Gammaherpesviruses are ubiquitous cancer-associated pathogens that usurp the B cell differentiation process to establish life-long latent infection in memory B cells. A unique feature of early gammaherpesvirus infection is the robust increase in differentiation of B cells that are not specific for viral antigens and instead encode antibodies that react with self-antigens and antigens of other species. Viral mechanisms that are involved in driving such irrelevant B cell differentiation are not known. Here, we show that gammaherpesvirus-encoded conserved protein kinase and host IL-1 signaling promote irrelevant B cell responses and gammaherpesvirus-driven germinal center responses, with the latter thought to be the target of viral transformation.
KEYWORDS: B cell responses, IL-1, gammaherpesvirus, germinal center, orf36, protein kinase
ABSTRACT
Gammaherpesviruses are ubiquitous pathogens that are associated with B cell lymphomas. In the early stages of chronic infection, these viruses infect naive B cells and subsequently usurp the B cell differentiation process through the germinal center response to ensure latent infection of long-lived memory B cells. A unique feature of early gammaherpesvirus chronic infection is a robust differentiation of irrelevant, virus-nonspecific B cells with reactivities against self-antigens and antigens of other species. In contrast, protective, virus-specific humoral responses do not reach peak levels until a much later time. While several host factors are known to either promote or selectively restrict gammaherpesvirus-driven germinal center response, viral mechanisms that contribute to the irrelevant B cell response have not been defined. In this report we show that the expression and the enzymatic activity of the gammaherpesvirus-encoded conserved protein kinase selectively facilitates the irrelevant, but not virus-specific, B cell responses. Further, we show that lack of interleukin-1 (IL-1) receptor attenuates gammaherpesvirus-driven B cell differentiation and viral reactivation. Because germinal center B cells are thought to be the target of malignant transformation during gammaherpesvirus-driven lymphomagenesis, identification of host and viral factors that promote germinal center responses during gammaherpesvirus infection may offer an insight into the mechanism of gammaherpesvirus pathogenesis.
IMPORTANCE Gammaherpesviruses are ubiquitous cancer-associated pathogens that usurp the B cell differentiation process to establish life-long latent infection in memory B cells. A unique feature of early gammaherpesvirus infection is the robust increase in differentiation of B cells that are not specific for viral antigens and instead encode antibodies that react with self-antigens and antigens of other species. Viral mechanisms that are involved in driving such irrelevant B cell differentiation are not known. Here, we show that gammaherpesvirus-encoded conserved protein kinase and host IL-1 signaling promote irrelevant B cell responses and gammaherpesvirus-driven germinal center responses, with the latter thought to be the target of viral transformation.
INTRODUCTION
Gammaherpesviruses establish life-long infection in >95% of adults worldwide and are associated with the development of lymphoproliferative diseases and other malignancies (1). Further, these viruses may promote select autoimmune diseases. Gammaherpesviruses take advantage of B cell differentiation to establish a latent viral reservoir in memory B cells (2–4). Specifically, Epstein-Barr virus (EBV) and murid gammaherpesvirus 68 (MHV68, also named γHV68 or murid herpesvirus-4) infect naive B cells, with subsequent induction of a robust germinal center response that includes both virus-infected and uninfected germinal center B cells (2, 5, 6). Importantly, this unique germinal center reaction encompasses a virus-specific B cell response, along with robust irrelevant, virus-nonspecific B cell differentiation. As a result of the latter, there is a rapid, albeit transient, increase in the titers of class-switched polyclonal antibodies with reactivities against self-antigens and antigens of other species (7, 8). In fact, the presence of high levels of antibodies against horse red blood cells (and, historically, sheep and bovine antigens) is a highly specific (99%) diagnostic of a recent EBV infection (9). In contrast, protective humoral responses directed against EBV and MHV68 antigens arise with delayed kinetics and may in fact be suppressed by these viruses (9–12).
Despite the unusual nature of the gammaherpesvirus-induced germinal center response, only a few factors involved in this response have been identified. CD4 T cells, including T follicular helper (Tfh) T cells, are necessary for the MHV68-induced germinal center response, in part via expression of interleukin-21 (IL-21) (7, 13, 14). In contrast, we identified interferon regulatory factor-1 (IRF-1) as a selective negative regulator of the gammaherpesvirus-induced germinal center response and a potential suppressor of EBV-associated lymphoproliferative disease (15). However, the relative contributions of these host factors to protective versus irrelevant B cell responses have not been defined. Further, gammaherpesvirus mechanisms that promote irrelevant B cell differentiation remain unknown.
The robust, irrelevant B cell differentiation induced by the gammaherpesvirus infection is likely to precede both lymphomagenesis and autoimmune diseases in a susceptible host. The germinal center stage of B cell differentiation is arguably the most treacherous from the perspective of cellular transformation, as germinal center B cells undergo rapid cellular division with concomitant downregulation of major tumor suppressors (16) and expression of enzymes that directly mutagenize the genome (17, 18). The induction of broad germinal center responses coupled to the high frequency of infected germinal center B cells likely contributes to viral lymphomagenesis, as evidenced by the observation that a majority of EBV-driven B cell lymphomas are of germinal center or post-germinal center differentiation stage (19). Further, induction of self-reactive antibodies during early EBV infection, a transient event in most individuals, may contribute to autoimmune disease in a susceptible host.
In this study, we show that expression and enzymatic activity of the conserved gammaherpesvirus protein kinase facilitated irrelevant B cell differentiation during the establishment of chronic infection. In contrast, protective, virus-specific B cell responses were not affected by the expression or enzymatic activity of the gammaherpesvirus protein kinase. Further, we show that the absence of IL-1 signaling led to the decrease in germinal center responses and irrelevant B cell differentiation induced by a wild-type but not by a viral-kinase-deficient gammaherpesvirus. Thus, we have used a combination of viral and host genetics to functionally separate the protective and irrelevant B cell responses driven by gammaherpesvirus infection.
RESULTS
Viral protein kinase expression and enzymatic activity are critical for the establishment of splenic gammaherpesvirus latency following a low-dose infection.
All herpesviruses, including EBV and MHV68, encode a protein kinase, with higher similarity among the protein kinases expressed within a specific herpesvirus family. Unfortunately, due to significant species specificity, it is not known how conserved protein kinases encoded by human gammaherpesviruses affect chronic infection. Because of the intrinsically druggable nature of kinases, a better understanding of how gammaherpesvirus kinases regulate in vivo infection may allow the development of new antiviral therapies, as pioneered by maribavir, the first inhibitor of betaherpesvirus protein kinase tested in the clinic (20).
