In the absence of an effective vaccine, alternative approaches to block HIV-1 infection and transmission with commensal bacteria expressing antiviral proteins are being considered. This report provides a proof-of-concept by using Lactobacillus bacteria stably expressing the HIV-1 receptor CD4 to capture and neutralize HIV-1 in vitro and in a humanized mouse model. The stable expression of antiviral proteins, such as CD4, following genomic integration of the corresponding genes into this Lactobacillus strain may contribute to the prevention of HIV-1 sexual transmission.
KEYWORDS: CD4, chromosomal integrative expression, HIV infection, Lactobacillus acidophilus ATCC 4356, bacterial engineering, humanized mice
ABSTRACT
Lactobacillus bacteria are potential delivery vehicles for biopharmaceutical molecules because they are well-recognized as safe microorganisms that naturally inhabit the human body. The goal of this study was to employ these lactobacilli to combat human immunodeficiency virus type 1 (HIV-1) infection and transmission. By using a chromosomal integration method, we engineered Lactobacillus acidophilus ATCC 4356 to display human CD4, the HIV-1 receptor, on the cell surface. Since human CD4 can bind to any infectious HIV-1 particles, the engineered lactobacilli can potentially capture HIV-1 of different subtypes and prevent infection. Our data demonstrate that the CD4-carrying bacteria are able to adsorb HIV-1 particles and reduce infection significantly in vitro and also block intrarectal HIV-1 infection in a humanized mouse model in preliminary tests in vivo. Our results support the potential of this approach to decrease the efficiency of HIV-1 sexual transmission.
IMPORTANCE In the absence of an effective vaccine, alternative approaches to block HIV-1 infection and transmission with commensal bacteria expressing antiviral proteins are being considered. This report provides a proof-of-concept by using Lactobacillus bacteria stably expressing the HIV-1 receptor CD4 to capture and neutralize HIV-1 in vitro and in a humanized mouse model. The stable expression of antiviral proteins, such as CD4, following genomic integration of the corresponding genes into this Lactobacillus strain may contribute to the prevention of HIV-1 sexual transmission.
INTRODUCTION
Lactic acid bacteria (LAB) are generally recognized as safe (GRAS) microorganisms in the human microbiota and have been widely used as probiotics for human health supplements. More importantly, these bacteria can be genetically manipulated for treating or preventing human diseases, which has opened an avenue for therapeutic use of these probiotic bacteria (1–3). Since probiotic bacteria naturally reside in the mucosal cavities of the human body, they can be used as a live mucosal-based delivery vehicle for therapeutics or vaccines against viral infections (4–8). HIV-1 infection is transmitted mainly through the mucosa of the vagina or rectum, in which commensal Lactobacillus bacteria exist in a large quantity. These commensal probiotic bacteria might be utilized to combat HIV-1 infection and transmission. Bacteria equipped with anti-HIV properties, such as the ability to adsorb or neutralize the invading viral particles at the port-of-entry, may effectively prevent infection. Moreover, since these bacteria can colonize the human body, the efficacy of prevention can be prolonged and eventually become a long-term strategy. In the absence of an effective HIV-1 vaccine, the probiotic Lactobacillus offers a potential opportunity to prevent HIV-1 acquisition.
There are some reports exploring this probiotic bacterial approach against HIV-1 infection. Several inhibitors have been tested, including forms of the receptor CD4 (9–11), fusion inhibitors (10, 12), a natural bacterial lectin inhibitor cyanovirin-N (CV-N) (13, 14), neutralizing antibodies (15), and a CCR5 antagonist (16). The human CD4 molecule, which is the primary HIV-1 receptor, binds to HIV-1 gp120 with high affinity. CD4 should effectively capture all infectious particles from different HIV-1 strains and prevent infection. As CD4 is a human molecule, immune reaction and inflammation are expected to be minimal. Thus, CD4 appears to be a good choice as an HIV-1 inhibitor for bacterial surface display in this approach.
Despite its theoretical appeal, there are some major challenges to develop this novel and unconventional antiviral approach, including bacterial engineering, inhibitor expression, and strain colonization. One challenge is engineering a stable inhibitor-producing strain. As required for clinical use or even testing in animal models, the engineered strain should be genetically stable and able to express the inhibitor(s) consistently. In general, plasmid transformation is a relatively easy method for engineering, but plasmid loss occurs readily from the engineered strains, especially when used in vivo without antibiotics. To overcome this problem, a chromosomal integration method has been used to engineer the bacteria. In this report, we utilize this integration method to directly insert the inhibitor gene encoding human CD4 into the genome of a commonly used Lactobacillus acidophilus ATCC 4356 strain to test the protective efficacy in a humanized mouse model. As this strain is closely related to Lactobacillus helveticus R0052 (17), which has been demonstrated to be a safe and good colonizer of the human body (18), it can potentially be directly advanced to clinical trials.
RESULTS
Construction of CD4 surface display cassette for Lactobacillus bacteria genomic integration.
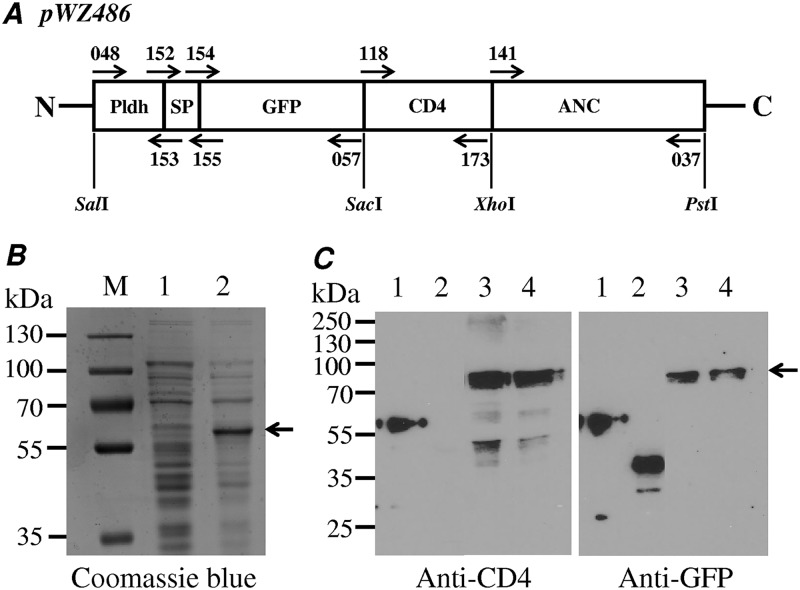
The insertion gene cassette for CD4 surface display was constructed based on two vectors, namely, pTRKH3-ldhGFP (19) and pLP401T (20, 21), widely used for Lactobacillus engineering. To achieve better surface expression of the CD4 molecule, we optimized different functional elements, including promoters, signal peptides, anchor motifs, reporter genes, and the linker length between CD4 and the protein marker. We chose GFP as the fusion protein marker, and the pTRKH3-ldhGFP vector was used as the backbone for our insert gene cassette construction. The PrtP anchor and Tcbh terminator from the vector pL401T were transferred to pTRKH3-ldhGFP for CD4 expression. We also added the signal peptide sequence (SPysirk), which was cloned from the YSIRK gene encoding a cell wall anchor protein with the LPXTG motif from Lactobacillus crispatus ST1 (22–24) (Fig. 1A). Both the short anchor and long anchor linker of the PrtP enzyme have been successfully applied to achieve surface-anchored expression of heterologous genes, with the longer anchor exhibiting higher efficacy for cell wall anchoring than the short anchor (25). In the expression vector pWZ486, GFP-CD4 was expected to be expressed as a fusion protein of 57 kDa with a flexible linker GSG and two more residues (EL) encoded by the SacI site (Fig. 1A).
FIG 1.
Cloning and expression of CD4-GFP fusion protein in L. acidophilus/pWZ486 transformants. (A) Construct of pWZ486 including promoter (Pldh), signal peptide (SP), protein marker (GFP), inhibitor gene (CD4), and anchor gene (ANC). Arrows indicate the primers (see Table 2). (B) Coomassie blue-stained gel showing the expression of the GFP-CD4 fusion protein expression in E. coli DE cells, 1 without IPTG and 2 with IPTG (1 mM/ml). (C) Western blots of protein expression in L. acidophilus/pWZ486, showing the results with an anti-CD4 and an anti-GFP antibody. 1, GFP-CD4 fusion protein control from E. coli strain; 2, GFP only control (pWZ521); and 3 and 4, GFP-CD4-ANC fusion protein.
Before testing the CD4-GFP fusion expression in Lactobacillus bacteria, we initially tested its expression in Escherichia coli BL21(DE3) with the vector pET28a and the resulting plasmid pWZ427. This transformant was induced by isopropyl-β-d-thiogalactopyranoside (IPTG) and the cell lysate was analyzed by SDS-PAGE with Coomassie brilliant blue staining. One clear band with the predicted molecular weight of about 57 kDa for the CD4-GFP fusion protein was observed (Fig. 1B), and this sample was subsequently used as the positive control of the CD4-GFP fusion protein for further Western blot analysis in Lactobacillus bacteria.
