Abstract
Background:
Hepatitis B is one of the major causes of mortality among viral diseases. To reduce morbidity rate and increase knowledge of people about potential risk factors, the aim of this study was to determine the prevalence of hepatitis B among the general population and the risk factors associated with hepatitis B virus (HBV) infection in Isfahan, Iran.
Materials and Methods:
In a case–control study, 314 HBV-infected patients and 557 healthy participants were recruited. Data on demographics, immunization history, medical history, family medical history, life history, therapeutic factors, and behavioral risk factors were collected through a standard checklist. Chi-square and logistic regression were used for univariate and multivariable analyses.
Results:
Our results showed that among sociodemographic variables, higher age, being male, lower economic status, and lower educational attainments increased the risk of affecting by HBV (odds ratio [OR] >1, P < 0.001); furthermore, Iranian and no immigrant people showed higher significant risk of being affected by HBV. Multivariable logistic regression showed among medical, blood, and behavioral risk factors, family history of hepatitis (OR: 10.56; 95% confidence interval [CI]: 4.56–24.86), dental treatment history (OR: 4.30; 95% CI: 1.41–13.10), and hospitalization (OR: 2.94; 95% CI: 1.72–5.00).
Conclusion:
Our results demonstrated that there are still several risk factors for hepatitis B surface antigen infection among the Iranian adult population. Immunization programs should continue and focus on high-risk adults, and interventions should be directed toward to reduce risk factors associated with hepatitis B.
Keywords: Hepatitis-B virus, risk factors, viral infection
INTRODUCTION
Hepatitis B virus (HBV) infection is still a major public health problem worldwide[1] and considered as the main underlying etiology of chronic liver disease in Iran, a developing country.[2]
In Iran, there are almost 2 million patients with chronic hepatitis B, of which about 350,000 are active carriers and 5–6 thousand people die annually due to complications of the disease including hepatic cirrhosis and hepatocellular carcinoma with various frequencies in different areas.[3,4]
Based on what has been mentioned above, importance of having enough knowledge about regional- and age-specific prevalence of hepatitis B infection in order to programming control efforts, disease prevention, and vaccination schedules and also assessing disease burden on community is more obvious. Global studies in this manner are limited in some areas, developing countries in specific. On the other hand, by generation transition and also vaccination provision, the information about epidemiologic factors of HBV need revision.[1,5]
Most HBV infections in the developing countries result from determinants including household contact, vertical transmission hemodialysis, transmission from a surgeon,[6] and the receipt of organs or blood products.[7] Given these different ways for HBV infection to occur among general population in different parts of the world, control of hepatitis B is one of the highest priorities in majority of developing countries including Iran. As no study has been conducted to determine the risk factors of HBV infection on a regional level in Iran, the current study aimed at investigating a wide variety of potential risk factors of hepatitis B in Isfahan, Iran. Knowledge of such findings can help local policymakers for enhancing local preventive strategies within national and regional immunization programs if needed.[8,9]
MATERIALS AND METHODS
Study design and participants
This is a case–control study conducted on 871 persons resident in Isfahan, Center of Iran from 2015 to 2017. Among them, 314 persons infected with HBV based on laboratory findings including positive hepatitis B surface antigen (HBSAg) and/or anti-hepatitis B core antibody (anti-HBc). HBV was diagnosed based on the following criteria: HBsAg and antibody to anti-hepatitis B core antibody of the immunoglobulin M class and acute-onset elevation (0.45 U/L) of serum alanine aminotransferase levels. To differentiate people infected with HB from acute exacerbation of other chronic infection, the absence of serum HBsAg and anti-HBc before recruiting in the study was ascertained by checking medical records. Patients with acute hepatitis A, hepatitis E, hepatitis-C virus infection and/or coinfection, and drug- or alcohol-induced hepatitis were excluded from the study. All cases had been referred to the Infectious Disease Research Center (affiliated to Isfahan University of Medical Sciences).