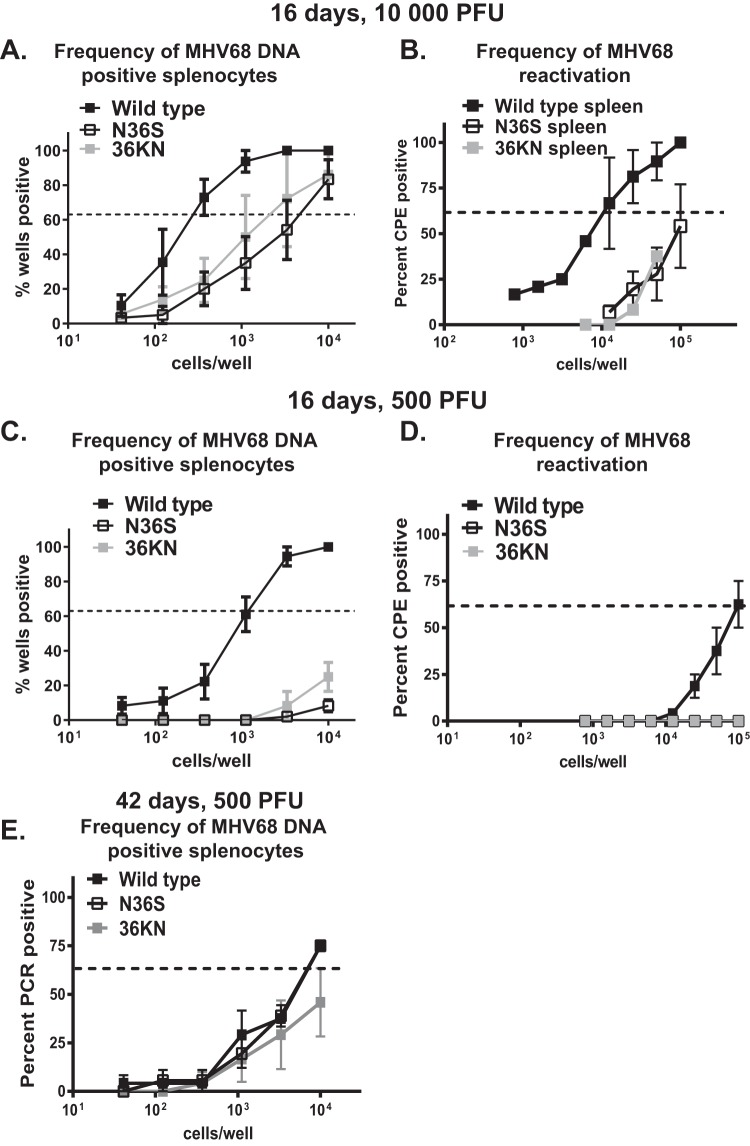
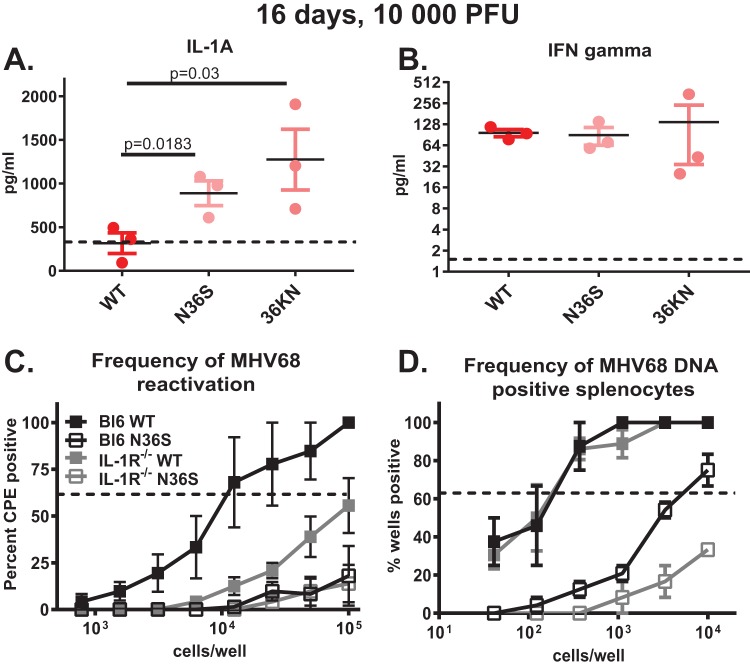
MHV68 is a natural rodent gammaherpesvirus that is closely related to the known human gammaherpesviruses and offers a robust animal model of chronic infection and viral pathogenesis (21–23). Capitalizing on the viral genetics of the MHV68 system, we used two MHV68 mutant viruses to interfere with the viral protein kinase (encoded by orf36): (i) an N36S mutant with a translational stop engineered into the orf36 gene that prevents protein kinase expression; (ii) a 36KN mutant that expresses an enzymatically null viral protein kinase due to a single amino acid substitution in the catalytic domain (24). Consistent with our previous observations (25), animals infected with a high dose (104 PFU) of either orf36 mutant virus displayed decreased frequency of latent infection and ex vivo reactivation from splenocytes although splenic infection was established in all three groups (Fig. 1A and B). Because a high infection dose can mask the phenotypes of viral genes (26, 27), we wanted to determine the extent to which viral protein kinase facilitates the establishment of MHV68 latency following a lower viral inoculum. In contrast to high-dose infection, very few MHV68 positive splenocytes were detected in mice infected with a low dose (500 PFU) of either orf36 mutant virus (Fig. 1C). Correspondingly, MHV68 reactivation was below the level of detection in splenocytes harvested from either N36S- or 36KN-infected animals (Fig. 1D). Thus, expression and enzymatic activity of the viral protein kinase were important for the early splenic colonization following low-dose, but not high-dose, intranasal infection.
FIG 1.
Viral protein kinase expression and enzymatic activity are critical for the establishment of gammaherpesvirus latency following a low-dose infection. (A to D). BL6 mice were intranasally infected with indicated doses of wild-type MHV68 or viral protein kinase mutant virus (N36S mutant lacking expression of kinase or 36KN mutant that expresses an enzymatically inactive viral kinase). Splenocytes were harvested at 16 days postinfection and subjected to limiting dilution assays (as described in Materials and Methods) to determine the frequency of MHV68 DNA-positive cells (i.e., latently infected cells) (A and C), or cells reactivating MHV68 ex vivo (B and D). Studies shown in panel E were extended to 42 days postinfection. Each experimental group consisted of 3 to 5 animals; data were pooled from 3 to 5 independent experiments. Here and in limiting dilution assays presented in Fig. 3 and 5, the dotted line is drawn at 62.5% and the x coordinate of intersection of this line with the sigmoid graph represents an inverse of frequency of positive events. CPE, cytopathic effect.
The peak number of latently infected splenocytes observed at 16 days post-MHV68 infection contracts between 16 and 42 days as infection transitions to a stable long-term phase. Even under conditions of highly attenuated early latency following inoculation with low doses of orf36 mutant viruses, all three groups of infected mice displayed similar frequencies of latently infected splenocytes at 42 days postinfection (Fig. 1E). As expected, no reactivation was observed in splenocytes of all three experimental groups at 42 days postinfection. Thus, in contrast to the early defect in viral colonization, the viral kinase or its enzymatic activity did not significantly contribute to the long-term maintenance of low-level splenic latency in an immunocompetent host.
Viral protein kinase expression and enzymatic activity facilitate the gammaherpesvirus-driven germinal center response.
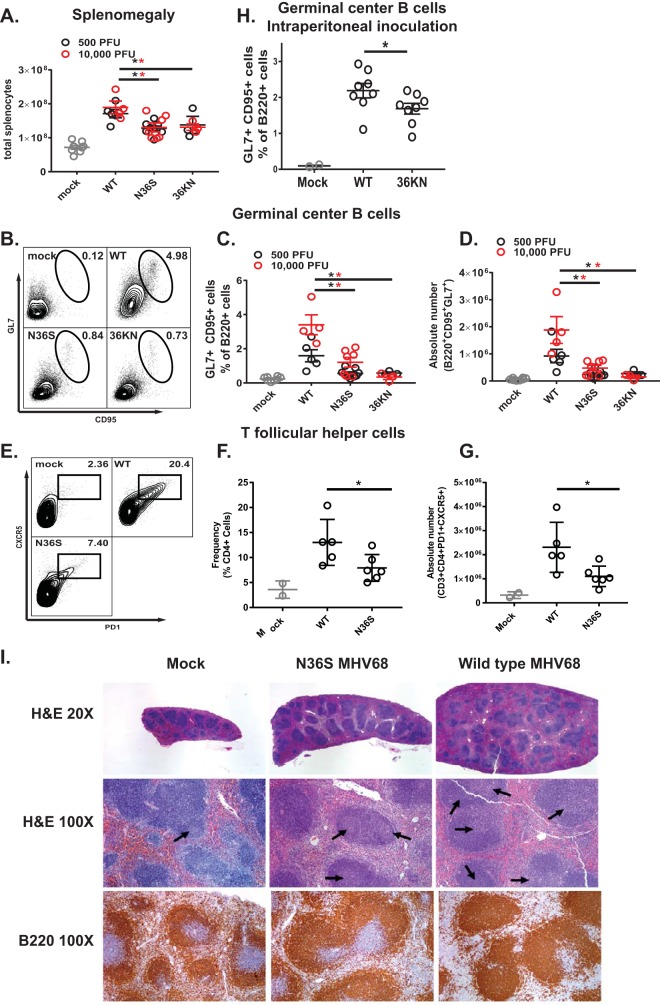
The germinal center response induced during the establishment of MHV68 infection coincides with and contributes to splenomegaly (28), a clinical finding also observed in young adults recently infected with EBV (29). Interestingly, mice infected with the N36S or 36KN mutant had attenuated splenomegaly at 16 days postinfection, as quantified by the number of nucleated cells/spleen (Fig. 2A). This attenuated splenomegaly was not rescued by increasing the inoculum dose to 104 PFU, a condition that allows orf36 mutants to colonize the spleen (Fig. 1A and B).
FIG 2.
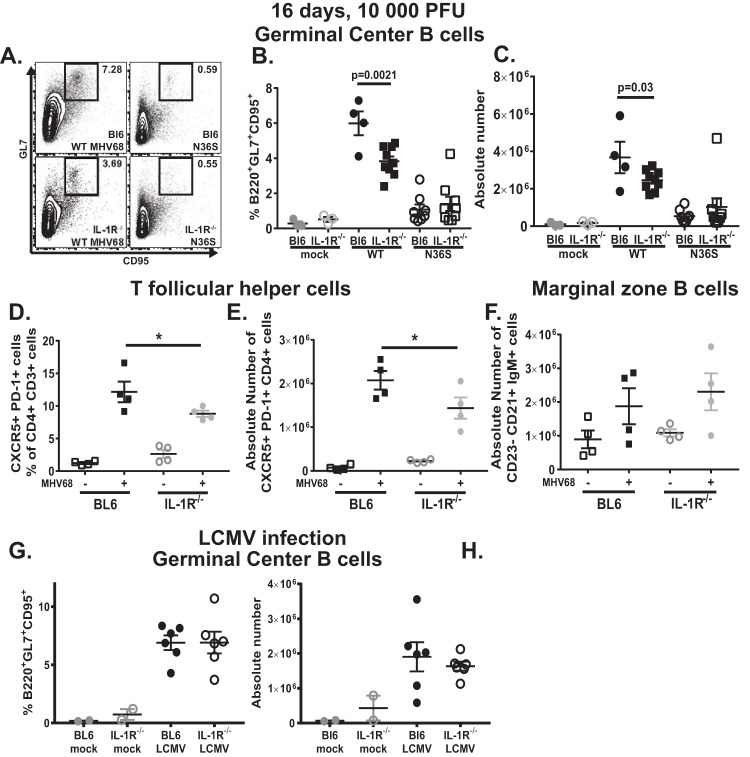
Viral protein kinase expression and enzymatic activity facilitate gammaherpesvirus-induced splenomegaly and germinal center response. For the experiments shown in panels A to G, mice were intranasally infected as described in the legend of Fig. 1 with the indicated doses of the virus, and splenocytes were analyzed at 16 days postinfection. each symbol represents an individual mouse (except in panels B and E). For the experiment shown in panel H, BL6 mice were intraperitoneally infected with 104 PFU of wild-type MHV68 or the 36KN mutant. (A) Total number of nucleated cells/spleen. (B, C, D, and H) Germinal center B cells were defined as B220+ GL7+ CD95+ cells and were gated as shown in panel B. Relative frequencies and absolute numbers are shown. (E to G) T follicular helper cells were defined as CD3+ CD4+ CXCR5+ PD1+ cells and gated as shown in panel E. Relative frequencies and absolute numbers are shown. *, P < 0.05. Data were pooled from 2 to 4 independent experiments. (I) Spleens were collected at 16 days following mock infection or infection with 104 PFU of wild-type MHV68 or the N36S mutant. Paraffin-embedded 5-μm sections of mouse organs were stained with hematoxylin and eosin or processed for immunohistochemistry using antibody against a B cell marker (B220). Histology was evaluated by a board-certified hematopathologist (H. Olteanu). Arrows indicate germinal centers. WT, wild type.
