The reemergence of Zika virus (ZIKV) has caused a global public health crisis since 2016, and there are currently no vaccines or antiviral drugs to prevent or treat ZIKV infection. However, considerable advances have been made in understanding the biology and pathogenesis of ZIKV infection. In particular, various animal models have been successfully established to mimic ZIKV infection and its associated neurological diseases and to evaluate potential countermeasures. However, the clinical symptoms in these mouse and nonhuman primate models are different from the common clinical manifestations seen in human ZIKV patients; in particular, dermatological manifestations are rarely recapitulated in these animal models. Here, we developed a new animal model of ZIKV infection in tree shrews, a rat-sized, primate-related mammal. In vitro and in vivo characterization of ZIKV infection in tree shrews established a direct link between ZIKV infection and the immune responses and dermatological manifestations. The tree shrew model described here, as well as other available animal models, provides a valuable platform to study ZIKV pathogenesis and to evaluate vaccines and therapeutics.
KEYWORDS: animal model, pathogenesis, Zika virus
ABSTRACT
Animal models of Zika virus (ZIKV) infection have recently been established in mice, guinea pigs, and nonhuman primates. Tree shrews (Tupaia belangeri) are an emerging experimental animal in biomedical applications, but their susceptibility to ZIKV infection has not been explored. In the present study, we show that subcutaneous inoculation of ZIKV led to rapid viremia and viral secretion in saliva, as well as to typical dermatological manifestations characterized by massive diffuse skin rash on the trunk. Global transcriptomic sequencing of peripheral blood mononuclear cells isolated from ZIKV-infected animals revealed systematic gene expression changes related to the inflammatory response and dermatological manifestations. Importantly, ZIKV infection readily triggered the production of high-titer neutralizing antibodies, thus preventing secondary homologous infection in tree shrews. However, neonatal tree shrews succumbed to ZIKV challenge upon intracerebral infection. The tree shrew model described here recapitulates the most common dermatological manifestations observed in ZIKV-infected patients and may greatly facilitate the elucidation of ZIKV pathogenesis and the development of novel vaccines and therapeutics.
IMPORTANCE The reemergence of Zika virus (ZIKV) has caused a global public health crisis since 2016, and there are currently no vaccines or antiviral drugs to prevent or treat ZIKV infection. However, considerable advances have been made in understanding the biology and pathogenesis of ZIKV infection. In particular, various animal models have been successfully established to mimic ZIKV infection and its associated neurological diseases and to evaluate potential countermeasures. However, the clinical symptoms in these mouse and nonhuman primate models are different from the common clinical manifestations seen in human ZIKV patients; in particular, dermatological manifestations are rarely recapitulated in these animal models. Here, we developed a new animal model of ZIKV infection in tree shrews, a rat-sized, primate-related mammal. In vitro and in vivo characterization of ZIKV infection in tree shrews established a direct link between ZIKV infection and the immune responses and dermatological manifestations. The tree shrew model described here, as well as other available animal models, provides a valuable platform to study ZIKV pathogenesis and to evaluate vaccines and therapeutics.
INTRODUCTION
Zika virus (ZIKV) is a positive-sense RNA virus within the genus Flavivirus in the Flaviviridae family, along with other important pathogens important to public health, including dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus (TBEV). During the past half century after ZIKV was first isolated in 1947 (1), ZIKV infection in human beings has been reported only occasionally in a few African and Asian countries. In most patients, ZIKV infection is characterized by fever, rash, arthralgia, conjunctivitis, and muscle aches, symptoms similar to those caused by many other mosquito-borne flaviviruses. However, during recent outbreaks in the Americas, ZIKV has reemerged as a global health threat because of its potential to generate explosive epidemics and its ability to cause severe congenital diseases in the context of maternal infection during pregnancy (2).
To date, a variety of animal models that mimic aspects of ZIKV infection in humans have been established (3–7). In particular, accumulating evidence from pregnant mice and nonhuman primate (NHP) models has demonstrated that ZIKV infection causes pathological changes in the placenta and fetal brain (8–14). We and others have shown that ZIKV preferentially infects and targets neural progenitor cells (NPCs) and leads to extensive cell death and reduced cell proliferation, resulting in cortical thinning and microcephaly in offspring mice (8, 10, 11). Recently, several groups have developed pregnant NHP models that recapitulate many characteristics of congenital ZIKV infection, including consistent neuropathology in the fetal brain and spinal cord (9, 12–14). In addition, several groups have developed models of intravaginal ZIKV transmission in mice to investigate the consequences of sexual transmission of ZIKV to females and developing fetuses (15, 16). Similarly, Yockey et al. found that the vaginal tract is a susceptible site for ZIKV replication and that infection of pregnant mice could result in intrauterine growth retardation of the fetus (16). More recently, a guinea pig model that recapitulates close-contact transmission of ZIKV was established (17).
These animal models have greatly augmented our understanding of ZIKV pathogenesis and accelerated the discovery and development of antiviral drugs and vaccines. Interestingly, however, although devastating microcephaly and severe developmental abnormalities were observed in fetuses during recent epidemics, the clinical symptoms in adult patients—even in infected pregnant women—were mild, and some patients were even asymptomatic (2, 18). The most common clinical symptoms observed in ZIKV patients in the Americas include fever, arthralgia, conjunctivitis, muscle aches, and skin rash (18, 19). Miner et al. recently demonstrated that ZIKV infection can result in conjunctivitis and panuveitis in Ifnar−/− mice (20). However, a more relevant animal model that recapitulates other common clinical symptoms in adult ZIKV patients remains of high priority.
The tree shrew (Tupaia belangeri) is a rat-sized mammal that is widely distributed in Southeast Asia and in South and Southwest China. It has a small body size (100 to 150 g); a low maintenance cost; a short reproductive cycle (∼7 to 8 months), gestation period (∼40 to 45 days), and life span (6 to 8 years); and a high brain-to-body mass ratio (21). Whole-genome sequencing and phylogenomic analyses have revealed that tree shrews are more closely related to NHPs than to other related mammals, particularly commonly used experimental animals, such as rats and mice (21, 22). In addition, the extensive characterization of key factors and signaling pathways in the immune and nervous systems has shown that tree shrews possess features that are both conserved and unique relative to those of primates (23). More recently, the successful genetic manipulation of the tree shrew has opened a new avenue for the wide usage of this animal in biomedical research (24). Therefore, tree shrews have been explored as experimental animal models for biomedical applications, e.g., myopia, depression, breast cancer, alcohol-induced or nonalcoholic fatty liver diseases, and viral infections (21).
In the present study, we showed that both primary cells and neonatal tree shrews are susceptible and responsive to ZIKV infection. Interestingly, adult tree shrews developed systemic infection upon subcutaneous (s.c.) challenge and mounted a protective immune response. In particular, the dermatological manifestations in ZIKV-infected tree shrews were similar to those in human ZIKV patients. Thus, the tree shrew model described here provides a unique tool to study ZIKV pathogenesis and test potential countermeasures under development.
RESULTS
Tree shrew primary cells are susceptible to ZIKV infection.