After the apparent production of the CD4-GFP fusion protein in E. coli, the constructed cassette vector pWZ486 was transformed into L. acidophilus by electroporation. The expression of the CD4-GFP anchor fusion protein was detected by Western blot analysis with antibodies against CD4 or GFP. Two positive bands with the expected sizes were observed after blotting with either CD4 or GFP antibodies, indicating that both CD4 and GFP were expressed (Fig. 1C). The appearance of two positive bands was most likely due to the presence of protein isoforms before and after signal peptide cleavage during protein secretion and anchoring, with expected sizes around 78 kDa and 75 kDa, respectively. In negative-control transformants of the vector pWZ521 containing only GFP, positive bands of the expected size were observed only with the anti-GFP antibody and not the anti-CD4 antibody. The positive control, the CD4-GFP fusion protein expressed in E. coli, was detected by both anti-CD4 and anti-GFP antibodies. These results demonstrated that CD4 was expressed in L. acidophilus transformed by the pWZ486 vector. To verify whether the GFP-CD4 fusion protein expressed by the vector pWZ486 could be displayed on the surface of the L. acidophilus strain, flow cytometry was carried out to identify the presence of the CD4 protein on the cell wall (see Fig. 4).
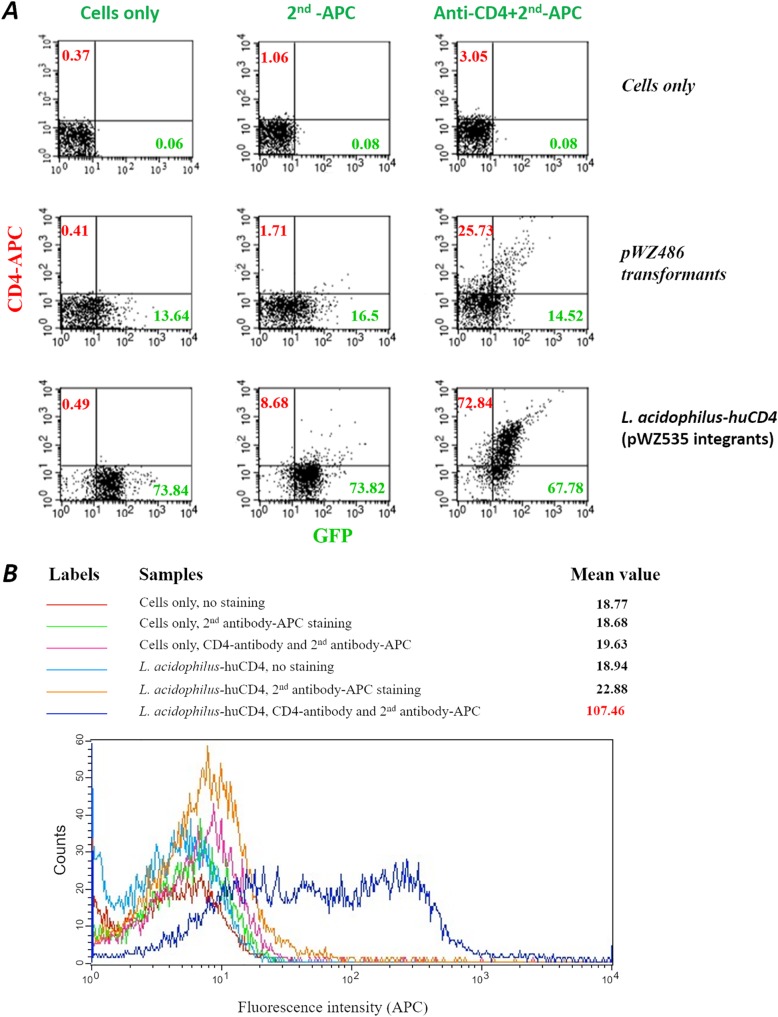
FIG 4.
Flow cytometry assay of CD4 surface display in L. acidophilus. (A) GFP, green on the x axis; CD4, red on the y axis, which was detected with a conjugate of our anti-CD4 polyclonal antibody and allophycocyanin (APC). Cells only were used as the negative controls. (B) Histograms of CD4 expression in L. acidophilus. The fluorescence intensity is plotted on the x axis.
Chromosomal integration of the CD4 expression cassette in L. acidophilus.
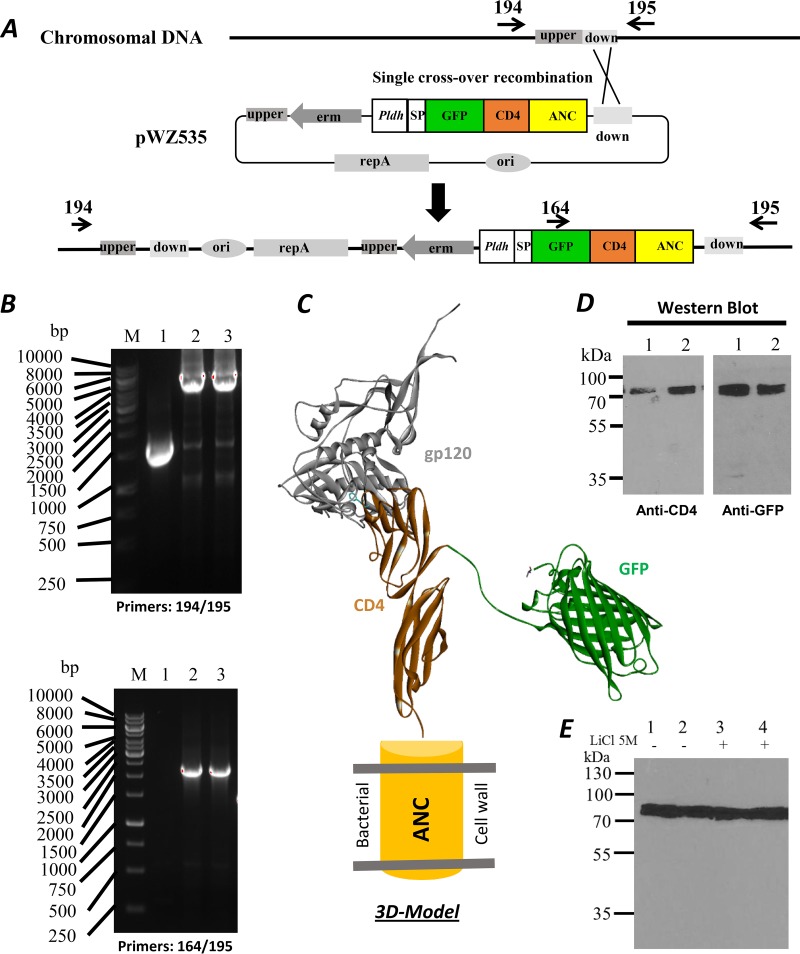
The chromosomal integrative vector pWZ535 for CD4-GFP surface-anchored expression was constructed as described in the Materials and Methods and Fig. 2A. The pWZ535-transformed L. acidophilus colonies were used for genomic DNA extraction. Primers corresponding to the upstream (194), downstream (195), or internal sequences of GFP (164) were designed for PCR verification of the integrated colonies. For the wild-type cells, a 1.0-kb fragment was amplified with the primer pair 194 and 195, but no product was obtained with the primer pair 164 and 195, as there was no GFP fragment in its chromosome (Fig. 2B, upper and lower panels, lane 1). In contrast, 8.2-kb or 2.0-kb fragments were amplified from pWZ535 integrants with primers 194 and 195 or 164 and 195, respectively (Fig. 2B, upper and lower panels, lanes 2 and 3). The results indicate that the complete plasmids were correctly integrated into the chromosome of L. acidophilus through homologous recombination. The clones with integrated vector DNA did not exhibit any observable growth defects or morphological changes. CD4 and GFP expression in both pWZ535 integrants were demonstrated by Western blot analysis with anti-CD4 and anti-GFP antibodies, respectively (Fig. 2D).
FIG 2.
Engineering of chromosomal integration for surface display. (A) Construction maps for chromosomal integration. The location of the down homologous sequence region was marked between the primers 194 and 195. (B) The DNA agarose gels showing PCR-amplified DNA fragments from the integrated L. acidophilus strain; 1, wild-type strain and 2 and 3 from two positive colonies; upper gel with the primers 194 and 195, lower gel with the primers 164 and 195. (C) 3D model of fusion surface display. The model was derived from crystal structures of the HIV-1 gp120-CD4 complex (PDB 3JWD) (42) and GFP (PDB 1GFL) (43). (D) Western blots with anti-CD4 and anti-GFP antibodies of the fusion protein in L. acidophilus. (E) Western blot showing the presence of the GFP-CD4 fusion protein after a 5 M LiCl wash. The GFP-CD4 fusion protein could not be removed from the cell by a 5 M LiCl wash. Members of the anchored family of surface proteins can only be released by enzymatic degradation of peptidoglycan.