Because the germinal center response contributes to splenomegaly and expansion of the viral latent reservoir, germinal center B cells were analyzed next (Fig. 2B). As expected, infection with wild-type MHV68 increased the frequency and absolute number of germinal center B cells (Fig. 2C and D); this increase was greater in mice infected with a high dose than in those infected with a low dose of wild-type MHV68. In contrast, the frequency and absolute number of germinal center B cells were significantly attenuated in mice infected with either the N36S or 36KN viral mutant compared to the wild-type virus infection (Fig. 2C and D), with no effect of increasing the inoculum dose to 104 PFU. Similar to results observed for germinal center B cells, the frequency and absolute numbers of CD4 T follicular helper cells were decreased in mice infected with the N36S mutant compared to the wild-type virus infection (Fig. 2E to G) (104 PFU inoculum).
Considering decreased frequency of MHV68 DNA-positive splenocytes following high-dose intranasal infection with either orf36 mutant virus (Fig. 1A), it was possible that the decreased germinal center response was simply a reflection of attenuated virus infection. To address this possibility, mice were intraperitoneally inoculated with 104 PFU of wild-type MHV68 or the 36KN mutant, experimental conditions that we previously showed to result in equivalent frequencies of MHV68 DNA-positive splenocytes in wild-type MHV68- and 36KN-infected mice (25). In spite of similar frequencies of latently infected splenocytes, the frequency of germinal center B cells was decreased in 36KN-infected spleens in contrast to wild-type MHV68-infected spleens (Fig. 3H). Thus, expression and enzymatic activity of orf36 were required for efficient induction of germinal center responses following MHV68 infection, independent of the route of inoculation or efficiency of the establishment of splenic latency.
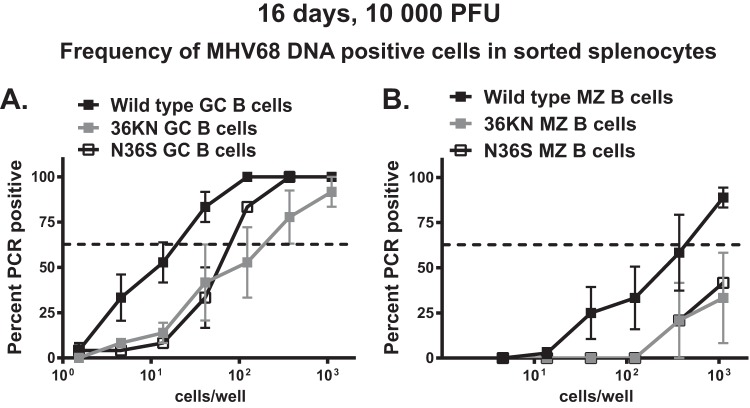
FIG 3.
Viral protein kinase facilitates MHV68 infection of germinal center B cells. BL6 mice were intranasally infected with 104 PFU of indicated viruses, and splenocytes were harvested at 16 days postinfection and sorted into germinal center B cells (A) and marginal zone (MZ) B cells (B). These populations were defined using markers described in Materials and Methods. The frequency of MHV68 DNA-positive cells was determined in each sorted population using limiting dilution nested PCR. Data were pooled from 2 to 4 independent experiments.
Gammaherpesvirus infection is associated with profound changes in splenic architecture due to the polyclonal immune activation and expansion of germinal center responses. To assess the extent to which viral protein kinase regulated such changes, spleens from infected animals were examined histologically by a board-certified hematopathologist (H. Olteanu) (Fig. 2I). A representative spleen from the mock-infected group was grossly normal in size and microscopically characterized by a preserved red pulp (RP) and white pulp (WP) architecture, with an RP/WP ratio of approximately 2:1. The WP showed antibody B220-positive (B220+) B-cell rich lymphoid follicles with rare germinal centers (1.7/10× objective field, as indicated by arrows on the hematoxylin and eosin [H&E] stain) and a normal distribution of periarteriolar lymphoid sheaths (PALS) composed primarily of CD3+ T cells (data not shown). Infection with wild-type MHV68 resulted in an increase in spleen size (5 to 6 times larger than spleens of mock-infected mice) but preservation of the RP/WP ratio (2:1). Importantly, there was evidence of follicular hyperplasia, with an increased number of follicles with germinal centers (average, 4.8/10× objective field; range 3 to 6 per 10× field), and variable PALS hyperplasia and plasmacytosis. Finally, the mice infected with the N36S viral kinase mutant demonstrated an intermediate response, with splenomegaly (2 to 3 times larger than spleens of mock-infected mice but smaller than those of wild-type MHV68-infected animals), and a preserved RP/WP ratio of approximately 2:1. The follicular hyperplasia was evident in this group, as well, as shown by the increased number of follicles with germinal centers (average, 2.6/10× objective field; range, 1 to 4 per 10× objective field), albeit hyperplasia was attenuated compared to the level with the wild-type infection. In summary, expression of the gammaherpesvirus protein kinase supported changes in splenic architecture associated with induction of germinal center responses.
Viral protein kinase facilitates MHV68 infection of germinal center and marginal zone B cells.
MHV68 infects transitional B cells (30), and we have previously shown that both the N36S and the 36KN MHV68 mutants can infect immature B cells, albeit at a lower frequency (25). However, it was not clear whether expression or enzymatic activity of the viral kinase was important for infection of germinal center B cells that host the largest proportion of latent MHV68 during the early stage of chronic infection. Thus, the frequency of MHV68 DNA-positive cells was measured in sorted germinal center or marginal-zone B cells, with the latter population representing another relevant reservoir of latent virus during the establishment of viral latency (31, 32). In these studies, only mice infected with a high virus dose (104 PFU) were analyzed due to the significant attenuation of splenic infection following low-dose inoculation of orf36 mutants (Fig. 1C).
The frequencies of MHV68 DNA-positive germinal center B cells were decreased in mice infected with either orf36 mutant virus compared to levels with wild-type virus (Fig. 3A) (7- to 11-fold). Consistent with germinal center B cells supporting a majority of MHV68 latent reservoir at this time postinfection, the observed decrease in the infection of germinal center B cells was proportional to the overall decrease in the frequency of MHV68 DNA-positive total splenocytes in the absence of functional orf36 (Fig. 1A) (8- to 15-fold compared to the level for wild-type MHV68). Similar attenuated frequency of infection of orf36 mutant viruses was also observed in sorted marginal zone B cells (Fig. 3B). Thus, expression and enzymatic activity of orf36 facilitated infection of germinal center and marginal zone B cells.
Viral protein kinase expression and enzymatic activity support gammaherpesvirus-driven production of irrelevant but not virus-specific class-switched antibodies.
Having observed attenuated germinal center response in mice infected with orf36 MHV68 mutants, we next wanted to examine functional consequences of such attenuation. MHV68 infection is characterized by an increase in total immunoglobulin serum titers that peak around 16 days postinfection (7). In contrast, generation of MHV68-specific antibodies occurs with slower kinetics, with peak titers observed by 35 to 40 days postinfection; this protective virus-specific B cell response may be suppressed by MHV68 (10–12). The difference between rapidly peaking total immunoglobulin levels and a delayed increase of virus-specific antibodies reflects a robust, albeit transient, rise in the titers of class-switched antibodies directed against irrelevant, nonviral antigens, including multiple self-antigens (7).