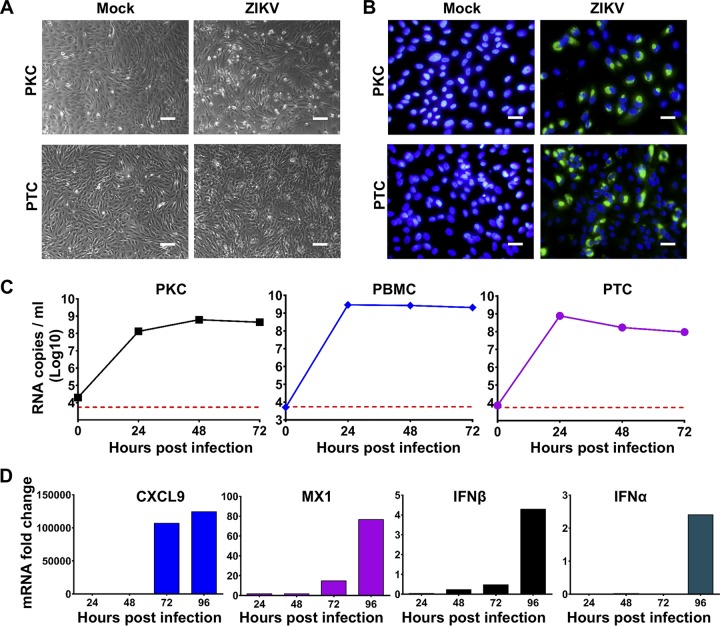
Tree shrew primary cells have been used for viral infection (25–27), an approach that led to the discovery of the hepatitis B virus (HBV) and hepatitis D virus (HDV) functional receptor sodium-taurocholate cotransporting polypeptide (NTCP) (27). In addition, human primary kidney cells (PKCs) and peripheral blood mononuclear cells (PBMCs) are susceptible to ZIKV infection (28), and the testis has been demonstrated as a potent target organ of ZIKV (29–31). Here, we first determined the susceptibility of various primary cells from tree shrews to ZIKV infection. As shown in Fig. 1A and B, ZIKV infection with a contemporary strain, GZ01, resulted in an appreciable cytopathic effect (CPE) in PKCs and primary testis cells (PTCs), and ZIKV-specific antigens were detected in both PKCs and PTCs by an indirect immunofluorescence assay (IFA) using human convalescent-phase serum from a ZIKV patient. Growth curve analysis showed that ZIKV genomic RNA replicated efficiently in all three primary cell types, with levels peaking at 48 h postinfection in PKCs and at 24 h postinfection in PTCs and PBMCs (Fig. 1C). Additionally, we assessed the induction of innate antiviral immunity-related genes in ZIKV-infected PBMCs and showed that ZIKV infection readily upregulated the mRNA levels of CXCL9, MX1, IFN-β and TNF-α in a time-dependent manner (Fig. 1D). Taken together, these results demonstrate that ZIKV infects and replicates robustly in multiple types of primary cells from tree shrews.
FIG 1.
Tree shrew primary cells are susceptible to ZIKV infection. All primary cells were isolated from a 5-month-old tree shrew and cultured according to the standard protocol. All primary cells were infected with ZIKV at a multiplicity of infection (MOI) of 1. (A) The cytopathic effects (CPEs) in primary kidney cells (PKCs) and primary testis cells (PTCs) were assessed by imaging at 48 h postinfection (hpi). (B) Immunostaining assays were performed at 48 hpi with ZIKV-specific antibodies. Positive viral antigens are shown in green, and 4′,6-diamidino-2-phenylindole (DAPI) is shown in blue. (C) The viral loads at the indicated time points were determined by reverse transcription quantitative PCR (RT-qPCR). The dotted line indicates the detection limit of the RT-qPCR assay. (D) The mRNA levels of selected genes in infected PBMCs at the indicated time points were determined using RT-qPCR. Each assay was performed at least two times with experimental triplicates. Bar, 50 μm.
ZIKV infection causes cutaneous damage in tree shrews.
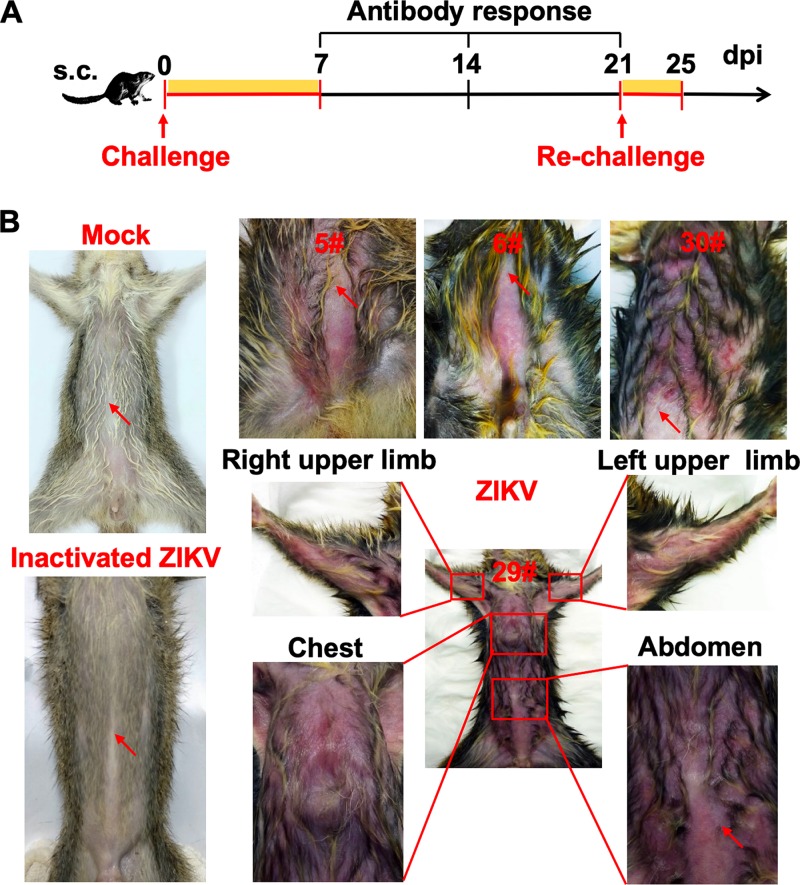
Then, we assessed the susceptibility of adult tree shrews to ZIKV infection. Groups of 5-month-old tree shrews (n = 26) were challenged with 106 PFU of ZIKV strain GZ01 via the s.c. route (Fig. 2A). The same doses of heat-activated ZIKV and phosphate-buffered saline (PBS) were used as controls. As expected, all animals that received PBS or inactivated ZIKV showed no clinical symptoms (Fig. 2B). Strikingly, nearly all ZIKV-infected tree shrews (24/26, 92%) readily developed a skin rash, one of the most common symptoms in ZIKV-infected patients. The abdomen and chest are the predominant sites of dermatological manifestations, and a heavy skin rash spread to the trunk and upper limbs in a few infected animals (Fig. 2B). Skin petechiae were also observed on the abdomen of several ZIKV-infected animals. Most animals (21/26, 70%) developed a rash at 2 days postinfection (dpi), but the rash disappeared completely at 3 dpi. In addition to dermatological manifestations, no additional clinical symptoms, such as fever, weight loss, or other neurological and behavioral abnormalities, were observed in ZIKV-infected animals throughout the observation period.
FIG 2.
ZIKV infection causes dermatological manifestations in adult tree shrews. (A) Study design and experimental parameters. Twenty-six tree shrews were inoculated via the s.c. route with 106 PFU of ZIKV strain GZ01. The yellow box indicates the time course for clinical observations and virological assays. (B) Representative images of dermatological manifestations in ZIKV-inoculated tree shrews. Heat-inactivated ZIKV and PBS were used as the controls. Red arrows denote the injection sites.
