Abstract
Bilateral deafness with concurrent vestibular dysfunction was first reported in the Doberman pinscher in 1980. Here, we identify a coding mutation in the MYO7A gene that is perfectly associated with the disorder. The lack of visual deficits in affected dogs suggests that, like rodents but unlike humans, MYO7A is not required for retinal function. DNA testing of the mutation will enable dog breeders to manage the incidence of this genetic defect.
Résumé
La surdité bilatérale avec dysfonctionnement vestibulaire concomitant a été rapporté pour la première fois chez le Doberman pinscher en 1980. Ici nous identifions une mutation codante dans le gène MYO7A qui est associée parfaitement avec cette condition. L’absence de défaut rétinien chez les chiens atteints suggère que, comme chez les rongeurs mais contrairement aux humains, MYO7A n’est pas requis pour la fonction rétinienne. Les tests d’ADN pour la mutation vont permettre aux éleveurs de chiens de gérer l’incidence de ce défaut génétique.
(Traduit par Docteur Serge Messier)
Introduction
In veterinary medicine, congenital bilateral deafness with concurrent vestibular loss was first reported in the 1980s in the Doberman pinscher (1,2); it was only characterized clinically more than a decade later (3). Today, it is colloquially referred to as “dings” in the Doberman pinscher breeding community. Based on anecdotal segregation in Doberman pinscher bloodlines, the disease has been suggested to have an autosomal recessive mode of inheritance (3). Affected dogs show early-onset vestibular dysfunction, which becomes apparent as the puppy first begins to locomote. Signs include exaggerated side-to-side head/neck excursions, body falling, and head tilt (3). Once the eyes open, it becomes apparent that the affected puppy lacks the vestibulo-ocular reflex and exhibits post-rotational nystagmus (3). Variable loss of the righting reflex is observed early, though this tends to improve with age, along with the wide head/neck excursions and limb/body ataxia (3). Brainstem auditory evoked responses (BAERs) reflect complete sensorineural hearing loss, consistent with the absence of a startle reflex (3). Importantly, no visual abnormalities are observed (3), making the canine phenotype similar to that observed in shaker-1 mice (4).
Although deafness with vestibular dysfunction (DVD) in Doberman pinschers has been suspected to have an autosomal recessive pattern of inheritance, a genetic mutation has not been identified. Here we map DVD to a locus on CFA21 in the canine genome. We leverage whole genome sequencing to identify a candidate mutation in the canine MYO7A gene. We also discuss these findings in the context of human and mouse hearing and balance impairments caused by mutations in this unconventional myosin. Lastly, we present a DNA test that can inform breeding decisions to reduce disease incidence in the Doberman pinscher population.
Materials and methods
Ethics statement
All canine samples were provided by private dog owners who consented to our use of de-identified data for research purposes in accordance with IACUC #11-02-002 (Van Andel Research Institute).
Subjects, sample recruitment, and DNA
Affected individuals and non-affected controls were purebred Doberman pinschers from Canada and the USA. Only animals with both bilateral deafness and concurrent vestibular dysfunction were used for this study. Clinical signs were confirmed by complete neurologic examinations, behavioral indicators of deafness (e.g., absent startle reflex), BAERs, and/or owner questionnaires. Behavioral evaluation involved actual and historical reporting of the animal’s response to noise stimuli when the observer was remote from the animal’s visual field. Neurologic examination included assessment of gait, mentation, cranial nerve function, proprioception, and spinal reflexes. Brainstem auditory evoked response testing was performed using a Cadwell Sierra Wedge II electrodiagnostic system using ear inserts to deliver click stimuli. The BAER was elicited using 1000 repetitions of a 0.1 ms alternating air conducted click stimulus at 80 dB nHL. Each ear was evaluated separately. White noise was delivered at 40 dB nHL to the ear not being evaluated. Disposable stainless-steel subdermal needle electrodes were used with the active electrode placed at the location of the occipital vertex, the reference electrode just rostral to the tragus of the ear being evaluated, and the ground electrode just rostral to the tragus of the ear not being evaluated. Animals were not sedated for the procedure. Animals evaluated by BAER were of a broad age range (6 wk to 9 y). Amplifier filter settings included a low-pass filter set at 100 Hz and high-pass filter set at 3 kHz. Cochlear pathology was confirmed histologically in one case. Inner ear tissue was collected from an affected puppy within 30 min of euthanasia and placed in 10% neutral buffered formalin. The tissue was decalcified, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E).
Blood or saliva samples were collected from cases and controls for DNA analysis using standard methods of nucleic acid extraction. Samples for population genetics analysis were collected with buccal swabs and extracted as previously described (5).
