Abstract
Osteoarthritis, the leading cause of chronic joint pain, is studied through different animal models, but none of them is ideal in terms of reliability and translational value. In this pilot study of female rats, 3 surgical models of osteoarthritic pain, i.e., destabilization of the medial meniscus (DMM), cranial cruciate ligament transection (CCLT), and the combination of both surgical models (COMBO) and 1 chemical model [intra-articular injection of monosodium iodoacetate (MIA)] were compared for their impact on functional pain outcomes [static weight-bearing (SWB) and punctate tactile paw withdrawal threshold (PWT)] and spinal neuropeptides [substance P (SP), calcitonin gene-related peptide (CGRP), bradykinin (BK), and somatostatin (SST)]. Six rats were assigned to each model group and a sham group. Both the chemical model (MIA) and surgical COMBO model induced functional alterations in SWB and PWT, with the changes being more persistent in the surgical combination group. Both models also produced an increase in levels of pro-nociceptive and anti-nociceptive neuropeptides at different timepoints. Pain comparison with the MIA model showed the advantage of a surgical model, especially the combination of the DMM and CCLT models, whereas each surgical model alone only led to temporary functional alterations and no change in neuropeptidomics.
Résumé
L’arthrose, la principale cause de douleur chronique articulaire, est étudiée à travers différents modèles animaux, mais aucun d’eux n’est idéal en termes de fiabilité et de valeur translationnelle. Trois modèles chirurgicaux de douleur arthrosique, c’est-à-dire, la déstabilisation du ménisque médial, la transsection du ligament croisé crânial et la combinaison des deux, ainsi qu’un modèle chimique (injection intraarticulaire de mono-iodoacétate de sodium) ont été comparés dans cette étude pilote chez des rattes quant à leurs impacts sur les évaluations fonctionnelles de la douleur (distribution pondérale statique, évaluation ponctuelle de l’allodynie tactile) et les neuropeptides spinaux (substance P, peptide relié au gène de la calcitonine, bradykinine et somatostatine). Six rats ont été assignés à chacun des modèles et un groupe Sham. Autant le modèle du mono-iodoacétate de sodium que celui de la combinaison chirurgicale ont tous les deux induits des altérations fonctionnelles de la distribution pondérale statique et du seuil de retrait de la patte suite à une stimulation ponctuelle tactile, mais avec des changements plus persistants dans le groupe de la combinaison chirurgicale. Ces deux modèles ont également engendré une augmentation des niveaux en neuropeptides pro-nociceptifs et anti-nociceptifs à différents moments. Un intérêt du modèle chirurgical a été démontré suite à la comparaison de la douleur avec le modèle du mono-iodoacétate de sodium, en particulier la déstabilisation du ménisque médial combinée à la transsection du ligament croisé crânial, tandis que les inductions chirurgicales unique entraînaient des altérations fonctionnelles temporaires avec aucun changement neuropeptidomique.
(Traduit par les auteurs)
Introduction
Osteoarthritis (OA) is one of the most frequent causes of chronic joint pain, resulting in tremendous decreases in productivity and economic losses (1). Osteoarthritis is also the most common chronic degenerative disease affecting pet animals in western society (2,3). Very few reliable and valid tools are available to clinicians in veterinary medicine for measuring pain (4). Given the great complexity of the pain phenomenon, it is therefore essential to develop experimental animal models that reliably mimic pain and face validity to the clinical condition.
Different animal models have been used to investigate pain mechanisms and test potential treatment. The ideal animal model should be reliable, valid, and offer the best translational value possible (5,6). The most commonly used model for evaluating analgesia in OA is the murine (rat) intra-articular (IA) injection of monosodium iodoacetate (MIA). The pathology develops very rapidly and dose-dependent structural alterations have been described (7); for review, see (8,9). Changes in weight-bearing (10) and centralized pain have been documented as functional outcomes (11–14). Moreover, in terms of weight-bearing, the MIA model’s progression is bimodal and has been reported to be highly variable (15,16). The rapidly developing osteoarthritis (OA) that occurs with the MIA model is clearly different from slowly developing natural OA. This often limits the assessment of the disease at one timepoint (17). The MIA model also displays substantial differences from human OA in gene arrays (18) and relies on a disease mechanism that is different from natural OA, which could limit the predictability of the therapeutic effect of analgesics.
Alternative models to MIA must be explored because of the many limitations of the MIA model and its poor translation to naturally developing OA in humans. Models of joint destabilization have therefore been used in dogs, sheep, rabbits, and guinea pigs and, more extensively, for pain research in rodents, e.g., partial or total medial meniscectomy, medial meniscotomy, destabilization of medial meniscus (DMM), and cranial cruciate ligament transection (CCLT). Until now, none of the surgical models in rats has satisfied all the desired criteria of reliability (reproducibility, repeatability), validity (face, construct predictive), and translational value (5,11,12,19–26).
