Abstract
In Korea, for the past 30 years (1987–present), porcine epidemic diarrhea (PED) has been established as an endemic situation in which multiple genogroups of classical G1 and G2b, and the recently introduced pandemic G2a, coexisted. Because of the dynamic nature of the virus, continuous field monitoring for PEDV strains is required. This study is the first to reveal prevalence of PEDV in 9 sampling provinces, with an overall detection rate of 6.70%. Porcine endemic diarrhea virus (PEDV) was present in pigs of all ages, especially in the non-PED vaccinated groups. The highest detection rate was in the finisher group (2.34%), followed by that in the newborn group (1.56%). Secondly, using Sanger sequencing, this study recovered a complete genome (28 005 nucleotides long) of NB1 strain from a farm severely affected by PED. Analyses of nucleotide and deduced amino acid sequences showed that NB1 differed from 18 other Korean PEDV mostly in 4 protein coding genes: ORF1a, ORF1b, S, and N. Two amino acid substitutions (V635E and Y681Q) in the COE and S1D neutralizing epitopes of NB1 resulted in antigenic index alteration of the adjacent sites, one of which contributed to a mutation that escaped neutralizing antibodies.
Résumé
En Corée, pour les 30 dernières années (1987 à ce jour), la diarrhée épidémique porcine (DEP) s’est établie comme une situation endémique dans laquelle de multiples génogroupes des classiques G1 et G2b, ainsi que le G2a pandémique récemment introduit, ont coexisté. Étant donné la nature dynamique du virus, un suivi continu sur le terrain des souches de DEP est requis. La présente étude est la première à révéler la prévalence de DEP dans neuf provinces échantillonnées, avec un taux de détection global de 6,70 %. Le virus de la DEP (VDEP) était présent chez les porcs de tout âge, spécialement dans les groupes d’animaux non-vaccinés contre la DEP. Les animaux dans le groupe en finition avaient taux de détection le plus élevé (2,34 %), suivi par ceux du groupe des nouveau-nés (1,56 %). Deuxièmement, en utilisant le séquençage de Sanger, nous avons récupéré un génome complet (28 005 nucléotides de long) de la souche NB1 sur une ferme sévèrement affectée par la DEP. L’analyse des nucléotides et des séquences d’acides aminés déduites a montré que NB1 différaient de 18 autres VDEP coréens principalement dans quatre gènes codant pour protéines: ORF1a, ORF1b, S, et N. Deux substitutions d’acides aminés (V635E et Y681Q) dans les épitopes neutralisants COE et S1D de NB1 ont résulté en une altération de l’index antigénique des sites adjacents, dont l’un contribuait à une mutation qui échappait aux anticorps neutralisants.
(Traduit par Docteur Serge Messier)
Introduction
Porcine epidemic diarrhea virus (PEDV) is a single-stranded, positive-sense RNA virus of the genus Alphacoronavirus, family Coronaviridae (1). The PEDV genome contains at least 7 open reading frames (ORF) including ORF1a/1b, spike (S), ORF3, envelop (E), membrane (M), and nucleocapsid (N) in a 5′–3′ order (2,3). It has been reported that PEDV evolves at the rate of 6.2 × 10−4 substitutions/site/year (4) and displays high levels of genetic variation in several coding regions, especially in the S gene (4–7). The S gene encodes for spike protein, which is divided into S1 and S2 domains. The outer surface of the S1 domain is responsible for binding to the host-specific receptor, while the S2 involves in the fusion process between the viral and cellular membranes (4). To date, several neutralizing epitopes on the spike protein were mapped, such as: S10, S1B (8), COE (within S1B region) (9), S1D (10), and 2C10 (11), etc.