Whole genome single-nucleotide polymorphism (SNP) genotyping and data analysis
Genome-wide SNP genotyping was performed with the CanineHD BeadChip array (Illumina, La Jolla, California, USA). Raw fluorescence intensity data generated from a BeadArray Reader (Illumina) were converted to curated genotypes with GenomeStudio Software (Illumina) using the pre-set genotypic cluster algorithms. Single-nucleotide polymorphism genotype data were analyzed as previously described (5) using PLINK (6).
Briefly, the data were filtered to exclude: i) individual dogs with call rates lower than 98%, ii) SNPs with minor allele frequency < 0.05, iii) SNPs with call rates < 99.8%, and (iv) SNPs that did not pass the test for Hardy-Weinberg equilibrium in the control cohort (P < 0.05). P-values were adjusted for multiple tests and the genome-wide significance threshold (6.4 × 10−7) was set by permutation of case-control labels (100 000 iterations). Allele and genotype frequencies for the deletion were from direct counts of sampled Doberman pinscher dogs. Additionally, 8628 SNPs were withheld from analysis for failure to replicate on duplicate samples. The quality control analysis yielded 78 223 informative SNPs for association analysis. Relatedness amongst individuals was visually assessed with a multidimensional scaling plot produced in PLINK (6). The software tool EMMAX (6) was used for association analysis while controlling for population substructure that could arise from cryptic relatedness. Haploview (7) was used to infer the ancestral haplotype upon which the MYO7A mutation originated.
Whole genome sequencing and data analysis
Sequencing was performed on 2 lanes of a HiSeq instrument (Illumina) using a paired-end library prepared from blood-derived genomic DNA. Raw reads were mapped to the CanFam3.1 reference assembly using the short-read aligner Bowtie 2 (8). Sequence variants were called using SAMtools (9). DNA variants were annotated and assessed for presumptive function using snpEff (10), and a probability of functional impact was estimated using Poly-Phen2 (11).
Sequencing-based testing of the MYO7A mutation
Primers were designed to amplify the presumptive causal mutation in MYO7A (forward: AATGTTTCTGGGGCACTGAAG, reverse: AGGAGGGCTGGAAGTAGGAGA). A T3 sequence primer tag (AACCCTCACTAAAGGGA) was appended to the 5′ end of the reverse primer to facilitate parallel DNA sequencing of the polymerase chain reaction (PCR) product. The PCR produced a 314 bp product (Chr21: 21 562 996 to 21 563 309). Sanger-based DNA sequencing of PCR products was performed by Quintara Biosciences (Boston, Massachusetts, USA).
Results
Clinical phenotypes
Clinical observation of affected Doberman pinscher puppies confirmed both bilateral vestibular dysfunction and bilateral deafness, consistent with previous reports (1,3). As puppies first began to locomote and suckle, vestibular dysfunction was demonstrated by wide side-to-side head/neck excursions, body falling, a “bobble-head” appearance, and variable head tilting. Affected puppies also failed to exhibit a normal vestibulo-ocular reflex. Startle responses were absent and BAERs indicated complete sensorineural deafness (Figure 1). Cochlear defects were confirmed by histological examination of inner ear tissue, which showed a complete degeneration of the organ of Corti, without involvement of the stria vascularis, consistent with neuroepithelial degeneration (Figure 2). The wide head/neck excursions, head tilt, and limb/body ataxia indicative of vestibular dysfunction improved with age, but the bilateral deafness was complete and permanent. No visual defects were observed.