The objectives of this pilot study were: i) to evaluate functional pain outcomes and spinal neuropeptides in 3 surgical rat models of OA pain, i.e., DMM, CCLT, and the combination of both (COMBO); and ii) to compare those results with the MIA model. We hypothesized that surgical induction of OA would be accompanied by changes in functional pain outcomes and quantifiable neurophysiological modifications compatible with the establishment of a chronic non-physiologic pain.
Materials and methods
Animals
The study protocol was approved by the Université de Montréal Animal Care and Use Committee (No. rech-1766), in accordance with the guidelines of the Canadian Council on Animal Care. Female Sprague-Dawley rats (N = 35) were obtained from Charles River Canada (St.-Constant, Québec). Mean ± standard deviation body weight was 389 ± 35 g and ages ranged from 4 to 6 mo (skeletal maturity). The study was conducted in an enriched environment and care, which included 2 rats per cage, with toys, cardboard boxes, pipes, and fruit crunchy treats, according to the facility’s standardized operating procedure (SOP AC7011-3).
Group description
Using the randomized block design, rats (n = 6 per group) were assigned to 1 of the 5 model groups that included 3 different surgical OA models, 1 sham surgical model, and 1 MIA model. Groups were as follows: i) sham; ii) DMM; iii) CCLT; iv) COMBO for the combination of DMM and CCLT models; and v) MIA. An independent group (n = 5) of naive rats was added for measuring the concentration of neuropeptides in their spinal cord.
Induction of osteoarthritis
Anesthesia and analgesia
For the 4 surgical groups, on day (D) 0, 1.0 mg/kg body weight (BW) of buprenorphine (Buprenorphine SR; Chiron Compounding Pharmacy, Guelph, Ontario) was administered intramuscularly as premedication and anesthesia was induced 40 min later with isoflurane (IsoFlo; Abbott Animal Health, Montreal, Quebec) in oxygen in an induction box and maintained with 2% isoflurane in oxygen mixture with a face mask. At the end of the surgical procedures, a peri-incisional bupivacaine 0.25% block (Marcaine; McKesson Canada, St.-Laurent, Quebec) at a dose of 0.05 to 0.1 mL per site (< 1 mg/kg BW) was administered. A similar procedure was conducted for the MIA group, with the exception of the periarticular bupivacaine block. Based on the manufacturer’s label (Chiron Compounding Pharmacy), the sustained-release opioid-based analgesia is expected to last for 72 h.
Intra-articular MIA injection
In the subjects of the MIA group, an IA injection of 2 mg of MIA (Sigma-Aldrich, St. Louis, Missouri, USA) dissolved in 50 μL of 0.9% sterile saline was administered through the right infrapatellar ligament using a previously described technique (7,12,15,27).
Surgical procedures
All procedures were carried out on the right stifle. Briefly, after aseptic surgical technique, a medial parapatellar arthrotomy was conducted, the patella was luxated laterally, and the designated procedure was carried out. The patella was then anatomically reduced and the surgical site was closed in 3 successive planes using 5-0 polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey, USA). In the subjects of the sham group, all IA structures were left intact after the arthrotomy. In the animals of the DMM group, the medial cranial meniscotibial ligament was identified and transected using a #12 blade as previously described (21,28). In the rats of the CCLT group, the cranial cruciate ligament was transected with adapted instrumentation as previously described (29). In the subjects of the COMBO group, the DMM was carried out first, followed by the CCLT procedure.
Functional pain outcomes
Functional pain was evaluated by using distribution of static weight-bearing (SWB) and tactile sensitivity (von Frey). Before evaluation, the rats were acclimatized to the evaluation environments at D −14, D −7, D −5, and D −3, according to a recent validation in rats (15). Assessment timepoints differed among the surgical groups (D −1 = baseline, D14, D28, and D42) and the MIA group (D −1 = baseline, D3, D7, D14, and D21). Both functional evaluation observers were completely blinded to OA induction and experimental design.
Weight distribution through the right and left stifle was assessed using an Incapacitance Meter (IITC Life Science, Woodland Hills, California, USA) to measure SWB distribution in the 2 hind limbs as previously described (15,16). The weight applied (force) by the animal for each hind limb was measured and analyzed in grams, but expressed in percentage of total body weight (%BW) in order to normalize the data for each individual.
Tactile sensitivity was then assessed using the Electronic von Frey Anesthesiometer (IITC Life Science) with a standardized filament (0.7 mm2 polypropylene Supertip) to obtain the punctate tactile paw withdrawal threshold (PWT), expressed in grams. Both hind paws were tested 3 times in a random order and with a refractory period of 1 min between each trial (15,16).
Euthanasia and spinal cord collection
Rats were euthanized by decapitation following isoflurane overdose (after the last functional evaluation day, D21 for the MIA and D42 for the surgical groups), after which the whole spinal cord was collected using a saline flush technique (15,16,30). The spinal cords from naive rats were also collected to obtain comparative values for each neuropeptide from normal rats. Samples were snap frozen in cold hexane and stored individually at −80°C pending neuropeptidomic analysis.