We utilized the anchor protein containing the LPXTG motif that covalently links the fusion protein to peptidoglycan (23, 24) so that it cannot be easily removed from the cell wall. To confirm this anchoring, we treated the bacteria with 5 M LiCl. The bacterial samples were treated with or without a 5 M LiCl solution for 15 min at 37°C. Then, bacteria were washed with phosphate-buffered saline (PBS) and boiled in SDS sample buffer for Western blotting with an anti-CD4 antibody. There were no significant differences between the LiCl-treated and untreated samples (Fig. 2E), consistent with the CD4-based fusion protein being covalently linked to the cell wall through peptidoglycan.
Based on these results, we conclude that the CD4 expression cassette was inserted into the chromosomal DNA of L. acidophilus and the CD4 fusion protein was successfully expressed. We refer to this engineered strain as L. acidophilus-huCD4. A three-dimensional (3D) molecular model of the CD4-GFP anchor fusion protein on the surface of the bacterium was created and is shown in Fig. 2C binding to the HIV-1 gp120 envelope glycoprotein.
Characterization of CD4 surface display on the L. acidophilus strain.
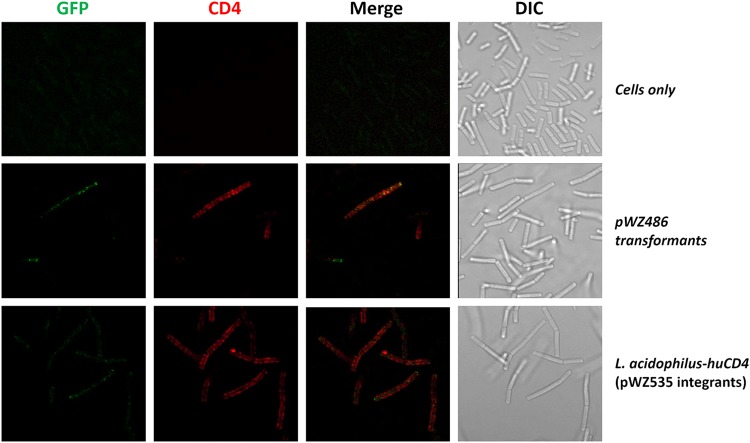
To confirm whether the CD4 and GFP components of the CD4-GFP fusion protein are displayed on the surface of L. acidophilus-huCD4, the bacteria were stained with fluorescently conjugated anti-CD4 antibodies (Fig. 3). Both GFP (green fluorescence) and CD4 (red fluorescence) were detected on the cell surface. The merged panel of Fig. 3 indicates that the two proteins are closely positioned on the bacterial cell surface.
FIG 3.
Microscopy of CD4 surface display in L. acidophilus. GFP, green; CD4, red which was conjugated with anti-CD4 polyclonal antibody and allophycocyanin (APC); DIC, differential interference contrast. WT cells were only used as the negative controls.
To further quantify the GFP- and CD4-positive bacteria, flow cytometry was used to analyze the bacterial cells. Control bacterial cells had no significant fluorescence levels when stained with the anti-CD4 antibody and goat anti-rabbit IgG conjugated with allophycocyanin (APC), goat anti-rabbit IgG conjugated with APC only, or without staining (Fig. 4A), indicating low background immunostaining with these antibodies. The L. acidophilus-huCD4 strain with the integrated pWZ535 DNA showed a high percentage of GFP-positive (67.8%) and CD4-positive (72.8%) bacteria. A transformant control strain, pWZ486, showed much lower positive rates for both GFP and CD4 (Fig. 4A), indicating that the plasmid transformant is not as stable as the integrants. In addition, the histogram of the integrated pWZ535 strain from the flow cytometry data showed a drastic shift of the CD4-APC fluorescence intensity (mean value of 107.46). These results strongly suggest that the CD4 molecules were presented efficiently on the surface of the L. acidophilus-huCD4 cells (Fig. 4B).
Taken together, the data indicate that the CD4 molecule is displayed on the surface of the L. acidophilus-huCD4 bacterium in a covalent linkage with the cell wall.
Functional characterization of the L. acidophilus-huCD4 strain.
Two methods were used to evaluate the ability of the CD4-carrying bacteria to inactivate HIV-1 in vitro.
HIV particle adsorption.
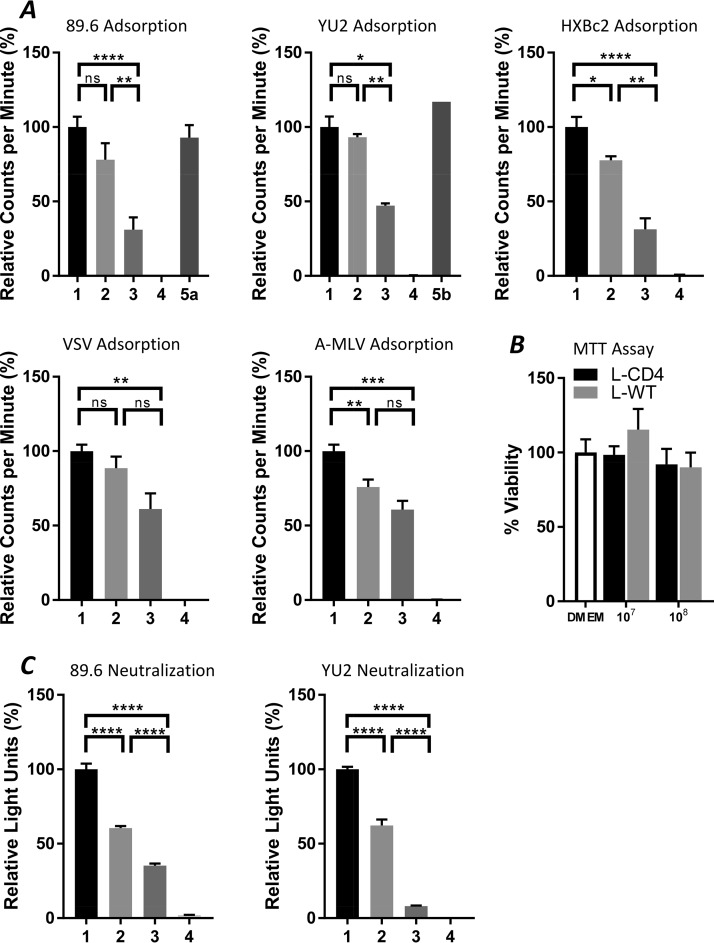
The adsorption method involves directly testing the bacteria to determine whether they can capture HIV-1 particles. If the CD4 molecule is in a correct conformation and exposed on the surface of the bacterium, it should hypothetically be able to bind to gp120 of the HIV-1 particle and capture the virus. We mixed the bacteria and the viruses together and incubated for 1 hour at 37°C, and then precipitated the bacterial cells by a low-speed spin. The captured viruses should be removed with the bacterial pellet. The amount of virus in the supernatant was measured using a reverse transcription (RT) assay. The adsorption results are shown in Fig. 5A. The bacteria engineered to express CD4 reduced the amount of virus significantly (∼50%) but the wild-type bacteria used as a control did not. The adsorption of HIV-1 particles by the L. acidophilus-huCD4 bacteria was eliminated by the addition of either soluble CD4 (sCD4) or an anti-CD4 antibody. Control viruses with the envelope glycoproteins of vesicular stomatitis virus (VSV) or the amphotropic murine leukemia virus (A-MLV) were not significantly adsorbed by L. acidophilus-huCD4 compared with the wild-type bacteria. These results suggest that the CD4-expressing bacteria can adsorb HIV-1 particles.
FIG 5.
Adsorption and neutralization of the engineered L. acidophilus-huCD4 strain. (A) Virus adsorption (HIV-1 dual-tropic R5X4 strain 89.6, R5 strain YU2, and X4 strain HXBc2; non-HIV strains, VSV and A-MLV). (B) Cytotoxicity of bacterial supernatants evaluated in an 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The bacterial concentrations were 107 or 108/ml. (C) Virus neutralization (HIV-1 strain 89.6 and YU2). Samples: 1, virus only as a positive control; 2, wild-type strain L. acidophilus; 3, engineered strain L. acidophilus-huCD4; 4, DMEM, negative controls (without viruses or bacteria); 5a, engineered strain L. acidophilus-huCD4 plus sCD4 (50 µg/ml); 5b, engineered strain L. acidophilus-huCD4 plus anti-CD4 mAb (30 µg/ml). The statistical significance was determined either by using the Holm-Sidak t test method with alpha of 5.0% or by the Student’s t test *P < 0.05, **P < 0.01, ****P < 0.0001; ns, not significant.
HIV-1 neutralization.