A total of 557 controls were randomly recruited from the source population from which the cases arose at the time of acute hepatitis B (AHB) diagnosis among who their negative HBV infection was proved by laboratory test of negative HBsAg.
A written informed consent which contains information give them assurance about the confidentiality of data was obtained from all participants.
Study instruments and assessment of variables
Patients infected with HBV and the controls were interviewed by trained research assistants using a standard checklist requesting information up to 3 months before the diagnosis of HBV infection. Information regarding sociodemographic characteristics, including age, gender, educational level, nationality, ethnicity, urban or rural area of residence, immigrant or being native, economic status through question about income, marital status, blood group, travel to abroad; (2) blood-related risk factors including history of blood transfusion, piercing, cupping, skin cutting in laboratory (needle stick), acupuncture, and dialysis; and (3) medical- and behavioral-related risk factors (iatrogenic) including history of hospitalization, surgery, cesarean section, stillbirth and abortion, history of organ transplantation, immune deficiency, history of drug injection, dental treatment history, circumcision for male, history of liver diseases, history of infectious diseases, sexually transmitted diseases and other disorders, history of high-risk behaviors including intravenous drug addiction, sharing needles, high-risk sexual behavior (adultery), tattooing and being injured in fighting or accidents history of hepatitis in family members and first-degree relatives and history of jaundice, and imprisonment experienced by participants or spouse.
Statistical analyses
The data were entered (double entry) and analyzed using the Statistical Package for the Social Sciences (SPSS16.0 for Windows, IBM, United States). Categorical and continues variables were reported as frequency (percentage) and mean ± standard deviation, respectively. The Chi-square and independent sample t-test were used to determine the differences in categorical and continuous variables, respectively. The odds ratio (OR) and 95% confidence interval (CI) based on multivariable logistic regression were computed for representing the association of potential risk factors of infected with HBV. To determine the factors contributing independently to AHB, forward stepwise multivariable logistic regression method was used on those independent variables that were significantly associated with HBV in univariate analyses at P < 0.05. Finally, those variables were considered a statistically significantly associated with HBV when P < 0.05.
RESULTS