Neuropeptidomics
In the present study, substance P (SP), calcitonin gene-related peptide (CGRP), bradykinin (BK), and somatostatin (SST) were analyzed by high performance liquid chromatography-mass spectrometry and expressed in fmol/mg of whole spinal cord homogenates [1:5 w/v in 0.25% trifluoroacetic acid (TFA) solution] as previously described (15,16).
Statistical analysis
The %BW and PWT data were expressed as the average of each paw measure in triplicate. When deemed necessary, asymmetry index was used to statistically confirm the impressions given by the graphs on SWB and PWT.
The normality of the data (Shapiro-Wilk test) and the homogeneity of variance were confirmed using the absolute values of the residuals of the mixed model, when appropriate. Unless indicated otherwise, hypotheses were 2-sided and alpha-value was set at 0.05. For each model, the first tested hypothesis was that there was at least 1 evaluation day when the outcome was different from the baseline. Based on the changes observed with the MIA rat model, pre-test analyses for such a limited sample size (n = 6) conducted to a power of > 89.1% for SWB and > 81.4% for PWT to detect a significant within-time (intra-group) difference compared to baseline (15,16). A linear-mixed model for repeated measures was used for detecting intra-group differences. Multiple comparisons were carried out using the Dunnett procedure. The surgical models that presented a significant change over time were then compared (the MIA model was excluded for inter-group testing because of its different timepoints of assessment).
The second hypothesis was that at least 1 model differed. Pre-test power analyses for inter-group difference with such sample size were low (< 30%). The alpha-value was set at 0.1 at that time to maximize the chances of significant results, keeping in mind that this was a comparative pilot study setting. When the goal of the study is to find an effect that could lead to a promising scientific discovery, it is acceptable to set a higher alpha value. This increases the power and consequently decreases the risk of Type-II error, but it also increases the chances of making a Type-I error (31). Data were processed using a linear-mixed model for repeated measures, except for the neuropeptides data, which were analyzed with the unpaired exact Wilcoxon test following a non-parametric Kruskal-Wallis 1-way analysis of variance. Tukey adjustment was used to obtain adjusted (adj) P-values for multiple comparisons.
Results
All animals completed the study and there were no significant complications after the surgical procedures or IA injections. Collection of the spinal cord was unsuccessful in 1 rat of the MIA group.
Functional pain outcomes
Static weight-bearing (SWB)
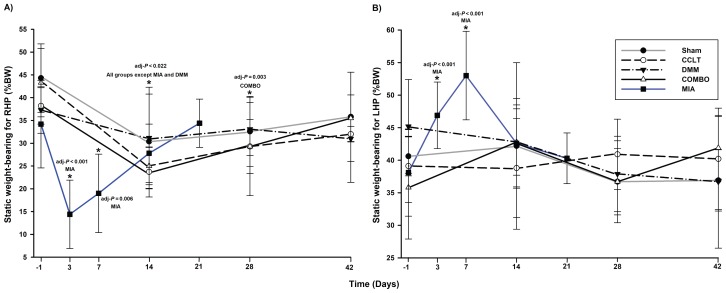
The values from all groups except DMM rats showed a significant change over time for the right-hind paw (RHP) %BW (Table I). Values decreased for all other groups at the second evaluation timepoint and tended to increase afterwards (Figure 1A). Within the surgical models, only the COMBO model data were still significantly lower than the baseline values at D28 (Table I). A Type III-day effect was noted (P = 0.004) for the surgical models, no group effect, and this indicated that globally, an alteration in the %BW of the RHP was detected over time, but the analysis was not sensitive enough to detect the apparently more severe change in the COMBO group (Figure 1A). Interestingly, there was an increase in the %BW of the left (non-affected) hind limb in the MIA group at D3 and D7 (Figure 1B) and the asymmetric SWB distribution confirmed a significant weight shift to the left side for these timepoints (P < 0.001). This phenomenon was not observed in the surgical groups (Figure 1B) and the symmetric distribution of SWB was not significantly different from baseline.
Table I.
Testing time effect and specific comparison versus baseline of static weight-bearing (SWB) for the right-hind paw (RHP).
| Experimental groups | Type-III test of fixed effects ProbF | Day | Adjusted P-value (differences of least squares mean, standard error) |
|---|---|---|---|
| Sham | 0.041 | 14 | 0.022 (−13.93, 4.61) |
| CCLT | 0.028 | 14 | 0.006 (−14.49, 2.98) |
| DMM | 0.599 | ||
| COMBO | <0.001 | 14 | <0.001 (−18.33, 2.83) |
| 28 | 0.003 (−14.01, 3.60) | ||
| MIA | <0.001 | 3 | <0.001 (−19.74, 4.11) |
| 7 | 0.006 (−15.17, 4.19) |
Bold indicates a significant difference.