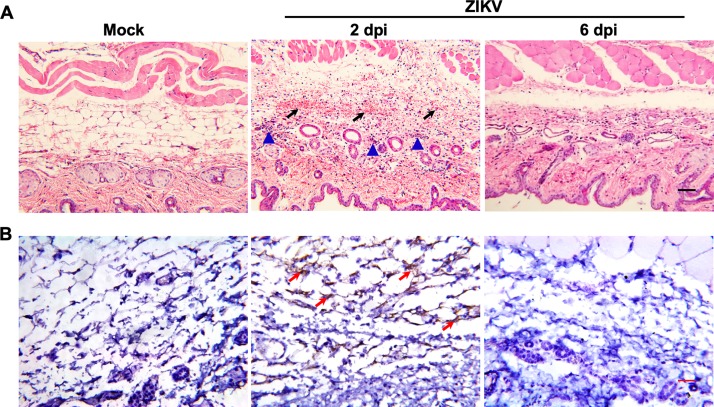
Histopathological examination of the skin tissues from ZIKV-infected tree shrews showed apparent cutaneous lesions characterized by massive hemorrhage with abundant infiltration of inflammatory cells in the hypodermis; these lesions developed at 2 dpi, but no cutaneous lesions were observed at 6 dpi (Fig. 3A). More importantly, ZIKV genomic RNA in situ hybridization (RNA ISH) revealed abundant ZIKV-specific RNA in the hypodermis of skin tissue sections from ZIKV-infected animals at 2 dpi, while all samples were negative at 6 dpi (Fig. 3B). Together, these results indicate that ZIKV infection in adult tree shrews causes apparent dermatological manifestations characterized by cutaneous damage and in situ viral replication.
FIG 3.
Histopathological and virological characterization assays in the skin of tree shrews. Skin tissues were collected from ZIKV-infected tree shrews at the indicated time points. (A) Histopathological characterization was performed by hematoxylin and eosin (H&E) staining. The arrows denote areas of hemorrhage, and the triangles denote inflammatory cell infiltration. (B) ZIKV genome RNA ISH was performed with a ZIKV-specific probe. Brown-colored staining indicates positive results (arrows). Bar, 50 μm.
ZIKV establishes systemic infection in tree shrews.
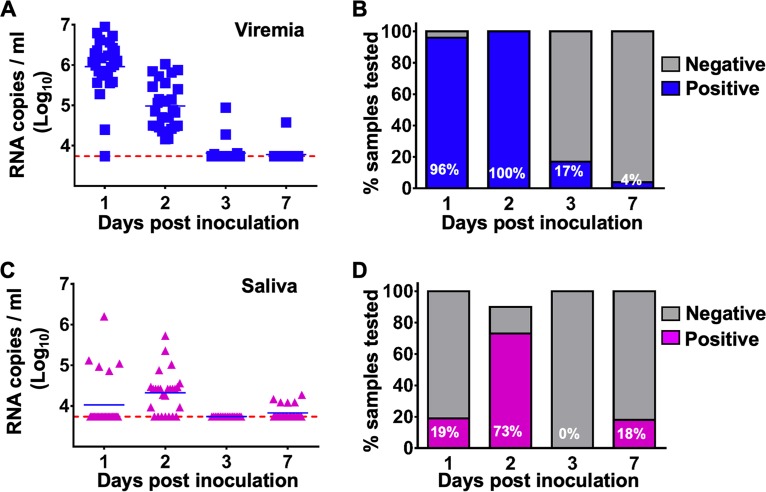
To further profile ZIKV infection dynamics in tree shrews, blood and major body fluids, including saliva, urine, and tears, were collected from infected animals and subjected to virological assays. Following s.c. inoculation, ZIKV viremia was readily detected in all animals (n = 26) at 1 dpi, and the viral loads ranged from 104.40 to 106.02 RNA copies/ml. However, viremia persisted for only 2 days and became undetectable at 3 dpi in most animals (20/24, 83%), with the exception of one animal that still exhibited delayed viremia at a low level at 7 dpi (Fig. 4A and B). Additionally, to confirm the production of infectious progeny ZIKV virions, sera from ZIKV-infected animals were subjected to virus isolation attempts in mosquito C6/36 cells. ZIKV virions were directly recovered from selected serum samples with high viral loads (Table 1).
FIG 4.
ZIKV establishes systemic infection in tree shrews. Viral loads in serum and saliva from ZIKV-infected tree shrews were determined by RT-qPCR. (A and B) The kinetics and time courses of viremia in all ZIKV-infected animals. (C and D) The kinetics and time courses of the viral load in saliva in all infected animals. The detection limit is indicated by the dotted line.
TABLE 1.
Virus isolation results using serum samples from ZIKV-infected tree shrews
| Sample no. | Initial viral loads |
After passage in C6/36 cells |
Result assessmenta | ||
|---|---|---|---|---|---|
| CT value | Log RNA copies/ml | CT value | Log RNA copies/ml | ||
| 01 | 28.94 | 6.37 | 34.83 | 4.62 | Negative |
| 13 | 31.22 | 5.74 | 37.48 | 3.89 | Negative |
| 14 | 30.87 | 5.84 | 33.23 | 5.07 | Negative |
| 17 | 31.92 | 5.55 | 32.98 | 5.14 | Negative |
| 20 | 30.45 | 5.95 | 22.59 | 8.03 | Positive |
| 21 | 29.92 | 6.10 | 16.85 | 9.63 | Positive |
| 22 | 28.74 | 6.42 | 16.83 | 9.63 | Positive |
| 23 | 28.98 | 6.35 | 19.66 | 8.85 | Positive |
| 24 | 30.02 | 6.07 | 17.61 | 9.42 | Positive |
| 25 | 30.31 | 5.99 | 33.39 | 5.02 | Negative |
An increase in the RNA copy number of at least 100-fold was defined as positive for virus isolation (in bold).
Interestingly, the secretion of ZIKV RNA in saliva was obviously delayed with respect to the viremia kinetics; ZIKV RNA became detectable only at 2 dpi in most animals (19/26, 73%) and disappeared at 3 dpi. The mean viral loads and duration in saliva were appreciably lower than those in sera (Fig. 4C and D). Interestingly, the shedding of viral RNA in urine and tears was not detectable in most animals after s.c. inoculation. These results indicated that viremia and viral RNA shedding in saliva could serve as indicators for ZIKV infection in tree shrews.
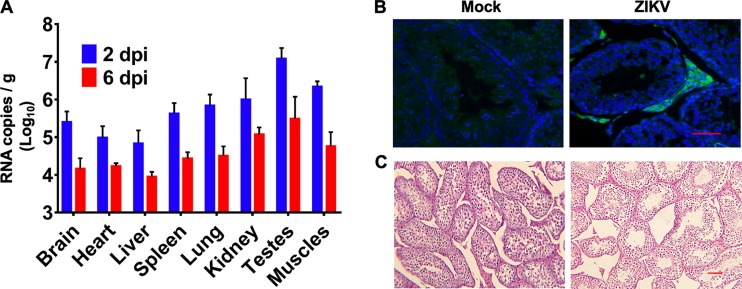
To determine the tissue tropism of ZIKV in tree shrews, necropsy was performed at 2 and 6 dpi. Interestingly, reverse transcription quantitative PCR (RT-qPCR) analysis showed the presence of ZIKV RNA in the brain and multiple peripheral organs, including the spleen, intestines, testes, and muscles, at 2 dpi; however, the viral loads decreased sharply at 6 dpi (Fig. 5A). The highest viral load was observed in the testes, consistent with observations in other animal models (17, 32). Then, we performed immunostaining of testis tissue with convalescence serum from a recovered ZIKV patient (33). ZIKV antigens were predominantly detected in the Leydig cells of the testis, consistent with previous findings in mice (31) and guinea pigs (17) (Fig. 5B). Moreover, substantial pathological changes characterized by structural abnormalities in the seminiferous tubules were observed in the testes of ZIKV-infected tree shrews (Fig. 5C). Collectively, these results suggest that ZIKV can infect tree shrews and establish systemic infection involving multiple organs.
FIG 5.
Tissue distribution of ZIKV in tree shrews. (A) ZIKV RNA loads in the indicated tissues from ZIKV-infected animals (n = 4) at 2 and 6 dpi were determined by RT-qPCR. (B) Histopathological changes in the testes were examined by H&E staining at 2 dpi. (C) Immunostaining of testis tissue harvested at 2 dpi was performed with anti-ZIKV antibodies (green) and DAPI (blue). Bar, 50 μm.