We tested the ability of the integrated bacteria to neutralize HIV-1. The viral neutralization experiment was carried out by using HIV-1 target cells (TZM-bl cells) in a 96-well plate. In this assay, the viral replication activity is proportional to the measured luciferase activity. To avoid bacterial toxicity, the bacteria were removed after a 1-hour incubation at 37°C of the virus-bacterium mixture, before adding the supernatants to the target cells. No toxicity of medium conditioned by Lactobacillus cultures for the TZM-bl target cells was observed (Fig. 5B). The neutralization results are shown in Fig. 5C. The engineered bacterial strain reduced the HIV-1 infection significantly, about 90% and 70% for YU2 and 89.6 strains, respectively. These results suggest that the bacteria with surface-displayed CD4 are able to inhibit HIV-1 infection in a cell culture system. In addition, some nonspecific binding of the viruses to the control wild-type bacteria was also observed (Fig. 5).
Protective efficacy of L. acidophilus-huCD4 strain in a humanized BLT mouse model.
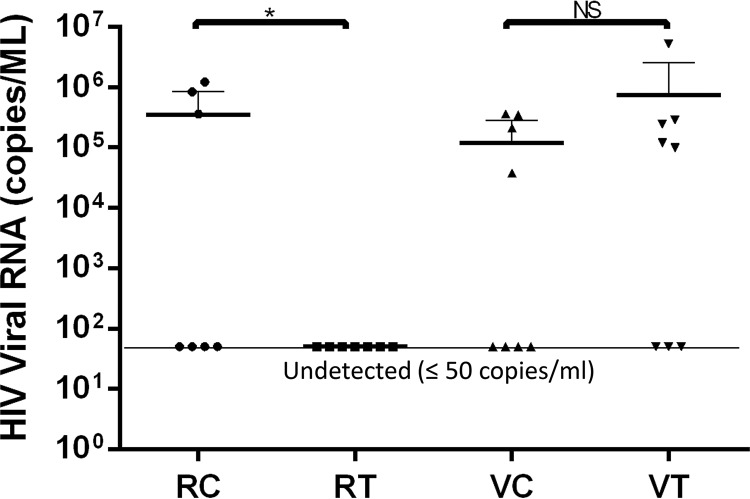
Humanized bone marrow, liver, and thymus (BLT) mice were generated from NOD/SCID/IL2rγ mice and are able to be infected directly by primary HIV-1 viruses. Thus, we can test the efficacy of protection from HIV-1 infection by the CD4-carrying bacteria. The prophylactic testing was designed to mimic the two principal natural routes, vaginal and rectal, of HIV-1 infection. For each challenge route, eight mice in the treatment group and eight mice in the control group were tested. The infections were evaluated by real-time quantitative reverse transcription-PCR (qRT-PCR). The results are shown in Table 1. The infection rate for the intrarectally challenged control group was 43%, whereas the treatment group was completely protected. Thus, the protection efficacy was 57% for intrarectal challenge. The vaginal challenge treatment group did not show any protection, with the infection rate similar to that of the control group (Table 1). Statistical analysis of these data suggested that the treatment group exhibited a significant level of protection against intrarectal challenge compared with the control group; in contrast, there was no significant difference in protective efficacy against vaginal challenge between the treatment and control groups (Fig. 6). All infected animals exhibited comparable viral loads, suggesting that the engineered bacteria blocked HIV-1 infection locally at the intrarectal site of infection but not systemically. Potential explanations for the apparent difference in protective efficacy in the rectal and vaginal challenges are discussed below.
TABLE 1.
Infections of humanized mice model
| Animal by groupa | Infection route | Viral load (copies/ml) | No. infected | Rate (%) |
|---|---|---|---|---|
| RC | ||||
| 241293 | Rectal control | 3.58E+05 | ||
| 241294 | Rectal control | Undetected | ||
| 241295 | Rectal control | Undetected | 3 | 43 |
| 241296 | Rectal control | Undetected | ||
| 241299 | Rectal control | 8.36E+05 | ||
| 241300 | Rectal control | 1.23E+06 | ||
| 241302 | Rectal control | Undetected | ||
| RT | ||||
| 241281 | Rectal treatment | Undetected | ||
| 241282 | Rectal treatment | Undetected | ||
| 241288 | Rectal treatment | Undetected | ||
| 241289 | Rectal treatment | Undetected | 0 | 0 |
| 241301 | Rectal treatment | Undetected | ||
| 241304 | Rectal treatment | Undetected | ||
| 241309 | Rectal treatment | Undetected | ||
| 241312 | Rectal treatment | Undetected | ||
| VC | ||||
| 241271 | Vaginal control | 2.09E+05 | ||
| 241272 | Vaginal control | 9.75E+04 | ||
| 241275 | Vaginal control | 3.60E+05 | ||
| 241278 | Vaginal control | Undetected | 4 | 50 |
| 241313 | Vaginal control | Undetected | ||
| 241314 | Vaginal control | 3.44E+05 | ||
| 241315 | Vaginal control | Undetected | ||
| 241318 | Vaginal control | Undetected | ||
| VT | ||||
| 241319 | Vaginal treatment | 5.19E+06 | ||
| 241320 | Vaginal treatment | Undetected | ||
| 241323 | Vaginal treatment | Undetected | ||
| 241324 | Vaginal treatment | 1.19E+05 | 5 | 63 |
| 241325 | Vaginal treatment | 9.93E+04 | ||
| 241326 | Vaginal treatment | 2.45E+05 | ||
| 241328 | Vaginal treatment | 2.88E+05 | ||
| 241329 | Vaginal treatment | Undetected |
RC, rectal control group to receive bacterial vector only; RT, rectal treatment group to receive hCD4+ bacteria; VC, vaginal control group to receive bacterial vector only; VT, vaginal treatment group: to receive hCD4+ bacteria.
FIG 6.
Analysis of HIV-1 challenge of humanized mice treated with the engineered L. acidophilus strain. Animal groups: RC, rectal control group (received the bacterial vector only); RT, rectal treatment group (received hCD4+ bacteria); VC, vaginal control group; VT, vaginal treatment group. Undetected viral RNA copy number was set at ≤50 copies/ml for analysis. The statistical significance was determined by using the Prism unpaired one-tailed t test at a P value of <0.05. Significance was determined by the Student’s t test. *P < 0.05, **P < 0.01, ****P < 0.0001; NS, not significant.
DISCUSSION
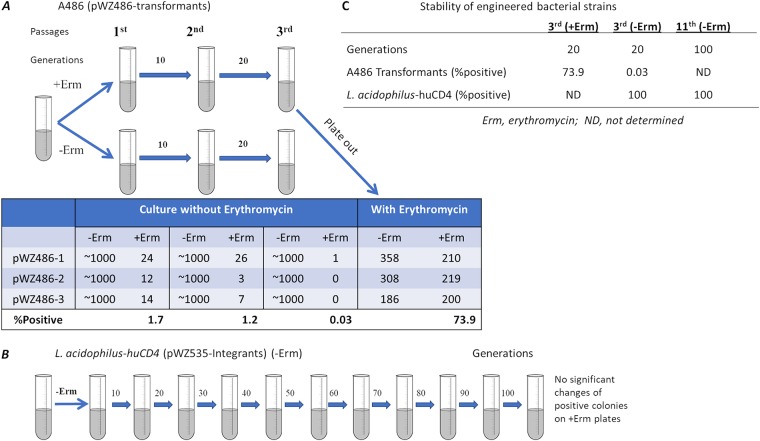
Genetic stability of the engineered bacteria is critical for the feasibility of using this approach for HIV-1 prophylaxis and other applications. Genomic integration appears to be required to generate stable engineered bacterial strains. In general, plasmid transformants are not stable because the plasmid can be lost easily. We have tested the stability of the plasmid-containing transformants and integrants. Without antibiotic pressure, the plasmid transformant strain lost the CD4 gene after 20 generations, but the integrant strain (L. acidophilus-huCD4) retained the gene for more than 100 generations (Fig. 7). These results recommend the use of stable integrants for the testing of applications in vivo on the path to clinical trials. In this report, we took advantage of the stability of the bacterial strains with a chromosomal integrated antiviral gene to test feasibility in humanized mice.
FIG 7.
Stability of the engineered Lactobacillus strains. The pWZ486 transformant strain A486 (A) was passaged in the absence or presence of erythromycin (Erm), and colonies counted on erythromycin plates. In the bacteria cultured without erythromycin selection, the A486 plasmid was lost by the third passage. In contrast, L. acidophilus-huCD4 strain (B), with the integrated pWZ535 DNA, maintained erythromycin resistance after 11 passages in erythromycin-negative medium. (C) Stability of engineered bacterial strains.