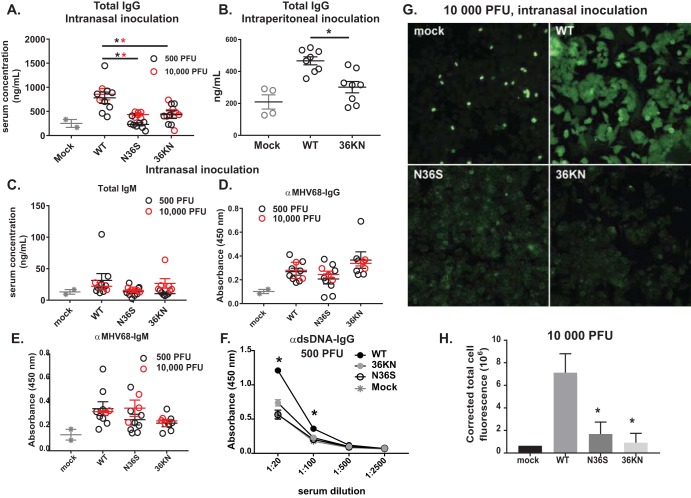
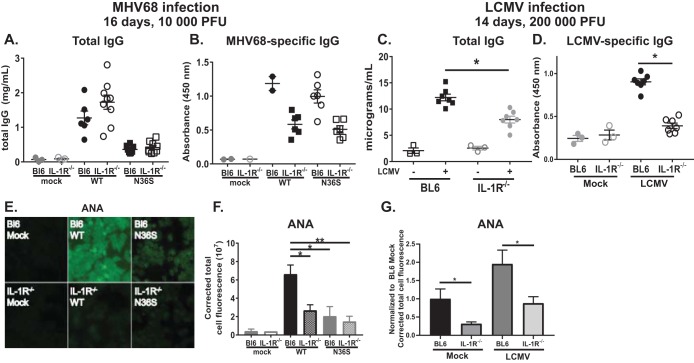
Consistent with the fact that antibody isotype switching occurs during B cell differentiation through the germinal centers, wild-type MHV68 infection increased levels of total IgG in the serum, independent of the inoculation dose (Fig. 4A). Consistent with the decreased germinal center responses (Fig. 2C and D), serum IgG titers were attenuated in mice infected with either orf36 MHV68 mutant (Fig. 4A). Similarly, a decreased germinal center response following intraperitoneal infection with the 36KN mutant (Fig. 2H) correlated with decreased total IgG serum titers in 36KN-infected mice (Fig. 4B). In contrast, total IgM levels were similar in all infected animals (Fig. 4C). Thus, orf36 expression and enzymatic activity facilitated an increase in total serum IgG but not IgM levels during infection.
FIG 4.
Viral protein kinase expression and enzymatic activity selectively facilitate gammaherpesvirus-driven production of irrelevant class-switched antibodies. BL6 mice were intranasally (A and C to H) or intraperitoneally (B) infected as described in the legend of Fig. 1 with the indicated doses of viruses, and serum was collected at 16 days postinfection. Sera were subjected to ELISA (A to F) as indicated or ANA analyses (G) using anti-mouse IgG fluorophore-conjugated antibody (H) Quantification of fluorescence levels of ANA slides. Data were pooled for 3 to 5 animals in each group. In panels A to E, each symbol represents an individual animal. *, P < 0.05.
Having observed a selective decrease in total serum IgG levels during orf36 mutant infection, MHV68-specific antibodies were assessed next. Surprisingly, levels of accumulated MHV68-specific IgG antibodies were similar in all infected mice (Fig. 4D). Levels of MHV68-specific IgM were also not affected by the expression or enzymatic activity of the viral kinase (Fig. 4E). Of note, the very low levels of infection in the spleens of mice infected with 500 PFU of orf36 mutant viruses (Fig. 1C) did not affect the generation of an MHV68-specific humoral response at 16 days postinfection (Fig. 4D and E). Thus, expression and enzymatic activity of viral protein kinase did not modify MHV68-specific B cell responses.
MHV68 infection leads to a transient rise in the titers of irrelevant antibodies directed against multiple self-antigens (8). Having observed similar MHV68-specific IgG titers along with decreased total serum IgG levels in mice infected with orf36 mutant viruses, we wanted to test the hypothesis that viral protein kinase selectively facilitates irrelevant B cell differentiation. Due to what is presumed to be random differentiation of bystander B cells, antibodies of many specificities can be produced, making the examination of this irrelevant response challenging. However, antibodies with specificity against double-stranded DNA (dsDNA) are induced in a significant proportion of MHV68-infected mice and have been successfully used to assess irrelevant B cell differentiation during infection (7, 8). As expected, the titers of anti-dsDNA antibodies increased following low-dose infection with wild-type MHV68 (Fig. 4F). However, titers of these antibodies were not induced in mice infected with either orf36 MHV68 mutant (Fig. 4F). Similar phenotypes were found following high-dose infection (data not shown). Thus, orf36 expression promoted the generation of autoreactive anti-dsDNA antibodies.
To measure irrelevant B cell differentiation in a more comprehensive manner, mouse serum collected at 16 days postinfection with a high dose of all three viruses was used to react with monolayers of Hep-2 cells, using a modified clinical assay employed for diagnosis of autoimmune diseases (antinuclear antibody [ANA] panel). Consistent with previous studies (8), sera harvested from wild-type virus-infected mice generated a significantly higher fluorescence signal than control sera from uninfected animals (Fig. 4G and H), revealing the generation of polyclonal class-switched antibodies against multiple self-antigens. In contrast, a significantly lower fluorescence signal was generated when Hep-2 cells were exposed to sera collected from mice infected with either orf36 mutant virus and probed for mouse IgG binding (Fig. 4G and H). Thus, expression and enzymatic activity of viral protein kinase facilitated the generation of irrelevant class-switched polyclonal antibodies directed against self-antigens, with very little if any effect on the protective virus-specific B cell responses.
IL-1 signaling supports MHV68 reactivation during the establishment of chronic infection.
Having observed decreased germinal center responses in mice infected with the orf36 mutant viruses, we next wanted to probe the mechanism underlying the orf36-dependent phenotypes. One possibility was that orf36 functions in cis to directly promote expansion and/or survival of infected germinal center B cells. However, given that the majority of gammaherpesvirus-driven germinal center B cells are virus negative, even following wild-type MHV68 infection (Fig. 3A), and that the cytokine milieu is known to shape humoral responses, including antibody isotype and diversity of antigen recognition, we reasoned that a soluble factor might contribute to the irrelevant B cell differentiation seen during gammaherpesvirus infection. To determine the extent to which orf36 expression and enzymatic activity modify the systemic cytokine milieu during infection, sera from mice infected with 104 PFU of either wild-type MHV68 or the N36S or 36KN virus mutant was screened for differential expression of cytokines and chemokines. Of the 31 cytokines and chemokines tested, IL-1α was the only analyte that was differentially expressed to higher levels in the serum of orf36 mutant-infected mice (Fig. 5A). In contrast, levels of IL-1β, a related cytokine that shares the receptor with IL-1α, were at or below the level of detection under all experimental conditions (data not shown). Other measured cytokines were either not induced by infection or were similarly increased in all infected groups, the latter exemplified by gamma interferon (IFN-γ) levels (Fig. 5B).
FIG 5.
IL-1 signaling supports MHV68 reactivation during the establishment of chronic infection. (A and B) BL6 mice were infected with 104 PFU of the indicated viruses, and serum was collected at 16 days postinfection. Levels of the indicated cytokines were measured as a part of the 31-plex assay by Eve Technologies. The dotted line represents the level of each cytokine observed in the sera of mock-infected mice. Each symbol represents an individual animal. (C and D) BL6 or IL-1R1−/− mice were intranasally infected with 104 PFU of wild-type MHV68 or the N36S viral mutant, and frequency of viral reactivation or viral DNA-positive splenocytes was determined at 16 days postinfection. Data were pooled from 2 to 4 independent experiments. CPE, cytopathic effect.
A ubiquitously expressed IL-1 receptor 1 (IL-1R1) binds both IL-1α and IL-1β and is required for signaling mediated by these two cytokines (33). Consistent with very low to undetectable levels of IL-1β observed in sera of infected mice in our screen, MHV68 opposes production of IL-1β in vitro, and chronic MHV68 infection is not affected by the genetic deficiency of caspase-1 required to generate active IL-1β, but not IL-1α (34). Thus, to test the relevance of elevated IL-1α levels observed in orf36 mutant-infected mice, parameters of latent MHV68 infection were examined in mice genetically deficient in IL-1R1 (33). IL-1R1 deficiency resulted in a significant decrease in reactivation of wild-type MHV68 (Fig. 5C). Intriguingly, the frequency of wild-type MHV68 DNA-positive splenocytes was not affected by IL-1R1 expression (Fig. 5D). In contrast, low frequency of N36S mutant reactivation was not further affected in IL-1R1−/− mice (Fig. 5C), whereas the frequency of latently infected splenocytes was further reduced in the absence of IL-1R1 (Fig. 5D). Thus, IL-1 signaling supported MHV68 reactivation during the establishment of chronic infection.
IL-1R1 signaling selectively supports gammaherpesvirus-induced germinal center response.
