycan supersite of vulnerability (MAbs PGT128 and PGT135) were intact (n = 3; Fig. 1F shows representative traces). In addition, binding of V2-glycan MAb PG9 (which binds both monomeric and trimeric Env) was observed. MAbs PG16, PGT145, and CAP256 VRC26_08 are trimer-specific antibodies that bind the V2 apex and have been shown to neutralize cell entry of the CAP256 SU virus (84). Weak binding of PG16 and CAP256 VRC26_08 was detected (Fig. 1G). PGT145 failed to bind, but this could be due to the much lower affinity of this MAb for CAP256 SU (84). The ELISA was positive for 447-52D; this MAb binds the V3 loop of gp120, which is considered nonneutralizing and a measure for monomeric Env and badly folded trimeric Env, suggesting that there is impaired folding of soluble Env (Fig. 1H). A second nonneutralizing MAb, F105, which binds the CD4 binding site of monomeric Env, was also positive (Fig. 1H). The His-tagged Env control protein, BG505 SOSIP.664-His, had a similar binding profile for MAbs PGT128, VRC01, and F105 (Fig. 1I and K) but much better binding for the trimer-specific MAbs PG16, PGT145, and CAP256 VRC26_08 (n = 1 [Fig. 1J]). No background binding of MAbs in the ELISA setup was observed (n = 1 [data not shown]). In all, these data confirm that a portion of CAP256 soluble trimeric Env is in a native-like state.
Secreted Env and/or Gag expression was confirmed in media of HEK293T cells cotransfected with DNA Env+DNA GagM, DNA Env, or DNA GagM vaccines, suggesting secretion of both proteins (Fig. 2A). Furthermore, both Gag and Env expression was observed within the same cells by confocal microscopy (Fig. 2B). Similar results were obtained for cells infected with the different rMVA vaccines (Fig. 2C and D).
FIG 2.
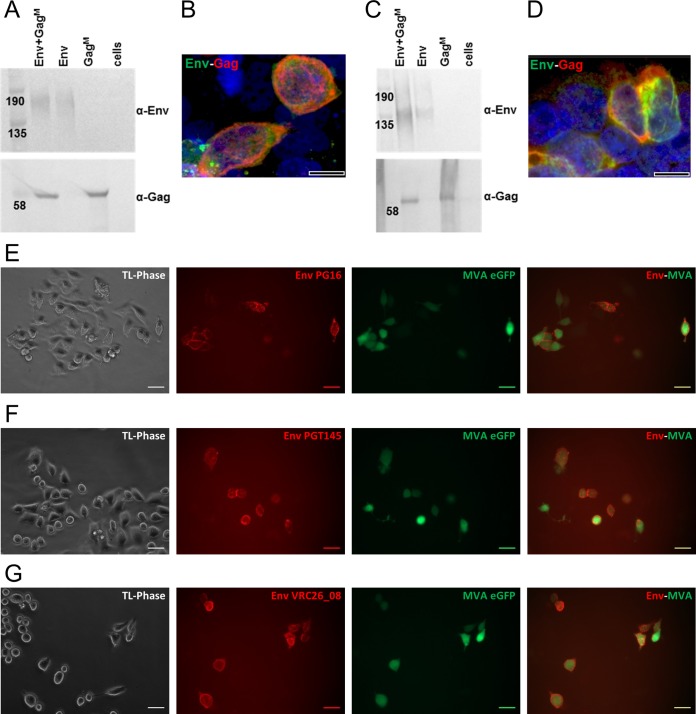
DNA and rMVA vaccine characterization. Western blots demonstrate in vitro secretion of Env and Gag protein from HEK293 cells after DNA vaccine transfection (A) or MVA vaccine infection (C). “Cells” refers to untransfected and uninfected cells. Membranes were cut in half and the top was probed with anti-Env antibodies and the bottom with anti-Gag antibodies. Confocal images show that both Env (green, Cy3) and Gag (red, Alexa Fluor 647) were expressed in the same cell when DNA vaccines were cotransfected (B) or when infected with rMVA containing both gp150 and GagM (D). Scale bars in confocal images represent 10 µm. (E to G) Live-cell staining of HeLa cells infected with MVA Env, using MAbs PG16 (E), PGT145 (F), and CAP256 VRC26_08 (G), which specifically detect native-like, trimeric Env. HeLa cells infected with rMVA were visualized by their eGFP expression (green). MAbs were detected with anti-human IgG-Cy3 (red). TL-Phase, transmitted light, phase contrast. Scale bars represent 20 µm.
The quaternary structure of Env expressed from the rMVA vaccines was further characterized by infecting HeLa cells with rMVA Env or rMVA Env+GagM and staining live cells using human MAbs visualized using an anti-human IgG-Cy3. Cells infected with rMVA Env or rMVA Env+GagM were identified by the expression of eGFP from rMVA. All the MAbs bound cells infected with rMVA Env to some degree (Table 1). Of note, binding by MAbs recognizing native-like trimers (PG16, PGT145, and CAP256 VRC26_08) (76, 84–87) was observed (Fig. 2E to G). Similar results were obtained for rMVA Env+GagM (Table 1) and DNA vaccines (Table 1). For DNA vaccines, expression of Env by transfected cells was identified using an anti-Env goat polyclonal antibody visualized with an anti-goat IgG-fluorescein isothiocyanate (FITC). Binding of the different MAbs to DNA and rMVA vaccines is summarized in Table 1. Untransfected and uninfected cells were negative in this assay (Fig. 2E to G and data not shown).
TABLE 1.
Characterization of the Env expressed on the surface of cells infected with DNA and MVA vaccinesa
| Antibody | Neutralization | Epitope | Native-like trimer | Live-cell mapping |
||
|---|---|---|---|---|---|---|
| MVA Env | MVA Env+GagM | DNV Env | ||||
| PGT128 | Broad | V3-glycan supersite | x | √ | √ | √ |
| PGT135 | Broad | V3-glycan supersite | x | √ | √ | √ |
| 447-52D | Narrow | V3 | x | √ | √ | √ |
| VRC01 | Broad | CD4 binding site | x | √ | √ | √ |
| F105 | Narrow | CD4 binding site | x | √ | √ | √ |
| PG9 | Broad | V2 apex | x | √ | √ | √ |
| PG16 | Broad | V2 apex | Yes | √ | √ | √ |
| PGT145 | Broad | V2 apex | Yes | √ | √ | √ |
| CAP256 VRC26_08 | Broad | V2 apex | Yes | √ | √ | √ |
| 10E8 | Broad | MPER | x | √ | √ | √ |
Summary of Env MAbs which show positive binding in a live-cell staining assay to HeLa cells infected or transfected with MVA Env, MVA Env+GagM, and DNA Env. √, positive for binding. x, antibody does not only bind Env in the native, trimeric state.
In vitro virus-like particle formation of CAP256 Env and mosaic Gag vaccines.
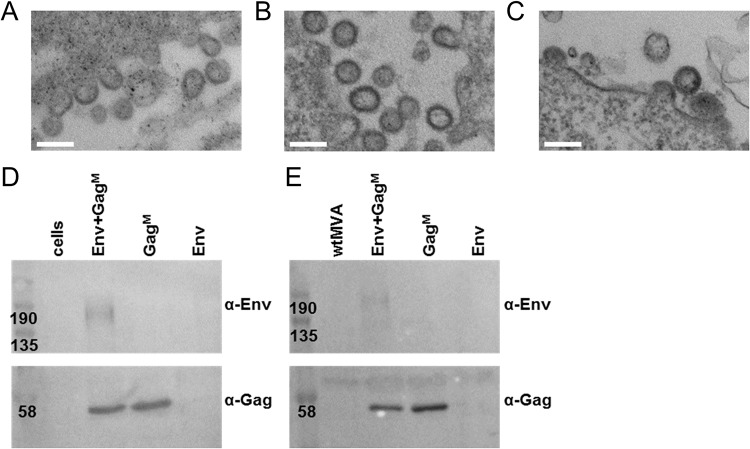
Expression of the mosaic Gag from both DNA and MVA vaccines has already been shown to lead to the formation of Gag VLPs (71) and again was observed in this study (Fig. 3A to C). Inclusion of Env into these DNA or rMVA vaccines also resulted in VLP formation as assessed by electron microscopy (Fig. 3B and C). Gag VLPs were isolated in a two-step OptiPrep gradient centrifugation protocol for further characterization. For DNA Env+GagM and rMVA Env+GagM vaccines, both Gag and Env protein could be detected in the same fraction by Western blotting, suggesting that Env was associated with Gag in VLPs (Fig. 3D and E).
FIG 3.
In vitro formation of virus-like particles (VLPs) from DNA and rMVA vaccines. VLPs, observed by transmission electron microscopy (TEM), after transfection of RK13 cells with DNA GagM (A) or cotransfection with DNA Env plus DNA GagM vaccines (B) are shown. (C) RK13 cells infected with rMVA Env+GagM at a multiplicity of infection (MOI) of 1 for 48 h. Scale bars represent 200 nm. VLPs from DNA (D) or rMVA (E) vaccines were isolated in a two-step OptiPrep gradient centrifugation protocol and analyzed by Western blotting. Fractions were isolated from untransfected cells (cells), cells transfected with DNA or infected with MVA vaccines expressing Env and GagM (Env+GagM), GagM alone (GagM), or Env alone (Env), and cells infected with wild-type MVA (wtMVA). (α-Env, anti-gp120; α-Gag, anti-p24). Membranes were cut in half and the top was probed with α-Env and the bottom with α-Gag.