Notes: The best structure for each group of the covariance model was assessed using a graphical method (plots of covariance versus lag in time between pairs of observation compared to different covariance model) and using information criteria that measure the relative fit of competing covariance models: normal distribution, compound symmetry covariance structure (sham, DMM, and MIA groups); heterogeneous compound symmetry covariance structure (CCLT group); and type-1 auto-regressive covariance structure (COMBO group). For the baseline-to-specific-day comparison, adjusted P-value for multiple comparisons was obtained using the Dunnett procedure.
CCLT — cranial cruciate ligament transection; DMM — destabilization of medial meniscus; COMBO — combination of both CCLT and DMM models; MIA — intra-articular injection of monosodium iodoacetate.
Figure 1.
Percentage of body weight (%BW) (mean ± standard deviation) for A) right-hind paw (RHP) and B) left-hind paw (LHP) for static weight-bearing (SWB) by day (D). Time is distributed differently for the surgical groups (D −1, D14, D28, and D42) and the MIA group (D −1, D3, D7, D14, and D21). *a day when there is a statistically significant decreased value compared to its baseline (see Table I for details). CCLT — cranial cruciate ligament transection; DMM — destabilization of medial meniscus; COMBO — combination of CCLT and DDM models; MIA — intra-articular injection of monosodium iodoacetate.
Tactile sensitivity (von Frey)
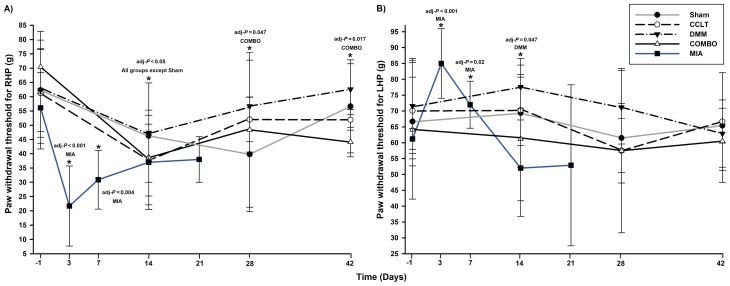
For the PWT in the RHP, changes in time were significant for all groups except the sham group (Table II). At the second evaluation timepoint, values decreased for all other groups and tended to increase afterwards (Figure 2A). The COMBO group continued to show a significantly persistent decrease in the PWT values for the RHP until the last evaluation day (Table II). A Type-III effect of the day (P = 0.014) and group (P = 0.064) was present when surgical models were compared using normal distribution compound symmetry with heterogeneous day covariance structure mixed model. This indicated that, globally, an alteration in PWT was detected over time and the statistical analysis was sensitive enough to detect a larger alteration in the COMBO group than in the DMM group (P = 0.053). Interestingly, the difference among groups was not significant for the COMBO group when compared to the CCLT and sham groups (Figure 2A). There was a simultaneous increase in the PWT of the LHP and decrease of PWT of the RHP on D14 for the DMM group and on D3 and D7 for the MIA group (Figure 2B). The asymmetric distribution of the PWT showed a significant weight shift to the left side at D3 (P < 0.001) and at D7 (P = 0.01) for the MIA group only. This was not observed in the surgical groups and the symmetric distribution of PWT was not significantly different from baseline.
Table II.
Testing time effect and specific comparison versus baseline of the paw withdrawal threshold (PWT) for the righthind paw (RHP).
| Experimental groups | Type-III test of fixed effects ProbF | Day | Adjusted P-value (differences of least squares mean, standard error) |
|---|---|---|---|
| Sham | 0.061 | ||
| CCLT | 0.036 | 14 | 0.014 (−23.47, 6.90) |
| DMM | 0.049 | 14 | 0.043 (−14.81, 5.52) |
| 14 | 0.005 (−31.64, 8.32) | ||
| COMBO | 0.009 | 28 | 0.047 (−21.97, 7.96) |
| 42 | 0.017 (−26.26, 8.14) | ||
| 3 | <0.001 (−34.34, 5.68) | ||
| MIA | <0.001 | 7 | 0.004 (−25.20, 6.83) |
| 14 | 0.049 (−19.07, 7.29) |
Bold indicates a significant difference.
Notes: The best structure of the covariance model for each group was assessed using a graphical method (plots of covariance versus lag in time between pairs of observation compared to different covariance model) and using information criteria that measure the relative fit of competing covariance models: normal distribution, compound symmetry covariance structure (sham, CCLT, DMM, COMBO, and MIA groups). For the baseline-to-specific-day comparison, adjusted P-value for multiple comparisons was obtained using the Dunnett procedure.
CCLT — cranial cruciate ligament transection; DMM — destabilization of medial meniscus; COMBO — combination of both CCLT and DMM models; MIA — intra-articular injection of monosodium iodoacetate.
Figure 2.
Paw withdrawal threshold (PWT) (mean ± standard deviation) for A) right-hind paw (RHP) and B) left-hind paw (LHP) by day (D). Time is distributed differently for the surgical groups (D −1, D14, D28, and D42) and the MIA group (D −1, D3, D7, D14, and D21). *a day when there is a statistically significant decreased value compared to its baseline (see Table II for details). CCLT — cranial cruciate ligament transection; DMM — destabilization of medial meniscus; COMBO — combination of CCLT and DMM models; MIA — intra-articular injection of monosodium iodoacetate.