Porcine epidemic diarrhea virus is highly enteropathogenic and causes acute diarrhea in swine, which severely affects piglets (12,13). The disease was reported for the first time in 1976 (14). However, following the first outbreak in the USA in 2013 (15), PED has emerged in almost all swine-producing countries (16). In Asia, Korea was one of the earliest PED-affected countries, with a report of a retrospective clinical case in 1987 (17). Since then, PEDV has been identified as an endemic infection with annual outbreaks (18). Over a long period of time (~1987 to 2017), PEDV in Korea was shown to belong to different genogroups: the continuously circulating classical G1 and G2b, and the recently identified pandemic G2a (19). Because of the presence of the pandemic G2a PEDV strain, which is distantly related to the classical PED vaccine strain (20), further studies about molecular and biological characterization are required to develop vaccines and continuously monitor field PEDV strains. With the goal of contributing to the understanding of molecular evolution of PEDV in Korea, this study characterized the genome of NB1 field strain, which was from a PED-affected pig farm.
Materials and methods
Samples
Intestine and fecal samples from pigs showing signs of diarrhea (n = 642) were randomly collected between January 2016 and May 2017 from 59 commercial farms across 9 provinces in south Korea and were screened for the presence of PEDV. Age groups ranged from suckling to sow pigs. Samples were eluted in phosphate-buffered saline (PBS) at pH 7.2 and stored at −20°C until use. We focused on the NB farm (Gyeongbuk province), which was the most severely affected farm with 100% mortality compared with the average mortality rate of 20% to 50%. No previous history of vaccination against PEDV was present in this farm.
Detection of PEDV and co-viral infections
Total RNA was extracted using TRIzol LS (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions. The RNA was then converted into cDNA with the use of random hexamers and the RNA to cDNA EcoDry premix kit (Clontech, Otsu, Japan) following the manufacturer’s protocol. All polymerase chain reactions (PCR) were done using a premix (AccuPower ProFi Taq PCR PreMix; Bioneer, Daejeon, Korea). For PEDV detection, the PEDV-specific primers PEDV-460F [5′-AATGGCAACAACAGGTCC-3′] and PEDV-947R [5′-GCATCAACACCTTTTTCGAC-3′] were designed, which amplified a 488-base pair (bp) region of the nucleocapsid protein coding gene. The thermal profile used in PCR reactions consisted of an initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 7 min. Specific primers and PCR conditions reported previously were used for the detection of common pig diarrhea viruses including PDCoV (21), kobuvirus (22), and rotavirus (23). Additionally, TGEV was detected using the i-TGEV/PEDV detection kit (iNtRON, Korea). The sensitivities of the conventional reverse transcription (RT)-PCR were 102.5TCID50/mL for TGEV, 101.8TCID50/mL for rotavirus, 101.8TCID50/mL for PDCoV, and 101.4TCID50/mL for kobuvirus. The lowest viral concentrations detected by RT-PCR were 101.0TCID50/mL for PEDV. Non-specific reactions were not observed when other viruses, bacteria, and cells were used to access the specificity of RT-PCR.
Complete genome sequencing of NB1 strain
To perform a genetic characterization of a field PEDV strain (namely NB1) from the NB farm (the farm most severely affected by PED), the full-length genome of the virus was sequenced using a primer walking method, which utilized 26 overlapping primer pairs (24). Amplification of the S and N genes was not successful using one set of previously reported primers (24) primers. However, using another set of primers reported (25), the S and N genes of NB1 were successfully amplified. All PCR products were purified via gel extraction and further processed for T-vector A tail (TA) cloning and transformation. The complete genome sequence of NB1 was registered under the GenBank accession number MF281416.
Molecular characterization of NB1
All known PEDV protein coding sequences of NB1 and of 18 Korean PEDV with complete genome sequence available in GenBank were compared (supplementary alignment S1–S7; available from corresponding authors upon request). Amino acid sequence alignment was done using multiple sequence alignment based on fast Fourier transform (MAFFT) (26) with default options. Mismatches in amino acid sequence alignment were visualized and images generated using the highlighter tool from the website https://www.hiv.lanl.gov/content/sequence/HIV/HIVTools.html
Neutralizing epitopes of the spike protein of PEDV, COE, and S1D have been determined (9,10). Jameson–Wolf antigenic index implemented using computer software (Lasergene Protean software; DNASTAR, Madison, Wisconsin, USA) to predict whether amino acid substitutions would affect antigenic properties of neutralizing epitopes. The antigenic index calculated for each amino acid site was plotted using computer software (Microsoft Excel, Microsoft Office 2016 version; Microsoft, Redmond, Washington, USA).