Rabbit immunization and serum anti-Env antibody characterization.
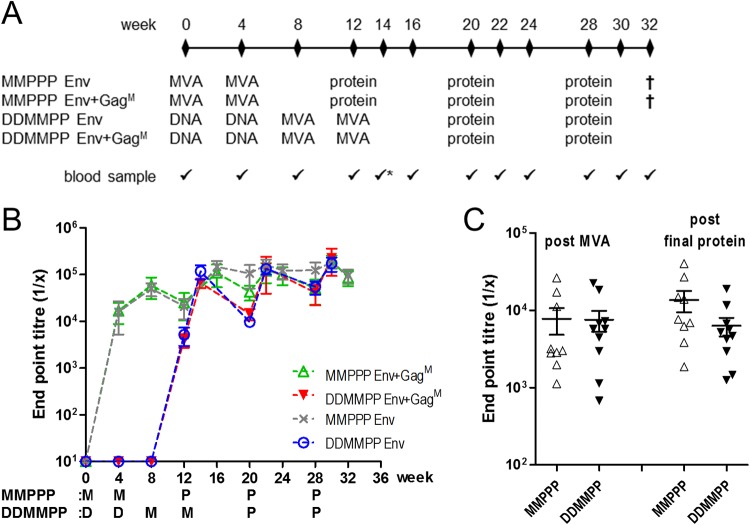
The immunogenicity of the different vaccines was investigated by inoculating 4 groups of rabbits with different regimens (Fig. 4A). The first two groups received 108 PFU of rMVA Env or rMVA Env+GagM intramuscularly at weeks 0 and 4, followed by three SEC-purified soluble, trimeric Env protein boosts (40 µg in AlhydroGel) at weeks 12, 20, and 28 (MMPPP). The other two groups received 100 µg of DNA Env or 100 µg of DNA Env plus 100 µg of DNA GagM plasmid at weeks 0 and 4, followed by two inoculations of 108 PFU of rMVA Env or rMVA Env+GagM (matching the respective DNA primes) at weeks 8 and 12 and two protein boosts with 40 µg of SEC-purified soluble, trimeric Env protein at weeks 20 and 28 (DDMMPP). AlhydroGel was selected as an adjuvant because it was previously shown by us to elicit a superior immune response compared to that with unadjuvanted or the MF59-equivalent AddaVax adjuvanted GNL purified soluble Env protein (80).
FIG 4.
Rabbit immunization protocol and serum characterization. (A) Immunization regimen for the four different rabbit groups. DNA (D) and rMVA (M) vaccines expressed either gp150 and GagM or gp150 alone. DNA and MVA vaccines for DDMMPP were matched. All groups were boosted with soluble Env (P). (B) Time course of soluble CAP256 Env binding ELISA for rabbit sera. When no binding was observed, the endpoint titer was plotted as 10. (C) CAP256 V1V2 loop scaffold binding ELISA of rabbit sera after the second MVA inoculation (post MVA) or after the second protein boost (DDMMPP) or third protein boost (MMPPP) (post final protein). All data are presented as group averages ± SEMs.
Anti-Env antibody titers in rabbit sera for the different time points were measured in an autologous Env binding assay (Fig. 4B). Both Env+GagM and Env DNA vaccines failed to elicit anti-Env antibodies, which developed only after rMVA boosting. For both rMVA Env+GagM and rMVA Env, anti-Env antibodies were elicited after the first rMVA inoculation, and although protein boosting appeared to increase Env binding titers, there were no significant differences in the peak titers after MVA (week 8 for MMPPP or week 14 for DDMMPP) and protein (weeks 22 and 30) boosting. Env binding antibody titers were boosted after each inoculation and plateaued around a dilution of 1 × 10−5 for all 4 groups tested. Inclusion of GagM in the DNA or rMVA vaccines did not affect Env binding antibody titers in any of the regimens (two-way analysis of variance [ANOVA]).
Serum antibody binding against the autologous CAP256 SU WT V1V2 loop, which was presented on a scaffold to retain the natively folded state (88), was measured after the second MVA inoculation (week 6 for MMPPP and week 14 for DDMMPP) and 2 weeks after the final protein boost (week 30). The data from the two different groups in each regimen were combined (Fig. 4C). No significant differences in endpoint titers were observed between MMPPP and DDMMPP regimens or between post-MVA and post-final protein inoculations (two-way ANOVA). Similarly, when all groups were compared individually, inclusion of GagM in the DNA or rMVA vaccines did not affect Env V1V2 loop binding antibody titers (two-way ANOVA).
Neutralization of HIV-1 pseudotype virions.
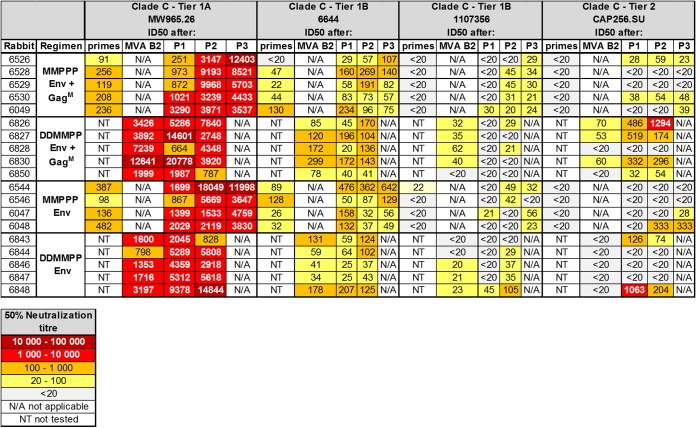
Rabbit sera were tested for neutralizing activity (reciprocal plasma/serum dilution causing a 50% reduction of relative light units [ID50]) against a panel of Env-pseudotyped viruses at selected time points (Table 2). In line with rMVA vaccines inducing anti-Env binding antibodies, neutralization titers for tier 1A virus MW965.26 and tier 1B virus 6644 were observed after the second rMVA inoculation (Table 2, MMPPP “primes” and DDMMPP “MVA B2”) in all groups. For MW965.26 these titers were significantly higher for the combined data of the two groups in the DDMMPP regimen than for the MMPPP groups (Student t test, P < 0.01). Protein boosting increased titers for MW965.26 in all four groups, and following the first protein boost, these titers were again significantly higher for the DDMMPP regimen (Student t test, P < 0.05). In line with this increased immunogenicity of the DDMMPP regimen, neutralizing antibodies against tier 1B 1107356 pseudovirions developed after MVA inoculation, whereas they developed only after protein boosting for the MMPPP regimen. Most encouragingly in these studies, all regimens induced vaccine-matched tier 2 (CAP256 SU) neutralization. For MMPPP groups, low-titer tier 2 neutralization was observed in the sera of 3/5 animals for MMPPP Env+GagM (ranging between 1:23 and 1:59) and 2/4 for MMPPP Env (1:28 and 1:333) after the third Env protein boost. These tier 2 neutralization titers were further enhanced by DNA priming, with 4/5 rabbits for DDMMPPP Env+GagM (ranging between 1:54 and 1:1,294) and 2/5 rabbits for DDMMPPP Env (1:74 and 1:204) developing these antibodies after the second Env protein boost. For 5/10 of the animals these titers were above the threshold required for 50% protection (1:105) in NHP models (89).
TABLE 2.
Serum neutralization measured by the TZM-bl assaya
The serum tested was taken 4 weeks after the second MVA prime for MMPPPP (primes) or 2 weeks after the second MVA boost for DDMMPP (MVA B2) and 2 weeks after each protein inoculation (P1, P2, and P3). Neutralization titers of serum at week 0 were negative for all viruses tested. All serum from all time points were negative in the MuLV negative-control neutralization assay. The 50% neutralization titers are color-coded to reflect their potency range as indicated. Titers below 20 are considered nonneutralizing and not color-coded.
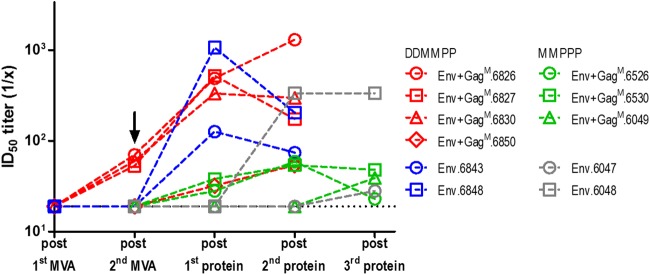
Some interesting observations were made in a longitudinal analysis of the individual animals that developed autologous tier 2 neutralizing antibodies. In comparing the groups receiving Env+GagM with those receiving Env alone, it was observed that more animals developed autologous tier 2 neutralization when Gag was included in the vaccines, with 3 out of 5 for MMPPP Env+GagM (60%), compared to 2 out of 4 for MMPPP Env (50%); this was even more pronounced for DDMMPP regimens, with 4 out of 5 (80%) for Env+GagM, compared to 2 out of 5 for Env alone (40%) (Fig. 5). However, these differences were not statistically different. Furthermore, in 5 out of the 7 animals that developed autologous tier 2 neutralizing antibodies, this occurred at an earlier time point when Gag was included in the vaccine, with only 1 animal having a delayed response. For the MMPPP regimen, autologous neutralization appeared after the first protein boost, whereas for the DDMMPP regimen, in 3 out of 4 rabbits, tier 2 neutralization of CAP256 SU developed after the second MVA inoculation, without the need for a protein boost (Fig. 5, arrow).