Neuropeptidomics
Compared to the COMBO group, all other surgical groups (CCLT, DMM, and sham) presented significantly lower values for CGRP (sham adj-P = 0.002; CCLT adj-P = 0.007; DMM adj-P < 0.001) (Table III). In the last 3 groups, the spinal content of CGRP was not different than in the naive rats, but it was different in the COMBO group. The concentration of SST in the COMBO group was also significantly higher than in the naive, sham, and CCLT groups (adj-P = 0.009, 0.088, and 0.017, respectively). The spinal concentrations of SP and BK presented a Type-III significant group effect (P = 0.095 and 0.028, respectively), but the analysis was not sensitive enough to detect any difference among the surgical groups. Compared to the naive rats, however, only the COMBO in the surgical groups presented a higher concentration in spinal content of SP. Values of all neuropeptides, except SP (P = 0.476), were significantly higher in the MIA model than in the COMBO group (adj-P < 0.02).
Table III.
Comparison among groups of concentrations of spinal neuropeptides (mean ± standard deviation) in surgical and chemical models of osteoarthritis pain in rats.
| Experimental groups | n | Neuropeptides (fmol/mg) | |||
|---|---|---|---|---|---|
|
| |||||
| SP | CGRP | BK | SST | ||
| Naive | 5 | 83 ± 17a | 464 ± 92a | 209 ± 41a | 227 ± 39a |
| Sham | 6 | 112 ± 12a,b | 569 ± 42a | 213 ± 15a | 339 ± 23b |
| CCLT | 6 | 118 ± 18a,b | 593 ± 58a | 183 ± 15a | 325 ± 28b |
| DMM | 6 | 104 ± 16a,b | 546 ± 42a | 191 ± 14a | 351 ± 23b,c |
| COMBO | 6 | 135 ± 31b | 725 ± 105b | 195 ± 20a | 379 ± 45c |
| MIA | 5 | 147 ± 11b | 1065 ± 153c | 354 ± 12b | 722 ± 44d |
Statistically significant difference shown in bold. Different letters indicate statistically significant between-groups difference.
Notes: Between-group comparison was conducted using a non-parametric Kruskal-Wallis 1-way analysis of variance with post-hoc analysis, when required, using the unpaired exact Wilcoxon test. Tukey adjustment was used to obtain adjusted P-values for multiple comparisons.
SP — substance P; CGRP — calcitonin gene-related peptide; BK — bradykinin; SST — somatostatin; CCLT — cranial cruciate ligament transection; DMM — destabilization of medial meniscus; COMBO — combination of CCLT and DMM models; MIA — intra-articular injection of monosodium iodoacetate.
Discussion
In the search for an animal model of osteoarthritic pain that would allow the best analgesic therapeutic evaluation and translation to other species, this study provided some interesting preliminary comparisons between the MIA chemical model and different surgical models, particularly the COMBO model. Because the duration of evaluation was different for the chemical model (MIA) and surgical models (up to D21 for MIA and up to D42 for surgical models), as well as the different distribution of timepoints, the comparison between the MIA and COMBO models calls for caution. Nevertheless, the main results are that: both the MIA and COMBO models induced functional alterations in %BW and PWT, with these changes lasting longer in the COMBO group; both the MIA and COMBO models induced an increased change in levels of pro-nociceptive (CGRP and SP) and anti-nociceptive (SST) neuropeptides; and the behavioral expression of pain was limited in the surgical CCLT and DMM models, which corresponded to an absence of change in spinal neuropeptidomics.
Functional pain outcomes
This pilot study highlighted a limitation in the CCLT and DMM models, as their functional alterations were of short duration and the change in concentration of spinal neuropeptides was not significant compared to the sham group. Interestingly, the functional changes induced by the CCLT and DMM models were not that different from those in the sham group. The sham procedure was not totally neutral, with a within-time significant change in static weight-bearing (SWB) in the right-hind paw (RHP) at D14 and some differences in spinal concentrations of neuropeptides compared to the naive group (Table III). Similar changes in sensory sensitivity and operant testing were observed with the IA injection of 0.9% sodium chloride (NaCl) in the sham group, and were confirmed by the spinal neuropeptidomics (15,16). This suggests that the functional alterations in the CCLT and DMM models were most likely due to surgical trauma associated with the arthrotomy and not to significant biomechanical instability. It could be argued that the CCLT and DMM models could have shown alterations resulting from biomechanical instability if the rats had been more mobile and active, which was not part of the current study design (32). These models could therefore remain of interest in specific study settings. The changes induced by both the MIA model and COMBO surgery model, however, led to changes in the biomechanical (static weight-bearing), sensory (paw withdrawal threshold), and nociceptive neuropeptides in the same research context.