Results
Detection of PEDV and common enteric viruses
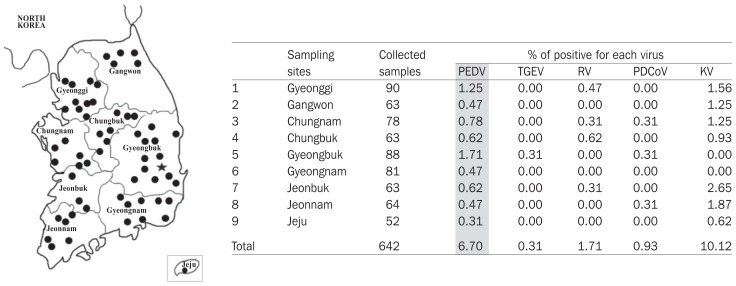
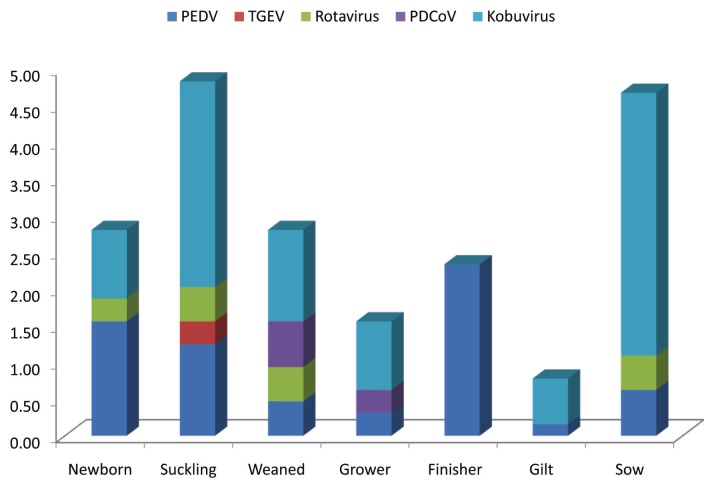
As shown in Figure 1, PEDV was detected from samples collected from all 9 sampling provinces between January 2016 and May 2017. The overall detection rate of PEDV was 6.70% (43 positive samples/642 tested samples). Besides PEDV, 4 common enteric viruses of swine were found, of which porcine kobuvirus was the most frequent pathogen (10.12%). The RT-PCR results, summarized based on age group of pigs, are shown in Figure 2. The PEDV was detected in all age groups, of which the highest detection rate was in the finisher group (2.34%), followed by that in the newborn and suckling groups (1.56% and 1.25%, respectively). Although at a low rate (0.16% and 0.62%), the virus was also found in gilts and sows, respectively.
Figure 1.
Sampling locations and detection rate of common enteric viruses. Sampling sites (filled circles) in each province are indicated in the left panel. Location of the NB farm is indicated by a filled star. Right panel shows reverse transcription-polymerase chain reaction (RT-PCR) results for viruses including porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), rotaviruses (RV), porcine deltacoronavirus (PDCoV), and porcine kobuvirus (KV).
Figure 2.
Detection rate of common enteric viruses based on age group. Samples were sorted into 7 groups including newborn (< 7 d), suckling (< 30 d), weaned (30 to 60 d), grower (60 to 90 d), finisher (≥ 90 d), gilt, and sow.
Genetic characterization of a field strain from a farm severely affected by PED
Among the 58 swine farms investigated, this study focused on the NB farm (indicated by a star, Figure 1), which was severely affected by PED. This farm is a single-site production system (separated into farrow-to-grower and grower-to-finisher units). The suckling group in this farm was reported to exhibit profuse diarrhea with mortality rates being approximately 100%. Except for PEDV-positive samples, all intestine samples of piglets collected from the NB farm were negative for TGEV, PDCoV, rotavirus, and kobuvirus. Because of the large genome size, this study was only able to obtain one PEDV complete genome from that farm.