FIG 5.
Serum neutralization measured by the TZM-bl assay. Longitudinal, tier 2 neutralizing antibody responses to autologous CAP256.SU pseudovirion from serum of individual rabbits are shown. The arrow indicates autologous tier 2 neutralization after the second rMVA boost in the DDMMPP regimen in rabbits receiving Env+GagM vaccines. For the MMPPP regimen, no neutralization assay was performed after the first rMVA vaccine. The dotted black line represents assay detection limit (1/20 dilution), all data points below the detection limit are plotted as 19.
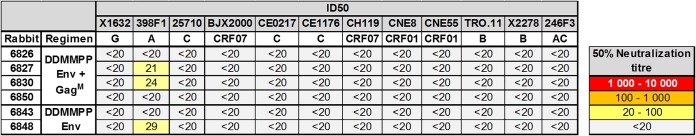
As autologous tier 2 neutralization was highest in animals treated with the DDMMPP regimen, week 30 sera from these particular rabbits were tested against a global panel of 10 tier 2 HIV-1 Env pseudoviruses. Three out of 6 animals developed a low titer neutralization response against clade A 398F1 (ranging between 1:20 and 1:29) (Table 3).
TABLE 3.
Serum neutralization of global tier 2 panela
The 50% neutralization titers are color-coded to reflect their potency range as indicated. Titers below 20 are considered nonneutralizing and not color-coded.
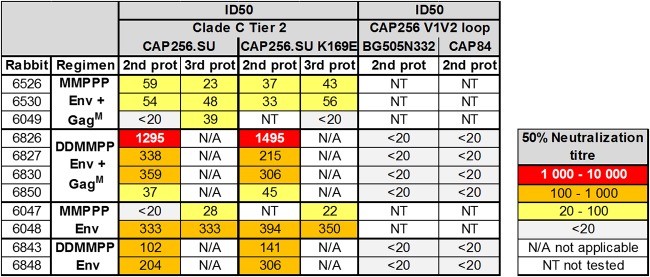
Serum from rabbits with autologous tier 2 neutralization titers at week 30 was used to investigate the possible site of neutralization within the Env sequence. A K169E mutation within CAP256 SU Env leads to the loss of binding of the native-like Env trimer-specific MAbs PG16 and CAP256 VRC26_08 (76, 77, 84). When sera were tested against CAP256 SU K169E pseudovirions, no differences were observed compared to CAP256 SU (Table 4). In line with this, chimeras formed by replacing the V1V2 region of two heterologous viruses, BG0505N332+ and CAP84, with that of CAP256 SU were not neutralized, indicating that the tier 2 NAbs elicited in this study probably did not target the V1V2 region.
TABLE 4.
Characterization of serum neutralization epitopea
Lack of neutralization of CAP256.SU K169E or neutralization of pseudovirions containing the V1V2 loop from CAP256 SU in heterologous CAP84 and BG505 backbones would reflect targeting of the trimer apex. The 50% neutralization titers are color-coded to reflect their potency range as indicated. Titers below 20 are considered nonneutralizing and not color-coded.
DISCUSSION
An effective HIV-1 vaccine will need to induce a range of immune responses, including polyfunctional nonneutralizing antibody responses, broadly neutralizing antibody responses, and high frequencies of polyfunctional cytotoxic T cell responses to multiple epitopes (90–94). In a previous study, we showed that mice primed with DNA and boosted with MVA vaccines expressing HIV-1 subtype C mosaic Gag developed a strong, Gag-specific cellular immune response (71). The aim of the present study was to build on these results by developing an improved vaccine regimen for the elicitation of neutralizing antibodies against HIV-1 Env which could be tested in a rabbit model. The Env protein from the superinfecting CAP256 SU virus, which is thought to have elicited V1V2 bNAbs in CAPRISA donor 256, was selected as an immunogen (76, 77). A DNA vaccine vector with enhanced expression of transgenes due to a novel enhancer element from porcine circovirus type 1 was used to prime the immune response. This DNA vaccine has been demonstrated to markedly improve immunogenicity in mice and to allow significant dose sparing (71, 81). To the best of our knowledge, this is the first time DNA and MVA vaccines expressing a gp150 HIV-1 envelope protein containing a flexible glycine linker and I559P mutation have been utilized. Other groups have developed viral vaccines that express the soluble gp140 envelope protein containing a flexible linker (54), gp140 antigens with a mutated cleavage site and a trimerization domain at the C terminus (95), or gp150 and gp160 antigens with and without mutated cleavage sites (96–98). DNA and MVA vaccines expressing HIV-1 Env containing this flexible linker are more suitable than the SOSIP constructs for heterologous prime-boost immunizations where endogenous furin is limited and cleavage is inefficient, as no cleavage is required. Use of a flexible linker also ensures that the gp120 portion of the protein is not lost, leaving only gp41 stumps on the surface of VLPs. Lastly, inclusion of the transmembrane region and a portion of the cytoplasmic tail allows for the incorporation of the envelope protein into the cell membrane, which could also improve the stability and conformation of the Env protein.
The quaternary structure of CAP256 envelope expressed by cells transfected or infected with the DNA and MVA vaccines, respectively, was characterized by live-cell staining with a selection of bNAbs and MAbs. V2 binding site and trimer-specific bNAbs PGT145, PG16, and CAP256 VRC26_08 bound to CAP256 envelope expressed on the surface of the cells, as did bNAbs PGT128 and PGT135, which recognize the V3-glycan supersite. VRC01, which recognizes the CD4 binding site, also bound to cells expressing the modified CAP256 Env. The binding of these MAbs to our modified CAP256 Env suggests that a proportion of the Env protein is correctly folded in a native-like trimeric structure. The binding of F105, which recognizes disordered trimers, indicates that some nonnative-like envelope is also produced. This is not surprising, as Capucci et al. also reported a mixture of native-like and nonnative-like trimers expressed from simian adenovirus and MVA vectors (expressing the BG505 SOSIP.664 antigen) (54). Three out of four rabbits immunized with the simian adenovirus vaccine, followed by MVA and then ISCOMATRIX-adjuvanted BG505s trimer, developed autologous tier 2 NAbs. Similarly, in our study, four out of five rabbits vaccinated with the DDMMPP regimen developed autologous tier 2 NAbs to the CAP256 pseudovirus.
The three vaccines were tested in two different regimens (MMPPP and DDMMPP). Both regimens elicited autologous tier 2 neutralizing antibodies (Table 2) and high titers of binding antibodies to both the matching CAP256 Env and a CAP256 V1V2 loop scaffold (Fig. 4B and C). Neutralizing antibodies against HIV-1 have been shown to protect in nonhuman primates (NHPs) and are therefore seen as an important component of an HIV vaccine (99–101).
Higher mean peak titers of tier 2 NAbs were obtained for the DDMMPP regimen than for the MMPPP regimen, although these differences were not significant, probably due to the low numbers of animals used in the study. Some rabbits vaccinated with the DDMMPP regimen also developed low levels of neutralizing antibodies to clade A pseudovirus 398F1 (Table 3). These findings correlate with other studies which have shown that DNA primes a good humoral response. The addition of a DNA prime to MVA vaccination regimens also increases the magnitude and quality of T cell responses (102–104). Addition of DNA-C priming in the EV01 phase I trial increased IgG antibody responses against Env from 27% in the group vaccinated with NYVAC alone to 75% in the DNA+NYVAC group (102). The DNA prime also significantly boosted the T cell responses.
The inclusion of Gag in the DNA and MVA vaccines resulted in earlier development of tier 2 autologous neutralizing antibodies for both vaccination regimens. In addition, a higher proportion of the rabbits primed with vaccines that included Gag developed tier 2 autologous NAbs than those primed with DNA and MVA vaccines expressing Env alone. It is also interesting that three out of five rabbits developed low levels of autologous tier 2 NAbs after vaccination with the DNA and MVA vaccines when Gag was included in the vaccines, whereas rabbits vaccinated with DNA and MVA vaccines that did not express Gag (i.e., Env alone) developed tier 2 NAbs only after the first protein boost. This could be due simply to the improved adjuvant properties of a relatively large VLP, or it may be due to the stabilization of the Env in a more native-like conformation on the envelope of the Gag VLPs. It should be noted that the licensed vaccines against hepatitis B virus, rotavirus, and human papillomavirus are all VLPs, indicating that this is a highly effective mode of vaccine delivery (61, 62). Tong et al. (63) showed that the surface of HIV-1-derived VLPs often contains uncleaved gp160 and gp41 stumps that promote the development of nonneutralizing responses. The removal of these nonfunctional Envs with protease treatment resulted in VLPs that were better able to induce tier 2 NAbs (63, 96). As the Env used in this study contains a flexible linker between the gp120 and gp41, there is no necessity for cleavage, and thus, it is highly unlikely that there will be any exposed gp41 stumps on the surface of our VLPs. Ingale et al. (57) showed that SOSIP or NFL trimers presented on the surface of nanoparticles were better at activating germinal center B cells and inducing B cell receptor signaling and activation than soluble Env, further supporting the use of a particle-based HIV vaccine. The Env presented on the surface of these nanoparticles also showed a trend toward eliciting better neutralizing antibody responses than the soluble Env (57).