Having assessed MHV68 latency in IL-1R1−/− mice, B cell responses were examined next. The magnitude of germinal center B cell expansion in BL6 mice eclipsed that of IL-1R1−/− mice in both the frequency and absolute number following infection with wild-type MHV68 (Fig. 6A to C). In contrast to that observed for wild-type MHV68, germinal center B cell expansion was equally attenuated following infection of BL6 and IL-1R1−/− mice with the N36S MHV68 mutant virus (Fig. 6B and C). Consistent with attenuated abundance of germinal center B cells, the frequency and absolute number of T follicular helper cells were decreased in IL-1R1−/− mice compared to the levels in BL6 mice infected with wild-type MHV68 (Fig. 6D and E). In contrast, the frequency and absolute numbers of marginal zone B cells were similar in BL6 and IL-1R1−/− mice following wild-type MHV68 infection (Fig. 6F; also data not shown), consistent with the unique, germinal center-independent differentiation of marginal zone B cells.
FIG 6.
IL-1R1 signaling selectively supports germinal center response induced by wild-type gammaherpesvirus infection. (A to F) BL6 or IL-1R1−/− mice were intranasally infected with 104 PFU of wild-type MHV68 or the N36S viral mutant, and analyses of germinal center B cells (A to C), T follicular helper cells (D and E), and marginal zone B cells (F) were performed at 16 days postinfection using the same flow cytometry approaches as described in the legend of Fig. 2. (G and H) BL6 or IL-1R1−/− mice were intraperitoneally infected with 2 × 105 PFU of LCMV Armstrong. Germinal center B cells were analyzed in the spleens at 14 days postinfection. Each symbol represents an individual mouse. *, P < 0.05.
Interestingly, germinal center B cell responses were similar in BL6 and IL-1R1−/− mice infected with lymphocytic choriomeningitis virus (LCMV), an unrelated RNA virus (Fig. 6G and H), indicating that the role of IL-1 signaling in driving germinal center responses is selectively important during gammaherpesvirus infection. In summary, IL-1R1 signaling selectively promoted germinal center responses induced by wild-type MHV68 but not by an unrelated RNA virus. IL-1R1 signaling also had no effect on the low levels of germinal center B cells induced by infection with the N36S mutant.
IL-1R1 expression supports self-antigen-directed and MHV68- and LCMV-specific class-switched B cell responses during early gammaherpesvirus latency.
Having observed decreased germinal center responses in IL-1R1−/− mice infected with wild-type MHV68, we next determined the outcome of such cellular differences by measuring immunoglobulin levels and specificity. Interestingly, total serum IgG levels were not affected by IL-1R1 expression in wild-type MHV68-infected mice (Fig. 7A), in spite of decreased germinal center responses that normally support antibody isotype switching. As expected, based on the similarly low magnitudes of germinal center responses, serum IgG levels were the same in BL6 and IL-1R1−/− mice infected with the N36S mutant (Fig. 7A).
FIG 7.
IL-1R1 expression supports self-antigen-directed and MHV68- and LCMV-specific class-switched B cell responses during early gammaherpesvirus latency. (A, B, E, and F) BL6 or IL-1R1−/− mice were intranasally infected with 104 PFU of wild-type MHV68 or the N36S viral mutant, and analyses of immune responses were performed at 16 days postinfection. (A and B) Levels of total or MHV68-specific IgG were measured by ELISA. (C, D, and G) BL6 or IL-1R1−/− mice were infected with LCMV as described in the legend of Fig. 6. Sera were collected at 14 days postinfection. (C and D) Levels of total or LCMV-specific IgG were measured by ELISA. (G) Levels of self-directed antibodies were measured by ANA analyses. (E and F) ANA analyses measured levels of polyclonal self-reactive class-switched antibodies. Quantification of the fluorescence of ANA slides is shown in panel F. Each symbol represents an individual animal in panels A, B, C, and D. Data in F and G were pooled from 3 to 8 mice per group. *, P < 0.05; **, P < 0.01.
Subsequently, the humoral response was analyzed with respect to MHV68 specificity. Interestingly, titers of MHV68-specific class-switched antibodies were decreased in IL-1R1−/− mice irrespective of the orf36 status of MHV68 infection (Fig. 7B). These decreased virus-specific antibody responses did not result in increased levels of latency or reactivation of MHV68 infection in IL-1R1−/− mice (Fig. 5C and D), consistent with the dispensable nature of MHV68-specific antibody in the control of chronic infection in an immunocompetent host (35).
Having observed decreased titers of MHV68-specific IgG antibodies in IL-1R1−/− mice, we next wanted to determine whether this phenotype is selective for gammaherpesvirus infection as generation of influenza virus-specific class-switched antibodies occurs independently of IL-1 signaling (36). Intriguingly, IgG responses against LCMV were also decreased in IL-1R1−/− mice (Fig. 7D). However, in contrast to what we observed in MHV68-infected mice, IL-1R1−/− mice infected with LCMV had decreased total IgG levels compared to levels in infected BL6 mice (Fig. 7C).
Finally, generation of polyclonal antibodies against self-antigens was assessed in BL6 and IL-1R1−/− mice following wild-type MHV68 or N36S mutant infection. Levels of self-directed class-switched antibodies were decreased in IL-1R1−/− mice compared to those in BL6 mice following infection with wild-type MHV68 (Fig. 7E and F). In contrast, IL-1R1 deficiency had no effect on the lower levels of self-directed antibodies induced in N36S-infected mice. The robust induction of self-directed antibodies seen following wild-type MHV68 infection of BL6 mice (10- to 15-fold) was not recapitulated by LCMV infection, where the class-switched self-directed antibodies were induced, at best, 2.5-fold (Fig. 7G). However, IL-1 signaling did contribute to the generation of low levels of class-switched self-directed antibodies both at baseline and following LCMV infection (Fig. 7G). In summary, in contrast to that observed for influenza virus infection, signaling through IL-1R1 facilitated generation of both virus-specific and self-directed class-switched antibodies in the context of MHV68 and LCMV infections.
DISCUSSION
The MHV68-encoded orf36 belongs to a family of conserved gammaherpesvirus protein kinases, biology of which remains poorly understood in the context of chronic infection. In this study, we found that both expression and enzymatic activity of MHV68 protein kinase facilitate the germinal center and the irrelevant B cell response during early phases of chronic gammaherpesvirus infection. We have taken advantage of viral genetics to demonstrate that generation of the protective antiviral humoral response and of irrelevant, virus-nonspecific B cell differentiation are two separate processes, with the viral protein kinase selectively important for the latter. We also found that IL-1 receptor signaling is proviral in the context of gammaherpesvirus infection and facilitates generation of germinal center responses and virus-specific responses along with irrelevant class-switched humoral responses.
Germinal center B cells are believed to be the target of malignant transformation for many types of EBV-induced lymphomas (19). Considering our discovery that the conserved gammaherpesvirus protein kinase facilitates germinal center responses during early infection, it is tempting to speculate that the gammaherpesvirus protein kinase may also contribute to viral lymphomagenesis. Indeed, an elegant study by Anders et al. convincingly demonstrated that expression of a related protein kinase encoded by human Kaposi’s sarcoma-associated herpesvirus (KSHV) is sufficient to induce B cell lymphomas in transgenic animals (37). Importantly, KSHV protein kinase transgenic mice displayed elevated numbers of germinal center B cells and developed viral protein kinase-positive B cell lymphomas that were of germinal center or post-germinal center origin. Thus, our and the published study suggest that the ability of gammaherpesvirus protein kinases to facilitate germinal center responses that eventually lead to viral lymphomagenesis is a feature that is conserved across species.
How does gammaherpesvirus protein kinase facilitate an irrelevant B cell response?
All gammaherpesviruses encode a protein kinase. A substantial body of research demonstrated that multiple cellular and viral proteins can be phosphorylated by these conserved kinases in vitro, with some overlap of substrates (24, 38–42). In contrast, the role these viral kinases play in vivo along with the identity of kinase substrates in distinct cell types remains enigmatic, in part due to the exquisite host restriction of human gammaherpesviruses. Here, we demonstrate that MHV68 protein kinase promotes irrelevant, but not virus-specific, humoral responses during the establishment of chronic infection.
A trivial explanation of the observed phenotypes is that decreased acute replication of orf36 mutant viruses in vivo (24, 39) leads to attenuated levels of latent infection and, subsequently, a subdued germinal center response of the host. While attenuated infection is a plausible mechanism underlying the observed orf36 phenotypes, we believe that it is not the only mechanism at play. First, increasing the virus inoculum 20-fold resulted in an increase in germinal center responses in wild-type MHV68-infected but not orf36 mutant-infected mice (Fig. 2B and C), in spite of profound differences in the abilities of orf36 mutants to colonize the spleen under the low- and high-inoculum conditions (Fig. 1A and C). Second, generation of MHV68-specific antibodies was not attenuated in the absence of orf36 (Fig. 4D and E), supporting the idea that physiological anti-MHV68 responses are regulated by mechanisms that are distinct from those driving the irrelevant B cell differentiation. Third, we previously showed that intraperitoneal inoculation of the 36KN mutant (104 PFU) results in a frequency of MHV68 DNA-positive splenocytes similar to that following wild-type MHV68 inoculation (25). In spite of similar latent loads in the spleen, both germinal center B cell responses and total IgG levels were decreased in mice infected with the 36KN mutant (Fig. 2H and 4B), supporting the idea that attenuated infection is not the only mechanism underlying effects of orf36 on the gammaherpesvirus-driven germinal center response. Finally, a linear relationship between gammaherpesvirus latency parameters and host responses is not likely to be a universal feature as attenuated wild-type MHV68 latency observed in mice with B cell-specific STAT3 deficiency had no effect on the magnitude of germinal center response (43). Similarly, attenuated splenic latency of the M2-deficient MHV68 mutant did not translate into the attenuation of the germinal center response following high-dose inoculation (44, 45).