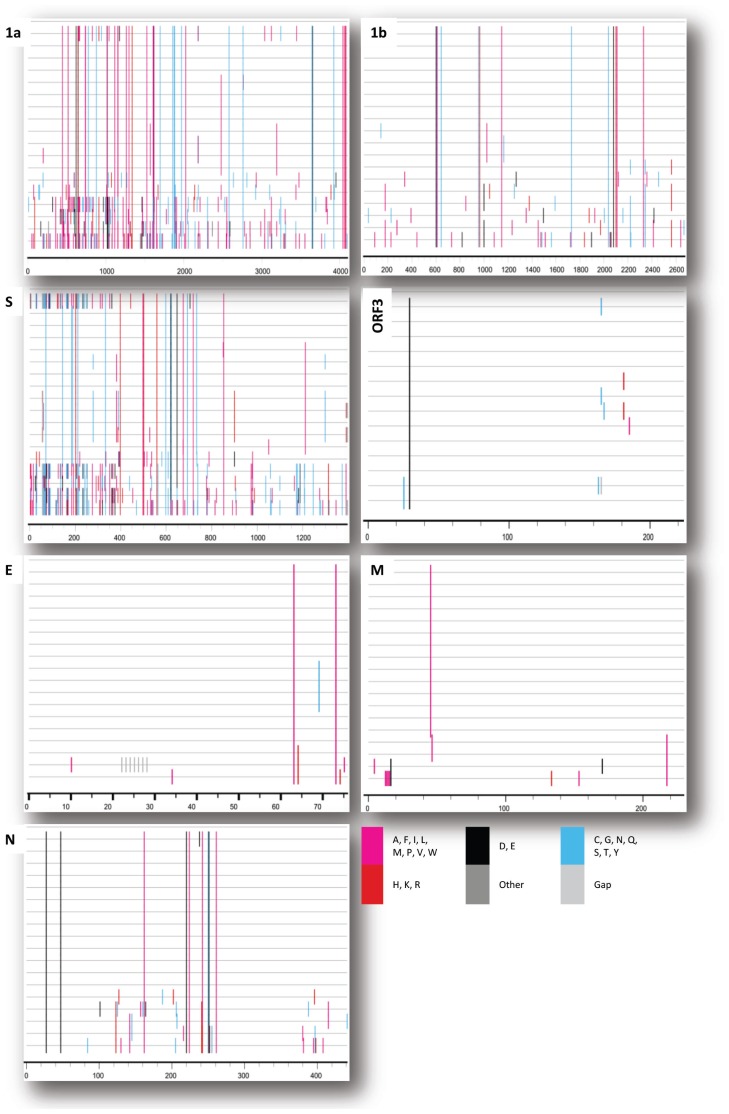
The complete genome sequence of NB1 (MF281416) is 28 005 nucleotides long. The genomic arrangement and corresponding nucleotide positions are as follows: 5′ un-translated region (nt 1 to 266), ORF1a (nt 266 to 12574), ORF1b (nt 12574 to 20610), spike (S, nt 20607 to 24764), ORF3 (nt 24764 to 25438), envelope (E, nt 25419 to 25649), membrane (M, nt 25657 to 26337), nucleocapsid (N, nt 26349 to 27674), and 3′ un-translated region (nt 27676 to 28,005). Detailed comparison of the deduced amino acid sequence of 7 coding regions (ORF1a, ORF1b, S, ORF3, E, M, and N) between NB1 and 18 Korean PEDV strains is shown in Figure 3.
Figure 3.
Amino acid polymorphisms in NB1 (top line) and 18 other Korean PEDV strains (remaining lines). Mismatches in amino acid sequence alignment were visualized and images generated using the highlighter tool. Amino acids differing from those in NB1 are highlighted with the symbol “|” in a color depending on amino acid identity.