The structure of the His-tagged CAP256 gp140 protein was assessed by capture ELISA and detection with the same bNAbs and MAbs (Fig. 1). Again, both trimeric and nonnative species of Env were detected. Trimer-specific bNAbs PG16 and CAP256 VRC26_08 (Fig. 1G) recognized the CAP256 protein, but so did MAb F105 (Fig. 1H). In future studies, these nonnative trimers could be removed using negative selection with the F105 antibody. Additional mutations could also be introduced to improve the stability (105).
The CAP256 SU Env elicited potent V2-directed bNAbs during infection. Such antibodies typically recognize a lysine-rich region (residues 168 to 171) on Env and also interact with a glycan at N160 (78, 79, 88, 106). Furthermore, immunization of rabbits with a viral isolate, CRF250, that shares V2 apex sequence similarity with CAP256 SU (both contain a glycan hole at the V2 apex, which may enhance reactivity with V2 precursors) has recently been shown to elicit tier 2 autologous antibodies. Two of these NAbs were targeted to the lysine-rich basic region in the V2 apex, although these were shown not be to trimer specific, as they were adsorbed by gp120 proteins. In our study, we observed no neutralization of pseudoviruses containing V1V2 of CAP256 in heterologous CAP84 and BG505 backbones, and no effect was observed when point mutations were introduced into the C strand of the V2 region. This indicates that the tier 2 autologous NAbs elicited by this vaccine regimen targeted a region of the envelope other than V2. Further neutralization assays utilizing different chimeras and mutations will need to be carried out to determine the binding site(s) of the tier 2 NAbs elicited in this study.
The GOVX-B11 subtype B DNA prime-MVA boost vaccines, which have been tested in clinical trials and shown to be safe and immunogenic, are similar in design to the vaccines used in this study (97, 107). That DNA vaccine expresses Gag VLPs containing gp160 Env and granulocyte-macrophage colony-stimulating factor (GM-CSF), and the MVA vaccine expresses Gag VLPs containing gp150 Env. These envelopes do not contain modifications such as those used in the SOSIP or NFL trimers to improve native trimer formation and stability. The vaccines were found to elicit higher titers of antibodies to gp41 than gp120 and low levels of tier 2 neutralizing antibodies, in only 15% of vaccinees. The flexible linker and I559P mutation used in our vaccines should prevent the dissociation of the gp120 and gp41 subunits and better stabilize the Env trimer, consequently leading to improved antibody responses.
Although the rMVA used in this study contained the K1L vaccinia virus host range gene, which makes the virus replication competent in RK13 cells, it did not appear to result in replication in the rabbits, as evidenced by the monitoring of the weight and well-being of the animals. It is not known if the inclusion of K1L would affect immunogenicity of MVA.
Further studies are planned to optimize our vaccination regimen. These include (i) the use of Pharmajet, needle-free devices for inoculation of the DNA and, possibly, MVA vaccines, (ii) the use of different adjuvants, such as ISCOMATRIX, with the protein boosts, and (iii) subcutaneous rather than intramuscular inoculation. It would also be desirable to shorten the vaccination regimen to DMP as opposed to DDMMPP if a comparative immune response can be elicited. Such modifications may be amenable to sequential immunization strategies in which the priming antigen is followed by a series of immunogens to broaden the neutralizing antibody response (90, 91, 108).
The vaccines tested in this study have been shown to elicit high-titer binding antibodies to the V1V2 region of CAP256 Env and robust tier 2 neutralizing antibodies in rabbits. Previously, the DNA and MVA vaccines expressing HIV-1 GagM were shown to elicit strong T cell responses in mice (71). In addition, the induction of polyfunctional, nonneutralizing antibody responses should be assessed, as this type of response has been shown to play a critical role in protection in nonhuman primate challenge studies and to correlate with protection in the RV144 trial (90). The excellent responses demonstrated in this heterologous prime-boost regimen in a rabbit model justifies further testing of these novel candidate HIV-1 vaccines. Accordingly, plans are under way to test these vaccines in nonhuman primates.
MATERIALS AND METHODS
Antibodies, plasmids, cell lines, media, and reagents.
Goat anti-HIV-1 gp160 (MRC ADP 72 408/5104), rabbit anti-HIV-1 p24 (Gag) (ARP 432), donkey anti-goat IgG-Cy3 or -FITC, and donkey anti-rabbit IgG-Alexa Fluor 647 (Life Technologies) were used for immunofluorescence assays. Goat anti-human IgG (Fc specific)-Cy3 antibody (Sigma) was used for live-cell staining. Goat anti-HIV-1 gp120 (Bio-Rad; 5000-0557), goat anti-HIV-1 p24 (Gag) (Bio-Rad; 4999-9007), and mouse monoclonal anti-goat/sheep IgG-alkaline phosphatase (AP) GT34 (Sigma) were used for Western blotting. Anti-HIV-1 Env human monoclonal antibodies (MAbs) PG9, PG16, PGT128, PGT135, PGT145, CAP256 VRC26_08, VRC01, 10E8, F105, and 447-52D were expressed in FreeStyle 293F cells (Life Technologies) using the PEIMAX transfection reagent (Polysciences). Monoclonal antibodies were purified from cell-free supernatants after 6 days using protein A affinity chromatography (77). CAP256 SU V1V2 scaffolded proteins were purified by affinity chromatography using a nickel-nitrilotriacetic acid (Ni-NTA) column as described previously by Gorman et al. (78). Env control protein BG505 SOSIP.664-His trimers were purified by affinity chromatography using a Ni-Sepharose column as described previously (46).
HeLa, RK13, BHK-21, HEK293, and HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) plus l-glutamine (Lonza) plus 10% fetal calf serum plus 1× penicillin-streptomycin (Pen-Strep) (both from Gibco).
The mammalian expression plasmid pTHCapR was used as a backbone for all DNA vaccines (81), and pTJDNA4 containing mosaic gag (gagM) was described earlier (71). All DNA vaccines were synthesized by Aldevron.
CAP256 gp150-FL-IP cloning.
The sequence of CAP256 SU gp160 (clone CAP256.206sp.032.C9) has been previously described (GenBank accession number KF241776.1) (84). This gene sequence was derived from that of the superinfecting virus isolated from patient CAP256 in the CAPRISA 002 cohort (76). The Env sequence was altered as follows and is schematically represented in Fig. 1A: the native leader (signal peptide [SP]) was removed and replaced with the human tissue plasminogen activator (TPA) leader sequence, the furin cleavage site was replaced with a flexible linker sequence (FL) (55), and an I548P mutation equivalent to the I559P in the SOSIP trimers was introduced to improve the trimerization of gp41 (44). Finally, the sequence was truncated to gp150 (amino acid [aa] 730) for MVA and DNA vaccines to increase expression and stability (109). Any potential poxvirus transcriptional termination signals (TTTTTNT) were removed from the coding sequence, and a poxvirus transcriptional termination sequence was added directly after the stop codon (TGA) of the Env gene. The Env sequences were human codon optimized and synthesized by GenScript. The DNA expression vector was called pMExT gp150-FL-IP (plasmid for mammalian expression with TPA leader). The TPA gp150-FL-IP was subcloned into the pox transfer vector.
Recombinant MVA CAP256 Env constructs.
Wild-type modified vaccinia virus (VACV) Ankara (MVA) and recombinant MVA-GagM (71, 72) were used for targeted integration of CAP256 gp150-FL-IP. A transfer vector was created in which, in between flanking sequences of the MVA G1L-I8R locus, a selection cassette containing eGFP under the control of the VACV p7.5 promoter, K1L under the control of the VACV pSS promoter, and TPA-gp150-FL-IP under the control of the VACV mH5 promoter were inserted to form the plasmid Shuttle and Selection for Pox Expression (pSSPEx) CAP256 gp150-FL-IP. This plasmid allows for positive selection of integration into MVA via K1L selection in RK13 cells and identification of recombinant virus by eGFP expression.