The significant weight shift to the left-hind paw (LHP) in the MIA group could be interpreted as an early-occurring but non-persistent biomechanical change since the %BW values for the MIA group did not change from baseline values after D7. This phenomenon was not observed in the surgical groups and could constitute a major difference between the MIA and surgical models. This contralateral weight shift could indicate major discomfort in the affected right-hind paw (RHP), with the rat seeking to relieve this acute inflammatory insult, while the more progressive damage in the COMBO model did not produce such intense and early pain.
The increase in the pain withdrawal threshold (PWT) in the LHP at the same time as the decrease in values for the RHP could be explained by 2 hypotheses. First, the significant weight shift to the LHP at D3 and D7 in the MIA model could be responsible for the animal being “less responsive” in lifting its left paw. This reluctance to put its weight on the painful (right) limb during the inflammatory phase of the MIA model (7,12) artificially increases the PWT on the contralateral limb (33). The early occurrence of such a shift in SWB and PWT values in the MIA model could also reflect early peripheral sensitization, leading subsequently or concomitantly to central sensitization. Second, diffuse descending pain inhibition mechanisms (8,34,35) could be activated very efficiently by the initial strong inflammation present in the MIA-treated stifle and be less intense as time passes and inflammation subsides. While it is uncertain at this point if the biomechanical, neurological, and/or inflammatory components are responsible for these results, it constitutes a significant difference between the MIA and the surgical models.
The changes persisted until the last evaluation timepoint for the PWT on the RHP in the COMBO group and only until D14 in the MIA group. This could indicate the potential for a more persistent tactile allodynia to be induced in the COMBO model than in the other models in this study. The group effect on the PWT in the RHP showed that sensitization was more severe in the COMBO model than in the DMM model because it induced more tactile allodynia. It would be expected that the COMBO model would also be more severe than the CCLT model, although the difference was not statistically significant in this study, likely because of a low statistical power (Type-II statistical error). The same explanation applies for the absence of statistical difference among surgical groups for SWB on the RHP.
Neuropeptidomics
As neuromodulators, SP and CGRP are important mediators in peripheral and central sensitization in inflammatory arthritis and OA (15,16,36). Concentrations of both SP and CGRP were higher in the COMBO model than in the other surgical models, but only CGRP reached statistical significance. Calcitonin gene-related peptide (CGRP) is accepted as an important mediator in subchondral (37) and central signalling of OA pain using the MIA model (11,15) and a surgical model (11) in rats. This suggests that neuronal plasticity was also induced at the central level for the COMBO model.
The significantly lower concentration of SST in rats in the sham and CCLT groups compared to the COMBO group is interesting as it could indicate that the COMBO model has a greater potential to induce allodynia. Somatostatin (SST) has not been evaluated specifically in osteoarthritic conditions, but has been studied primarily in inflammatory conditions such as rheumatoid arthritis and asthma (38). With the hypothesis that the inflammatory component of the disease is likely to be a major contributor to pathological pain, it would be expected that, if a model causes more inflammation, it could induce more allodynia. Additionally, such a change in the spinal content of SST could be associated with increased descending nociceptive inhibition (38,39). This phenomenon of increased inflammation and concomitant inhibitory pain modulation could be monitored by the quantification of SST in a research setting. Moreover, all experimental OA groups presented higher spinal content of SST compared to the naive group. This was the only tested neuropeptide for which such differences were detected. The gradation in spinal content of SST in the different surgical and MIA groups and the previously reported relationship between spinal content of SST and the severity of cartilage lesions induced by different MIA doses (including in the sham group) (16) suggests that SST is the most sensitive biomarker in quantifying OA pain. Finally, bradykinin (BK) has been studied in multiple species and is reported to be involved in OA pain (40). However, it was not possible to detect significant changes in BK in the present study.
The significantly higher values of CGRP, SST, and BK in the MIA model could indicate that it causes more pain and has a greater potential for inducing allodynia. The comparison with the COMBO group is limited, however, since the time frame for both groups was different, as was the time of spinal cord collection. It will probably take time for significant articular lesions and neuronal plasticity to develop in both the MIA and the surgical models (5,41). Previous studies of surgical models showed that it could take at least 6 wk for structural changes to occur after CCLT and meniscectomy (20), but it was faster for DMM with micro-computed tomography detection (21–23). The medial meniscal tear (meniscotomy) model (5,19,42) induced structural alterations and mechanical allodynia similar to the evolution of the PWT observed with the COMBO model in the present study. This was also the case for (partial or total) medial meniscectomy alone (12,25) or combined with CCLT (11,26), which presented similar evolution as the COMBO model for gait analysis and mechanical allodynia. In 1 study (25), the surgical model induced more structural and pain functional alterations than the MIA, whereas it was the opposite in another study (11), although the MIA dose used was very different, 1 (25) versus 3 mg (11), which could explain the apparent divergence. The maximum potential for inducing pathological pain might not have been reached at D42 with the COMBO model (11).