It was clear that the deduced amino acid sequence of NB1 differed from the 18 Korean PEDVs mostly in polyprotein (encoded by ORF1a, ORF1b), S protein, and N protein (Figure 3). In sharp contrast, a few differences were observed in ORF3, E, and M proteins. Of note, in 6 out of 7 nonstructural/structural proteins (except for M protein), NB1 had 81 unique amino acid residues distributed in the following order: ORF1a, 39; ORF1b, 13; S, 17; ORF3, 1; M, 0; and N, 9. Among 81 unique amino acids, 34 amino acids resulted in change in physical properties (polar to non-polar or vice versa) comparing NB1 and the 18 Korean PEDV. Details are shown in supplementary alignment S1–S7 and Table S1 (available from corresponding authors upon request).
Neutralizing epitope variations in the spike protein of the NB1 strain
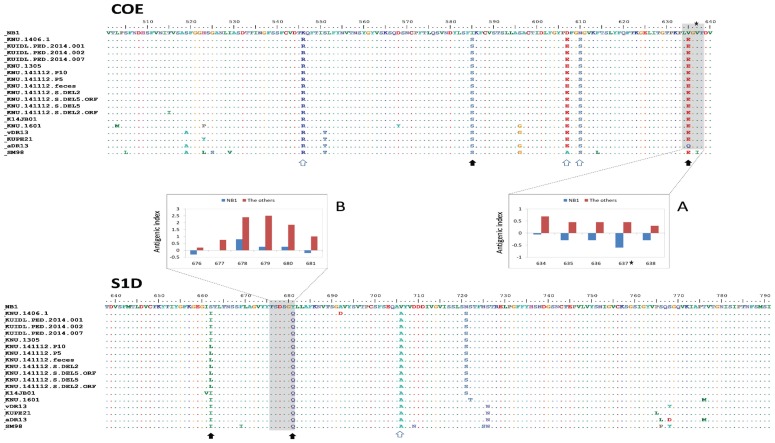
Figure 4 shows amino acid alignment of COE and S1D epitopes between NB1 and 18 Korean PEDV strains. There were 2 regions in the COE and S1D of NB1 had negative or lower antigenic index in comparison with the other PEDV. In the COE, 2 out of 5 unique substitutions in NB1 resulted in physical property change (hydrophilic–hydrophobic). The amino acid substitution V635E significantly altered antigenic indexes of 4 adjacent sites (inserted panel A). In the S1D epitope, 2 out of 3 unique substitutions in NB1 resulted in physical property change (hydrophilic–hydrophobic). Of these, the amino acid substitution Y681Q was also found to alter antigenic indexes of 4 adjacent sites (inserted panel B).
Figure 4.
Amino acid substitutions in neutralizing epitopes in NB1 and 18 field Korean strains. Amino acid position was numbered based on NB1 epitopes COE (aa 501–640) and S1D (aa 638–791). Unique amino acids in NB1 are indicated with empty arrows, while those with physical property changes (polar to non-polar or vice versa) are indicated with filled arrows. Shaded areas in COE and S1D are regions where antigenic indexes (inserted panels) were different due to amino acid variations between NB1 and the other Korean PEDV.
Discussion
Since the first clinical case in 1987, PED emerged in Korea as an endemic disease with annual outbreaks (18). Currently, PEDV is not only found in domestic pigs but also in wild boar at an incidence rate of 9.75% (27). Therefore, it was plausible to detect the virus in all 9 sampling provinces in this study (Figure 1). The overall PEDV detection rate of 6.70% in this report is considered low because it is comparable to the detection rate of 7.80% in 2008 (6 y prior to re-emergence of PED in 2013) (6). However, this study focused only on pigs with symptoms of diarrhea. Consequently, real incidence of PEDV in the field might be higher than that reported here. Because the modified live PED vaccine is used in Korea and our RT-PCR method was not able to distinguish between field and vaccine strains, it was suspected that the 6.70% PEDV-positive pigs might include vaccinated pigs. However, based on the following reasons, the percentage of PED vaccinated pigs could be neglected. First, due to reduction in vaccine efficacy following the emergence of the pandemic genogroup 2a in 2013 (20), the usage of the attenuated genogroup 1-based vaccine decreased. Second, the PED vaccine was applied only to the sow group. Thus, the data shown in Figure 2 not only reflected the presence of PEDV in non-PED vaccinated groups (from newborn to gilt), but also implied that PEDV actively circulated in a farm.