ZIKV induces a robust immune response and protects against secondary homologous challenge.
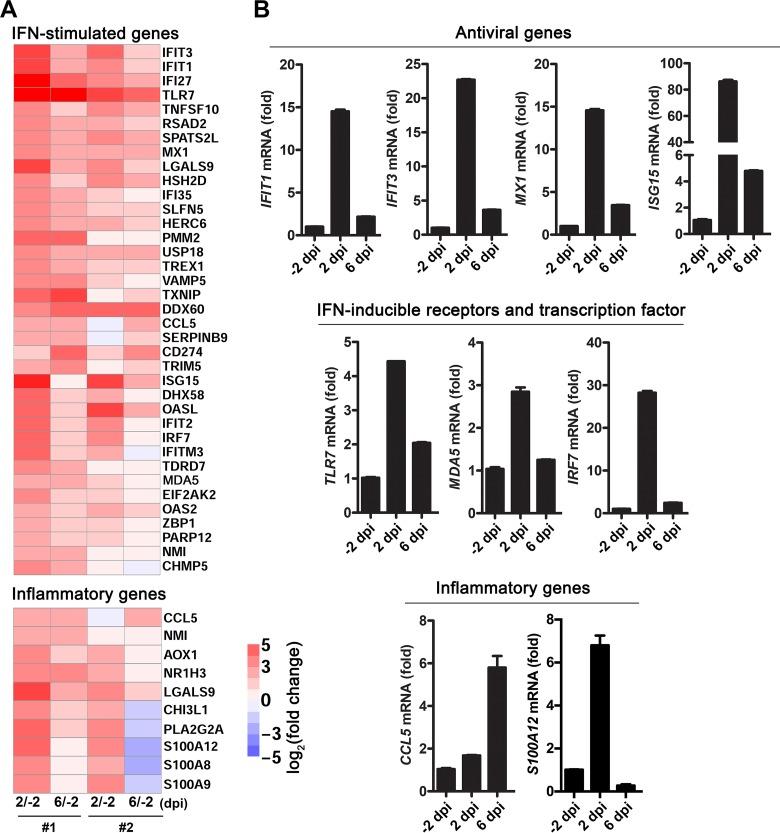
To further characterize the immune responses induced by ZIKV infection, global transcriptomic profiles in PBMCs from ZIKV-infected tree shrews (n = 2) were analyzed by deep sequencing. Notably, differential expression analysis showed that numerous antiviral interferon-stimulated genes (ISGs) and inflammatory genes were significantly induced in PBMCs at 2 and 6 dpi relative to their expression before infection (−2 dpi) (Fig. 6A). The expression of representative antiviral genes (e.g., IFIT1, IFIT3, MX1, and ISG15), IFN-inducible RNA sensors (TLR7 and MDA5), and the transcription factor IRF7, as well as inflammatory genes, such as CCL5 and S100A12, was further validated by RT-qPCR. Except for CCL5, all these validated genes were significantly induced in ZIKV-infected tree shrews at 2 dpi, while the expression of CCL5 peaked at 6 dpi (Fig. 6B). These results suggest that antiviral immunity and inflammatory responses are efficiently triggered in infected tree shrews. In particular, the induction of some inflammatory genes, such as the damage-associated molecular pattern gene S100A12, may be related to the dermatological manifestations observed in ZIKV-infected tree shrews (34, 35).
FIG 6.
Global transcriptomic analysis in PBMCs from ZIKV-infected tree shrews. (A) RNA-seq analysis of antiviral ISGs and inflammatory genes in ZIKV-infected PBMCs isolated from two tree shrews at −2, 2, and 6 dpi. (B) RT-qPCR validation of the expression of antiviral genes, IFN-inducible receptors and transcription factors, as well as inflammatory genes, in ZIKV-infected PBMCs described in panel A. The data are shown as the means ± standard deviations (SDs) (n = 3).
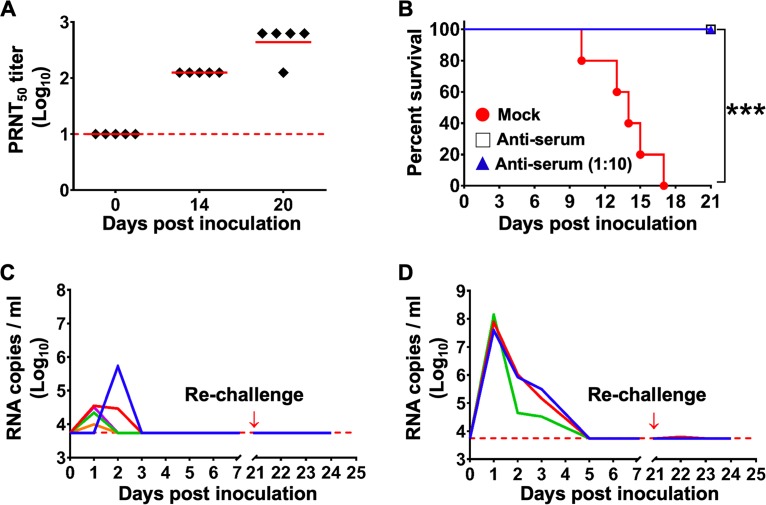
Furthermore, we determined the kinetics of ZIKV-specific neutralizing antibodies in ZIKV-inoculated animals. The 50% plaque reduction neutralization test (PRNT50) showed that ZIKV infection induced the production of a high level of neutralizing antibodies against ZIKV; the geometric mean titer was 534.80 at 20 dpi (Fig. 7A). To further confirm the in vivo protection profile of antiserum from infected animals, we performed passive transfer experiments in an established suckling model (36). Upon intracerebral ZIKV challenge, control mice developed typical neurological symptoms and died 10 to 17 dpi (Fig. 7B). However, antisera from infected tree shrews and 10-fold dilutions of antisera conferred full protection against lethal ZIKV challenge, and all mice survived without developing any clinical symptoms (Fig. 7B). Then, to validate whether primary ZIKV infection could induce protective immunity, groups of tree shrews (n = 8) infected with 105 or 106 PFU of ZIKV were rechallenged with the same dose of homologous ZIKV at 21 days post primary infection. Notably, all animals were fully protected, and no animal developed a skin rash or any other apparent clinical symptoms. In addition, ZIKV RNA levels remained negative in all rechallenged animals (Fig. 7C and D). Collectively, these results show that ZIKV infection induces both innate and adaptive immune responses in tree shrews and confers full protection against secondary homologous challenge.
FIG 7.
ZIKV infection triggers protective immunity against secondary homologous infection. (A) Neutralizing antibody kinetics in ZIKV-infected animals. Dotted lines represent the limits of detection. (B) Passive transfer protection experiments were performed in 1-day-old suckling BALB/c mice using antisera and 10-fold dilutions (n = 5) of antisera from ZIKV-infected tree shrews (n = 5). PBS was included as the mock control (n = 5). The Kaplan-Meier survival curves were analyzed by the log-rank test. *** denotes a P value of less than 0.01. (C and D) All tree shrews that received 105 or 106 PFU of ZIKV were rechallenged with the same dose of ZIKV at 21 days post primary infection. Viremia was monitored for 7 days after each challenge. The limit of detection is indicated by the dotted line.
ZIKV is neurovirulent in neonatal tree shrews.