Gammaherpesvirus protein kinase is traditionally thought of as a lytic protein that ought not to be expressed during latent infection. However, many gammaherpesvirus genes are expressed during both lytic and latent infection; thus, the kinase may also be selectively expressed in a subpopulation of latently infected cells, including germinal center B cells. In fact, transcription from the MHV68 orf6 locus that encodes a single-stranded DNA binding protein critical for lytic viral DNA replication was demonstrated in germinal center B cells of infected animals, despite the latent nature of viral infection in this cellular population (32). However, the specific presence of orf6 mRNA remains to be confirmed as the analyses of the published study did not use a strand-specific approach. Interestingly, EBV lytic genes are important for EBV-dependent transformation in humanized mouse models that develop latently infected lymphomas (46); however, expression of EBV protein kinase has not been assessed in infected and/or malignant B cells from humanized mice. It is possible that the traditional, tissue culture-based classification of latent versus lytic gammaherpesvirus genes may be too inflexible to reflect the roles of these genes during in vivo infection.
To consider potential molecular mechanisms underlying the phenotypes observed in this study, we turned our attention to orf36 targets that operate in infected primary cells and affect viral replication. We showed that ataxia telangiectasia mutated (ATM) kinase was required to amplify serine 139 phosphorylation of H2AX by MHV68 orf36 and that ATM expression facilitated replication of wild-type but not orf36-deficient MHV68 in primary macrophages (39). This proviral effect of ATM expression is entirely due to the ability of ATM to attenuate type I IFN signaling and not to its role in the DNA damage response (47). Importantly, germinal center responses were equivalently induced in control and ATM−/− mice (data not shown), indicating that activation of ATM by orf36 is not involved in the phenotypes observed in the current study.
Hwang et al. demonstrated that orf36 can target IRF-3 to attenuate type I IFN signaling and the partial rescue of orf36 mutant MHV68 in IFNAR−/− mice (24). This orf36-mediated attenuation of type I IFN is likely to feed into the orf36–IL-1 cross talk, as type I IFN opposes IL-1 signaling by increasing expression of the IL-1 receptor antagonist (IL-1Ra) (48, 49) and intracellular SOCS3 (suppressor of cytokine signaling 30) (50). IL-1Ra is a secreted decoy receptor that binds IL-1 and prevents its interaction with the cell surface-associated signaling receptor. Similarly, an IFN-dependent increase in SOCS3 results in the dissociation of the TRAF6/TAK1 complex that mediates IL-1 receptor signaling (50). Thus, it is possible that increased type I IFN expression and/or signaling in mice infected with the orf36 mutant viruses may be, along with other mechanisms, attenuating IL-1 signaling.
How does IL-1 signaling regulate the B cell response and promote gammaherpesvirus infection?
IL-1α serum levels were selectively increased in mice infected with either the N36S or 36KN mutant compared to levels with wild-type MHV68 infection (Fig. 5A). Further, IL-1R1 deficiency led to attenuation of the gammaherpesvirus-induced germinal center response (Fig. 6B and D) and viral reactivation (Fig. 5C), indicating that IL-1 signaling plays a proviral role during MHV68 infection. IL-1R1 binds IL-1α or IL-1β to initiate signaling. Inflammasome assembly and subsequent caspase 1 activation play a critical role in the generation of active IL-1β but not IL-1α. MHV68 infection inhibits generation of active IL-1β; further, lack of caspase 1 expression has no effect on MHV68 replication in primary macrophage cultures or chronic infection in vivo (34). Accordingly, in our study, IL-1β levels were at or below baseline levels in BL6 mice infected with wild-type MHV68 or either orf36 mutant virus (data not shown). Collectively, this suggests that IL-1β-mediated signaling is not likely to be responsible for phenotypes we have observed in IL-1R1−/− mice. IL-1α is distinct from IL-1β as it is constitutively expressed by hematopoietic and some nonhematopoietic cells and can act as an alarmin following cell lysis without further processing (51, 52). Interestingly, B cells and monocytes are unique in having the ability to display active IL-1α on the cell surface without cell lysis (53). The relative contribution of IL-1α versus that of IL-1β to the observed phenotypes remains to be defined in the future studies.
The contribution of IL-1R1 signaling to antibody responses remains contentious. Naive IL-1R1−/− mice (on a mixed BL6/129 background) displayed normal immunoglobulin levels at baseline and responded to 2,4-dinitrophenol (DNP)-Ficoll or trinitrophenyl-keyhole limpet hemocyanin (TNP-KLH) immunization with antibody titers that were similar to those observed in control mice (33). Similarly, influenza virus infection of IL-1R1−/− mice (on a BL6 background) resulted in titers of influenza virus-specific IgG antibodies comparable to those of BL6 mice (36). In contrast, combined deficiency of IL-1α and IL-1β (on a BALB/c background) reduced antibody production in response to sheep red blood cell immunization due to an ∼2-fold decrease in the expression of CD40L on T cells (54). Further, IL-1β and an intact inflammasome supported the generation of optimal antibody responses against live, but not heat-killed, bacteria (55). In our study, IL-1R1 deficiency (on the BL6 background) attenuated generation of MHV68-specific antibodies regardless of viral kinase expression (Fig. 7B) and, intriguingly, also resulted in decreased levels of LCMV-specific IgG responses (Fig. 7D). These findings suggest that either IL-1 signaling is also proviral during LCMV infection (an unlikely hypothesis considering uncontrolled LCMV infection in mice lacking MyD88 [56], an adaptor protein downstream of several receptors, including IL-1R1) or that humoral responses against MHV68 and LCMV are generated via a similar mechanism that is distinct from that utilized to generate anti-influenza virus antibodies.
Intriguingly, we observed similar total IgG serum titers in BL6 and IL-1R1−/− mice following wild-type MHV68 infection in spite of decreased germinal center B cells and virus-specific and self-directed antibodies. It is possible that the antibodies produced in the absence of IL-1 receptor either had very low affinity for the antigens we tested or had specificity for self-antigens and/or viral antigens that were not present in our assays. An alternative, nonexclusive hypothesis is that IL-1 signaling may also restrict germinal center response-independent class switching in MHV68-infected mice that can be initiated via several mechanisms, including changes in the cytokine milieu.
In contrast, total IgG titers were decreased in LCMV-infected IL-1R1−/− mice (Fig. 7C), in spite of equivalent germinal center responses. This was likely a reflection of a significant decrease in LCMV-specific IgG antibody titers (Fig. 7D) as self-directed antibody responses were minimally induced in LCMV-infected animals and were, therefore, unlikely to be a significant component of the total IgG titers generated in the LCMV-infected mice. Thus, in spite of some similarities of B cell phenotypes observed following MHV68 and LCMV infection of IL-1R1−/− mice, it is also clear that gammaherpesvirus-induced germinal center and humoral responses are qualitatively and quantitatively distinct from the physiological response to other viral infections. Given the germinal center or post-germinal center stage of differentiation of many EBV B cell lymphomas (19), identification of the mechanisms underlying the unique features of the gammaherpesvirus-driven germinal center response is an important goal.
MATERIALS AND METHODS
Mice and infections.
All mice were housed and bred in a specific-pathogen-free barrier facility in accordance with federal and institutional guidelines. All experimental manipulations of mice were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (AUA971). C57BL/6J (designated BL6 throughout the manuscript) and IL-1R1−/−mice were originally obtained from Jackson Laboratories (Bar Harbor, ME). Virus stocks were prepared, and titers were determined on NIH 3T12 cells. N36S, 36KN, and the parental control virus retaining a single LoxP site (referred to as wild type) were previously described (24). Infections were performed by intranasal inoculation of 3 to 5 mice per group at 6 to 7 weeks of age under light anesthesia. Mice were inoculated with 500 or 104 PFU of corresponding virus or sterile carrier (mock) in an inoculum volume of 15 μl per mouse. Virus was diluted in sterile serum-free Dulbecco’s modified Eagle’s medium (Corning, Tewksbury, MA). For LCMV infections, BL6 or IL-1R1−/− mice were intraperitoneally infected with 2 × 105 PFU of LCMV Armstrong. The virus was prepared by a single passage on BHK21 cells, and viral titer was determined by plaque assay on Vero cells.
Limiting dilution assays.
Splenocytes harvested at 16 or 42 days postinfection were pooled within each experimental group and subjected to limiting dilution reactivation and nested PCR assays as previously described (25). To determine the frequency of cells reactivating virus ex vivo, serial 2-fold dilutions of splenocyte suspensions harvested from infected mice were plated onto monolayers of mouse embryonic fibroblasts (MEFs) at 24 replicates per dilution. In order to control for any preformed infectious virus, 2-fold serial dilutions of mechanically disrupted splenocytes or peritoneal cells were plated as described above. Cytopathic effect (CPE) was scored at 21 days postplating. Similarly, to determine the frequency of MHV68 DNA-positive (latently infected) splenocytes, serial dilutions of pooled splenocytes were subjected to two rounds of nested PCR with MHV68-specific primers. For some analyses, splenocytes were first sorted into populations of interest, and sorted cells were subjected to limiting dilution PCR assays to quantify the frequency of infected cells.