Since 2013, a new pattern of PEDV emerged in Korea, of which a mixture of classical genogroups (G1 and G2b) and pandemic G2a was detected (19). Consequently, comparing genetic sequences between field strains of each genogroup is a topic of interest. The result shown in Figure 3 revealed that ORF1a/1b, S protein, and N protein contained the most diversity in amino acids between NB1 and 18 Korean PEDV strains. Generally, that result agreed with previous publications (4,28–30) which showed several high variation regions occurring across the PEDV genome, including ORF1 (regions encoding nsp2, nsp3), S (especially the S1 domain-encoding region), and ORF3 genes. Despite the fact that 14 out of 18 strains compared with NB1 belonged to genogroup G2 (data not shown), NB1 still displayed 81 unique amino acids across the genome. This finding highlighted the importance of continuous monitoring of field PEDV strains for updating molecular evolution of the virus.
The S1 domain of PEDV is comprised of 4 core regions, S10 to S1CD (8,31). The binding of antibodies to either N-terminal sialic acid binding region (S10) or the protein receptor binding domain (S1B, containing the COE epitope) resulted in neutralization (8). However, mutant virus could escape neutralizing activities by single amino acid substitution in S1B (V638G, according to the position of GDU strain, KU985230) (8). Though that escape mutant was not observed in NB1 strain (indicated by a star, Figure 4), amino acid substitutions from glutamate (E) to valine (V) at position 635 (equivalence to position 636 of GDU strain) of NB1 strain significantly altered antigenic indexes of 4 adjacent sites (including the escape mutant position, inserted panel A). However, with the current experiment data, the significance of that prediction is not easy to comprehend.
In conclusion, the NB1 strain, recovered from a farm severely affected by PED, displayed significant genetic differences in 4 protein coding genes (ORF1a, ORF1b, S, and N) in comparison with 18 other Korean PEDV strains. Several amino acid substitutions in the COE and S1D neutralizing epitopes of NB1 resulted in antigenic index alteration of the adjacent sites, one of which contributed to mutation that escaped neutralizing antibodies.
Acknowledgments
The authors thank Hye Jung Yang and Jung Ah Kim for their technical assistance.
Funding
This study was supported by a grant (No. PJ011184) from the BioGreen 21 Programs Rural Development Administration, and Bioindustry Technology Development Program (No. 114055031SB010), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
In addition, this work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Advanced Production Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (315005-3).
References
- 1.Song D, Park B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kocherhans R, Bridgen A, Ackermann M, Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte M, Gelfi J, Lambert P, Rasschaert D, Laude H. Genome organization of porcine epidemic diarrhoea virus. In: Laude H, Vautherot J-F, editors. Coronaviruses: Molecular Biology and Virus-Host Interactions. Boston, Massachusetts: Springer; 1993. pp. 55–60. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis MC, Lam HC, Zhang Y, et al. Genomic and evolutionary inferences between American and global strains of porcine epidemic diarrhea virus. Prev Vet Med. 2016;123:175–184. doi: 10.1016/j.prevetmed.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Huo JY, Chen L, et al. Genetic variation analysis of reemerging porcine epidemic diarrhea virus prevailing in central China from 2010 to 2011. Virus Genes. 2013;46:337–344. doi: 10.1007/s11262-012-0867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Kim S, Song D, Park B. Novel porcine epidemic diarrhea virus variant with large genomic deletion, South Korea. Emerg Infect Dis. 2014;20:2089–2092. doi: 10.3201/eid2012.131642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diep NV, Norimine J, Sueyoshi M, Lan NT, Yamaguchi R. Novel porcine epidemic diarrhea virus (PEDV) variants with large deletions in the spike (S) gene coexist with PEDV strains possessing an intact S gene in domestic pigs in Japan: A new disease situation. PLoS One. 2017;12:e0170126. doi: 10.1371/journal.pone.0170126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Li W, Lucio de Esesarte E, et al. Cell attachment domains of the porcine epidemic diarrhea virus spike protein are key targets of neutralizing antibodies. J Virol. 2017;91:00273–00217. doi: 10.1128/JVI.00273-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SH, Bae JL, Kang TJ, et al. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells. 2002;14:295–299. [PubMed] [Google Scholar]