Exploratory pilot study context
This study was conducted to determine the impact of 3 surgical models on pain outcomes compared to the MIA model, which is considered the gold standard for OA pain models in rats. Because the focus of the research was pain management, we restricted the evaluation to pain functional and neuropeptidomics outcomes. The idea was first to compare the validity of each model on pain outcomes to further pursue the development of the most valid pain model. The subsequent development of the most valid model would include structural changes, longer evaluation, as well as the influence of gender or hormonal effect, exercise, age, and strains of tested rats on the structural and pain functional and neuropeptidomics outcomes (43). By processing like this, we would follow the 3Rs rule (replacement, reduction, and refinement) and decrease the number of research animals used in terminal procedures.
The use of DMM was previously studied in mice (28,44) and rats (21–23) for structural and biomarker assessment. To our knowledge, this is the first application of this surgical model for pain assessment in rats. It was selected because of the ease of induction and standardization compared to the meniscectomy or medial meniscal tear, as well as the validity in induced structural alterations. Consequently, the COMBO model appears as a new surgical OA pain model in rats.
The rat species was selected for its ease of behavioral assessment and popularity in OA pain investigation, even though there are advantages to using other species, e.g., genetically modified animals for studying specific pathophysiological pathways in mice and anatomic and biomechanical translational value for large animals (45). In particular, the common use of the MIA OA pain model in rats has led to behavioral and neuropeptidomic characterization by our group (15,16) and others; for review, see (45).
Due to the results of this study, further investigation of the COMBO model is highly recommended. Only female rats were used in this pilot study because it is recognized that OA occurs more often in women (46,47). Following this pilot study, we recommend using 12 rats per group, which would create a statistical power of 80% with an alpha-value of 0.05, documenting an 8% difference in SWB and 10 g in the PWT. Those numbers reflect the difference documented between the sham and COMBO groups.
In conclusion, the surgical induction of OA was accompanied by quantifiable neurophysiological changes associated with pain, as shown by functional analysis, spinal neuropeptides, and comparison with the MIA model, which is the current gold standard of OA pain in rats. The COMBO model induces changes compatible with chronic pain that are more persistent than in the MIA model, which indicates its usefulness for evaluating therapeutic modalities. Moreover, the changes observed in the COMBO surgical model seem more progressive and consequently present a higher degree of face validity with naturally developing OA. Prospective studies with appropriate size groups (n = 12), different durations of followup, evaluation of the structural, functional, epigenomic, and neuroproteomic changes at multiple timepoints, as well as considering the possible influence of gender, age, and strains of rats studied would help to obtain a better characterization of the COMBO model. Validation with therapeutic intervention should also be carried out.
Acknowledgments
This work was funded in part by a Pfizer Neuropathic Pain Research Award (#WS386180) to Dr. Eric Troncy from Pfizer Canada, a Discovery Grant (#327158–2008, #441651–2013, #386637-2010) to Dr. Eric Troncy and Dr. Francis Beaudry, which supported salaries, as well as Collaborative Research and Development Grants (#RDCPJ 418399–2011, #RDCPJ 491953-2016) to Dr. Eric Troncy from the Natural Sciences and Engineering Research Council of Canada in partnership with ArthroLab Inc. for operations and salaries. In addition, an ongoing New Opportunities Fund Grant (#9483) and a Leader Opportunity Fund Grant (#24601) from the Canada Foundation for Innovation was used to purchase pain/function equipment. The authors thank staff at ArthroLab Inc. for their expertise supporting this work.
Footnotes
The authors declare that there are no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Shearer P. Epidemiology of orthopedic disease. Vet Focus. 2011;21:24–25. [Google Scholar]
- 3.Slingerland LI, Hazewinkel HA, Meij BP, Picavet P, Voorhout G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J. 2011;187:304–309. doi: 10.1016/j.tvjl.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Klinck MP, Mogil JS, Moreau M, et al. Translational pain assessment: Could natural animal models be the missing link? Pain. 2017;158:1633–1646. doi: 10.1097/j.pain.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 5.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–376. [PubMed] [Google Scholar]
- 6.Little CB, Smith MM. Animal models of osteoarthritis. Curr Rheumatol Rev. 2008;4:1–8. [Google Scholar]
- 7.Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. Mono-iodoacetate-induced experimental osteoarthritis: A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40:1670–1679. doi: 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 8.Eitner A, Hofmann GO, Schaible HG. Mechanisms of osteoarthritic pain. Studies in humans and experimental models. Front Mol Neurosci. 2017;10:349. doi: 10.3389/fnmol.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaible HG. Joint pain: Basic mechanisms. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Wall and Melzack’s Textbook of Pain. 6th ed. Philadelphia, Pennsylvania: Elsevier Saunders; 2013. pp. 609–619. [Google Scholar]