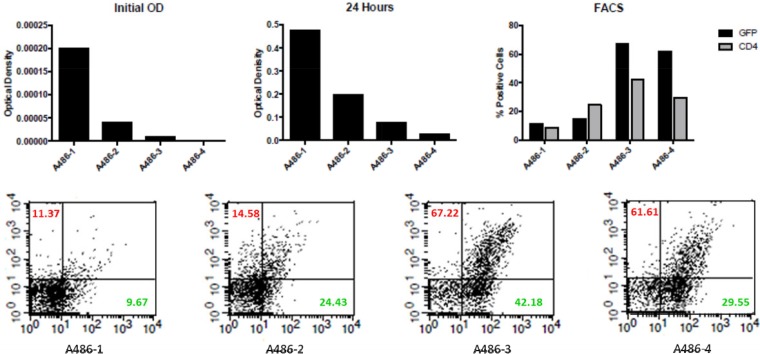
Considering the use of the more efficient ldh promoter, we noticed that the bacterial density is negatively correlated with the CD4 expression level on the surface of the bacteria in culture (Fig. 8). The CD4 surface display evaluated by flow cytometry was much higher when the bacteria were in the log growth phase but significantly decreased in the stationary growth phase. Whether this will be a problem in an in vivo environment is unknown, but the choice of promoter may need to be tailored to the biological entity produced in particular applications.
FIG 8.
Reverse correlation of bacterial density and surface expression of GFP and CD4 molecules. The pWZ486 transformant strain A486 in different initial concentrations (from high to low) is designated A486-1, A486-2, A486-3, and A486-4. The GFP and CD4 surface expression was evaluated by fluorescence-activated cell sorter (FACS), and the data are shown in the lower panel. CD4% is shown in red, and GFP% in green.
The choice of commensal bacterial strains is also important for the efficacy of this approach, based on the growth conditions and colonization capacities. In this report, we used the L. acidophilus ATCC 4356 strain, which is closely related to a commonly used probiotic strain, Lactobacillus helveticus R0052 (17, 26). Therefore, this strain can be easily tested in a clinical trial, but the colonization ability of modified variants has to be evaluated. The R0052 strain is resistant to gastric and bile acidity and, thus, is able to pass through the stomach and the upper gastrointestinal tract alive. Due to the presence of mucus-binding proteins, this bacterium can bind strongly to intestinal epithelial cells; this binding may be important for its competition with pathogens, stimulation of mucus production, and the modulation of the host immune system. This strain adheres to the intestinal epithelial cells, thereby maintaining the intestinal barrier of the gut, which inhibits pathogens and prevents infections from occurring. The strain is believed to improve lactose digestion and modulation of the immune system and is expected to be an ideal candidate vehicle for the delivery of bioactive molecules to the human mucosal surface to provide protective effects (18, 27).
Humanized mice could be a good animal model for directly testing the anti-HIV-1 efficacy of this live bacterial approach. Our challenge model employed a dose of approximately one animal infectious dose; although higher than the HIV-1 doses encountered during sexual transmission, this challenge dose allows a sufficient number of infections to occur. This model system could be improved by increasing the infection rate after intrarectal challenge with HIV-1. This may require repeated inoculations (the so-called multiple low-dose challenge method) used in nonhuman primate models (28, 29). Despite evidence of protection after intrarectal challenge, there was no apparent protective efficacy against vaginal challenge. One explanation is the low-pH environment of the vagina compared with the rectum. Recent reports have indicated that the pH in the human vagina averages 3.5 (range from 2.8 to 4.2) due to the predominance of Lactobacillus populations (30, 31). Such an acidic environment could destabilize the CD4 protein structure. There are two disulfide bonds in each domain of the CD4 molecule that are potentially susceptible to acidic attack. Additional studies are required to evaluate the effect of pH on the functionality of CD4 in our system. Specific adjustments may need to be made to allow this approach to be effective in vaginal compartments. Human CD4 appears to be a good inhibitor for bacterial surface display in this approach, as its advantages include broad coverage, high affinity, and low reactivity. Additional studies to address the long-term colonizing ability of L. acidophilus-huCD4 and its utility in HIV-1 prophylaxis are warranted.
CONCLUSIONS
The CD4 molecule was successfully displayed on the surface of the bacterial strain L. acidophilus ATCC 4356 using an integrative engineering approach. CD4 was stably expressed and was able to adsorb HIV-1 particles, neutralize the viruses, and block infection in tissue culture systems. In a humanized mouse model, the engineered CD4-carrying bacteria prevented HIV-1 infection after intrarectal but not intravaginal challenge. Further development of commensal probiotic bacteria engineered to inhibit HIV-1 infection may provide novel options for achieving control of the HIV-1 epidemic.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and primers used in this study are listed in Table 2. Escherichia coli strains were aerobically grown in LB broth at 37°C in a rotary shaker. Lactobacillus strains were grown in 5% CO2 in MRS (Oxoid) broth at 37°C without shaking. Solid medium was prepared by adding 1.5% (wt/vol) agar to the broth. The plasmid constructions were first established in E. coli cells and then transformed into Lactobacillus strains. The antibiotics used for E. coli were 100 µg/ml ampicillin, 100 µg/ml kanamycin, or 150 µg/ml erythromycin, while that used for Lactobacillus bacteria was 5 µg/ml erythromycin.
TABLE 2.
Strains, plasmids, and primers used in this studya
| Strain, plasmid, or primer | Relevant characteristic | Source or reference |
|---|---|---|
| Strain | ||
| L. crispatus ST1 | Used for the amplification of the anchor YSIRK sequence | ATCC |
| L. acidophilus ATCC 4356 | Host strain for fusion protein genomic integration | ATCC |
| E. coli BL21 (DE3) | Host strain for protein expression in Escherichia coli | Novagen |
| Plasmid | ||
| pTRKH3-ldhGFP | Cloning vector for protein expression in Lactobacillus | Addgene (19) |
| pLP401T | Cloning vector for protein expression in Lactobacillus | (21, 44) |
| pUC18 | Cloning vector in E. coli | NEB, Inc. |
| pET28a | Inducible expression vector in E. coli | Novagen |
| pWZ427 | pET28a-GFP-CD4 | This study |
| pWZ486 | pTRKH-Pldh-SP1-GFP-CD4-ANC, Ermr | This study |
| pWZ521 | pTRKH-Pldh-SP2-GFP | This study |
| pWZ528 | pUC18-Ermr | This study |
| pWZ531 | pUC18-Ermr-Upper-down (landing pad) | This study |
| pWZ535 | pUC18-Ermr-Pldh-SP1-GFP-CD4-ANC, Ampr, Ermr | This study |
| Primer | ||
| 001 | GCGGAATTCTGTTTTGAATTTTGTCATTGTCG | erm resistance gene, forward |
| 002 | GCGGAATTCTTAATTAATGAGACAGGTTTTAAGCAACTTGC | erm resistance gene, reverse |
| 037 | GCGCCTGCAGCTATTCTTCACGTTGTTTCCGTTTC | Long anchor of PrtP, reverse |
| 048 | GCGCGAATTCGCAGTCGACAAGCTTTTTAGTC | Pldh, forward |
| 057 | AATTCTCGAGTCCTGAGCCTTTGTATAGTTCATCCATG | GFP, reverse |
| 072 | GTATAATTATAGCACGACCTCTGATAAATATGAACATG | erm with mutated SacI site, forward |
| 073 | CATGTTCATATTTATCAGAGGTCGTGCTATAATTATAC | erm with mutated SacI site, reverse |
| 118 | GGCCGAGCTCAAGAAGGTTGTTTTAGGTAAGA | CD4, forward |
| 119 | GCTTTTCAAAAGGCTTCATCAGGAGGTAGTAAAGGAGAAG | CD4-GFP overlap, forward |
| 120 | GAAAAGTTCTTCTCCTTTACTACCTCCTGATGAAGCCTTTTGAAAAGC | CD4-GFP overlap, reverse |
| 141 | GCGACTCGAGGATAAGAAGACTTCGCTGC | Long anchor of PrtP, forward |
| 152 | CTAATAAAAAAGGAGACTTGACTTCCATGAAGCGACTTAAATTTTTAG | Pldh-SP1, forward |
| 153 | CTAAAAATTTAAGTCGCTTCATGAAGTCAAGTCTCCTTTTTTATTAG | Pldh-SP1, reverse |
| 154 | GCAGCAACCATAGAAAGCGGAGGTAGTAAAGGAGAAG | Pldh-SP1-GFP, forward |
| 155 | CTTCTCCTTTACTACCTCCGCTTTCTATGGTTGCTGC | Pldh-SP1-GFP, reverse |
| 164 | GGACGACGGGAACTACAAGAC | GFP, forward |
| 173 | GCGCCTGCAGTTACTCGAGTGATGAAGCCTTTTGAAAAGC | CD4, reverse |
| 184 | GCGCGTTAATTAATCCTTTAAACTCATCAAAAGCCAAATG | Upper pad, forward |
| 185 | GGCAGTTAATTAATCATTTCTTTTGATCAAAACACTTAC | Upper pad, reverse |
| 186 | GCGAAAGCTTGTTTAAACATTTTACTATCGCCAATG | Down pad, forward |
| 187 | GCGAAAGCTTGTTTAAACCTGCGGCTACTTGATTAGCTTTAG | Down pad, reverse |
| 194 | GTTAGGAAACCAAGCTCTGAC | Forward primer to test integration |
| 195 | GCCAGAATATGCTTGCGTCT | Reverse primer to test integration |
Ermr, erythromycin resistance gene; Ampr, ampicillin resistance gene; Pldh, promoter; SP, signal peptide; ANC, anchor domain; prtP, serine proteinase anchor domain.