Table 1 presents the comparisons of sociodemographic variables of the study participants between case and control groups. Two groups were similarly distributed in terms of marital status but significantly different in terms of other studied variables (P < 0.001). Majority of HBV patients were male (68.7%), had low income (75.3%), and had academic degree (55.8%). In Table 1, sociodemographic risk factors of HBV have been shown. As can be seen higher age, being male, lower economic status, and lower educational attainments increased the risk of affecting by HBV (OR > 1, P < 0.001); furthermore, Iranian and no immigrant people showed higher risk of being affected by HBV.
Table 1.
The comparison of sociodemographic characteristics between healthy and hepatic groups and their association with affecting by hepatitis-B virus
| Variable | Control group (n=557), n (%) | Hepatitis C positive (n=314), n (%) | P* | OR (95% CI for OR)** |
|---|---|---|---|---|
| Age | 34.58±11.04 | 37.21±1.66 | 0.002 | 1.023 (1.001-1.04) |
| Gender | ||||
| Female | 304 (57.3) | 95 (31.3) | <0.001 | 1 |
| Male | 227 (42.7) | 209 (68.7) | 3.79 (2.38-6.63) | |
| Economic status | ||||
| Low income (<1 million Tomans [about 270 $] per month) | 252 (51.3) | 225 (75.3) | <0.001 | 1 |
| Middle income (1-3 million Tomans [about 270-800 $] per month) | 209 (42.6) | 32 (4.3) | 0.2 (0.11-0.35) | |
| High income (>3 million Tomans [about 800 $] per month) | 29 (5.9) | 1 (0.2) | 0.62 (0.26-1.44) | |
| Marital status | ||||
| Married | 424 (77.4) | 249 (80.8) | 0.54 | 1 |
| Single | 119 (21.7) | 57 (18.5) | 0.55 (0.53-1.71) | |
| Widowed/divorced | 5 (0.9) | 2 (0.6) | 1.18 (0.25-5.63) | |
| Education | ||||
| Academic degree | 308 (55.8) | 44 (14.3) | <0.001 | 1 |
| Diploma (12 years formal education) | 149 (27) | 96 (31.2) | 3.97 (2.38-6.34) | |
| Less educated or illiterate | 50 (9.1) | 70 (22.7) | 8.23 (4.75-14.28) | |
| Nationality | ||||
| Iranian | 557 (100) | 279 (88.9) | <0.001 | 1 |
| Others | 0 | 35 (11.1) | 116.77 (11.24-1212.43) | |
| Ethnicity | ||||
| Fars | 485 (89.2) | 290 (96.3) | <0.001 | 1 |
| Others | 59 (10.8) | 11 (3.6) | 0.34 (0.16-0.72) | |
| Place of residence | ||||
| Urban | 527 (95.8) | 282 (89.8) | 0.001 | - |
| Rural | 23 (4.2) | 32 (10.2) | ||
| Migration | ||||
| No immigrant | 472 (91.5) | 266 (85.3) | 0.005 | 2.70 (1.13-6.47) |
| Immigrant | 44 (8.5) | 48 (4.7) | 1 |
*Resulted from independent t-test for continuous and Chi-square or Fisher’s exact test for categorical variables, **Resulted from multivariable logistic regression. CI=Confidence interval; OR=Odds ratio
Figure 1 presents the prevalence of HBV in males and females over the different age categories; significant difference in the prevalence between male and female in age categories has been found (P = 0.001).
Figure 1.
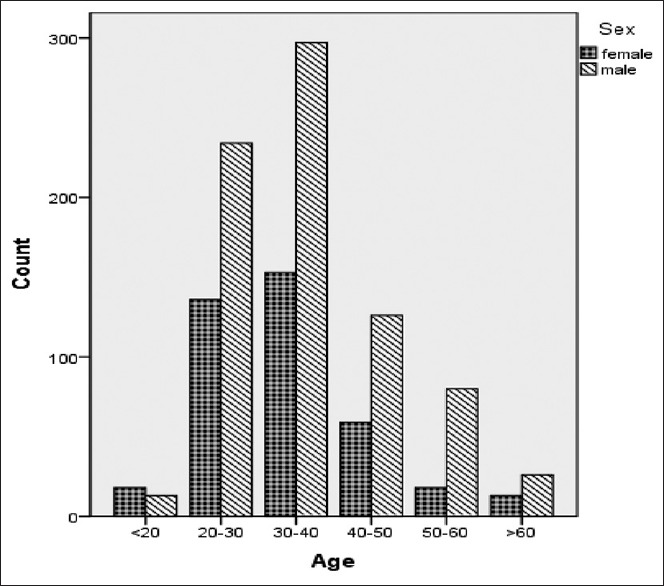
Age distribution of studied population
Table 2 presents the frequency distribution of medical, blood, and behavioral risk factors in healthy and HBV groups; we can see that the frequency of risk factors such as history of hospitalization, IV drug addiction, needle sharing, injection, prison records, and history of hepatitis in the family was significantly higher in HBV group, while jaundice and work experience in health centers were higher in control group (P < 0.05).
Table 2.