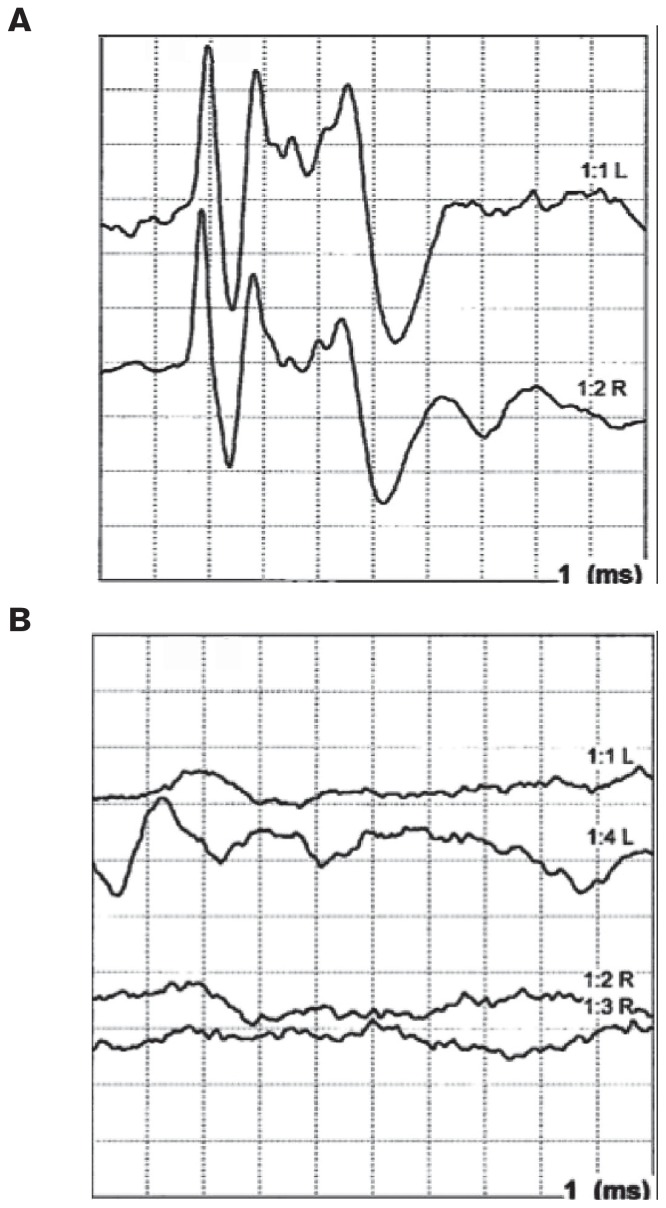
Figure 1.
Brainstem auditory evoked response (BAER) tracing from Doberman pinscher puppies without (A) and with (B) evidence of the deafness with vestibular dysfunction (DVD) phenotype. Note the absence of waveforms in (B), indicative of sensorineural hearing loss.
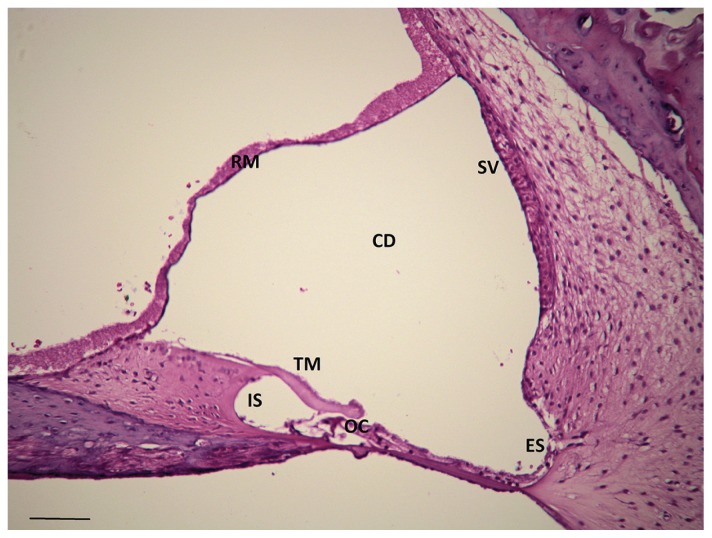
Figure 2.
Histologic appearance of a 10-week-old Doberman pinscher puppy with congenital sensorineural deafness and clinical evidence of bilateral peripheral vestibular disease. Note the degeneration of the organ of Corti. RM — Reisner’s membrane, TM — tectorial membrane, OC — organ of Corti, IS — internal sulcus, CD — cochlear duct, ES — external sulcus, SV — stria vascularis, H&E; scale bar = 100 μm.