Construction of the CD4 surface display plasmid.
The pTRKH3-ldhGFP vector (19) was used as the backbone for the construction of the recombinant vector. To assemble the CD4 expression cassette, the constitutive ldhL promoter from L. acidophilus ATCC 4356 and green fluorescence protein (GFP) from vector pTRKH3-ldhGFP and the signal peptide sequence of the YSIRK gene (GenBank accession number CBL49691) from L. crispatus ST1 genomic DNA (22) were individually amplified and then fused to a single DNA fragment with SalI and SacI sites by overlapping PCR. Human CD4 domain 1 and 2, with codons optimized for expression in Lactobacillus bacteria, were amplified with SacI and XhoI sites. The short anchor (117 amino acid [aa]) or the long anchor (244 aa) of the serine proteinase (PrtP) from L. casei was amplified from vector pLP401T (21, 32, 33) or synthesized as a whole length sequence by Eurofins with flanked XhoI and PstI sites, respectively. The transcription terminator (Tcbh) of the L. plantarum-conjugated bile acid hydrolase gene was amplified with flanked SalI-PstI and BglII sites. All these fragments were assembled and finally cloned into the SalI and BamHI sites in pTRKH3-ldhGFP to give CD4 anchor vector pWZ486, as depicted in Fig. 1A.
Electroporation.
Plasmids were transformed into the Lactobacillus bacteria by electroporation, as described by Majidzadeh Heravi (34) with minor modifications. Briefly, overnight cultures of Lactobacillus cells were diluted (1:50) into fresh MRS medium with 1% glycine and incubated at 37°C without shaking for 2 h. Cells were harvested and treated with 50 mM EDTA (pH 8.0) for 15 min, followed by two washes with ice-cold electroporation buffer (0.5 M sucrose), and resuspended in electroporation buffer (1/100 volume of the initial culture). A total of 50 µl of cells was mixed with plasmid DNA and incubated on ice for 15 min. The mixture was added to an ice-cold 0.2-cm GenePulser (Bio-Rad) cuvette and pulse was immediately applied at the conditions of 10 KV/cm, 200 Ω, and 25 µF. Cells were suspended in 1 ml MRS broth with 2 mM CaCl2 and 20 mM MgCl2 and then incubated at 37°C for 4 h. Cells were pooled on MRS plates with 5 µg/ml erythromycin and cultured for 2 to 3 days with 5% CO2. Clones were picked and grown in MRS medium with erythromycin. A total of 50 µl of cells was collected, washed once with 1 ml PBS, suspended in 50 µl of PBS, and then boiled for 10 min and subjected to PCR verification.
DNA manipulation.
DNA manipulations were performed as previously described (35). The restriction enzymes and T4 DNA ligase, calf intestinal alkaline phosphatase (New England Biolabs, MA, USA) and the high-fidelity DNA polymerase (TaKaRa, Japan) were used according to the manufacturer’s instructions. Amplified PCR products were separated on 1% agarose gels and purified using the QIAquick gel extraction kit (Qiagen). Plasmid DNA was purified from E. coli using the Qiagen mini/maxi kit (Qiagen). Genomic DNA from Lactobacillus bacteria was extracted using the GenElute bacterial genomic DNA kit (Sigma). All DNA constructs were verified by DNA sequencing (Eurofins).
Expression of CD4 in E. coli.
For fusion expression of GFP-CD4 in E. coli, the fusion DNA fragments were amplified by overlapping PCR with primer pairs 118 + 120 and 119 + 057 (Table 2), then digested with SacI and XhoI, and cloned into the expression vector pET28a (Invitrogen), resulting in pWZ427. Clones of the expression host E. coli BL21(DE3) containing pWZ427 were cultured to optical density at 600 nm (OD600) around 0.6 to 0.8 and then induced by 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 3 h at 37°C. After induction, the cells were collected by centrifugation, boiled in SDS sample buffer, and subjected to SDS-PAGE and Western blot analysis.
Chromosomal integration of Lactobacillus bacteria.
For the chromosomal integration, the E. coli cloning vector pUC18, which is not able to replicate in Lactobacillus bacteria, was used as the basis for the construction of the integration vectors for CD4 expression.
First, the erythromycin resistance gene (erm) from vector pLP401T was chosen as the selective marker of the integrated bacteria. To eliminate the internal SacI site to facilitate subsequent cloning procedures, the gene sequence was amplified through overlapping PCR with primer pairs 001 + 072 and 002 + 073, in which SacI site was mutated. The amplified fragment was digested with EcoRI and cloned into pUC18; the insertion direction was verified by restriction analysis to produce the vector pWZ528.
Second, the sequence in the middle of two reverse transcript genes in the L. acidophilus chromosome was chosen as the integrative site to achieve the lowest adverse effects on the host strain characteristics. Two chromosomal fragments as the homologous upper and down recombination sites were amplified with primer pairs 184 + 185 or 186 + 187, then digested with EcoRI or HindIII, respectively, and inserted into vector pWZ528 consequently. The insertion direction of the two fragments in the final vector pWZ531 was verified by PCR to be the same as the original chromosomal direction. To facilitate the exchange of the upper and down integration sites to other location and even other stains, two rare restriction sites, namely, PacI and PmeI, were inserted flanking the upper and down fragments, respectively.
Third, the entire CD4 expression cassette was digested out from vector pWZ486 with SalI and PstI and subcloned into the same sites of the vector pWZ531, giving the CD4 integrative vector pWZ535.
Western blot analysis.
Western blotting samples were run on a 10% SDS-polyacrylamide gel and stained with Coomassie blue to verify protein expression. For the detection of CD4 expression, the SDS-PAGE gel was further transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, USA) and the membrane was blocked with blocking buffer (5% skim milk in PBS) for 1 h at room temperature and reacted with primary antibody (2000-fold-diluted rabbit anti-CD4 polyclonal antibody; Santa Cruz) overnight at 4°C. The membrane was washed 3 times with wash buffer (0.1% Tween 20 in PBS) and incubated with a secondary antibody (5000-fold-diluted goat anti-rabbit IgG antibody conjugated with horseradish peroxidase [HRP]) for 1 h at room temperature. After washing 3 times with wash buffer, the membrane was developed with ECL substrate (Fisher). The GFP expression was detected with HRP-conjugated rabbit anti-GFP antibody (Santa Cruz) with the similar treatment of the membrane as described above.
Flow cytometry and indirect immunofluorescence microscopy of the CD4-expressing L. acidophilus cells.
Cells that reached a density of approximately 5 × 106 cells were collected by centrifugation. Cells were washed twice with 500 µl PBS and suspended in 100 µl PBS with 2 µl anti-CD4 polyclonal antibody (Santa Cruz). After incubation on ice with gentle shaking for 1 to 2 h, the bacteria were centrifuged at 7,000 × g for 3 min at 4°C and washed three times with 500 µl PBS. The cells were subsequently resuspended in 100 µl PBS with 2 µl of goat anti-rabbit IgG conjugated with APC allophycocyanin (APC; Santa Cruz) and incubated on ice with gentle shaking for 1 h. After collecting the bacteria by centrifugation at 7,000 × g for 3 min at 4°C and washing three times with 500 µl PBS, staining was analyzed by flow cytometry using fluorescence-activated cell sorter (FACS) Calibur (Becton, Dickinson), as described previously (36, 37). Images were collected using a Leica TCS-NT/SP confocal microscope (Leica Microsystems, Mannheim, Germany).
Generation of pseudotyped viruses.
The 293T cells were plated at a density of 2.0 × 106 cells in a 10-cm plate. Twenty-four hours later, the cells were transfected with an HIV-1 backbone plasmid, pSG3ΔEnv (NIH AIDS Reagent Program), and an Env expression plasmid using polyethyleneimine (PEI). One day following transfection, additional medium was added to the plate. Three days posttransfection, the supernatants were harvested and, after a short spin to remove cell debris, stored at −80°C. The viral titers were determined by reverse transcriptase assay.
Reverse transcriptase assay.
In duplicate, 500 µl of pseudovirus-containing supernatant was spun at 14,000 × g for 2 h at 4°C. Following the spin, the supernatant was removed and the viral pellet was resuspended in a Triton X-100-based suspension buffer and vortexed, followed by three rapid freeze-thaw cycles to lyse the virus. A total of 50 µl of reaction mix [oligo(dT) poly-A and 3H-dTTP; PerkinElmer] was added, and the samples were incubated at 37°C for 1 h in a heating block. Following incubation, the samples were pipetted onto cut squares of DEAE filtermat paper (PerkinElmer), followed by three 10-minute washes in 2× SSC (0.3 M NaCl plus 0.03 M sodium citrate) buffer, and one 10-second wash in 100% ethanol. The filters were dried at room temperature and then analyzed using a scintillation counter to quantify the incorporation of 3H-dTTP into cDNA. The average cpm values from each duplicate were then used to normalize the amount of virus-containing supernatant that was used in subsequent single-round viral entry assays.