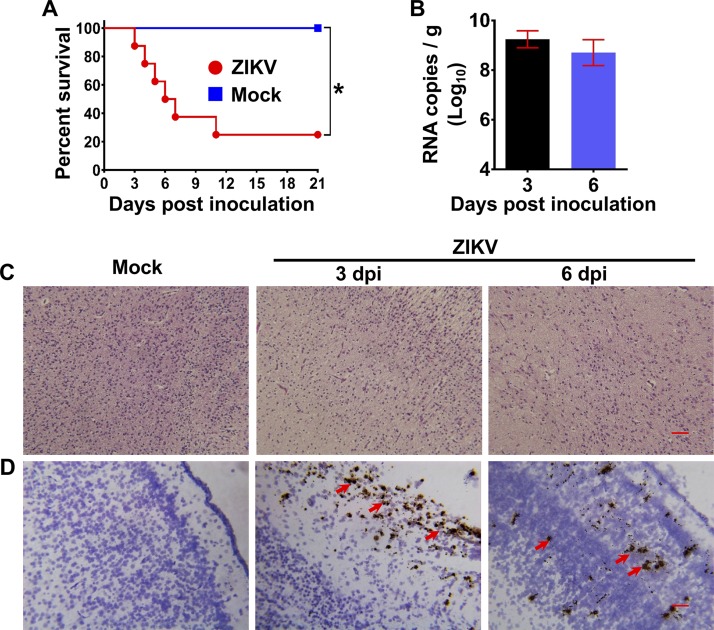
Neonatal mice have been widely used to study ZIKV neurovirulence and neuropathogenesis (8, 10). To determine whether neonatal tree shrews could also be used for neurovirulence tests, groups of 1-day-old neonatal tree shrews were subjected to intracerebral inoculation with 105 PFU of ZIKV. Strikingly, all ZIKV-inoculated neonatal animals (n = 8) succumbed to infection beginning at 3 dpi and exhibited a 75% mortality rate within 21 days (Fig. 8A). However, as expected, mock inoculation failed to kill any animals. We also assessed the brain size; there was no sign of microcephaly in the ZIKV-infected tree shrews compared with the mock-infected animals. However, high levels of ZIKV RNA were detected at 3 and 6 dpi in the brain of ZIKV-infected animals (Fig. 8B). Importantly, substantial pathological changes, including neuron destruction, were observed in the brains of infected neonatal tree shrews (Fig. 8C), and an RNA ISH assay revealed abundant ZIKV RNA in the cerebral cortex at 3 and 6 dpi (Fig. 8D). Together, these results suggest that ZIKV can efficiently infect neonatal tree shrews and replicate in the brain.
FIG 8.
Neurovirulence of ZIKV in neonatal tree shrews. (A) Survival curve for ZIKV-infected 1-day-old tree shrews. Groups of neonatal tree shrews (n = 8) were inoculated with 105 PFU of ZIKV by the intracerebral route. PBS was used as the mock control. The Kaplan-Meier survival curves were analyzed by the log-rank test. * denotes a P value of less than 0.05. (B) Viral RNA loads in brain of ZIKV-infected neonatal tree shrews at 3 and 6 dpi were determined by RT-qPCR. (C and D) Histopathological and ISH assays of brain sections obtained from ZIKV-infected neonatal tree shrews at 3 and 6 dpi. The red arrows indicate positive results. Bar, 50 μm.
DISCUSSION
Previously, tree shrews have been used to recapitulate infections by certain viruses, including H1N1 influenza virus (37), herpes simplex virus (38), retrovirus (39), and Epstein-Barr virus (40). In this study, we successfully recapitulated typical dermatological manifestations of ZIKV infection in adult tree shrews. Upon s.c. infection, a heavy rash, which was predominantly present on the lower abdomen, was observed within 2 days in most (24/26) tree shrews, which was synchronized with the viremia kinetics. The skin symptoms resolved within 3 days in all ZIKV-infected animals, and no other relevant symptoms, such as fever and weight loss, were observed. In general, the predominant clinical features in ZIKV patients include a pruritic descending macular or maculopapular rash generally lasting 3 to 11 days (41, 42). These unique dermatological symptoms observed in tree shrews have not been documented in other animal models, such as mice and guinea pigs (17). Recently, Dudley et al. reported that two of six rhesus macaques that received s.c. inoculation of ZIKV developed a very mild rash around the injection site at 1 dpi that persisted for 4 to 5 days (43). Another study showed that seven rhesus macaques infected with ZIKV by the s.c. route bilaterally on the hands and arms developed a transient rash on the arms and upper torso (44). In addition, minimal perivascular lymphocytic or lymphoplasmacytic inflammatory cell infiltrates were present in skin sections from the upper torso of two rhesus macaques (34). These casual skin reactions near the injection sites might have been immunological responses to the injectant. Our results showed that heat-inactivated ZIKV failed to cause any dermatological manifestation in tree shrews and that the skin rash in response to ZIKV infection diffusely covered multiple areas of the body. In particular, we detected viral RNA in the skin tissue of tree shrews (Fig. 3B), and human keratinocytes, dermal fibroblasts, and endothelial cells have been shown to be permissive for ZIKV infection (45). Because skin cells are the first to encounter ZIKV following s.c. injection or mosquito bites, the mechanism underlying ZIKV-induced skin damage deserves further investigation.
Viremia is well documented as a marker of flaviviral replication in vivo. We reported transient viremia, which peaked at 1 dpi (106.15 RNA copies/ml) and decreased below the detection limit at 3 dpi, in immunocompetent mice after s.c. infection with ZIKV (32). Moreover, sustained viremia was detected in all guinea pigs from 2 to 5 dpi, with peak titers of 105.4 to 106.9 RNA copies/ml at 3 dpi (17). In addition, the mean viremia duration in rhesus macaques was 6.6 days, and the peak titers of 104.16 to 105.82 RNA copies/ml occurred between 2 and 5 dpi (46). In our study, all tree shrews developed viremia at 2 dpi, with peak titers of 104.40 to 106.02 RNA copies/ml. Although the peak viremia titer was comparable, the viremia duration in tree shrews was much shorter than that in humans and NHPs, suggesting that tree shrews are somewhat resistant to ZIKV infection.
To understand the specific immune response induced in ZIKV-infected tree shrews, we further investigated the innate antiviral response in ZIKV-infected PBMCs. The transcriptome sequencing (RNA-seq) results showed that many antiviral ISGs were induced in ZIKV-infected cells. Previously, Xu et al. demonstrated that retinoic acid-inducible gene I (RIG-I) was lost in the Chinese tree shrew lineage and that MDA5 alone or MDA5 and LGP2 could replace RIG-I in sensing RNA viruses and triggering IFN production (23). Notably, innate antiviral response-related genes, including TLR7, MDA5, IRF7, CCL5, MX1, ISG15, IFIT1, and IFIT3, were significantly induced in ZIKV-infected tree shrews. The innate immune responses in ZIKV-infected tree shrews are similar to those in infected cell lines, including A549 cells (47). Interestingly, the NS5 protein of ZIKV has been reported to target human STAT2 to inhibit the IFN response but not mouse STAT2 (48). Recently, Siddharthan et al. reported that STAT2 knockout hamsters are lethally susceptible to ZIKV infection (6). Our RNA-seq analysis of samples from ZIKV-infected tree shrews failed to detect STAT2 transcripts, although transcripts of other IFN-regulated transcriptional activators, including STAT1, STAT3, and STAT5, were detectable. The way in which ZIKV interacts with the innate immune system of tree shrews deserves further investigation. Additionally, we found that although the S100A12 gene was highly expressed in the blood of tree shrews with active skin damage at 2 dpi, its expression decreased at 6 dpi with the disappearance of the rash. S100A12 is a proinflammatory protein that interacts with the receptor for advanced glycation end products (RAGE) and has been recognized as a potential marker of inflammatory skin diseases (34, 35). However, the underlying mechanism linking S100A12 and ZIKV-related dermatological manifestations deserves further investigation.
In summary, ZIKV infection in adult tree shrews leads to robust viral secretion in sera and saliva, as well as cutaneous inflammation and dermatological manifestations. This novel tree shrew animal model provides not only an opportunity to study ZIKV but also a useful platform to evaluate the efficacy of potent drugs that alleviate symptoms caused by ZIKV.
MATERIALS AND METHODS
Ethics statement.