Genome-wide association identifies a single genomic locus
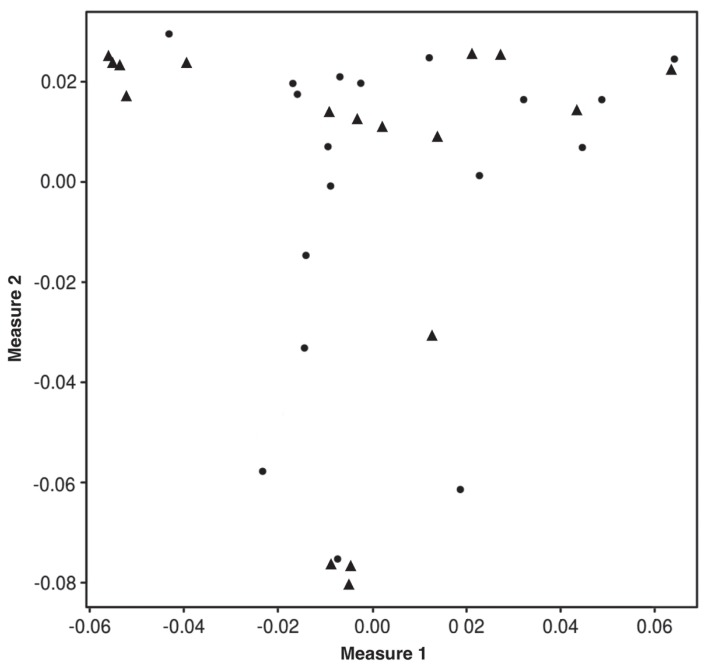
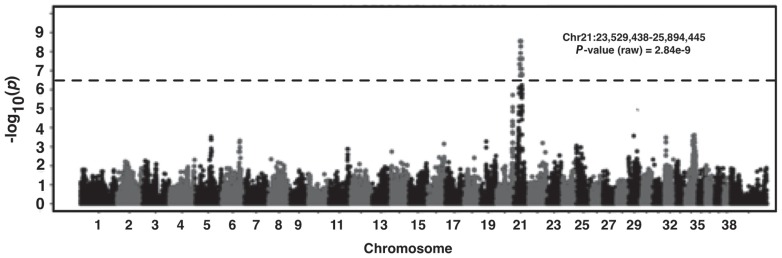
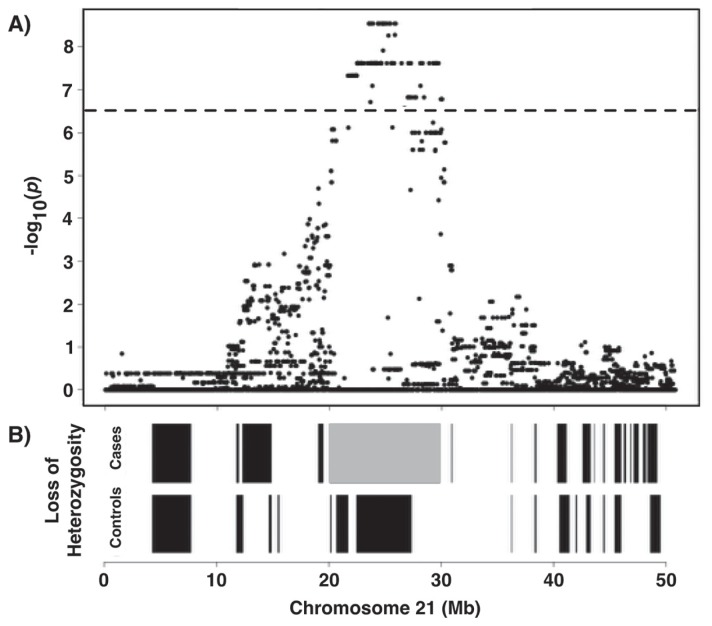
We endeavored to map the genetic basis of DVD. Genome-wide array-based SNP genotyping (174 376 SNP markers) was performed on 17 cases and 17 controls to generate data for association analysis. Dogs were selected for genotyping with respect to pedigrees and bloodlines to ensure there was no relatedness within the 3 generations. The average call rate across genotyped individuals was 97% ± 1.2%, indicating the arrays and the DNA samples were of high quality. A multidimensional scaling plot based on genotype sharing (i.e., a measure of relatedness) indicated that, although cases may have been more related to one another than controls, both cohorts were adequately sampled from a common interbreeding population, which minimized stratification and did not confound subsequent genetic mapping (Figure 3). Genotype data were subjected to quality control filtering and the cleaned data were analyzed for genome-wide association. A single statistically significant association was found on CFA21, with the strongest signal between 23.5 to 25.8 Mb (PRaw < 10−9; Figure 4).
Figure 3.
Multidimensional scaling plot depicting genotype-based relatedness among cases (▲) and controls (●).
Figure 4.
Manhattan plot showing results of genome-wide association study analysis with EMMAX of a 17:17 case-control genotype dataset. A 2.3 Mb locus on CFA21 achieved statistical significance after correcting for multiple marker testing (as reflected by the horizontal line, which depicts the threshold for genome-wide significance).
Genotypic and haplotypic analyses fine-mapped the interval and suggested recessive inheritance
All cases were homozygous for an extensive shared haplotype at the locus, consistent with an autosomal recessive mode of inheritance. Single-nucleotide polymorphism autozygosity in this haplotype stretched for 9 MB (Chr21: 20.5 to 29.5 Mb) among cases and centered on the peak of association (24.0 Mb; Figure 5). This implied that a single mutation was shared by descent from a common ancestor (i.e., founder effect) and that the causal mutation was of relatively recent origin.
Figure 5.
A — Associated locus on CFA21 (20 073 102 to 29 889 027). B — SNP autozygosity stretching over a 9 Mb interval (gray block) centered on the peak region of association.