Comparison of distribution frequency of risk factors between two groups (healthy group and the group with hepatitis)
| Control group, n (%) | Hepatitis-B group, n (%) | P | OR (95% CI) | |
|---|---|---|---|---|
| Hospitalization | ||||
| No | 309 (59.1) | 156 (49.8) | 0.009 | 1 |
| Yes | 214 (40.9) | 157 (50.2) | 2.94 (1.72-5.00) | |
| Undergoing surgery | ||||
| No | 291 (54.9) | 192 (61.5) | 0.060 | - |
| Yes | 239 (45.1) | 120 (38.5) | - | |
| Wound in dispute | ||||
| No | 493 (95.7) | 77 (100) | 0.065 | |
| Yes | 22 (4.3) | 0 | - | |
| Wound in accident/accident injuries | ||||
| No | 475 (90.3) | 271 (88.3) | 0.355 | - |
| Yes | 51 (9.7) | 36 (11.7) | - | |
| Blood transfusion | ||||
| No | 474 (91) | 262 (84.8) | 0.065 | - |
| Yes | 47 (9) | 47 (15.2) | - | |
| Organ transplant | ||||
| No | 521 (98.7) | 296 (99.3) | 0.384 | - |
| Yes | 7 (1.3) | 2 (0.7) | - | |
| Blood type/blood group | ||||
| A | 147 (31.6) | 17 (28.8) | 0.444 | - |
| B | 89 (19.1) | 12 (20.3) | - | |
| AB | 51 (11) | 3 (5.1) | - | |
| O | 178 (38.3) | 27 (45.8) | - | |
| RH factor | ||||
| Positive | 324 (87.8) | 49 (86) | 0.695 | - |
| Negative | 45 (12.2) | 8 (14) | - | |
| Jaundice | ||||
| No | 443 (94.1) | 266 (97.8) | 0.019 | 1 |
| Yes | 28 (5.9) | 6 (2.2) | 0.34 (0.07-1.79) | |
| IV drug addiction | ||||
| No | 466 (87.1) | 160 (51.4) | <0.0001 | 1 |
| Yes | 69 (12.9) | 151 (48.6) | 0.84 (0.36-1.91) | |
| Needle sharing | ||||
| No | 526 (100) | 303 (98.4) | 0.003 | - |
| Yes | 0 | 5 (1.6) | Notcomputable | |
| Sexual relationship (with someone other than spouse) | ||||
| No | 482 (91.1) | 288 (92.6) | 0.451 | - |
| Yes | 47 (8.9) | 23 (7.4) | - | |
| Spouse’s jaundice | ||||
| No | 426 (98.6) | 255 (97.36) | 0.227 | - |
| Yes | 6 (1.4) | 7 (2.7) | - | |
| Having a weak immune system | ||||
| No | 487 (94.4) | 301 (99.7) | <0.0001 | - |
| Yes | 29 (5.6) | 1 (0.3) | 0.19 (0.02-1.67) | |
| Drug injection | ||||
| No | 424 (88) | 234 (89.7) | 0.490 | - |
| Yes | 58 (12) | 27 (10.3) | - | |
| Prison records | ||||
| No | 514 (97.7) | 288 (93.8) | 0.004 | 1 |
| Yes | 12 (2.3) | 19 (6.2) | 1.53 (0.43-5.71) | |
| Spouse’s prison records | ||||
| No | 485 (99.4) | 236 (98.7) | 0.370 | - |
| Yes | 3 (0.6) | 3 (1.3) | - | |
| Dialysis | ||||
| No | 509 (97.7) | 302 (99.3) | 0.078 | - |
| Yes | 12 (2.3) | 2 (0.7) | - | |
| Dental treatment | ||||
| No | 82 (15.1) | 25 (8) | 0.002 | 1 |
| Yes | 461 (84.9) | 289 (92) | 4.30 (1.41-13.10) | |
| Tattooing | ||||
| No | 494 (93.4) | 290 (93.2) | 0.939 | - |
| Yes | 35 (6.6) | 21 (6.8) | - | |
| Cupping | ||||
| No | 427 (79.5) | 255 (82.3) | 0.338 | - |
| Yes | 109 (20.5) | 55 (17.7) | - | |
| Skin cuts in laboratory | ||||
| No | 455 (86.7) | 270 (86.5) | 0.958 | - |
| Yes | 70 (13.3) | 42 (13.5) | - | |
| Work experience in health centers | ||||
| No | 474 (92.4) | 299 (99.7) | <0.0001 | 1 |
| Yes | 39 (7.6) | 1 (0.3) | Not computable | |
| STIs | ||||
| No | 504 (96.9) | 16 (100) | 0.476 | - |
| Yes | 16 (3.6) | 0 | - | |
| Acupuncture | ||||
| No | 509 (95.5) | 13 (100) | 0.434 | - |
| Yes | 24 (4.5) | 0 | - | |
| Hepatitis in family | ||||
| No | 508 (96) | 224 (72.5) | <0.0001 | 1 |
| Yes | 21 (4) | 85 (27.5) | 10.56 (4.56-24.86) |
STIs=Sexually transmitted infections; IV=Intravenous; RH=Rhesus; CI=Confidence interval; OR=Odds ratio
Those independent variables which were significant in univariate analyses were entered in multivariable logistic regression analysis. Results showed that among studied potential medical, blood, and behavioral risk factors, family history of hepatitis (OR: 10.56; 95% CI: 4.56–24.86), history of hospitalization (OR: 2.94; 95% CI (1.72–5.00), and dental treatment history (OR: 4.30; 95% CI: 1.41–13.10) were in significant association with HBV infection.