Chinese tree shrews (Tupaia belangeri chinensis) (F1 generation) were obtained from the Kunming Primate Research Center at the Kunming Institute of Zoology, Chinese Academy of Sciences. All animal experiments were performed in strict accordance with the guidelines of the Chinese Regulations of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China) and Laboratory Animal—Requirements of Environment and Housing Facilities (GB 14925-2010, National Laboratory Animal Standardization Technical Committee). All procedures were performed under sodium pentobarbital anesthesia by trained technicians, and all efforts were made to ameliorate the welfare and to minimize animal suffering in accordance with the recommendations of the Weatherall Report on the use of nonhuman primates. The experimental protocols were approved by the Animal Experiment Committee of the Laboratory Animal Center, Academy of Military Medical Sciences (AMMS), China (IACUC-13-2016-001).
Viruses and cells.
ZIKV strain GZ01 (GenBank accession number KU820898) was originally isolated from a Chinese male patient who returned from Venezuela (33). ZIKV stocks were propagated in Aedes albopictus C6/36 cells and titrated by a plaque-forming assay in BHK-21, cells as previously described (46, 49). All experiments involving infectious ZIKV were conducted in biosafety level 2 (BSL2) facilities.
PBMCs were isolated using Ficoll-Hypaque separation and suspended in RPMI 1640 medium containing l-glutamine and supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. PKCs and PTCs were isolated and cultured by a standard protocol. Briefly, kidney and testis tissues were washed in PBS solution and cut into pieces after stripping off the tunica albuginea. The tissue pieces were then incubated with 5% collagenase type IV at 37°C for 60 min with gentle agitation, washed twice in PBS, and incubated with 0.25% trypsin at 37°C for <10 min, until most cells were dispersed. Digestion was terminated by adding 10% FBS, and the cell suspensions were centrifuged at 1,000 rpm for 5 min to remove the supernatant. The cell pellets were resuspended in 1% bovine serum albumin (BSA), and clumps were removed by filtration through a 100-μm nylon cell strainer (BD, USA). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10 ng/ml animal-free recombinant human epidermal growth factor (EGF; Peprotech, USA). All cells were maintained in an atmosphere of 37°C and 5% CO2.
Animal experiments.
For the primary infection experiment, 5-month-old tree shrews were inoculated with 105 or 106 PFU of ZIKV GZ01 by the s.c. route. Heat-inactivated ZIKV and PBS were used as the controls. After inoculation, the animals were monitored daily for 14 days for body weight changes, rectal temperature, and clinical symptoms. Blood and major body fluids (saliva, tears, and urine) were collected at 1, 2, 3, and 7 dpi for viral load analysis. Briefly, blood was collected from the retroorbital sinus of each tree shrew, urine was collected from a container under the animal’s cage, saliva was obtained by running a sterile swab under the animal’s tongue, and tears were collected by gently running a sterile swab over the animal’s eyes. All samples were stored at −80°C until processing. For necropsy, all animals were anesthetized and perfused with PBS prior to the collection of samples for virological or histopathological assays.
For the rechallenge experiments, tree shrews initially infected with 105 or 106 PFU of ZIKV were then rechallenged with the same dose of homologous virus on day 21 after primary infection. Clinical symptoms were recorded, and viremia was monitored accordingly.
For the neurovirulence tests, a group of 1-day-old neonatal tree shrews was inoculated by the intracerebral route with 105 PFU of ZIKV. PBS was used as the control. The animals were observed daily for signs of illness and mortality. At 3 and 6 dpi, the neonatal tree shrews were anesthetized, and selected organs were dissected and subjected to RT-qPCR, histological assays, and RNA ISH assays, as described below.
For the passive transfer experiments, antisera from ZIKV-infected tree shrews and 10-fold dilutions of antisera were mixed with an equal volume of solution containing 103 PFU/ml of ZIKV strain GZ01. After incubation at 37°C for 1 h, the antiserum-virus mixture was injected intracerebrally into 1-day-old suckling BALB/c mice. PBS was used as the mock control. The animals were monitored daily for 3 weeks for clinical signs of infection, including ruffled hair, a hunched back, paralysis, and death. The Kaplan-Meier survival curves were analyzed by the log-rank test.
RNA isolation and RT-qPCR.
Total RNA was extracted from cells, tissues, or virus by using a PureLink RNA minikit (Life Technologies, USA) according to the manufacturer’s recommendations. Using virus-specific primers and a probe described previously (46, 50), RT-qPCR was carried out with a One-Step PrimeScript RT-PCR kit (TaKaRa, Japan) on a LightCycler system (Roche, USA). The viral titer was then adjusted for volume and organ weight to report the organ loads as RNA copies/ml and RNA copies/gram. In addition, SYBR green RT-qPCR mix (TaKaRa, Japan) was used to determine the mRNA levels of various cytokines. All RT-qPCR primer sequences are available on request.
Histopathological and immunofluorescence staining.
For the histopathological assay, various tissue samples were collected from tree shrews and fixed in 4% neutral-buffered formaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Images were captured using an Olympus BX51 microscope equipped with a DP72 camera.
For immunostaining, various tissues were fixed in 4% paraformaldehyde for 48 h and dehydrated in 30% sucrose for 24 or 48 h. The fixed tissues were frozen in tissue-freezing medium and sectioned into 40-μm slices. The cryosections were blocked at room temperature (RT) for 1 h in 3% BSA, 10% FBS, and 0.2% Triton X-100 in PBS and were then incubated with ZIKV human convalescence serum (1:500 dilution) from a recovered patient (51) at 4°C overnight. The cryosections were then washed with 0.2% Triton X-100 in PBS (3 × 10 min), incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (ZSGB-Bio, China) at RT for 1 h, and washed three times. The cryosections were counterstained with hematoxylin.
ISH assay.
ZIKV genome RNA ISH assays were performed with an RNAscope 2.5 assay (Advanced Cell Diagnostics, USA) according to the manufacturer’s instructions. Briefly, paraffin-embedded tissue sections were deparaffinized by incubation for 60 min at 60°C. Endogenous peroxidase activity was quenched with hydrogen peroxide for 10 min at RT. Slides were then boiled for 15 min in RNAscope target retrieval reagents and incubated for 30 min in RNAscope Protease Plus before probe hybridization. The probe targeting ZIKV RNA was synthesized by Advanced Cell Diagnostics (catalog no. 467871). Tissues were counterstained with Gill’s hematoxylin and visualized with standard bright-field microscopy.
Virus isolation.
Serum samples from infected tree shrews were inoculated into C6/36 mosquito cells and maintained in RPMI 1640 medium (Life Technologies, USA) supplemented with 2% FBS (Life Technologies) at 28°C in 5% CO2. Six days later, RNA was extracted from the culture supernatant and analyzed by RT-qPCR as described above. An increase in the RNA copy number of at least 1,000-fold was deemed positive for virus isolation.
Neutralizing antibody assays.
Neutralizing antibody titers were determined by a constant virus-serum dilution 50% plaque reduction neutralization test (PRNT50), as previously described (49). Briefly, serial 5-fold dilutions of inactivated serum were mixed with equal volumes of ZIKV in DMEM supplemented with 2% FBS. After incubation at 37°C for 1 h, virus-antibody mixtures were added to plates containing BHK-21 cells. The concentration of infectious virus was determined using the plaque assay described above. The endpoint neutralization titer was calculated according to the Reed-Muench method.
RNA-seq analysis.