Next-generation sequencing identifies a nonsynonymous SNP in MYO7A
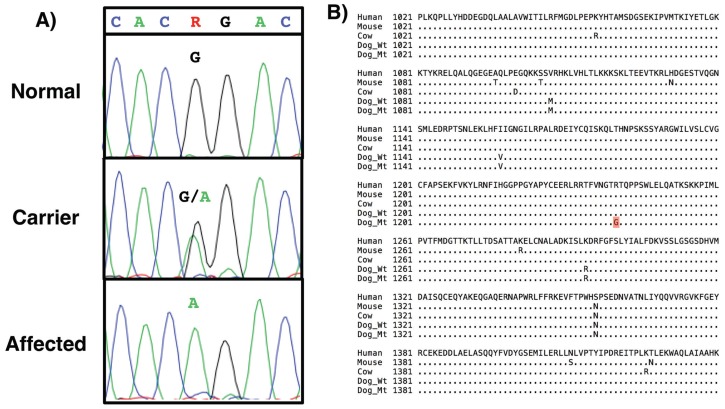
The extensive linkage disequilibrium of the associated haplotype and the difficulty in expanding numbers for a case cohort suggested that progress by fine-scale mapping would be experimentally difficult. We therefore chose to perform whole genome sequencing to produce a list of DNA variants, among which might be a compelling candidate for the causal mutation. The DNA of an affected dog homozygous for the entire risk haplotype was sequenced to an average depth across the genome of 15× coverage. The DNA variants corresponding to the mapped interval were mined from the data and assessed for putative functional significance. A point mutation in exon 28 of MYO7A was identified at genomic base coordinate 21 563 111. This coding base is highly conserved across species (phastcons = 0.99). Gain- and loss-of-function mutations in the MYO7A gene cause both syndromic and non-syndromic deafness in humans and rodent models (12–14), making the sequence variant a strong candidate for the causal mutation. The mutation was confirmed by conventional DNA sequencing (Figure 6A). We refer to the mutation as MYO7AG3719A, denoting the position of the mutation within the gene’s protein-coding sequence.
Figure 6.
A — Electropherogram results from Sanger-based DNA sequencing confirming the MYO7AG3719A mutation. The assay shows full discrimination of the 3 genotypic classes, which correspond to normal (G/G), carrier (G/A), and affected (A/A). B — Alignment of partial MYO7A protein sequences from dog (Wt — wild type, Mt — mutant) as well as human, mouse, and cow. MYO7A is highly conserved across the entire protein (97%) as well as in the region shown (residues 1021 to 1440 of 2177 amino acid total) harboring the mutated residue R1240Q (in red).
The MYO7AG3719A mutation predicted a non-conservative amino acid substitution. Specifically, the missense mutation substituted glutamine (neutral, polar) for arginine (positively charged, aliphatic) at residue 1240 in the MYO7A protein (R1240Q). Alignment of the MYO7A peptide sequences from multiple species showed the arginine residue that is affected by the mutation is highly conserved evolutionarily (Figure 6B) and thus probably important for protein structure and function. We evaluated the likelihood of the mutation to be causal using Poly-Phen2 (14), an algorithm that predicts the functional effect of amino acid substitutions resulting from coding mutations. The confidence was strong (1.00) that the R/Q change was “probably damaging” to protein structure/function.
Mutation assay confirms near perfect association and establishes a population genetics baseline
As the study progressed, 15 additional affected dogs were recruited. Some were relatives of cases included in the genomewide association study and did not represent fully independent data points. Nonetheless, all 15 affected dogs were homozygous for MYO7AG3719A, consistent with prior results. To estimate the frequency of the mutation in the gene pool, we genotyped a collection of buccal swab DNA samples, most of which had been collected from purebred Doberman pinschers prior to the DVD research. We predicted that no individuals would be homozygous for MYO7AG3719A. Of 632 dogs tested, none were homozygous. We found that 62 dogs were heterozygous for the mutation, suggesting an allele frequency of 4.9% (62/1224 chromosomes sampled) and a carrier frequency in the breed of nearly 10%. These estimates could be inflated owing to unknown ascertainment biases.
Discussion
We have identified a mutation in MYO7A that is strongly associated with DVD, an inherited bilateral deafness with vestibular dysfunction in the Doberman pinscher breed. The mutation in the protein-coding sequence of the gene suggests a highly damaging amino acid substitution that, based on in silico analysis, has a high probability of negatively impacting MYO7A protein function.