BHK-21 cells were transfected with pSSPEx CAP256 gp150-FL-IP 2 h after infection with either wild-type MVA or rMVA-GagM. Three days after infection and transfection, cells were freeze-thawed and the viral lysate was passaged in RK13 cells, a cell line permissive to MVA in the presence of K1L expression (82, 83). Single foci from RK13 cells were repeatedly selected and screened for correct integration of the targeting construct by PCR. Expression of GagM and/or Env was verified by Western blot analysis and immunofluorescence. Positive single foci were expanded in 3 hyperflasks and harvested after most RK13 cells had lifted. Three freeze-thaw cycles were performed, and supernatant containing rMVA virus was cleared by low-speed centrifugation. Remaining virus in the pellet was released by lysis with 0.1 mM Tris (pH 9), and the second supernatant was added to the previous one after another low-speed spin. rMVA was concentrated by centrifugation through a 36% sucrose cushion and resuspended in a total volume of 10 ml of PBS plus 10% glycerol. The generated rMVA GagM CAP256 gp150-FL-IP (rMVA Env+GagM) and rMVA CAP256 gp150-FL-IP (rMVA Env) were aliquoted and stored at −80°C for downstream usage. Titers were determined in BHK-21 cells, in which GFP-positive plaques were counted 48 h after infection of a serial dilution range. Titers for both rMVA Env+GagM and rMVA Env were >0.5 × 109 PFU/ml. High-titer stocks were screened for correct integration of the targeting construct by PCR and PCR sequencing (analyzed with CLC Main Workbench; Qiagen). Expression of mosaic Gag and/or Env was verified by Western blot analysis and immunofluorescence.
Soluble CAP256 Env protein.
A stable cell line in HEK293 cells expressing CAP256 gp140-FL-IP protein (soluble Env) was made as described previously by van Diepen et al. (80). A second stable cell line was generated in a similar fashion for a His-tagged gp140 (soluble Env-His) in which a 3×Ala plus 6×His motif was added after amino acid 653 of CAP256 gp140-FL-IP. Soluble Env and soluble Env-His were isolated from media of respective serum-starved stable cell lines (passaged >10 times) as described previously. In short, flasks were coated with poly-l-lysine, allowing repeat harvests. Agarose-conjugated lectin (Galanthus nivalis; Sigma) (GNL) affinity purification columns were made to capture soluble Env from supernatant 3 days after changing to serum-free conditions. Protein was eluted in PBS plus 1 M methyl α-d-mannopyranoside (Sigma). Fractions containing trimeric protein (as assessed by molecular weight) were isolated after size exclusion chromatography (SEC) on a Superdex 200 HiLoad 16/600 column (GE Healthcare Life Sciences). Purified soluble trimeric Env protein was aliquoted and stored at −80°C for downstream usage. Protein concentration was determined using a DC protein assay (Bio-Rad) against a bovine serum albumin (BSA) standard.
CAP256 GP140-FL-IP protein characterization.
To confirm isolation of soluble Env protein and trimers, precast NativePAGE Novex 3 to 12% bis-Tris protein gels (Life Technologies) were used for PAGE. Gels were either stained with Bio-Safe Coomassie (Bio-Rad) or blotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). For the latter, blots were incubated with goat anti-gp120 (1:1,000), followed by monoclonal anti-goat/sheep IgG-AP (1:10,000), and detected with 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium (NBT) phosphatase substrate (KPL). NativeMark unstained protein standard (Life Technologies) for native gel electrophoresis was used for estimating molecular weight. Liquid chromatography-mass spectrometry (CPGR, Cape Town) was used to confirm that soluble CAP256 Env was the foremost protein eluted off the lectin affinity purification column and in the trimeric fraction after SEC.
The trimeric fraction of soluble Env-His was used to characterize the antigenic structure. Ni-NTA 96 well plates (Qiagen) were coated with 200 ng/well of soluble, trimeric Env-His for 2 h, washed with PBS (3×), and blocked with 5% nonfat milk (Sigma) in PBS (block buffer) for 30 min (all at room temperature). Following PBS washing (3×), plates were incubated overnight at 4°C with 100 µl of serial dilutions (steps of 1:3) of anti-Env human monoclonal antibodies PG9, PG16, PGT128, PGT135, PGT145, CAP256 VRC26_08, VRC01, F105, and 447-52D in block buffer. Plates were washed with PBS (3×) and incubated with anti-human IgG HRP (1:10,000) (Dako) in block buffer. After the final PBS wash (3×), tetramethylbenzidene (TMB) ELISA substrate (Abcam) was added for detection and the reaction was stopped after 10 min with 100 µl of 1 N H2SO4. The signal was analyzed using a VersaMax ELISA microplate reader (Molecular Devices), which subtracted absorbance at 540 nm from 450 nm. The experiment was repeated for three different isolations (n = 3) and fitted to a four-parameter logistic regression curve (4PL curve) in GraphPad Prism 5.0.
Expression and colocalization of mosaic Gag and CAP256 gp150-FL-IP.
To characterize Gag and Env expression and colocalization from rMVA and DNA vaccines, HEK293T cells were infected with MVA or transfected with DNA vaccines. After 48 h (rMVA vaccines) or 72 h (DNA vaccines), the supernatant was harvested and cleared by a low-speed spin (5 min at 275 × g) for Western blot analysis.
For immunofluorescence, cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with methanol (1 min), and washed with PBS. Cells were blocked for 1 h at 37°C before being incubated with primary goat anti-gp160 and rabbit anti-p24 (both 1:500), diluted in blocking reagent, at 4°C overnight. After washing with PBS, secondary antibody (donkey anti-goat IgG-Cy3 and donkey anti-rabbit IgG Alexa Fluor 647, both at 1:500 in blocking reagent) was added for 1 h and coverslips were mounted with Mowiol after PBS washes. Cells were imaged on an LSM 880 Fast Airyscan (Zeiss) at the UCT Confocal & Light Microscope Imaging Facility. In figures, pseudocoloring for Cy3 is in red and that for Alexa Fluor 647 in green. Single images comprising 13 merged z-stacks were generated using Zeiss Zen software.
Live-cell staining of CAP256 gp150-FL-IP using human monoclonal anti-Env antibodies.
To assess the structural integrity of Env expressed from rMVA and DNA vaccines, HeLa cells were infected with rMVA vaccines or transfected with DNA vaccines and tested for binding to anti-Env human monoclonal antibodies PG9, PG16, PGT128, PGT135, PGT145, CAP256 VRC26_08, VRC01, 10E8, F105, and 447-52D. After 48 h (rMVA vaccines) or 72 h (DNA vaccines) medium on cells was replaced with medium containing 10 µg/ml of MAb and incubated at room temperature for 1 h. For cells transfected with DNA vaccines, wells were incubated with goat anti-gp160 (1:500 in medium) at room temperature for 0.5 h. Following 3× PBS washes, cells were incubated with medium containing anti-human IgG-Cy3 (1:500) (for rMVA vaccines) or anti-human IgG Cy3 plus anti-goat IgG FITC (both 1:500) (for DNA vaccines) at room temperature for 0.5 h, and after 3× PBS washes, cells were imaged in PBS on a Zeiss Microscope using Zeiss Zen software.
Detection of VLPs.
RK13 cells were infected with rMVA vaccines or transfected with DNA vaccines to investigate the formation of Gag virus-like particles (VLPs). After 48 h (rMVA vaccines) or 72 h (DNA vaccines) cells were collected by scraping, spun briefly, and washed, after which the pellets were washed with PBS and then were fixed overnight in 2.5% glutaraldehyde in PBS (4°C). The fixation agent was replaced with PBS, pellets were sent to the Microscopy & Microanalysis Unit at the University of KwaZulu-Natal for postprocessing as described earlier (71), and images were acquired on a JEOL 1010 platform.
HEK293T cells were infected with rMVA vaccines or transfected with DNA vaccines to characterize Env inclusion into VLPs. After 48 h (rMVA vaccines) or 72 h (DNA vaccines), VLPs were isolated from the supernatant of infected and transfected cells as follows. The supernatant was cleared with a low-speed spin (5 min at 275 × g) and run on an OptiPrep cushion (12, 24, and 60%) at ∼110,000 × g and 4°C for 90 min. A visible band (or equivalent) around the 12% to 24% interface was isolated and subsequently run overnight at ∼110,000 × g and 4°C on an OptiPrep gradient (18, 21, 24, 30, 36, and 42%). The only visible band (or equivalent) around 45 mm from the bottom was isolated. Bands from the cushion and gradient were analyzed for the presence of mosaic Gag or Env by Western blotting following separation on 8% denaturing SDS-PAGE gels. Western blotting was performed as described above, with both primary antibodies diluted at 1:1,000 and detected with AP-linked secondary antibodies (1:10,000).
Rabbit immunization.
Female New Zealand White rabbits were housed in the animal facility of the Health Sciences Faculty at the University of Cape Town (UCT). All the animal procedures were approved by the UCT Animal Research Ethics Committee (reference UCT AEC 014-030 and 015-051) and performed by a trained animal technologist. Four groups, with 5 rabbits each, were selected to compare direct rMVA priming (rMVA Env+GagM or rMVA Env) with groups receiving DNA primes (DNA Env+GagM or DNA Env), followed by matching rMVA. All animals were boosted with trimeric, soluble CAP256 Env protein. DNA and rMVA priming vaccines were administered intramuscularly in the hind leg at weeks 0 and 4. DNA primes consisted of 100 µg of pTJDNA4 mixed with 100 µg of pMExT CAP256 gp150-FL-IP (both at 1 mg/ml) or 100 µg of pMExT CAP256 gp150-FL-IP (DDMMPP). Recombinant MVA was administered intramuscularly in the hind leg at weeks 0 and 4 as priming vaccines (MMPPP) or at weeks 8 and 12 as booster vaccines (DDMMPP) at 108 PFU in 500 µl of PBS. For MMPPP groups, rabbits received 40 µg of trimeric soluble Env protein in 500 µl of 1:1 (vol/vol) Alhydrogel adjuvant in the same fashion at weeks 12, 20, and 28 to boost the immune response, whereas animals in the DDMMPP group received the protein boost at weeks 20 and 28. All animals were bled every 2 to 4 weeks from when inoculations started. Week 0 bleeds were used as prebleeds. In total, three animals died due to unrelated causes.