- 10.Pomonis JD, Boulet JM, Gottshall SL, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114:339–346. doi: 10.1016/j.pain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Ferland CE, Laverty S, Beaudry B, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97:603–610. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Fernihough J, Gentry C, Malcangio M, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Im HJ, Kim JS, Li X, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62:2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: Basic studies in animal models. Osteoarthritis Cartilage. 2013;21:1308–1315. doi: 10.1016/j.joca.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otis C, Gervais J, Guillot M, et al. Concurrent validity of different functional and neuroproteomic pain assessment methods in the rat osteoarthritis monosodium iodoacetate (MIA) model. Arthritis Res Ther. 2016;18:150. doi: 10.1186/s13075-016-1047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otis C, Guillot M, Moreau M, et al. Spinal neuropeptide modulation, functional assessment and cartilage lesions in a monosodium iodoacetate rat model of osteoarthritis. Neuropeptides. 2017;65:56–62. doi: 10.1016/j.npep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Hummel M, Whiteside GT. Measuring and realizing the translational significance of preclinical in vivo studies of painful osteoarthritis. Osteoarthritis Cartilage. 2017;25:376–384. doi: 10.1016/j.joca.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Barve RA, Minnerly JC, Weiss DJ, et al. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: Relevance to human disease. Osteoarthritis Cartilage. 2007;15:1190–1198. doi: 10.1016/j.joca.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Bove SE, Laemont KD, Brooker RM, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14:1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Iijima H, Aoyama T, Ito A, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22:1036–1043. doi: 10.1016/j.joca.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Iijima H, Aoyama T, Ito A, et al. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthritis Cartilage. 2016;24:1092–1102. doi: 10.1016/j.joca.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Iijima H, Aoyama T, Tajino J, et al. Subchondral plate porosity colocalizes with the point of mechanical load during ambulation in a rat knee model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2016;24:354–363. doi: 10.1016/j.joca.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis” — The need for consensus? Osteoarthritis Cartilage. 2012;20:261–267. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Mapp PI, Sagar DR, Ashraf S, et al. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage. 2013;21:1336–1345. doi: 10.1016/j.joca.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai HC, Chen TL, Chen YP, Chen RM. Traumatic osteoarthritis-induced persistent mechanical hyperalgesia in a rat model of anterior cruciate ligament transection plus a medial meniscectomy. J Pain Res. 2018;11:41–50. doi: 10.2147/JPR.S154038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007;87:349–359. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Williams J, Felten D, Peterson R, O’Connor B. Effects of surgically induced instability on rat knee articular cartilage. J Anat. 1982;134:103–109. [PMC free article] [PubMed] [Google Scholar]
- 30.Ferland CE, Pailleux F, Vachon P, Beaudry F. Determination of specific neuropeptides modulation time course in a rat model of osteoarthritis pain by liquid chromatography ion trap mass spectrometry. Neuropeptides. 2011;45:423–429. doi: 10.1016/j.npep.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Curran-Everett D, Benos DJ American Physiological Society. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Endocrinol Metab. 2004;287:E189–191. doi: 10.1152/ajpendo.00213.2004. [DOI] [PubMed] [Google Scholar]
- 32.Appleton CT, McErlain DD, Pitelka V, et al. Forced mobilization accelerates pathogenesis: Characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9:R13. doi: 10.1186/ar2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haywood AR, Hathway GJ, Chapman V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-25581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol. 2005;17:624–628. doi: 10.1097/01.bor.0000172800.49120.97. [DOI] [PubMed] [Google Scholar]
- 35.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 36.Schaible HG, Richter F, Ebersberger S, et al. Joint pain. Exp Brain Res. 2009;196:153–162. doi: 10.1007/s00221-009-1782-9. [DOI] [PubMed] [Google Scholar]
- 37.Aso K, Izumi M, Sugimura N, Okanoue Y, Ushida T, Ikeuchi M. Nociceptive phenotype alterations of dorsal root ganglia neurons innervating the subchondral bone in osteoarthritic rat knee joints. Osteoarthritis Cartilage. 2016;24:1596–1603. doi: 10.1016/j.joca.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Pintér E, Helyes Z, Szolcsányi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther. 2006;112:440–456. doi: 10.1016/j.pharmthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Bär KJ, Schurigt U, Scholze A, et al. The expression and localization of somatostatin receptors in dorsal root ganglion neurons of normal and monoarthritic rats. Neuroscience. 2004;127:197–206. doi: 10.1016/j.neuroscience.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 40.Meini S, Maggi CA. Knee osteoarthritis: A role for bradykinin? Inflamm Res. 2008;57:351–361. doi: 10.1007/s00011-007-7204-1. [DOI] [PubMed] [Google Scholar]
- 41.Orita S, Ishikawa T, Miyagi M, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brederson JD, Chu KL, Xu J, et al. Characterization and comparison of rat monosodium iodoacetate and medial meniscal tear models of osteoarthritic pain. J Orthop Res. 2018 doi: 10.1002/jor.23869. [DOI] [PubMed] [Google Scholar]
- 43.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Inglis JJ, McNamee KE, Chia SL, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008;58:3110–3119. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 45.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 47.Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134–138. doi: 10.1016/j.rehab.2016.01.006. [DOI] [PubMed] [Google Scholar]