HIV absorption.
Pseudotyped single-round HIV-1, VSV, and AMLV viruses made from 293T cells were used for the experiment, and the virus titers were measured by reverse transcriptase (RT) activity. The engineered or wild-type bacteria (∼5 × 107/ml) were mixed with the viruses (virus stocks, ∼200 kcpm/ml) in a 1.5-ml microcentrifuge tube. The bacterium and virus mixtures were incubated for 1 h at 37°C with rocking. In some experiments, either soluble CD4 (sCD4) (50 µg/ml) (NIH AIDS Reagent Program) or the QS4120 anti-CD4 monoclonal antibody (30 µg/ml) (EMD Millipore) was added to the bacterium-virus mixture. Then, the tubes were spun for 1 min at full speed to remove the bacteria. The supernatants were collected and the viral titers were determined by RT.
MTT cytotoxicity assay.
The MTT assay was performed to determine the cytotoxicity of Lactobacillus cell cultures for the TZM-bl cells used in the neutralization assays. TZM-bl cells were seeded in a 96-well plate at 6,000 cells/well and incubated overnight. The cells were washed with PBS, and then 50 µl of Lactobacillus-cultured Dulbecco’s modified Eagle medium (DMEM) was added to the cells. The Lactobacillus-cultured DMEM was prepared with wild-type L. acidophilus and L. acidophilus-huCD4. Bacteria were incubated in DMEM at 37°C for 1 h. The bacteria were pelleted and the supernatants were applied to the TZM-bl cells. After an overnight incubation, 150 μl of fresh DMEM was added to the cells. Twenty-four hours later, the medium was removed and the cells were washed once with PBS. A 5-mg/ml solution of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2 H-tetrazolium bromide (Sigma-Aldrich) was added to each well. Plates were incubated at 37°C for 4 h. The precipitate was dissolved in 100 μl DMSO and absorbance was measured on an enzyme-linked immunosorbent assay (ELISA) plate reader at 570 nm.
HIV-1 neutralization analysis.
Pseudotyped HIV-1 viruses for single-round infection were used in the engineered bacterial neutralization assay. TZM-bl cells which express CD4, CCR5, and CXCR4 were used for the target cells, which allow viral infectivity to be evaluated by measuring the luciferase activity (38). The TZM-bl cells were set at a density of 8.0 × 103 per well in a 96-well plate. The control bacteria (L. acidophilus cells only) or CD4-expressing integrants were cultured to early log phase (OD600, 0.1 to 0.2), and 5 × 107 cells (approximately 5 × 108 cells per OD600) were collected. Cells were washed and resuspended in DMEM. The incubation of mixed bacteria and viruses was prepared in a tube for triplicates of each well with 2,500 RT units of viral stock in a final 100-µl infection volume. The tubes with mixed bacteria and viruses were incubated with rocking for 60 min at 37°C. After centrifugation at 10,000 rpm for 3 min to spin down the bacteria, supernatants were transferred to new Eppendorf tubes and loaded into the wells of TZM-bl cells for infection at a final volume of 100 μl. Three days postinfection, the supernatants were removed, the cells were washed once with PBS, and the cells were lysed in 1× passive lysis buffer and frozen at −80°C. The plates were then thawed, and luciferase activity was measured using the Veritas luminometer and beetle luciferin substrate (Promega).
LiCl treatment.
To verify whether the expressed CD4 is covalently anchored on the cell wall, cells (5 × 107) were incubated with 100 µl of 5 M LiCl for 15 min and then cells were washed with PBS and resuspended in SDS sample buffer for Western blot analysis. The cell samples used in Fig. 2E were subjected to incubation with a 5 M LiCl solution for 15 min at 37°C. Cells were then washed with PBS, boiled in SDS sample buffer, loaded for SDS-PAGE and Western blot. There was no significant difference between treated and untreated samples, indicating that CD4 was covalently linked to the cell wall through peptidoglycan (39).
Generation of BLT humanized mice.
Bone marrow, liver, and thymus (BLT) humanized mice were generated by implantation of human fetal thymic grafts and adoptive transfer of autologous hematopoietic stem cells (CD34) into NOD/SCID/IL2Rγ−/− (NSG) mice, as described previously (40). For evaluation, flow cytometry was used to detect hCD45, hCD4, and hCD8 cells. Prior to HIV-1 challenge, the BLT mice were treated with 2 mg of medroxyprogesterone subcutaneously to synchronize the estrus cycle of the female mice, allowing their vaginal epithelium to be at a comparable thickness at the time of HIV-1 challenge (41). Animal work was approved by the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts General Hospital.
Bacterial inoculation and HIV-1 challenge of BLT humanized mice.
A total of 32 BLT humanized mice, half males and half females, were used for this experiment. Four groups (8 mice per group) were divided into the rectal challenge route group (male mice) and vaginal challenge groups (female mice) as follows: (i) rectal control group, to receive bacterial vector only (RC); (ii) rectal treatment group, to receive hCD4+ bacteria (RT); (iii) vaginal control group, to receive bacterial vector only (VC); and (iv) vaginal treatment group, to receive hCD4+ bacteria (VT). One mouse (241287) was found dead prior to the start of the experiment and was therefore removed from the RC group.
Bacterial administration was done by direct atraumatic application of 20 µl of the bacterial samples (1010/ml, frozen samples) into the rectum or vagina. Two hours after bacterial inoculation, the viral challenge followed. All mice were anesthetized with isoflurane inhalation during the experimental process, and their body was kept in an inverted position for 4 min after the bacterial and viral inoculation. In addition, in order to decrease feces/urine during the experiment, all mouse feeding was stopped for 2 h before and after the bacterial and viral inoculation. Note that for vaginal groups of mice, in order to synchronize/prolong the estrous phase for bacterial inoculation, they were injected subcutaneously with progesterone (2 mg/mouse; Depo-Provera, Pharmacia & Upjohn Diagnostics) in a 100-µl volume 5 days before the bacterial inoculation. Viral rectal or vaginal challenges matched the route of bacterial inoculations. HIV-1 JR-CSF (105 50% tissue culture infective dose [TCID50]) in 10 µl PBS was directly applied intravaginally or rectally using a pipette. Blood samples for viral load detection were collected at day 0 before bacterial inoculation/viral challenge and days 14 and 28 postchallenge.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism software (version 6.0). To analyze the engineered bacterial neutralizing activities against HIV-1 viruses, the statistical significances were determined by using the Holm-Sidak t test method with alpha of 5.000%. For humanized mouse data, the statistical significances were determined by using the unpaired one-tailed t test at P value of <0.05.
ACKNOWLEDGMENTS
This study was supported by grants (51783) from the Bill and Melinda Gates Foundation to S.-H.X. D.B. was an NIH Ruth L. Kirschstein Fellow (5T32AI06547-8).
We thank You Zhou of the Microscopy Core Facility for his help with the study. We also thank Danielle Shea of the Nebraska Center for Virology Flow Cytometry Core Facility for her support of the flow cytometry analysis. We thank Peter Pouwels for sending us the plasmid pLP401T.