Flow cytometry.
Single-cell suspensions prepared from individual spleens were resuspended in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] plus 2% fetal calf serum [FCS] and 0.05% sodium azide) at 1 × 107 cells/ml. A total of 1 × 106 cells were prestained with Fc block (24G2) and then incubated with an optimal amount of antibody conjugate (eFluor450, fluorescein isothiocyanate [FITC], r-phycoerythrin [PE], PE-Cy7, or allophycocyanin [APC]). Antibodies to the following molecules were purchased from BioLegend (San Diego, CA): Pacific Blue B220/CD45R (RA3-6B2), FITC GL7, and PE CD95. To stain for T follicular helper cells, a triple amplification stain was employed for CXCR5 detection. Prior to staining, single-cell suspensions were incubated with Fc block as described above. The first antibody treatment was an optimized concentration of rat anti-mouse CXCR5 antibody which was diluted in FACS buffer supplemented with 2% normal mouse serum. Following washing, the cells were incubated with a biotin-conjugated goat anti-rat antibody to amplify the signal. In the final step, cells were incubated with an APC-streptavidin conjugate and additional antibodies in the staining panel (Pacific Blue CD3, PE-Cy7 CD4, and FITC PD1). Antibodies were purchased from the following companies: BioLegend (San, Diego, CA), Pacific Blue CD3 and APC-streptavidin; eBioscience (San Diego, CA), PE-Cy7 CD4 and FITC PD1; BD Bioscience (San Jose, CA), rat anti-mouse CXCR5; Jackson Immunoresearch (West Grove, PA), biotin SP Affinipure goat anti-rat IgG(H+L).
Cell sorting.
Single-cell suspensions of splenocytes were prepared and prestained with Fc block (24G2) as described for flow cytometry. Antibodies used to distinguish germinal center B cells were the following: Pacific Blue B220/CD45R (RA3-6B2), FITC GL7, and PE CD95 (BioLegend, San Diego, CA). Antibodies used to distinguish marginal zone B cells were PE B220/CD45R (RA3-6B2), PE-Cy7 CD23 (B3B4), and Pacific Blue CD21 (7E9) (BioLegend, San Diego, CA). Sorting was performed on a BD FACSAria III cell sorter (BD Biosciences, San Jose, CA).
ELISA.
Total and MHV68-specific immunoglobulin levels were determined as previously described (57, 58). For LCMV-specific enzyme-linked immunosorbent assay (ELISA), LCMV lysate was created by infecting BHK cells at a multiplicity of infection (MOI) of 0.5 of LCMV clone 13. Following the appearance of CPE at 4 days postinfection, cells were sonicated, and the lysates were stored at −80˚C. ELISA plates were coated with 100 μl of LCMV lysate diluted 1:100 in ELISA coating buffer (BioLegend, San Diego, CA) overnight at 4°C. Following coating with LCMV lysate, the ELISA protocol followed the same steps as those described for the MHV68 ELISA (57, 58).
ANA panels.
An antinuclear antibody (ANA) test kit was purchased from Antibodies Incorporated (Davis, CA), and serum was quantitatively analyzed according to the manufacturer’s protocol. Diluted serum (1:40 in PBS) was incubated over slides coated with fixed HEp-2 cells. Slides were rinsed with PBS, followed by staining with anti-mouse IgG(H+L) Alexa Fluor-488 (ThermoScientific). Fluorescent images were captured using NIS Elements software. Corrected fluorescence was quantified using ImageJ software from a randomly chosen field of ∼20 cells in each sample.
Histology and immunohistochemistry.
Paraffin-embedded, hematoxylin- and eosin-stained 5-μm sections of mouse organs were prepared by the Children’s Research Institute Histology Core Lab. Immunohistochemistry was performed as previously described (15). Follicular hyperplasia was defined as the presence of frequent secondary follicles with prominent germinal centers and otherwise normal histologic features. Germinal centers were counted within a 10× objective field, and an average of 4 to 5 fields was reported.
Serum cytokine/chemokine screen.
Serum was collected from mock-treated or infected mice at 16 days postinfection and subjected to a 31-plex Mouse Cytokine Array/Chemokine Array (MD31) by Eve Technologies (Calgary, AB, Canada). The analyzed biomarkers included the following: eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, keratinocyte-derived chemokine (KC), LIF, LIX, monocyte chemoattractant protein 1 (MCP-1), macrophage colony-stimulating factor (M-CSF), MIG, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, RANTES, tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF).
Statistical analyses.
All statistical analyses were performed using GraphPad Prism software (San Diego, CA). Student's t test or nonparametric analyses were used based on data distribution.
ACKNOWLEDGMENTS
We are indebted to Stephen Gauld for thoughtful discussions and insight into this study. We thank Kyle Stoltz for support of this study. We thank the members of the Corbett and Cui laboratory for lively discussions of this and other studies and Bonnie Dittel for insightful comments and reviews of this study. Histology slides were prepared by the Children’s Research Institute Histology Core under the supervision of Christine Duris.
This study was supported by 18PRE33960455 (P.T.L.) and ACS Research Scholar Grants RSG-12-174-01-MPC, CA183593, and CA203923 (V.L.T.).
E.J.D., C.N.J., and V.L.T. designed the overall study and subsequent revisions and wrote the manuscript. E.J.D., C.N.J., K.E.J., P.T.L., and G.X. contributed to the design of the studies and performed the experiments. W.C. contributed to the design of the studies. H.O. directed histological studies, evaluated spleen histology, and contributed to manuscript preparation.
REFERENCES
- 1.Cesarman E. 2014. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol 9:349–372. doi: 10.1146/annurev-pathol-012513-104656. [DOI] [PubMed] [Google Scholar]
- 2.Flano E, Kim IJ, Woodland DL, Blackman MA. 2002. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J Exp Med 196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willer DO, Speck SH. 2005. Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40. J Virol 79:2891–2899. doi: 10.1128/JVI.79.5.2891-2899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. 1999. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med 190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roughan JE, Thorley-Lawson DA. 2009. The intersection of Epstein-Barr virus with the germinal center. J Virol 83:3968–3976. doi: 10.1128/JVI.02609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA. 2001. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol 1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 7.Sangster MY, Topham DJ, D'Costa S, Cardin RD, Marion TN, Myers LK, Doherty PC. 2000. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol 164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 8.Gauld SB, De Santis JL, Kulinski JM, McGraw JA, Leonardo SM, Ruder EA, Maier W, Tarakanova VL. 2013. Modulation of B-cell tolerance by murine gammaherpesvirus 68 infection: requirement for Orf73 viral gene expression and follicular helper T cells. Immunology 139:197–204. doi: 10.1111/imm.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleisher GR, Collins M, Fager S. 1983. Limitations of available tests for diagnosis of infectious mononucleosis. J Clin Microbiol 17:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matar CG, Anthony NR, O'Flaherty BM, Jacobs NT, Priyamvada L, Engwerda CR, Speck SH, Lamb TJ. 2015. Gammaherpesvirus co-infection with malaria suppresses anti-parasitic humoral immunity. PLoS Pathog 11:e1004858. doi: 10.1371/journal.ppat.1004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getahun A, Smith MJ, Kogut I, van Dyk LF, Cambier JC, 2012. Retention of anergy and inhibition of antibody responses during acute gamma herpesvirus 68 infection. J Immunol 189:2965–2974. doi: 10.4049/jimmunol.1201407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Getahun A, Wemlinger SM, Rudra P, Santiago ML, van Dyk LF, Cambier JC, 2017. Impaired B cell function during viral infections due to PTEN-mediated inhibition of the PI3K pathway. J Exp Med 214:931–941. doi: 10.1084/jem.20160972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins CM, Speck SH, 2014. Expansion of murine gammaherpesvirus latently infected B cells requires T follicular help. PLoS Pathog 10:e1004106. doi: 10.1371/journal.ppat.1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins CM, Speck SH, 2015. Interleukin 21 signaling in B cells is required for efficient establishment of murine gammaherpesvirus latency. PLoS Pathog 11:e1004831. doi: 10.1371/journal.ppat.1004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mboko WP, Olteanu H, Ray A, Xin G, Darrah EJ, Kumar SN, Kulinski JM, Cui W, Dittel BN, Gauld SB, Tarakanova VL, 2015. Tumor suppressor IRF-1 counteracts germinal center reaction driven by a cancer-associated gammaherpesvirus. J Virol 90:2818–2829. doi: 10.1128/JVI.02774-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan RT, Dalla-Favera R, 2004. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 17.Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, Honjo T, 1999. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem 274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, Bardwell PD, Woo CJ, Fan M, Shulman MJ, Scharff MD, 2002. Activation-induced cytidine deaminase turns on somatic hypermutation in hybridomas. Nature 415:802–806. doi: 10.1038/nature714. [DOI] [PubMed] [Google Scholar]