DISCUSSION
HBV infection is still a threat for human being health, particularly in the developing countries. This infection has a significantly high prevalence in society of Iran, a developing country where numerous carriers would experience morbidities and mortalities of HBV infection due to its liver associated dysfunctions. Thus, necessity of finding risk factors, early diagnosis, efficient vaccination, and early treatment of HBV is more clarified.[3]
In terms of assessing HBV-associated risk factors, we have conducted the current study among documented HBV-affected cases and compared their socioeconomical, clinical, and behavioral history with control group.
The findings of our study have shown that older age, male gender, and lower economical and educational states are statistically among socioeconomical risk factors of being affected by HBV. Another finding which was somewhat considerable was significant association of nativity with HBV presentation as we found native Iranian people were significantly more affected by HBV in comparison with emigrants. Clinical risk factors of HBV infection were the second part of our study that we assessed among our study population. Based on these findings, hospitalization, history of jaundice, history of intravenous drug use, history of needle sharing, immune deficiency, imprisonment, dental treatment, working in health-care system, and family history of HBV infection were independently risk factors of being affected by HBV. Furthermore, our evaluation did not show a significant relationship between history of transfusion, acupuncture, and HBV infection which can be due to paying attention to use sterile tools in blood products applications and related procedures.
Mehmet et al. in Turkey have conducted similar study in order to comparing HBV risk factors in rural versus urban areas. They presented higher rate of HBsAg-positive cases in rural areas in comparison to urban ones. Assessment of risk factors associated with HBV infection presented significant association of HBV with increased age and also lower level of education.[6]
The other meta-analysis conducted by Salehi-Vaziri et al. in community of Iran tried to assess HBV infection prevalence in general population of Iran. In their study that is latest revision of HBV infection in Iran, they found the prevalence of 2.2% in Iranian general population ranged from 2% in West to 4% in North. They have declared that male gender was significantly higher among cases with documented HBV infection.[2] Another study conducted in Karachi, Pakistan, tried to assess HBV infection risk factors among children. Statistical significant risk factors presented were the use of multiple injections due to hospitalization. This finding occurred while hepatitis-B vaccination is a part of health-care schedule provided by Pakistan Health Care Ministry. Other statistical findings were parental education that HBV-positive results were more among those with less educated parents.[10] The other study conducted in Pakistan on general population showed moderate-to-high prevalence of hepatitis B in community of Pakistan which was higher as compared to society of Iran, while both communities are among developing countries. They have presented needle use in health-care systems, intravenous drug abuse, blood transfusion, shaving by barbers, and transmission by spouse which were risk factors of HBV infection in Pakistan.[11] Multiple injections in health-care systems as a risk factor of HBV infection in the developing countries have been well documented previously as well.[12] Insisting on the use of sterile new needles in health-care systems in Iran may be the reason of this difference between risk factors of Iran versus Pakistan. Notable fact about our study is lack of association between spouses’ HBV infection and the rater spouse infection.[13] A comprehensive study in France which was done on 14,416 adults revealed that low socioeconomic status, intravenous drug use, <12 years of education, and male sex were significant risk factors for HBV infection.[14] Other meta-analysis assessed 21 studies worldwide presented that two-thirds of patients with HBV are male worldwide.[15] These findings are while a study conducted in Hong Kong presented no gender distribution significant dominance.[16]
Hemodialysis is among those factors that were not in significant association with HBV infection. In other words, our findings were not presenting history of hemodialysis as a risk factor for HBV infection while previous studies have declared this association. In a study conducted by Carrilho in Brazil, each month under hemodialysis was accompanied with approximate 1.5 times more occurrence of HBV infection.[17] The other study conducted in center of Brazil confirmed this association in addition to other risk factors including blood transfusion and male gender.[13]