Rabbit serum binding ELISAs.
To assess Env binding antibody titers in rabbit sera, a matching soluble CAP256 Env binding ELISA was performed as described earlier (80). In short, Nunc MaxiSorp flat-bottom 96-well plates (Sigma) were coated with 10 ng/well of soluble, trimeric Env. Rabbit sera were used in the primary incubation in a serial dilution range starting at 1:10. Anti-rabbit IgG-horseradish peroxidase (HRP) (1:5,000, Roche) was used for detection with TMB ELISA substrate (Abcam). The reaction was stopped after 10 min with 1 N H2SO4. The ELISA signal was analyzed using a VersaMax ELISA microplate reader (Molecular Devices), which subtracted absorbance values at 540 nm from values at 450 nm. ELISAs for the whole time course and each group were performed at the same time on duplicate plates. Duplicate data points were averaged and fitted to a four-parameter logistic regression curve (4PL curve) in GraphPad Prism 5.0. Antibody endpoint titers were calculated from 4PL curves with the threshold set as 4PL curve minimum + standard error of minimum for each time point. Data were plotted as the mean ± SEM for the whole group. ELISAs for binding to CAP256 SU V1V2 loop scaffold for week 0 and 2 weeks after the final MVA inoculation or protein boost were performed in a similar fashion, but wells were coated with 500 ng/well of protein.
Rabbit serum neutralization assay.
Rabbit sera from different time points were tested for their ability to inhibit entry of Env-pseudotyped virions into a reporter cell line. Neutralization was measured as a reduction in luciferase gene expression after a single round of infection of JC53bl-13 cells, also known as TZM-bl cells (NIH AIDS Research and Reference Reagent Program), with Env-pseudotyped viruses (MW965.26, 6644, CA146, 1107356, CAP37, CT349, Du156, 188146, and CAP256 SU). Serum from selected animals and time points were also tested against CAP256 SU K169E, BG505 N332_CAP256 V1V2-loop, CAP84_CAP256 V1V2-loop and a Tier 2 global panel (X1632, 398F1, 25710, BJX2000, CE0217, CE1176, CH119, CNE8, CNE55, TRO.11, X2278, and 246F3). Titer was calculated as the reciprocal plasma/serum dilution causing a 50% reduction of relative light units (ID50). Dilutions were started at 1:20. For graphs, data were plotted as 19 when the ID50 was <20. Murine leukemia virus (MuLV) was used as a negative control.
Statistical analysis.
All statistical analysis was performed in GraphPad Prism 5.0. Both one-way and two-way ANOVA were performed with Bonferroni post hoc testing. For the neutralization data, a titer value of 19 was used for data of <20.
ACKNOWLEDGMENTS
This work is based upon research supported by the South African Medical Research Council with funds received from the South African Department of Science and Technology and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation. The LSM 880 Airyscan confocal microscope used in this work was purchased using funds from the Wellcome Trust, grant reference number 108473/Z/15/Z.
A provisional patent has been applied for covering the recombinant MVA PA166530/P, “Recombinant MVA with modified HIV-1 env.” Authors listed on this patent include Michiel van Diepen, Rosamund Chapman, Nicola Douglass, Shireen Galant, Edward Rybicki, and Anna-Lise Williamson. The DNA vaccine utlized in this study is covered by patent WO2007054788A1, “Expression system incorporating a capsid promoter sequence as an enhancer of a cytomegalovirus promoter.” Authors listed on this patent include Edward Rybicki.
REFERENCES
- 1.Stephenson KE, Barouch DH. 2013. A global approach to HIV-1 vaccine development. Immunol Rev 254:295–304. doi: 10.1111/imr.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein JS, Bjorkman PJ. 2010. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog 6:e1000908. doi: 10.1371/journal.ppat.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 4.Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol 80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward AB, Wilson IA. 2015. Insights into the trimeric HIV-1 envelope glycoprotein structure. Trends Biochem Sci 40:101–107. doi: 10.1016/j.tibs.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, CAPRISA002 Study Team. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med 13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, Schuitemaker H. 2010. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J Infect Dis 201:1045–1053. doi: 10.1086/651144. [DOI] [PubMed] [Google Scholar]
- 15.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. 2014. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landais E, Huang X, Havenar-Daughton C, Murrell B, Price MA, Wickramasinghe L, Ramos A, Bian CB, Simek M, Allen S, Karita E, Kilembe W, Lakhi S, Inambao M, Kamali A, Sanders EJ, Anzala O, Edward V, Bekker LG, Tang J, Gilmour J, Kosakovsky-Pond SL, Phung P, Wrin T, Crotty S, Godzik A, Poignard P. 2016. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog 12:e1005369. doi: 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blish CA, Dogan OC, Derby NR, Nguyen MA, Chohan B, Richardson BA, Overbaugh J. 2008. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol 82:12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D, Abbink P, Schmidt SD, Wang K, Chen X, Joyce MG, Georgiev IS, Choe M, Kwong PD, Doria-Rose NA, Le K, Louder MK, Bailer RT, Moore PL, Korber B, Seaman MS., Abdool Karim SS, Morris L, Koup RA, Mascola JR, Burton DR, Barouch DH. 2017. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med 9:eaal1321. doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 20.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 22.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 24.van Gils MJ, Euler Z, Schweighardt B, Wrin T, Schuitemaker H. 2009. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS 23:2405–2414. doi: 10.1097/QAD.0b013e32833243e7. [DOI] [PubMed] [Google Scholar]
- 25.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, Kaya G, Krahling V, Kwilas S, Lemaitre B, Matthey A, Silvera P, Becker S, Fast PE, Moorthy V, Kieny MP, Kaiser L, Siegrist CA, VSV-Ebola Consortium. 2015. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 15:1156–1166. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wibmer CK, Moore PL, Morris L. 2015. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS 10:135–143. doi: 10.1097/COH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Taeye SW, Moore JP, Sanders RW. 2016. HIV-1 envelope trimer design and immunization strategies to induce broadly neutralizing antibodies. Trends Immunol doi: 10.1016/j.it.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M, Qiao ZS, Montefiori DC, Haynes BF, Reinherz EL, Liao HX. 2005. Comparison of HIV type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res Hum Retroviruses 21:58–67. doi: 10.1089/aid.2005.21.58. [DOI] [PubMed] [Google Scholar]
- 30.Beddows S, Franti M, Dey AK, Kirschner M, Iyer SP, Fisch DC, Ketas T, Yuste E, Desrosiers RC, Klasse PJ, Maddon PJ, Olson WC, Moore JP. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329–340. doi: 10.1016/j.virol.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Earl PL, Sugiura W, Montefiori DC, Broder CC, Lee SA, Wild C, Lifson J, Moss B. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J Virol 75:645–653. doi: 10.1128/JVI.75.2.645-653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earl PL, Broder CC, Long D, Lee SA, Peterson J, Chakrabarti S, Doms RW, Moss B. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol 68:3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava IK, Stamatatos L, Kan E, Vajdy M, Lian Y, Hilt S, Martin L, Vita C, Zhu P, Roux KH, Vojtech L, C Montefiori D, Donnelly J, Ulmer JB, Barnett SW. 2003. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J Virol 77:11244–11259. doi: 10.1128/JVI.77.20.11244-11259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CW, Chishti Y, Hussey RE, Reinherz EL. 2001. Expression, purification, and characterization of recombinant HIV gp140. The gp41 ectodomain of HIV or simian immunodeficiency virus is sufficient to maintain the retroviral envelope glycoprotein as a trimer. J Biol Chem 276:39577–39585. doi: 10.1074/jbc.M107147200. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Farzan M, Wyatt R, Sodroski J. 2000. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J Virol 74:5716–5725. doi: 10.1128/JVI.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, King SR, Montefiori DC, Nabel GJ. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol 76:5357–5368. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Florin L, Farzan M, Kolchinsky P, Kwong PD, Sodroski J, Wyatt R. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J Virol 74:4746–4754. doi: 10.1128/JVI.74.10.4746-4754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol 76:4634–4642. doi: 10.1128/JVI.76.9.4634-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ringe RP, Sanders RW, Yasmeen A, Kim HJ, Lee JH, Cupo A, Korzun J, Derking R, van Montfort T, Julien JP, Wilson IA, Klasse PJ, Ward AB, Moore JP. 2013. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc Natl Acad Sci U S A 110:18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, Klasse PJ. 2014. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology 11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binley JM, Sanders RW, Master A, Cayanan CS, Wiley CL, Schiffner L, Travis B, Kuhmann S, Burton DR, Hu SL, Olson WC, Moore JP. 2002. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J Virol 76:2606–2616. doi: 10.1128/JVI.76.6.2606-2616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol 74:627–643. doi: 10.1128/JVI.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, Kalyanaraman R, Paluch M, Berkhout B, Maddon PJ, Olson WC, Lu M, Moore JP. 2002. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol 76:8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klasse PJ, Depetris RS, Pejchal R, Julien JP, Khayat R, Lee JH, Marozsan AJ, Cupo A, Cocco N, Korzun J, Yasmeen A, Ward AB, Wilson IA, Sanders RW, Moore JP. 2013. Influences on trimerization and aggregation of soluble, cleaved HIV-1 SOSIP envelope glycoprotein. J Virol 87:9873–9885. doi: 10.1128/JVI.01226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, Crampton J, Hu J, Bontjer I, Torres JL, Arendt H, DeStefano J, Koff WC, Schuitemaker H, Eggink D, Berkhout B, Dean H, LaBranche C, Crotty S, Crispin M, Montefiori DC, Klasse PJ, Lee KK, Moore JP, Wilson IA, Ward AB, Sanders RW. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klasse PJ, LaBranche CC, Ketas TJ, Ozorowski G, Cupo A, Pugach P, Ringe RP, Golabek M, van Gils MJ, Guttman M, Lee KK, Wilson IA, Butera ST, Ward AB, Montefiori DC, Sanders RW, Moore JP. 2016. Sequential and simultaneous immunization of rabbits with HIV-1 envelope glycoprotein SOSIP.664 trimers from clades A, B and C. PLoS Pathog 12:e1005864. doi: 10.1371/journal.ppat.1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torrents de la Pena A, de Taeye SW, Sliepen K, LaBranche CC, Burger JA, Schermer EE, Montefiori DC, Moore JP, Klasse PJ, Sanders RW. 2018. Immunogenicity in rabbits of HIV-1 SOSIP trimers from clades A, B, and C, given individually, sequentially, or in combination. J Virol 92:e01957-17. doi: 10.1128/JVI.01957-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Pena A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR. 2017. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46:1073–1088.e6. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng Y, Tran K, Bale S, Kumar S, Guenaga J, Wilson R, de Val N, Arendt H, DeStefano J, Ward AB, Wyatt RT. 2016. Thermostability of well-ordered HIV spikes correlates with the elicitation of autologous tier 2 neutralizing antibodies. PLoS Pathog 12:e1005767. doi: 10.1371/journal.ppat.1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boliar S, Das S, Bansal M, Shukla BN, Patil S, Shrivastava T, Samal S, Goswami S, King CR, Bhattacharya J, Chakrabarti BK. 2015. An efficiently cleaved HIV-1 clade C Env selectively binds to neutralizing antibodies. PLoS One 10:e0122443. doi: 10.1371/journal.pone.0122443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capucci S, Wee EG, Schiffner T, LaBranche CC, Borthwick N, Cupo A, Dodd J, Dean H, Sattentau Q, Montefiori D, Klasse PJ, Sanders RW, Moore JP, Hanke T. 2017. HIV-1-neutralizing antibody induced by simian adenovirus- and poxvirus MVA-vectored BG505 native-like envelope trimers. PLoS One 12:e0181886. doi: 10.1371/journal.pone.0181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma SK, de Val N, Bale S, Guenaga J, Tran K, Feng Y, Dubrovskaya V, Ward AB, Wyatt RT. 2015. Cleavage-independent HIV-1 Env trimers engineered as soluble native spike mimetics for vaccine design. Cell Rep 11:539–550. doi: 10.1016/j.celrep.2015.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Murillo P, Tran K, Guenaga J, Lindgren G, Àdori M, Feng Y, Phad GE, Vázquez Bernat N, Bale S, Ingale J, Dubrovskaya V, O’Dell S, Pramanik L, Spångberg M, Corcoran M, Loré K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2017. Particulate array of well-ordered HIV clade C Env trimers elicits neutralizing antibodies that display a unique V2 cap approach. Immunity 46:804–817.e7. doi: 10.1016/j.immuni.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ingale J, Stano A, Guenaga J, Sharma SK, Nemazee D, Zwick MB, Wyatt RT. 2016. High-density array of well-ordered HIV-1 spikes on synthetic liposomal nanoparticles efficiently activate B cells. Cell Rep 15:1986–1999. doi: 10.1016/j.celrep.2016.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bale S, Goebrecht G, Stano A, Wilson R, Ota T, Tran K, Ingale J, Zwick MB, Wyatt RT. 2017. Covalent linkage of HIV-1 trimers to synthetic liposomes elicits improved B cell and antibody responses. J Virol 91:e00443-17. doi: 10.1128/JVI.00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sliepen K, Ozorowski G, Burger JA, van Montfort T, Stunnenberg M, LaBranche C, Montefiori DC, Moore JP, Ward AB, Sanders RW. 2015. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 12:82. doi: 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Georgiev IS, Joyce MG, Chen RE, Leung K, McKee K, Druz A, Van Galen JG, Kanekiyo M, Tsybovsky Y, Yang ES, Yang Y, Acharya P, Pancera M, Thomas PV, Wanninger T, Yassine HM, Baxa U, Doria-Rose NA, Cheng C, Graham BS, Mascola JR, Kwong PD. 2018. Two-component ferritin nanoparticles for multimerization of diverse trimeric antigens. ACS Infect Dis 4:788–796. doi: 10.1021/acsinfecdis.7b00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kushnir N, Streatfield SJ, Yusibov V. 2012. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. 2010. Virus-like particles in vaccine development. Expert Rev Vaccines 9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 63.Tong T, Crooks ET, Osawa K, Binley JM. 2012. HIV-1 virus-like particles bearing pure Env trimers expose neutralizing epitopes but occlude nonneutralizing epitopes. J Virol 86:3574–3587. doi: 10.1128/JVI.06938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med 13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 65.Prendergast A, Goodliffe H, Clapson M, Cross R, Tudor-Williams G, Riddell A, Daniels J, Williams A, Goulder P. 2011. Gag-specific CD4+ T-cell responses are associated with virological control of paediatric HIV-1 infection. AIDS 25:1329–1331. doi: 10.1097/QAD.0b013e3283478575. [DOI] [PubMed] [Google Scholar]
- 66.Williamson AL, Rybicki EP. 2016. Justification for the inclusion of Gag in HIV vaccine candidates. Expert Rev Vaccines 15:585–598. doi: 10.1586/14760584.2016.1129904. [DOI] [PubMed] [Google Scholar]
- 67.Mann JK, Ndung'u T. 2015. HIV-1 vaccine immunogen design strategies. Virol J 12:3. doi: 10.1186/s12985-014-0221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, Kuiken C, Haynes B, Letvin NL, Walker BD, Hahn BH, Korber BT. 2007. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med 13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 69.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, Quinn D, Parks RJ, Tsao CY, Carville A, Mansfield KG, Pavlakis GN, Felber BK, Haynes BF, Korber BT, Letvin NL. 2010. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med 16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chapman R, Jongwe TI, Douglass N, Chege G, Williamson AL. 2017. Heterologous prime-boost vaccination with DNA and MVA vaccines, expressing HIV-1 subtype C mosaic Gag virus-like particles, is highly immunogenic in mice. PLoS One 12:e0173352. doi: 10.1371/journal.pone.0173352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jongwe TI, Chapman R, Douglass N, Chetty S, Chege G, Williamson AL. 2016. HIV-1 subtype C mosaic gag expressed by BCG and MVA elicits persistent effector T cell responses in a prime-boost regimen in mice. PLoS One 11:e0159141. doi: 10.1371/journal.pone.0159141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, Mahmoud A, Pinheiro S, Gillespie G, Brown D, Loach V, Roberts J, Guimaraes-Walker A, Hayes P, Loughran K, Smith C, De Bont J, Verlinde C, Vooijs D, Schmidt C, Boaz M, Gilmour J, Fast P, Dorrell L, Hanke T, McMichael AJ. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol 80:4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joachim A, Nilsson C, Aboud S, Bakari M, Lyamuya EF, Robb ML, Marovich MA, Earl P, Moss B, Ochsenbauer C, Wahren B, Mhalu F, Sandstrom E, Biberfeld G, Ferrari G, Polonis VR. 2015. Potent functional antibody responses elicited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS One 10:e0118486. doi: 10.1371/journal.pone.0118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saade F, Gorski SA, Petrovsky N. 2012. Pushing the frontiers of T-cell vaccines: accurate measurement of human T-cell responses. Expert Rev Vaccines 11:1459–1470. doi: 10.1586/erv.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O'Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, NISC Comparative Sequencing Program, Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L, Mascola JR. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, Khoza T, Kitchin D, Botha G, Gorman J, Garrett NJ, Abdool Karim SS, Shapiro L, Williamson C, Kwong PD, Mascola JR, Morris L, Moore PL. 2015. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med 21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorman J, Soto C, Yang MM, Davenport TM, Guttman M, Bailer RT, Chambers M, Chuang GY, DeKosky BJ, Doria-Rose NA, Druz A, Ernandes MJ, Georgiev IS, Jarosinski MC, Joyce MG, Lemmin TM, Leung S, Louder MK, McDaniel JR, Narpala S, Pancera M, Stuckey J, Wu X, Yang Y, Zhang B, Zhou T, Program NCS, Mullikin JC, Baxa U, Georgiou G, McDermott AB, Bonsignori M, Haynes BF, Moore PL, Morris L, Lee KK, Shapiro L, Mascola JR, Kwong PD. 2016. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat Struct Mol Biol 23:81–90. doi: 10.1038/nsmb.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrabi R, Voss JE, Liang CH, Briney B, McCoy LE, Wu CY, Wong CH, Poignard P, Burton DR. 2015. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity 43:959–973. doi: 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Diepen MT, Chapman R, Moore PL, Margolin E, Hermanus T, Morris L, Ximba P, Rybicki EP, Williamson AL. 2018. The adjuvant AlhydroGel elicits higher antibody titres than AddaVax when combined with HIV-1 subtype C gp140 from CAP256. PLoS One 13:e0208310. doi: 10.1371/journal.pone.0208310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanzer FL, Shephard EG, Palmer KE, Burger M, Williamson AL, Rybicki EP. 2011. The porcine circovirus type 1 capsid gene promoter improves antigen expression and immunogenicity in a HIV-1 plasmid vaccine. Virol J 8:51. doi: 10.1186/1743-422X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer H, Sutter G, Mayr A. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 83.Perkus ME, Goebel SJ, Davis SW, Johnson GP, Limbach K, Norton EK, Paoletti E. 1990. Vaccinia virus host range genes. Virology 179:276–286. doi: 10.1016/0042-6822(90)90296-4. [DOI] [PubMed] [Google Scholar]
- 84.Moore PL, Sheward D, Nonyane M, Ranchobe N, Hermanus T, Gray ES, Abdool Karim SS, Williamson C, Morris L. 2013. Multiple pathways of escape from HIV broadly cross-neutralizing V2-dependent antibodies. J Virol 87:4882–4894. doi: 10.1128/JVI.03424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sok D, van Gils MJ, Pauthner M, Julien JP, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, Hua Y, Seaman MS, Moore JP, Ward AB, Wilson IA, Sanders RW, Burton DR. 2014. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A 111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O’Dell S, Patel N, Shahzad-Ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang G-Y, Diwanji D, Georgiev I, Do Kwon Y, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-Y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang L-X, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bricault CA, Kovacs JM, Badamchi-Zadeh A, McKee K, Shields JL, Gunn BM, Neubauer GH, Ghantous F, Jennings J, Gillis L, Perry J, Nkolola JP, Alter G, Chen B, Stephenson KE, Doria-Rose N, Mascola JR, Seaman MS, Barouch DH. 2018. Neutralizing antibody responses following long-term vaccination with HIV-1 Env gp140 in guinea pigs. J Virol 92:e00369-18. doi: 10.1128/JVI.00369-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen KW, Frahm N. 2017. Current views on the potential for development of a HIV vaccine. Expert Opin Biol Ther 17:295–303. doi: 10.1080/14712598.2017.1282457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol 169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 93.Johnston MI, Fauci AS. 2008. An HIV vaccine—challenges and prospects. N Engl J Med 359:888–890. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- 94.Plotkin SA. 2013. Complex correlates of protection after vaccination. Clin Infect Dis 56:1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 95.Nkolola JP, Peng H, Settembre EC, Freeman M, Grandpre LE, Devoy C, Lynch DM, La Porte A, Simmons NL, Bradley R, Montefiori DC, Seaman MS, Chen B, Barouch DH. 2010. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J Virol 84:3270–3279. doi: 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, Menis S, Huang X, Kulp D, Osawa K, Muranaka J, Stewart-Jones G, Destefano J, O’Dell S, LaBranche C, Robinson JE, Montefiori DC, McKee K, Du SX, Doria-Rose N, Kwong PD, Mascola JR, Zhu P, Schief WR, Wyatt RT, Whalen RG, Binley JM. 2015. Vaccine-elicited tier 2 HIV-1 neutralizing antibodies bind to quaternary epitopes involving glycan-deficient patches proximal to the CD4 binding site. PLoS Pathog 11:e1004932. doi: 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goepfert PA, Elizaga ML, Seaton K, Tomaras GD, Montefiori DC, Sato A, Hural J, DeRosa SC, Kalams SA, McElrath MJ, Keefer MC, Baden LR, Lama JR, Sanchez J, Mulligan MJ, Buchbinder SP, Hammer SM, Koblin BA, Pensiero M, Butler C, Moss B, Robinson HL, HVTN 205 Study Group, National Institutes of Allergy and Infectious Diseases HIV Vaccines Trials Network. 2014. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 210:99–110. doi: 10.1093/infdis/jiu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen X, Basu R, Sawant S, Beaumont D, Kwa SF, LaBranche C, Seaton KE, Yates NL, Montefiori DC, Ferrari G, Wyatt LS, Moss B, Alam SM, Haynes BF, Tomaras GD, Robinson HL. 2017. HIV-1 gp120 protein and MVAgp140 boost immunogens increase immunogenicity of a DNA/MVA HIV-1 vaccine. J Virol 91:e01077-17. doi: 10.1128/JVI.01077-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. 2017. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev 275:296–312. doi: 10.1111/imr.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Gils MJ, Sanders RW. 2013. Broadly neutralizing antibodies against HIV-1: templates for a vaccine. Virology 435:46–56. doi: 10.1016/j.virol.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, Schmidt SD, Mankoff Z, Wu L, Asokan M, Beil C, Lange C, Leuschner WD, Kruip J, Sendak R, Do Kwon Y, Zhou T, Chen X, Bailer RT, Wang K, Choe M, Tartaglia LJ, Barouch DH, O’Dell S, Todd J-P, Burton DR, Roederer M, Connors M, Koup RA, Kwong PD, Yang Z-y, Mascola JR, Nabel GJ. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Asbach B, Kliche A, Kostler J, Perdiguero B, Esteban M, Jacobs BL, Montefiori DC, LaBranche CC, Yates NL, Tomaras GD, Ferrari G, Foulds KE, Roederer M, Landucci G, Forthal DN, Seaman MS, Hawkins N, Self SG, Sato A, Gottardo R, Phogat S, Tartaglia J, Barnett SW, Burke B, Cristillo AD, Weiss DE, Francis J, Galmin L, Ding S, Heeney JL, Pantaleo G, Wagner R. 2016. Potential to streamline heterologous DNA prime and NYVAC/protein boost HIV vaccine regimens in rhesus macaques by employing improved antigens. J Virol 90:4133–4149. doi: 10.1128/JVI.03135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li SS, Kochar NK, Elizaga M, Hay CM, Wilson GJ, Cohen KW, De Rosa SC, Xu R, Ota-Setlik A, Morris D, Finak G, Allen M, Tieu HV, Frank I, Sobieszczyk ME, Hannaman D, Gottardo R, Gilbert PB, Tomaras GD, Corey L, Clarke DK, Egan MA, Eldridge JH, McElrath MJ, Frahm N, NIAID HIV Vaccine Trials Network. 2017. DNA priming increases frequency of T-cell responses to a vesicular stomatitis virus HIV vaccine with specific enhancement of CD8(+) T-cell responses by interleukin-12 plasmid DNA. Clin Vaccine Immunol 24:e00263-17. doi: 10.1128/CVI.00263-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McKay PF, Cope AV, Mann JF, Joseph S, Esteban M, Tatoud R, Carter D, Reed SG, Weber J, Shattock RJ. 2014. Glucopyranosyl lipid A adjuvant significantly enhances HIV specific T and B cell responses elicited by a DNA-MVA-protein vaccine regimen. PLoS One 9:e84707. doi: 10.1371/journal.pone.0084707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Taeye SW, de la Pena AT, Vecchione A, Scutigliani E, Sliepen K, Burger JA, van der Woude P, Schorcht A, Schermer EE, van Gils MJ, LaBranche CC, Montefiori DC, Wilson IA, Moore JP, Ward AB, Sanders RW. 2018. Stabilization of the gp120 V3 loop through hydrophobic interactions reduces the immunodominant V3-directed non-neutralizing response to HIV-1 envelope trimers. J Biol Chem 293:1688–1701. doi: 10.1074/jbc.RA117.000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, Parks R, Eudailey J, Lloyd KE, Blinn J, Alam SM, Haynes BF, Amin MN, Wang LX, Burton DR, Koff WC, Nabel GJ, Mascola JR, Bewley CA, Kwong PD. 2013. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol 20:804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, Hural J, DeRosa SC, DeFawe OD, Tomaras GD, Montefiori DC, Xu Y, Lai L, Kalams SA, Baden LR, Frey SE, Blattner WA, Wyatt LS, Moss B, Robinson HL, National Institute of Allergy and Infectious Diseases HIV Vaccine Trials Network. 2011. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 203:610–619. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrabi R, Bhiman JN, Burton DR. 2018. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr Opin Immunol 53:143–151. doi: 10.1016/j.coi.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wyatt LS, Earl PL, Xiao W, Americo JL, Cotter CA, Vogt J, Moss B. 2009. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. J Virol 83:7176–7184. doi: 10.1128/JVI.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]