- 19.Thorley-Lawson DA, Gross A, 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 20.Alain S, Revest M, Veyer D, Essig M, Rerolles JP, Rawlinson W, Mengelle C, Huynh A, Kamar N, Garrigue I, Kaminski H, Segard C, Presne C, Mazeron MC, Avettant-Fenoel V, Lecuit M, Lortholary O, Coaquette A, Hantz S, Leruez-Ville M, Ploy MC, 2013. Maribavir use in practice for cytomegalovirus infection in French transplantation centers. Transplant Proc 45:1603–1607. doi: 10.1016/j.transproceed.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 21.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA, 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol 71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- 22.Virgin HW, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH, 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol 71:5894–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarakanova VL, Suarez FS, Tibbetts SA, Jacoby M, Weck KE, Hess JH, Speck SH, Virgin HW, 2005. Murine gammaherpesvirus 68 infection induces lymphoproliferative disease and lymphoma in BALB β2 microglobulin deficient mice. J Virol 79:14668–14679. doi: 10.1128/JVI.79.23.14668-14679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang S, Kim KS, Flano E, Wu TT, Tong LM, Park AN, Song MJ, Sanchez DJ, O'Connell RM, Cheng G, Sun R, 2009. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe 5:166–178. doi: 10.1016/j.chom.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, Gauld SB, 2010. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology 405:50–61. doi: 10.1016/j.virol.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Jacoby MA, Virgin HW, Speck SH, 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J Virol 76:1790–1801. doi: 10.1128/JVI.76.4.1790-1801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herskowitz JH, Herskowitz J, Jacoby MA, Speck SH, 2005. The murine gammaherpesvirus 68 M2 gene is required for efficient reactivation from latently infected B cells. J Virol 79:2261–2273. doi: 10.1128/JVI.79.4.2261-2273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usherwood EJ, Ross AJ, Allen DJ, Nash AA, 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol 77:627–630. doi: 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- 29.Chetham MM, Roberts KB, 1991. Infectious mononucleosis in adolescents. Pediatr Ann 20:206–213. doi: 10.3928/0090-4481-19910401-10. [DOI] [PubMed] [Google Scholar]
- 30.Coleman CB, Nealy MS, Tibbetts SA, 2010. Immature and transitional B cells are latency reservoirs for a gammaherpesvirus. J Virol 84:13045–13052. doi: 10.1128/JVI.01455-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins CM, Boss JM, Speck SH, 2009. Identification of infected B-cell populations by using a recombinant murine gammaherpesvirus 68 expressing a fluorescent protein. J Virol 83:6484–6493. doi: 10.1128/JVI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marques S, Efstathiou S, Smith KG, Haury M, Simas JP, 2003. Selective gene expression of latent murine gammaherpesvirus 68 in B lymphocytes. J Virol 77:7308–7318. doi: 10.1128/JVI.77.13.7308-7318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ, 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol 159:3364–3371. [PubMed] [Google Scholar]
- 34.Cieniewicz B, Dong Q, Li G, Forrest JC, Mounce BC, Tarakanova VL, van der Velden A, Krug LT, 2015. Murine gammaherpesvirus 68 pathogenesis is independent of caspase-1 and caspase-11 in mice and impairs interleukin-1β production upon extrinsic stimulation in culture. J Virol 89:6562–6574. doi: 10.1128/JVI.00658-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClellan KB, Gangappa S, Speck SH, Virgin HW, 2006. Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses. PLoS Pathog 2:e58. doi: 10.1371/journal.ppat.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz N, Kurrer M, Bachmann MF, Kopf M, 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders PM, Montgomery ND, Montgomery SA, Bhatt AP, Dittmer DP, Damania B, 2018. Human herpesvirus-encoded kinase induces B cell lymphomas in vivo. J Clin Invest 128:2519–2534. doi: 10.1172/JCI97053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li R, Zhu J, Xie Z, Liao G, Liu J, Chen MR, Hu S, Woodard C, Lin J, Taverna SD, Desai P, Ambinder RF, Hayward GS, Qian J, Zhu H, Hayward SD, 2011. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe 10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarakanova VL, Leung-Pineda V, Hwang S, Yang CW, Matatall K, Basson M, Sun R, Piwnica-Worms H, Sleckman BP, Virgin HW, 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275–286. doi: 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen MR, Chang SJ, Huang HW, Chen JY, 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J Virol 74:3093–3104. doi: 10.1128/JVI.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asai R, Kato A, Kato K, Kanamori-Koyama M, Sugimoto K, Sairenji T, Nishiyama Y, Kawaguchi Y, 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J Virol 80:5125–5134. doi: 10.1128/JVI.02674-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang PC, Fitzgerald LD, Van GA, Izumiya Y, Ellison TJ, Wang DH, Ann DK, Luciw PA, Kung HJ, 2009. Kruppel-associated box domain-associated protein-1 as a latency regulator for Kaposi's sarcoma-associated herpesvirus and its modulation by the viral protein kinase. Cancer Res 69:5681–5689. doi: 10.1158/0008-5472.CAN-08-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy SS, Foreman HC, Sioux TO, Park GH, Poli V, Reich NC, Krug LT, 2016. Ablation of STAT3 in the B cell compartment restricts gammaherpesvirus latency in vivo. mBio 7:e00723-16. doi: 10.1128/mBio.00723-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rangaswamy US, O'Flaherty BM, Speck SH, 2014. Tyrosine 129 of the murine gammaherpesvirus M2 protein is critical for M2 function in vivo. PLoS One 9:e105197. doi: 10.1371/journal.pone.0105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrell S, Speck SH, 2017. Murine gammaherpesvirus M2 antigen modulates splenic B cell activation and terminal differentiation in vivo. PLoS Pathog 13:e1006543. doi: 10.1371/journal.ppat.1006543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma SD, Hegde S, Young KH, Sullivan R, Rajesh D, Zhou Y, Jankowska-Gan E, Burlingham WJ, Sun X, Gulley ML, Tang W, Gumperz JE, Kenney SC, 2011. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol 85:165–177. doi: 10.1128/JVI.01512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darrah EJ, Stoltz KP, Ledwith M, Tarakanova VL, 2017. ATM supports gammaherpesvirus replication by attenuating type I interferon pathway. Virology 510:137–146. doi: 10.1016/j.virol.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Blatt LM, Taylor MW, 1995. Type 1 interferon as an antiinflammatory agent: inhibition of lipopolysaccharide-induced interleukin-1 beta and induction of interleukin-1 receptor antagonist. J Interferon Cytokine Res 15:317–321. doi: 10.1089/jir.1995.15.317. [DOI] [PubMed] [Google Scholar]
- 49.Tilg H, Mier JW, Vogel W, Aulitzky WE, Wiedermann CJ, Vannier E, Huber C, Dinarello CA, 1993. Induction of circulating IL-1 receptor antagonist by IFN treatment. J Immunol 150:4687–4692. [PubMed] [Google Scholar]
- 50.Frobose H, Ronn SG, Heding PE, Mendoza H, Cohen P, Mandrup-Poulsen T, Billestrup N, 2006. Suppressor of cytokine Signaling-3 inhibits interleukin-1 signaling by targeting the TRAF-6/TAK1 complex. Mol Endocrinol 20:1587–1596. doi: 10.1210/me.2005-0301. [DOI] [PubMed] [Google Scholar]
- 51.Rider P, Carmi Y, Voronov E, Apte RN, 2013. Interleukin-1α. Semin Immunol 25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Garlanda C, Dinarello CA, Mantovani A, 2013. The interleukin-1 family: back to the future. Immunity 39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zola H, Flego L, Wong YT, Macardle PJ, Kenney JS, 1993. Direct demonstration of membrane IL-1 alpha on the surface of circulating B lymphocytes and monocytes. J Immunol 150:1755–1762. [PubMed] [Google Scholar]
- 54.Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y, 2001. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J Immunol 167:90–97. doi: 10.4049/jimmunol.167.1.90. [DOI] [PubMed] [Google Scholar]
- 55.Barbet G, Sander LE, Geswell M, Leonardi I, Cerutti A, Iliev I, Blander JM, 2018. Sensing microbial viability through bacterial RNA augments T follicular helper cell and antibody responses. Immunity 48:584–598. e585. doi: 10.1016/j.immuni.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartholdy C, Christensen JE, Grujic M, Christensen JP, Thomsen AR, 2009. T-cell intrinsic expression of MyD88 is required for sustained expansion of the virus-specific CD8+ T-cell population in LCMV-infected mice. J Gen Virol 90:423–431. doi: 10.1099/vir.0.004960-0. [DOI] [PubMed] [Google Scholar]
- 57.Kulinski JM, Darrah EJ, Broniowska KA, Mboko WP, Mounce BC, Malherbe LP, Corbett JA, Gauld SB, Tarakanova VL, 2015. ATM facilitates mouse gammaherpesvirus reactivation from myeloid cells during chronic infection. Virology 483:264–274. doi: 10.1016/j.virol.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darrah EJ, Kulinski JM, Mboko WP, Xin G, Malherbe LP, Gauld SB, Cui W, Tarakanova VL. 2017. B cell-specific expression of ataxia-telangiectasia mutated protein kinase promotes chronic gammaherpesvirus infection. J Virol 91:e01103-17. doi: 10.1128/JVI.01103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]