Older age was the other risk factor found to be in association with HBV infection. In fact, we found that HBV infection was significantly higher among older patients. This result can be attributed to length of exposure to virus and also initiation of sexual activity. The increasing vaccine coverage of younger age groups may also have contributed to the difference in the prevalence between the various age groups. Previous results showed that in recent years, the age of HBV infection has increased dramatically in the United States.[18]
Another factor associated with HBV infection was intravenous drug abuse. This association was presented in previous studies as well. Shirin et al. in a study conducted in Bangladesh presented this association. In their study, they found that not only history of intravenous drug abuse is in association with HBV infection occurrence but also duration of it is another considerable factor.[19] The other study conducted by Fuller et al. tried to assess risks of early initiation of intravenous drug abuse among adolescents and presented high risk of HBV infection among these adolescents that would encounter them with considerable health associated risks in their future life.[20] Garfein et al. assessed similar relation and presented significant association between both IVDU merely and its duration with HBV infection.[21] Studies of Meffre et al. in France as a developed country in Western Europe in 2006 and 2010 declared similar results about the effect of IVDU on HBV infection. It means that IVDU prevention should be considered steeply in health-care systems policies.[22]
Imprisonment was the rater significant risk factor of HBV infection in our study. This association was presented in a study conducted in 2009 in Tehran.[23] Other study in Mashhad confirmed this finding as well.[24] These similar results in studies of Iran show that the importance of giving concise attentions to the facts occur in prisons in Iran. It seems IVDU or even homosexuality plays a significant role in HBV occurrence in prison in Iran. In addition, other studies in other part such as Wales[14] and Bangladesh[19] presented similar associations.
Another risk factor of HBV infection found in our study was dental procedures. It seems that by concise considerations to use sterile equipment in dentistries as a part of health-care system rules in Iran, this factor should decrease significantly in the future. In an old study conducted in Britain, dental procedures were significantly in association with HBV infection.[25] Other studies confirmed this association as well.[26,27,28]
Working in health-care system is the ratter risk factor of HBV infection found in our study. Hypothesizes made including needlestick occurrence, sprinkling of infected fluid to eyes and exposure to infected body liquids and blood in laboratories. A study conducted by Allegranzi et al. tried to assess the prevalence of health-care system associated infections among these centers’ employees. Among infections, HBV had high prevalence. An important fact in their study was underestimation of these diseases in most of the centers even in developed countries. On the other hand, the occurrence of health-care system associated infections was considerably high in the developing countries.[29] Sorrell et al. presented that HBV as the most common underlying reason of end-stage liver disease worldwide is deeply in association with working in health-care systems. Thus, in this term, appropriate considerations are required.[30] This fact has been mentioned by other studies as well.[31]
The last risk factor found in our study was family history of HBV infection. As routine HBV vaccination has been initiated since three decades ago in Iran, this association may be attributed to vertical transmission occurs during vaginal delivery. On the other hand, as low socioeconomic was a risk factor of HBV infection, addiction and IVDU through needle sharing may be presented in multiple members of a family. In a study conducted in Turkey as another developing country with similar cultural patterns with Iran, history of HBV infection was among risk factors of HBV infection.[6] There are studies in which association of HBV-related hepatocellular carcinoma with positive family history of HBV infection has been presented as well.[32,33]
We reviewed a list of potential risk factors of HBV infection. Previous study has mentioned that vaccination in various populations would be a potential preventive factor that could have an important role in HBV infection incidence reduction. According to our recent report about economic burden of HBV in our country, we believe that better public knowledge about common risk factors would be a beneficial way to reduce health-care costs due to HBV infection.[5]
CONCLUSION
Our results demonstrated that there are still several risk factors for HBsAg infection among the Iranian adult population. Immunization programs should continue and focus on high-risk adults, and interventions should be directed toward to reduce risk factors associated with hepatitis B. Knowledge of such findings can help local policymakers for enhancing local preventive strategies within national and regional immunization programs if needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to Baghiatallah Research Center for Gastroenterology and Liver Disease employees.