REFERENCES
- 1.Riglar DT, Silver PA. 2018. Engineering bacteria for diagnostic and therapeutic applications. Nat Rev Microbiol 16:214–225. doi: 10.1038/nrmicro.2017.172. [DOI] [PubMed] [Google Scholar]
- 2.Landry BP, Tabor JJ. 2017. Engineering diagnostic and therapeutic gut bacteria. Microbiol Spectr 5. doi: 10.1128/microbiolspec.BAD-0020-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez-Humaran LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, Chatel JM, Langella P. 2013. Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol 16:278–283. doi: 10.1016/j.mib.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Pant N, Hultberg A, Zhao Y, Svensson L, Pan-Hammarstrom Q, Johansen K, Pouwels PH, Ruggeri FM, Hermans P, Frenken L, Boren T, Marcotte H, Hammarstrom L. 2006. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J Infect Dis 194:1580–1588. doi: 10.1086/508747. [DOI] [PubMed] [Google Scholar]
- 5.Wells JM, Mercenier A. 2008. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin MC, Pant N, Ladero V, Gunaydin G, Andersen KK, Alvarez B, Martinez N, Alvarez MA, Hammarstrom L, Marcotte H. 2011. Integrative expression system for delivery of antibody fragments by lactobacilli. Appl Environ Microbiol 77:2174–2179. doi: 10.1128/AEM.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajikawa A, Zhang L, Long J, Nordone S, Stoeker L, LaVoy A, Bumgardner S, Klaenhammer T, Dean G. 2012. Construction and immunological evaluation of dual cell surface display of HIV-1 gag and Salmonella enterica serovar Typhimurium FliC in Lactobacillus acidophilus for vaccine delivery. Clin Vaccine Immunol 19:1374–1381. doi: 10.1128/CVI.00049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Gao Z, Zhang Y, Pan L. 2016. Lactic acid bacteria as mucosal delivery vehicles: a realistic therapeutic option. Appl Microbiol Biotechnol 100:5691–5701. doi: 10.1007/s00253-016-7557-x. [DOI] [PubMed] [Google Scholar]
- 9.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, Lewicki JA, Lee PP. 2003. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci U S A 100:11672–11677. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu JJ, Reid G, Jiang Y, Turner MS, Tsai CC. 2007. Activity of HIV entry and fusion inhibitors expressed by the human vaginal colonizing probiotic Lactobacillus reuteri RC-14. Cell Microbiol 9:120–130. doi: 10.1111/j.1462-5822.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Lagenaur LA, Lee PP, Xu Q. 2008. Engineering of a human vaginal Lactobacillus strain for surface expression of two-domain CD4 molecules. Appl Environ Microbiol 74:4626–4635. doi: 10.1128/AEM.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pusch O, Kalyanaraman R, Tucker LD, Wells JM, Ramratnam B, Boden D. 2006. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS 20:1917–1922. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y, Tsai D, Rao SS, Hamer DH, Parks TP, Lee PP, Xu Q. 2006. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother 50:3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y, Yu R, Venzon D, Lee PP, Hamer DH. 2011. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol 4:648–657. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcobal A, Liu X, Zhang W, Dimitrov AS, Jia L, Lee PP, Fouts TR, Parks TP, Lagenaur LA. 2016. Expression of human immunodeficiency virus type 1 neutralizing antibody fragments using human vaginal Lactobacillus. AIDS Res Hum Retroviruses 32:964–971. doi: 10.1089/AID.2015.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vangelista L, Secchi M, Liu X, Bachi A, Jia L, Xu Q, Lusso P. 2010. Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrob Agents Chemother 54:2994–3001. doi: 10.1128/AAC.01492-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tompkins TA, Barreau G, Broadbent JR. 2012. Complete genome sequence of Lactobacillus helveticus R0052, a commercial probiotic strain. J Bacteriol 194:6349. doi: 10.1128/JB.01638-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster LM, Tompkins TA, Dahl WJ. 2011. A comprehensive post-market review of studies on a probiotic product containing Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011. Benef Microbes 2:319–334. doi: 10.3920/BM2011.0032. [DOI] [PubMed] [Google Scholar]
- 19.Lizier M, Sarra PG, Cauda R, Lucchini F. 2010. Comparison of expression vectors in Lactobacillus reuteri strains. FEMS Microbiol Lett 308:8–15. doi: 10.1111/j.1574-6968.2010.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouwels PH, Leer RJ, Boersma WJ. 1996. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigens. J Biotechnol 44:183–192. doi: 10.1016/0168-1656(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 21.Pouwels PH, Vriesema A, Martinez B, Tielen FJ, Seegers JF, Leer RJ, Jore J, Smit E. 2001. Lactobacilli as vehicles for targeting antigens to mucosal tissues by surface exposition of foreign antigens. Methods Enzymol 336:369–389. doi: 10.1016/S0076-6879(01)36602-8. [DOI] [PubMed] [Google Scholar]
- 22.Edelman SM, Lehti TA, Kainulainen V, Antikainen J, Kylväjä R, Baumann M, Westerlund-Wikström B, Korhonen TK. 2012. Identification of a high-molecular-mass Lactobacillus epithelium adhesin (LEA) of Lactobacillus crispatus ST1 that binds to stratified squamous epithelium. Microbiology 158:1713–1722. doi: 10.1099/mic.0.057216-0. [DOI] [PubMed] [Google Scholar]
- 23.Navarre WW, Schneewind O. 1994. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol 14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 24.Novick RP. 2000. Sortase: the surface protein anchoring transpeptidase and the LPXTG motif. Trends Microbiol 8:148–151. doi: 10.1016/S0966-842X(00)01741-8. [DOI] [PubMed] [Google Scholar]
- 25.Marcotte H, Kõll-Klais P, Hultberg A, Zhao Y, Gmür R, Mändar R, Mikelsaar M, Hammarström L. 2006. Expression of single-chain antibody against RgpA protease of Porphyromonas gingivalis in Lactobacillus. J Appl Microbiol 100:256–263. doi: 10.1111/j.1365-2672.2005.02786.x. [DOI] [PubMed] [Google Scholar]
- 26.Cremonesi P, Zottola T, Locatelli C, Pollera C, Castiglioni B, Scaccabarozzi L, Moroni P. 2013. Identification of virulence factors in 16S-23S rRNA intergenic spacer genotyped Staphylococcus aureus isolated from water buffaloes and small ruminants. J Dairy Sci 96:7666–7674. doi: 10.3168/jds.2013-6917. [DOI] [PubMed] [Google Scholar]
- 27.Alemka A, Clyne M, Shanahan F, Tompkins T, Corcionivoschi N, Bourke B. 2010. Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect Immun 78:2812–2822. doi: 10.1128/IAI.01249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler K, Morgan JS, Hanson DL, Adams D, Garcia-Lerma JG, Heneine W, Ellenberger D, Hendry RM, McNicholl J, Johnson WE, Kersh EN. 2013. Susceptibility to repeated, low-dose, rectal SHIVSF162P3 challenge is independent of TRIM5 genotype in rhesus macaques. AIDS Res Hum Retroviruses 29:1091–1094. doi: 10.1089/aid.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hanlon DE, Moench TR, Cone RA. 2013. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. 2018. Human vaginal pH and microbiota: an update. Gynecol Endocrinol 34:451–455. doi: 10.1080/09513590.2017.1407753. [DOI] [PubMed] [Google Scholar]
- 32.Koo OK, Amalaradjou MA, Bhunia AK. 2012. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PLoS One 7:e29277. doi: 10.1371/journal.pone.0029277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maassen CB, Laman JD, den Bak-Glashouwer MJ, Tielen FJ, van Holten-Neelen JC, Hoogteijling L, Antonissen C, Leer RJ, Pouwels PH, Boersma WJ, Shaw DM. 1999. Instruments for oral disease-intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 17:2117–2128. doi: 10.1016/S0264-410X(99)00010-9. [DOI] [PubMed] [Google Scholar]
- 34.Majidzadeh Heravi R, Roozbeh Nasiraii L, Sankian M, Kermanshahi H, Varasteh AR. 2012. Optimization and comparison of two electrotransformation methods for lactobacilli. Biotechnology 11:50–54. doi: 10.3923/biotech.2012.50.54. [DOI] [Google Scholar]
- 35.Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual, fourth ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 36.Gunasekera TS, Attfield PV, Veal DA. 2000. A flow cytometry method for rapid detection and enumeration of total bacteria in milk. Appl Environ Microbiol 66:1228–1232. doi: 10.1128/AEM.66.3.1228-1232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunasekera TS, Veal DA, Attfield PV. 2003. Potential for broad applications of flow cytometry and fluorescence techniques in microbiological and somatic cell analyses of milk. Int J Food Microbiol 85:269–279. doi: 10.1016/S0168-1605(02)00546-9. [DOI] [PubMed] [Google Scholar]
- 38.Smith AJ, Wietgrefe SW, Shang L, Reilly CS, Southern PJ, Perkey KE, Duan L, Kohler H, Muller S, Robinson J, Carlis JV, Li Q, Johnson RP, Haase AT. 2014. Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J Immunol 193:3126–3133. doi: 10.4049/jimmunol.1400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel SD, Reardon ME, Ton-That H. 2017. Anchoring of LPXTG-like proteins to the gram-positive cell wall envelope. Curr Top Microbiol Immunol 404:159–175. doi: 10.1007/82_2016_8. [DOI] [PubMed] [Google Scholar]
- 40.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. 2009. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol 83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Princiotto AM, Vrbanac VD, Melillo B, Park J, Tager AM, Smith AB 3rd, Sodroski J, Madani N. 2018. A small-molecule CD4-mimetic compound protects bone marrow-liver-thymus humanized mice from HIV-1 infection. J Infect Dis 218:471–475. doi: 10.1093/infdis/jiy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pancera M, Majeed S, Ban YE, Chen L, Huang CC, Kong L, Kwon YD, Stuckey J, Zhou T, Robinson JE, Schief WR, Sodroski J, Wyatt R, Kwong PD. 2010. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc Natl Acad Sci U S A 107:1166–1171. doi: 10.1073/pnas.0911004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang F, Moss LG, Phillips GN Jr, 1996. The molecular structure of green fluorescent protein. Nat Biotechnol 14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- 44.Pouwels PH, Leer RJ. 1993. Genetics of lactobacilli: plasmids and gene expression. Antonie Van Leeuwenhoek 64:85–107. [DOI] [PubMed] [Google Scholar]