The MYO7A gene encodes an unconventional myosin with important functions in sensory neuroepithelia (15). Upon examining the deafness variation database at the University of Iowa, of the 309 MYO7A mutations with known significance, 2 cause retinitis pigmentosa with mild hearing loss, 6 result in dominant non-syndromic deafness, 40 result in recessive non-syndromic deafness, and 261 result in human Usher syndrome type 1B (16). In humans, the MYO7A actin-based motor is expressed in the sensory cells of the cochlea, vestibule, and retina, and complete loss of function mutations cause Usher syndrome IB, which includes deafness, variable balance problems, and retinal degeneration. Specific alleles of human MYO7A have been found to cause recessive non-syndromic deafness (DFNB2) (4,17) and dominant audiovestibular defect without retinal abnormalities (DFNA11) (4). It has been hypothesized that these select mutations either affect cochlear- and vestibular-specific protein domains, or possibly that these mutations are hypomorphic (i.e., partially functional) and the retina is less sensitive to reduced function than the inner ear.
Tissue-specific thresholds for MYO7A function might explain the audiovestibular defects of DVD with an absence of visual phenotype. The non-conservative substitution in a highly conserved domain of MYO7A implies that the mutation is highly damaging, however. Another possible explanation is that canines, like rodents, may not require MYO7A function in the retina. The shaker-1 mouse, first characterized in 1929 (18), was shown to be a MYO7A mutant (19). The mutation we have identified may be nullisomic, causing the complete loss of function of the gene. Rodents do not require MYO7A function during development of the photoreceptor epithelium, possibly owing to functional redundancy with another unconventional myosin. A non-essential role for MYO7A in canines may similarly explain the lack of a retinal phenotype in DVD.
Until now, the shaker-1 mouse has been the only spontaneously occurring non-human mammal in which the MYO7A mutation causes DVD (20,21). To our knowledge, this is the first description of a disease-causing MYO7A mutation in a large mammalian species.
Deafness is defined as the inability to perceive sound. Deafness can be partial or complete, peripheral (i.e., involving outer, middle, and/or inner ear) or central (i.e., involving the central nervous system), and is classified as being congenital, acquired, and/or inherited (22–25). Deafness in animals, including humans, is further categorized as being the result of abnormalities in the conductive hearing pathway (i.e., conductive hearing loss), the sensorineural hearing pathway (i.e., sensorineural hearing loss), or a combination of the 2 (i.e., mixed hearing loss) (22). Though DVD has been known to be a congenital sensorineural deafness with suspected autosomal recessive pattern of inheritance, the present study confirms an autosomal recessive mode of inheritance due to mutation in MYO7A.
Aside from the categorization above, forms of deafness in human medicine are also categorized as being either syndromic (i.e., occurring with other clinical signs) or non-syndromic (i.e., occurring alone). Categorization of deafness as syndromic or non-syndromic may not be appropriate for individuals with MYO7A mutations, however. Specifically, MYO7A mutations causing hearing loss in humans are traditionally categorized as: i) syndromic [e.g., autosomal recessive Usher syndrome type 1B (12)] in which the patient experiences deafness, blindness (due to retinal degeneration), and vestibular dysfunction; or ii) non-syndromic [e.g., autosomal dominant DFNA11 and autosomal recessive DFNB2 (14)] in which the phenotype is hearing loss alone. However, some patients with DFNB2 may develop variable vestibular dysfunction and retinal disease (26,27). Our present work also supports that the MYO7A mutation, similar to that of the shaker-1 mouse (19), can result in deafness and vestibular dysfunction independent of blindness. Altogether, these findings suggest that other factors modify the phenotypic expression of MYO7A mutations.
Several mutations of MYO7A have been linked to deafness in humans (4). MYO7A codes for myosin VIIa protein, which is an unconventional actin-based protein that plays an important role in binding cargo proteins involved in endocytosis, cell-cell adhesion, and cochlear hair cell stereocilia tip link structural maintenance (28). Further, studies examining myosin VIIa-deficient mice (shaker-1) suggest that it is necessary for hair cell stereocilia development (20). The findings of the current study, taken together with histologic and previously reported scanning electron microscopic descriptions of organ of Corti (3), substantiate the necessity of myosin VIIa in cochlear hair cell development. Specifically, it has been demonstrated that cochlear outer hair cells, followed by inner hair cells, progressively degenerate with age in dogs (3). In addition to stereocilia development, myosin VIIa is important in cochlear hair cell structural integrity, especially with respect to cochlear hair cell stereocilia tiplinks (29). Cochlear hair cell stereocilia tiplinks are structures that play an integral role in the mechanical gaiting of the mechanoelectrical transduction (MET) channels of the cochlea. It is the opening of these MET channels that results in potassium influx and initial cell depolarization. Cellular depolarization is further augmented through the opening of voltage-gated calcium channels, ultimately leading to mechanoelectrical transduction of sound. Aside from reduced development of cochlear hair cells, hearing loss is also due to impaired mechanoelectrical transduction (29). This would explain profound hearing loss in dogs with DVD which are not completely deaf (3). Interestingly, profound vestibular deficits observed in dogs with DVD cannot be related to vestibular hair cell degeneration. Ultrastructural examination of hair cells of the vestibular labyrinth has failed to identify degeneration of hair cells within the maculae or cristae ampullares (3). As such, the vestibular deficits observed in DVD may be due to other causes of impaired mechanoelectrical transduction within the vestibular labyrinth and not simply because of vestibular labyrinth hair cell degeneration.