REFERENCES
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–9. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Salehi-Vaziri M, Sadeghi F, Almasi Hashiani A, Gholami Fesharaki M, Alavian SM. Hepatitis B virus infection in the general population of Iran: An updated systematic review and meta-analysis. Hepat Mon. 2016;16:e35577. doi: 10.5812/hepatmon.35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keshavarz K, Kebriaeezadeh A, Alavian SM, Akbari Sari A, Abedin Dorkoosh F, Keshvari M, et al. Economic burden of hepatitis B virus-related diseases: Evidence from Iran. Hepat Mon. 2015;15:e25854. doi: 10.5812/hepatmon.15(4)2015.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosravani A, Sarkari B, Negahban H, Sharifi A, Toori MA, Eilami O, et al. Hepatitis B infection among high risk population: A seroepidemiological survey in Southwest of Iran. BMC Infect Dis. 2012;12:378. doi: 10.1186/1471-2334-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 6.Mehmet D, Meliksah E, Serif Y, Gunay S, Tuncer O, Zeynep S, et al. Prevalence of hepatitis B infection in the Southeastern Region of Turkey: Comparison of risk factors for HBV infection in rural and urban areas. Jpn J Infect Dis. 2005;58:15–9. [PubMed] [Google Scholar]
- 7.Poortahmasebi V, Alavian SM, Keyvani H, Norouzi M, Mahmoodi M, Jazayeri SM, et al. Hepatic steatosis: Prevalence and host/viral risk factors in iranian patients with chronic hepatitis B infection. Asian Pac J Cancer Prev. 2014;15:3879–84. doi: 10.7314/apjcp.2014.15.9.3879. [DOI] [PubMed] [Google Scholar]
- 8.Harpaz R, Von Seidlein L, Averhoff FM, Tormey MP, Sinha SD, Kotsopoulou K, et al. Transmission of hepatitis B virus to multiple patients from a surgeon without evidence of inadequate infection control. N Engl J Med. 1996;334:549–54. doi: 10.1056/NEJM199602293340901. [DOI] [PubMed] [Google Scholar]
- 9.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 10.Jafri W, Jafri N, Yakoob J, Islam M, Tirmizi SF, Jafar T, et al. Hepatitis B and C: Prevalence and risk factors associated with seropositivity among children in Karachi, Pakistan. BMC Infect Dis. 2006;6:101. doi: 10.1186/1471-2334-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali SA, Donahue RM, Qureshi H, Vermund SH. Hepatitis B and hepatitis C in Pakistan: Prevalence and risk factors. Int J Infect Dis. 2009;13:9–19. doi: 10.1016/j.ijid.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira RC, Teles SA, Dias MA, Tavares VR, Silva SA, Gomes SA, et al. Hepatitis B virus infection profile in hemodialysis patients in central Brazil: Prevalence, risk factors, and genotypes. Mem Inst Oswaldo Cruz. 2006;101:689–92. doi: 10.1590/s0074-02762006000600019. [DOI] [PubMed] [Google Scholar]
- 14.Butler TG, Dolan KA, Ferson MJ, McGuinness LM, Brown PR, Robertson PW, et al. Hepatitis B and C in New South Wales prisons: Prevalence and risk factors. Med J Aust. 1997;166:127–30. doi: 10.5694/j.1326-5377.1997.tb140041.x. [DOI] [PubMed] [Google Scholar]
- 15.Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: Meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361–7. doi: 10.1111/j.1440-1746.2011.06801.x. [DOI] [PubMed] [Google Scholar]
- 16.Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–11. doi: 10.1038/sj.bjc.6601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrilho FJ, Moraes CR, Pinho JR, Mello IM, Bertolini DA, Lemos MF, et al. Hepatitis B virus infection in haemodialysis centres from Santa Catarina State, Southern Brazil. Predictive risk factors for infection and molecular epidemiology. BMC Public Health. 2004;4:13. doi: 10.1186/1471-2458-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein ST, Alter MJ, Williams IT, Moyer LA, Judson FN, Mottram K, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982-1998: Implications for vaccination programs. J Infect Dis. 2002;185:713–9. doi: 10.1086/339192. [DOI] [PubMed] [Google Scholar]