- 10.Sun DB, Feng L, Shi HY, et al. Spike protein region (aa 636–789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies. Acta Virol. 2007;51:149–156. [PubMed] [Google Scholar]
- 11.Cruz DJ, Kim CJ, Shin HJ. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize porcine epidemic diarrhea virus. Virus Res. 2008;132:192–196. doi: 10.1016/j.virusres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debouck P, Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am J Vet Res. 1980;41:219–223. [PubMed] [Google Scholar]
- 13.Jung K, Wang Q, Scheuer KA, Lu Z, Zhang Y, Saif LJ. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg Infect Dis. 2014;20:662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood EN. An apparently new syndrome of porcine epidemic diarrhoea. Vet Rec. 1977;100:243–244. doi: 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson GW, Hoang H, Schwartz KJ, et al. Emergence of porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- 16.Lee C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol J. 2015;12:193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park NY, Lee SY. Retrospective study of porcine epidemic diarrhea virus (PEDV) in Korea by in situ hybridization. Korean J Vet Res. 1997;37:809–816. [Google Scholar]
- 18.Yang DK, Kim HH, Lee SH, Yoon SS, Park JW, Cho IS. Isolation and characterization of a new porcine epidemic diarrhea virus variant that occurred in Korea in 2014. J Vet Sci. 2017;19:71–78. doi: 10.4142/jvs.2018.19.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung HC, Lee JH, Nguyen VG, et al. New emergence pattern with variant porcine epidemic diarrhea viruses, South Korea, 2012–2015. Virus Res. 2016;226:14–19. doi: 10.1016/j.virusres.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song D, Moon H, Kang B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin Exp Vaccine Res. 2015;4:166–176. doi: 10.7774/cevr.2015.4.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Chung HC, Nguyen VG, et al. Detection and phylogenetic analysis of porcine deltacoronavirus in Korean swine farms, 2015. Transbound Emerg Dis. 2016;63:248–252. doi: 10.1111/tbed.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter G, Egyed L. Bovine kobuvirus in europe. Emerg Infect Dis. 2009;15:822–823. doi: 10.3201/eid1505.081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MH, Jeoung HY, Park HR, Lim JA, Song JY, An DJ. Phylogenetic analysis of porcine astrovirus in domestic pigs and wild boars in South Korea. Virus Genes. 2013;46:175–181. doi: 10.1007/s11262-012-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Kou Q, Ge X, Zhou L, Guo X, Yang H. Phylogenetic analysis of porcine epidemic diarrhea virus field strains prevailing recently in China. Arch Virol. 2013;158:711–715. doi: 10.1007/s00705-012-1541-2. [DOI] [PubMed] [Google Scholar]
- 25.Chung HC, Nguyen VG, Moon HJ, et al. Isolation of porcine epidemic diarrhea virus during outbreaks in South Korea, 2013–2014. Emerg Infect Dis. 2015;21:2238–2240. doi: 10.3201/eid2112.150437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DU, Kwon T, Je SH, et al. Wild boars harboring porcine epidemic diarrhea virus (PEDV) may play an important role as a PEDV reservoir. Vet Microbiol. 2016;192:90–94. doi: 10.1016/j.vetmic.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Gauger PC, Stafne MR, et al. Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S-INDEL-variant strains in conventional neonatal piglets. J Gen Virol. 2016;97:1107–1121. doi: 10.1099/jgv.0.000419. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, Ma J, Wang Y, et al. Genomic and epidemiological characteristics provide new insights into the phylogeographical and spatiotemporal spread of porcine epidemic diarrhea virus in Asia. J Clin Microbiol. 2015;53:1484–1492. doi: 10.1128/JCM.02898-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F, Zhu Y, Wu M, et al. Comparative genomic analysis of classical and variant virulent parental/attenuated strains of porcine epidemic diarrhea virus. Viruses. 2015;7:5525–5538. doi: 10.3390/v7102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls AC, Tortorici MA, Bosch BJ, et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]