Of the animals that we genotyped, 2 were heterozygous for the DVD mutation; one was deaf without vestibular deficits and the other had vestibular deficits without hearing loss. It may be possible that there are modifier genes which play a role in the phenotypic expression of these animals possessing the MYO7A mutation.
Humans with Usher syndrome type 1B are affected by vision loss related to retinitis pigmentosa (30). Though it is unknown why retinal degeneration occurs, patients with MYO7A mutations have primary photoreceptor degeneration as opposed to primary retinal pigment epithelial or synaptic dysfunction (31). Non-human models of Usher syndrome 1B fail to demonstrate progressive retinal degeneration (30). Though not specifically examined in dogs with DVD, affected dogs do not appear to suffer from vision impairment (3). It is possible that dogs with DVD develop retinal degeneration, though many affected animals are euthanized at a young age and as such, they may not be examined at a sufficiently older age. Nevertheless, prospective ophthalmoscopic and/or electroretinographic evaluation of dogs with DVD is required to determine whether progressive retinal degeneration occurs over time. Alternatively, one must consider that dogs with DVD may be spared from retinal degeneration given some inherent biological difference compared with humans. Identifying such biological differences would potentially aid in developing therapies for humans with retinitis pigmentosa resulting from the MYO7A mutation.
Addendum
During the completion of the review process for this manuscript, a frameshift mutation in the protein tyrosine phosphatase receptor type Q (PTPRQ) gene was identified as a cause of unilateral deafness and unilateral peripheral vestibular dysfunction in a single Doberman pinscher puppy (32). Importantly, and as discussed by the authors of the manuscript, limitations of their study included that only one animal was identified, that the parents of the affected dog were not examined, and that the animal’s clinical signs were not consistent with those described by Wilkes and Palmer (3) (i.e., only unilateral signs were present). It could be that the phenotype of unilateral deafness and vestibular dysfunction in the Doberman pinscher they described were unique to the mutation in the PTPRQ gene. The overall low prevalence of the mutant PTPRQ allele (1.5%) may be simply due to it being a new spontaneous mutation occurring in a very specific pedigree, similar to a KIT mutation causing white patterning in a family of German shepherds, that was identified by 2 of the current authors (MWN and AR) (33).
Regardless, as in humans with heritable hearing loss, there are likely many forms of genetically determined deafness in dogs. Our results, taken together with the recent identification of a mutation in PTPRQ causing unilateral deafness in a Doberman pinscher, underscore the importance of educating breeders of purebred dogs that animals can have similar clinical diseases yet different genetic causes. As such, phenotypic examination and identification, and subsequent genetic investigation, are important, especially in animals derived from parents free of genetic mutations.
Acknowledgments
Research support was provided by the Van Andel Research Institute and the Translational Genomics Research Institute. Additional support was provided by private Doberman pinscher breeders and the non-profit organization, ProjectDog. We thank M. Huentelman and J. Corneveaux for the next generation sequencing and C. Kingsley for the computational biology. We also thank E. Boguslawski, J. Beck, L. Kefene, and Q. Shan for their technical assistance, and K. Davieds for the targeted sample recruitment. We thank D. Nadziejka for the comments on the manuscript.