PBMCs harvested at −2, 2, and 6 dpi (two replicates per group) were isolated using Ficoll-Hypaque separation, and total RNA was extracted by an RNeasy minikit (Qiagen, Germany) for global transcriptome analysis. To remove rRNA, 1 μg of RNA was input into a Ribo-Zero Magnetic Gold kit (Illumina, USA), followed by cleanup using Agencourt RNAClean XP beads (Beckman Coulter, USA), elution in 5 μl of RNA fragmentation and priming buffers mixed from the NEBNext Ultra directional RNA library prep kit for Illumina (NEB, USA). Library construction was performed per the vendor’s instructions, starting with the “RNA Fragmentation, Priming and First-Strand cDNA Synthesis” step. Library quality was evaluated on an Agilent 2100 bioanalyzer and quantified by quantitative PCR (qPCR) using a Library quantification kit-Illumina/universal (KAPA Biosystems, USA). Libraries were sequenced on an Illumina HiSeq 2500 instrument using single-end 150-bp reads. Quality control (QC) was completed with Skewer (v0.1.127). Then, low-quality data were filtered, adapters were removed, and reads were compiled. Cleaned reads were mapped to Chinese_treeshrew_KIZv2.gtf by using HISAT2 (52) (v2.0.4) with the default parameters; the reference genome was downloaded from the Tree Shrew Database (http://www.treeshrewdb.org/). Relative gene expression levels in PBMCs from individual tree shrews were calculated by dividing the reads per kilobase per million mapped reads (RPKM) values of the ZIKV-infected samples by the RPKM values of the corresponding uninfected samples. Differential expression analysis was performed by DESeq2 (53) with a fold change cutoff of 2 and a q-value cutoff of 0.05. Gene set enrichment analysis was performed by categories (54).
Statistical analysis.
All statistical data were analyzed with GraphPad Prism Software version 5.01 (GraphPad Software Inc., La Jolla, CA). Survival curves were compared by the log-rank test. Statistical evaluation was performed with Student’s unpaired t test. P values of less than 0.05 were considered significant.
Data availability.
All sequence data generated by RNA-seq have been deposited in the GEO database (accession no. GSE115093). All relevant data from this study are available from the authors upon request.
ACKNOWLEDGMENTS
We thank the veterinarians from the Laboratory Animal Center at the Academy of Military Medical Science for their excellent technical support. We thank Jing An (Capital Medical University) and other members of the Xia laboratory for insightful discussions and technical support.
This work was supported by the National Natural Science Foundation of China (NSFC) (grants U1702282, 81772176, 31770190, and 31670883), the National Key Research and Development Project of China (grant 2016YFD0500304), and the National Science and Technology Major Project of China (grant 2018ZX09711003-005-002). C.-F.Q. was supported by the Excellent Young Scientist Program (no. 81522025), the Innovative Research Group of the NSFC (no. 81621005), and a Newton Advanced Fellowship from the UK Academy of Medical Sciences (no. NAF003\1003). X.X. was supported by the Yunnan Innovative Team Project (2015HC030). F.M. was supported by the CAMS Initiative for Innovative Medicine (no. 2016-I2M-1-005). J.D. was supported by the Yunnan Science and Technology Talent and Platform Program (2017HC019).
We declare that we have no conflicts of interest.
C.-F.Q., X.X., N.-N.Z., and Y.-Q.D. conceived and designed this study. N.-N.Z., L.Z., Y.-Q.D., Y.F., F.M., Q.W., Q.Y., and Y.H. performed the experiments. C.-F.Q., X.X., Y.-Q.D., N.-N.Z., L.Z. Y.F., F.M., X.Q., X.S., F.-C.Z., G.W., and J.D. analyzed the data. C.-F.Q., X.X., Y.-Q.D., N.-N.Z., and F.M. wrote the paper, with contributions from all authors.
REFERENCES
- 1.Dick GW, Kitchen SF, Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Pierson TC, Diamond MS. 2018. The emergence of Zika virus and its new clinical syndromes. Nature 560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 3.Morrison TE, Diamond MS. 2017. Animal models of zika virus infection, pathogenesis, and immunity. J Virol 91:e00009-17. doi: 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darbellay J, Cox B, Lai K, Delgado-Ortega M, Wheler C, Wilson D, Walker S, Starrak G, Hockley D, Huang Y, Mutwiri G, Potter A, Gilmour M, Safronetz D, Gerdts V, Karniychuk U. 2017. Zika virus causes persistent infection in porcine conceptuses and may impair health in offspring. EBioMedicine 25:73–86. doi: 10.1016/j.ebiom.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LJ, Nasar F, Schellhase CW, Norris SL, Kimmel AE, Valdez SM, Wollen-Roberts SE, Shamblin JD, Sprague TR, Lugo-Roman LA, Jarman RG, Yoon IK, Alera MT, Bavari S, Pitt MLM, Haddow AD. 2018. Zika virus infection in Syrian golden hamsters and strain 13 guinea pigs. Am J Trop Med Hyg 98:864–867. doi: 10.4269/ajtmh.17-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddharthan V, Van Wettere AJ, Li R, Miao J, Wang Z, Morrey JD, Julander JG. 2017. Zika virus infection of adult and fetal STAT2 knock-out hamsters. Virology 507:89–95. doi: 10.1016/j.virol.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Wichgers Schreur PJ, van Keulen L, Anjema D, Kant J, Kortekaas J. 2018. Microencephaly in fetal piglets following in utero inoculation of Zika virus. Emerg Microbes Infect 7:42. doi: 10.1038/s41426-018-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. 2016. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res 26:645–654. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M Jr, Rajagopal L. 2016. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. 2016. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. 2016. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG. 2017. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13:e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, Hecht JL, Borducchi EN, Larocca RA, Peterson RL, Rinaldi W, Ferguson M, Didier PJ, Weiss D, Lewis MG, De La Barrera RA, Yang E, Warfield SK, Barouch DH. 2018. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell doi: 10.1016/j.cell.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams Waldorf KM, Nelson BR, Stencel-Baerenwald JE, Studholme C, Kapur RP, Armistead B, Walker CL, Merillat S, Vornhagen J, Tisoncik-Go J, Baldessari A, Coleman M, Dighe MK, Shaw DWW, Roby JA, Santana-Ufret V, Boldenow E, Li J, Gao X, Davis MA, Swanstrom JA, Jensen K, Widman DG, Baric RS, Medwid JT, Hanley KA, Ogle J, Gough GM, Lee W, English C, Durning WM, Thiel J, Gatenby C, Dewey EC, Fairgrieve MR, Hodge RD, Grant RF, Kuller L, Dobyns WB, Hevner RF, Gale M Jr, Rajagopal L. 2018. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med 24:368–374. doi: 10.1038/nm.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. 2016. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep 17:3091–3098. doi: 10.1016/j.celrep.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. 2016. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 166:1247–1256 e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng YQ, Zhang NN, Li XF, Wang YQ, Tian M, Qiu YF, Fan JW, Hao JN, Huang XY, Dong HL, Fan H, Wang YG, Zhang FC, Tong YG, Xu Z, Qin CF. 2017. Intranasal infection and contact transmission of Zika virus in guinea pigs. Nat Commun 8:1648. doi: 10.1038/s41467-017-01923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerbino-Neto J, Mesquita EC, Souza TML, Parreira V, Wittlin BB, Durovni B, Lemos MCF, Vizzoni A, Bispo de Filippis AM, Sampaio SA, Gonçalves B. d S, Bozza FA. 2016. Clinical manifestations of Zika virus infection, Rio de Janeiro, Brazil, 2015. Emerg Infect Dis 22:1318–1320. doi: 10.3201/eid2207.160375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazear HM, Diamond MS. 2016. Zika virus: new clinical syndromes and its emergence in the Western Hemisphere. J Virol 90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Ruckert C, Govero J, Noguchi KK, Ebel GD, Diamond MS, Apte RS. 2016. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep 16:3208–3218. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao YG. 2017. Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? Zool Res 38:118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Huang Z-Y, Cao C-C, Chen C-S, Chen Y-X, Fan D-D, He J, Hou H-L, Hu L, Hu X-T, Jiang X-T, Lai R, Lang Y-S, Liang B, Liao S-G, Mu D, Ma Y-Y, Niu Y-Y, Sun X-Q, Xia J-Q, Xiao J, Xiong Z-Q, Xu L, Yang L, Zhang Y, Zhao W, Zhao X-D, Zheng Y-T, Zhou J-M, Zhu Y-B, Zhang G-J, Wang J, Yao Y-G. 2013. Genome of the Chinese tree shrew. Nat Commun 4:1426. doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Yu D, Fan Y, Peng L, Wu Y, Yao YG. 2016. Loss of RIG-I leads to a functional replacement with MDA5 in the Chinese tree shrew. Proc Natl Acad Sci U S A 113:10950–10955. doi: 10.1073/pnas.1604939113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li CH, Yan LZ, Ban WZ, Tu Q, Wu Y, Wang L, Bi R, Ji S, Ma YH, Nie WH, Lv LB, Yao YG, Zhao XD, Zheng P. 2017. Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Res 27:241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong Y, Zhu Y, Xia X, Liu Y, Feng Y, Hua X, Chen Z, Ding H, Gao L, Wang Y, Feitelson MA, Zhao P, Qi ZT. 2011. Tupaia CD81, SR-BI, claudin-1, and occludin support hepatitis C virus infection. J Virol 85:2793–2802. doi: 10.1128/JVI.01818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, Feng YM, Feng Y, Lu C, Liu L, Sun X, Dai J, Xia X. 2015. Identification and characterization of liver microRNAs of the Chinese tree shrew via deep sequencing. Hepat Mon 15:e29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan H, Zhong GC, Xu GW, He WH, Jing ZY, Gao ZC, Huang Y, Qi YH, Peng B, Wang HM, Fu LR, Song M, Chen P, Gao WQ, Ren BJ, Sun YY, Cai T, Feng XF, Sui JH, Li WH. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michlmayr D, Andrade P, Gonzalez K, Balmaseda A, Harris E. 2017. CD14+CD16+ monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol 2:1462–1470. doi: 10.1038/s41564-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. 2016. Zika virus causes testis damage and leads to male infertility in mice. Cell 167:1511–1524.e10. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. 2016. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 22:1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, Homer RJ, Iwasaki A, Fikrig E. 2017. Zika virus causes testicular atrophy. Sci Adv 3:e1602899. doi: 10.1126/sciadv.1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang NN, Tian M, Deng YQ, Hao JN, Wang HJ, Huang XY, Li XF, Wang YG, Zhao LZ, Zhang FC, Qin CF. 2016. Characterization of the contemporary Zika virus in immunocompetent mice. Hum Vaccin Immunother 12:3107–3109. doi: 10.1080/21645515.2016.1219004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F-C, Li X-F, Deng Y-Q, Tong Y-G, Qin C-F. 2016. Excretion of infectious Zika virus in urine. Lancet Infect Dis 16:641–642. doi: 10.1016/s1473-3099(16)30070-6. [DOI] [PubMed] [Google Scholar]
- 34.Wilsmann-Theis D, Wagenpfeil J, Holzinger D, Roth J, Koch S, Schnautz S, Bieber T, Wenzel J. 2016. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J Eur Acad Dermatol Venereol 30:1165–1170. doi: 10.1111/jdv.13269. [DOI] [PubMed] [Google Scholar]
- 35.Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. 2012. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol 132:2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, Wang HJ, Wang Q, Liu ZY, Yuan L, Huang XY, Li G, Ye Q, Yang H, Shi L, Deng YQ, Qin CF, Xu Z. 2017. American strain of Zika virus causes more severe microcephaly than an old Asian strain in neonatal mice. EBioMedicine 25:95–105. doi: 10.1016/j.ebiom.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z-F. 2013. The tree shrew provides a useful alternative model for the study of influenza H1N1 virus. Virol J 10:111. doi: 10.1186/1743-422X-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Li Z, Wang E, Yang R, Xiao Y, Han H, Lang F, Li X, Xia Y, Gao F, Li Q, Fraser NW, Zhou J. 2016. Herpes simplex virus 1 infection of tree shrews differs from that of mice in the severity of acute infection and viral transcription in the peripheral nervous system. J Virol 90:790–804. doi: 10.1128/JVI.02258-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang F, Yu W, He Z. 2013. Foamy virus in the tree shrew Tupaia belangeri is highly related to simian foamy virus in Macaca mulatta. AIDS Res Hum Retroviruses 29:1177–1178. doi: 10.1089/AID.2013.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Yi X, Du L, Wang H, Tang J, Wang M, Qi C, Li H, Lai Y, Xia W, Tang A. 2017. A study of Epstein-Barr virus infection in the Chinese tree shrew(Tupaia belangeri chinensis). Virol J 14:193. doi: 10.1186/s12985-017-0859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobson JS, Levell NJ. 2018. Spotting Zika spots: descriptive features of the rash used in 66 published cases. Clin Exp Dermatol doi: 10.1111/ced.13733. [DOI] [PubMed] [Google Scholar]
- 42.Cosano-Quero A, Velasco-Tirado V, Sánchez Seco MP, Manzanedo-Bueno L, Belhassen-García M. 2018. Zika virus: cutaneous manifestations in 3 patients. Actas Dermosifiliogr 109:e13–e16. doi: 10.1016/j.ad.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O'Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O'Connor DH. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, DeFilippis VR, Denton M, Smith PP, Messer WB, Colgin LM, Ducore RM, Grigsby PL, Hennebold JD, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Wiley CA, Nelson JA, Streblow DN. 2017. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 13:e1006219. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau V-M, Choumet V, Briant L, Desprès P, Amara A, Yssel H, Missé D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, Zhang NN, Ye Q, Zhao H, Liu ZY, Fan H, An XP, Sun SH, Gao B, Fa YZ, Tong YG, Zhang FC, Gao GF, Cao WC, Shi PY, Qin CF. 2016. Characterization of a 2016 clinical isolate of Zika virus in non-human primates. EBioMedicine 12:170–177. doi: 10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Deng YQ, Wang S, Ma F, Aliyari R, Huang XY, Zhang NN, Watanabe M, Dong HL, Liu P, Li XF, Ye Q, Tian M, Hong S, Fan J, Zhao H, Li L, Vishlaghi N, Buth JE, Au C, Liu Y, Lu N, Du P, Qin FX, Zhang B, Gong D, Dai X, Sun R, Novitch BG, Xu Z, Qin CF, Cheng G. 2017. 25-Hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity 46:446–456. doi: 10.1016/j.immuni.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sánchez-Seco MP, Evans MJ, Best SM, García-Sastre A. 2016. Zika virus targets human STAT2 to inhibit type i interferon signaling. Cell Host Microbe 19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, Xiao H, Yan J, Shi Y, Qin CF, Qi J, Gao GF. 2016. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, Kalkeri R, Rayner J. 2016. Development of a Zika virus infection model in cynomolgus macaques. Front Microbiol 7:2028. doi: 10.3389/fmicb.2016.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Hong S, Deng YQ, Ye Q, Zhao LZ, Zhang FC, Qin CF, Xu Z. 2017. Transfer of convalescent serum to pregnant mice prevents Zika virus infection and microcephaly in offspring. Cell Res 27:158–160. doi: 10.1038/cr.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim T, Seo HD, Hennighausen L, Lee D, Kang K. 2018. Octopus-toolkit: a workflow to automate mining of public epigenomic and transcriptomic next-generation sequencing data. Nucleic Acids Res 46:e53. doi: 10.1093/nar/gky083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawrence M, Gentleman R. 2017. VariantTools: an extensible framework for developing and testing variant callers. Bioinformatics 33:3311–3313. doi: 10.1093/bioinformatics/btx450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequence data generated by RNA-seq have been deposited in the GEO database (accession no. GSE115093). All relevant data from this study are available from the authors upon request.