- 19.Shirin T, Ahmed T, Iqbal A, Islam M, Islam MN. Prevalence and risk factors of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infections among drug addicts in Bangladesh. J Health Popul Nutr. 2000;18:145–50. [PubMed] [Google Scholar]
- 20.Fuller CM, Vlahov D, Arria AM, Ompad DC, Garfein R, Strathdee SA, et al. Factors associated with adolescent initiation of injection drug use. Public Health Rep. 2001;116(Suppl 1):136–45. doi: 10.1093/phr/116.S1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: The prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–61. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meffre C, Le Strat Y, Delarocque-Astagneau E, Dubois F, Antona D, Lemasson JM, et al. Prevalence of hepatitis B and hepatitis C virus infections in france in 2004: Social factors are important predictors after adjusting for known risk factors. J Med Virol. 2010;82:546–55. doi: 10.1002/jmv.21734. [DOI] [PubMed] [Google Scholar]
- 23.Vahdani P, Hosseini-Moghaddam SM, Family A, Moheb-Dezfouli R. Prevalence of HBV, HCV, HIV and syphilis among homeless subjects older than fifteen years in Tehran. Arch Iran Med. 2009;12:483–7. [PubMed] [Google Scholar]
- 24.Rowhani-Rahbar A, Tabatabaei YA, Panahi M. Prevalence of common blood-borne infections among imprisoned injection drug users in Mashhad, North-East of Iran. Arch Iranian Med. 2004;7:190–4. [Google Scholar]
- 25.Polakoff S. Acute hepatitis B in patients in Britain related to previous operations and dental treatment. Br Med J (Clin Res Ed) 1986;293:33–6. doi: 10.1136/bmj.293.6538.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahboobi N, Porter SR, Karayiannis P, Alavian SM. Dental treatment as a risk factor for hepatitis B and C viral infection. A review of the recent literature. J Gastrointestin Liver Dis. 2013;22:79–86. [PubMed] [Google Scholar]
- 27.Mahboobi N, Agha-Hosseini F, Mahboobi N, Safari S, Lavanchy D, Alavian SM, et al. Hepatitis B virus infection in dentistry: A forgotten topic. J Viral Hepat. 2010;17:307–16. doi: 10.1111/j.1365-2893.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Guidelines for the Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection. World Health Organization. 2015 [PubMed] [Google Scholar]
- 29.Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377:228–41. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 30.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, et al. National institutes of health consensus development conference statement: Management of hepatitis B. Ann Intern Med. 2009;150:104–10. doi: 10.7326/0003-4819-150-2-200901200-00100. [DOI] [PubMed] [Google Scholar]
- 31.Dement JM, Epling C, Ostbye T, Pompeii LA, Hunt DL. Blood and body fluid exposure risks among health care workers: Results from the duke health and safety surveillance system. Am J Ind Med. 2004;46:637–48. doi: 10.1002/ajim.20106. [DOI] [PubMed] [Google Scholar]
- 32.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 33.Wei XL, Luo HY, Li CF, Jin Y, Zeng ZL, Ju HQ, et al. Hepatitis B virus infection is associated with younger median age at diagnosis and death in cancers. Int J Cancer. 2017;141:152–9. doi: 10.1002/ijc.30719. [DOI] [PubMed] [Google Scholar]