References
- 1.Chrisman CL. Vestibular diseases. Vet Clin North Am Small Anim Pract. 1980;10:103–129. doi: 10.1016/s0195-5616(80)50007-0. [DOI] [PubMed] [Google Scholar]
- 2.Skerritt GC. Head tilt in puppies. Veterinary Record. 1983;112:111. doi: 10.1136/vr.112.9.207-b. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes MK, Palmer AC. Congenital deafness and vestibular deficit in the dobermann. J Small Anim Pract. 1992;33:218–224. [Google Scholar]
- 4.Liu XZ, Walsh J, Mburu P, et al. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat Genet. 1997;16:188–190. doi: 10.1038/ng0697-188. [DOI] [PubMed] [Google Scholar]
- 5.Neff MW, Beck JS, Koeman JM, et al. Partial deletion of the sulfate transporter SLC13A1 is associated with an osteochondrodysplasia in the Miniature Poodle breed. PLoS One. 2012;7:e51917. doi: 10.1371/journal.pone.0051917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purcell S, Neale B, Todd-Brown K, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 8.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cingolani P, Platts A, Wang le L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toms M, Bitner-Glindzicz M, Webster A, Moosajee M. Usher syndrome: A review of the clinical phenotype, genes and therapeutic strategies. Expert Rev Ophthalmol. 2015;10:241–256. [Google Scholar]
- 13.Riazuddin S, Nazli S, Ahmed ZM, et al. Mutation spectrum of MYO7A and evaluation of a novel nonsyndromic deafness DFNB2 allele with residual function. Hum Mutat. 2008;29:502–511. doi: 10.1002/humu.20677. [DOI] [PubMed] [Google Scholar]
- 14.Bitner-Glindzicz M. Hereditary deafness and phenotyping in humans. Br Med Bull. 2002;63:73–94. doi: 10.1093/bmb/63.1.73. [DOI] [PubMed] [Google Scholar]
- 15.Libby RT, Steel KP. The roles of unconventional myosins in hearing and deafness. Essays Biochem. 2000;35:159–174. doi: 10.1042/bse0350159. [DOI] [PubMed] [Google Scholar]
- 16.Deafness Variation Database [database on the Internet] Iowa City: University of Iowa; c2017. [Last accessed September 29, 2018]. Available from: http://deafnessvariationdatabase.org/ [Google Scholar]
- 17.Weil D, Küssel P, Blanchard S, et al. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet. 1997;16:191–193. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- 18.Lord EM, Gates WmH. Shaker, a new mutation in the house mouse (Mus musculus) Am Nat. 1929;63:435–442. [Google Scholar]
- 19.Gibson F, Walsh J, Mburu P, et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 20.Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- 21.Hasson T, Walsh J, Cable J, Mooseker MS, Brown SD, Steel KP. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton. 1997;37:127–138. doi: 10.1002/(SICI)1097-0169(1997)37:2<127::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Strain GM. The genetics of deafness in domestic animals. Front Vet Sci. 2015;2:29. doi: 10.3389/fvets.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama JS, Lam ET, Ruhe AL, et al. Variation in genes related to cochlear biology is strongly associated with adult-onset deafness in border collies. PLoS Genet. 2012;8:e1002898. doi: 10.1371/journal.pgen.1002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb AA, Cullen CL. Coat color and coat color pattern-related neurologic and neuro-ophthalmic diseases. Can Vet J. 2010;51:653–657. [PMC free article] [PubMed] [Google Scholar]
- 25.Strain GM. Aetiology, prevalence and diagnosis of deafness in dogs and cats. Br Vet J. 1996;152:17–36. doi: 10.1016/s0007-1935(96)80083-2. [DOI] [PubMed] [Google Scholar]
- 26.Liu XZ. The clinical presentation of DFNB2. Adv Otorhinolaryngol. 2002;61:120–123. doi: 10.1159/000066823. [DOI] [PubMed] [Google Scholar]
- 27.Zina ZB, Masmoudi S, Ayadi H, et al. From DFNB2 to Usher syndrome: Variable expressivity of the same disease. Am J Med Genet. 2001;101:181–183. doi: 10.1002/ajmg.1335. [DOI] [PubMed] [Google Scholar]
- 28.Pan LZ, Zhang M. Structures of usher syndrome 1 proteins and their complexes. Physiology (Bethesda) 2012;27:25–42. doi: 10.1152/physiol.00037.2011. [DOI] [PubMed] [Google Scholar]
- 29.Kros CJ, Marcotti W, van Netten SM, et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with MYO7A mutations. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SG, Cideciyan AV, Gibbs D, et al. Retinal disease course in Usher syndrome 1B due to MYO7A mutations. Invest Ophthalmol Vis Sci. 2011;52:7924–7936. doi: 10.1167/iovs.11-8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson SG, Cideciyan AV, Aleman TS, et al. Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum Mol Genet. 2008;17:2405–2415. doi: 10.1093/hmg/ddn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guevar J, Olby NJ, Meurs KM, Yost O, Friedenberg SG. Deafness and vestibular dysfunction in a Doberman Pinscher puppy associated with a mutation in the PTPRQ gene. J Vet Intern Med. 2018;32:665–669. doi: 10.1111/jvim.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AK, Ruhe AL, Robertson KR, Loew ER, Williams DC, Neff MW. A de novo mutation in KIT causes white spotting in a subpopulation of German Shepherd dogs. Anim Genet. 2013;44:305–310. doi: 10.1111/age.12006. [DOI] [PubMed] [Google Scholar]