Abstract
Background
Inflammation is one of the most significant mechanisms of hepatic ischemia-reperfusion injury (IRI). Sufentanil has a protective effect against liver injury by reducing inflammatory response. In this study, we used a cellular hepatic ischemic/reoxygenated (IR) model to determine whether sufentanil preconditioning protects against hepatic IRI.
Material/Methods
The human normal liver cells line L-O2 was studied. The levels of glutamic oxaloacetic transaminase (AST), lactate dehydrogenase (LDH), malonaldehyde (MDA), and superoxide dismutase (SOD) were measured using corresponding assay kits. The protein levels of total and phosphorylated ERK1/2, JNK, and p38, and the expression of p65 and COX2 genes, were measured by Western blotting. The levels of inflammatory factors were examined by ELISA. The Cell Counting Kit-8 (CCK-8) was used to determine if the viability of L-O2 cells was affected by sufentanil. The effects of sufentanil on IR-induced cell apoptosis were examined by flow cytometry.
Results
IR-induced caused L-O2 cells to become rounded and to have a lower adhesive rate than normal cells. The levels of AST, LDH, and MDA were higher but the level of SOD was lower in the IR group than in the control group. The phosphorylated protein levels of ERK1/2, JNK, and p38, along with the expression of p65 and COX2, were upregulated in the IR group compared to the normal group. In addition, a variety of inflammatory factors were secreted in L-O2 cells after IR. The viability of L-O2 cells decreased and cell apoptosis increased significantly after IR treatment. All indexes of cell injury were reversed by sufentanil in a concentration-dependent manner.
Conclusions
Sufentanil stimulation triggers downregulation of inflammatory factors such as HIF-1α, TNF-α, IL-1β, and IL-6, possibly through suppressing the p38/ERK/JNK/NF-κB-p65/COX2 pathways, and thereby reduces the damage to IR hepatic cells.
MeSH Keywords: Inflammation, MAP Kinase Kinase Kinases, Reperfusion Injury, Sufentanil
Background
IRI refers to hepatic blood supply recovery after ischemia associated with hepatic surgery such as hepalobectomy, and hepatic transplantation further aggravates hepatic dysfunction and structural damage instead of ameliorating functional recovery [1]. It is vitally important significance to discover protection strategies since IRI can trigger a spectrum of organ abnormalities resulting in various post-operative complications [2].
Anesthetic preconditioning (APC) has been widely regarded as a promising protective approach because it has a remarkable ability to effectively reduce IRI by suppressing inflammation [3,4]. Fentanyl, a high-potency opiate, is widely prescribed to treat acute and chronic pain [5,6]. The mechanisms responsible for the analgesic effects of fentanyl have been extensively investigated [7–9]. Sufentanil, a derivative of fentanyl, is an opioid with high affinity to opioid receptors. Its analgesic potency is 8 times that of fentanyl, and it is the most potent opioid agonist known [10]. Compared with fentanyl, the elimination of the effect of sufentanil in vivo is faster, and the effect on respiratory function is less [11]. Other studies have shown that there is no obvious accumulation in tissues after use of sufentanil, and it is also easily cleared in adipose and muscle tissues. It has been well documented that inflammation results in pain [12,13]. Sufentanil has the advantages of strong analgesic effect, quick onset, and short waking time [11]. It has been previously concluded that narcotics have a protective effect against acute lethal liver injury by reducing the magnitude of inflammatory response [14], which is one of the initiating factors causing hepatic IRI to cause damage [15,16]. However, the effect of sufentanil on hepatic IRI is unclear. In this study, the effects of sufentanil on hepatic ischemic reperfusion injury was explored.
Inflammation is an important hallmark of hepatic IRI [17], and it has been previously reported that inflammatory stimuli increase expression of mitogen-activated protein kinases p38 [17], ERK1/2 [18], and JNK [19], as well as activating nuclear factor kappa B (NF-κB) and downstream inflammation-exacerbating factors COX2, TNF-α, IL-1β, and IL-6 [20]. ERK1/2, JNK, and p38 belong to the superfamily of serine/threonine kinases, which are mainly involved in activation of nuclear transcription factors controlling cell proliferation, differentiation, and apoptosis [21].
In our study, we found that sufentanil increased cell activity and decreased apoptosis of IR hepatocytes, accompanied by reduction of inflammatory factors such as HIF-1α, TNF-α, IL-1β, and IL-6, indicating the protective function of sufentanil against hepatic IRI. We also found that a protective role of sufentanil rooted in suppressing the p38/ERK1/2/JNK/NF-κB-p65/cox2 signaling pathway.
Material and Methods
Cell culture and pretreatment
The human normal liver cells line (L-O2) was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin (100U/ml), streptomycin (100 ug/ml), and 10% fetal bovine serum (FBS) at 37°C in a humidified incubator with 5% CO2. When the cell count reached (2×103) cells per well, cells were pretreated with sufentanil (Yichang Humanwell Pharmaceutical Co. Yichang, China) in various gradient concentrations (5, 10, 20, and 40 μM) for 24 h. To obtain ischemia-hypoxia/reoxygenation models, L-O2 cells were cultured with serum-free medium in a sealed container with 95% N2 and 5% CO2 at a flow rate of 10 L/min for 5 min, and then were incubated in a hypoxic environment of 1% O2 at 37°C for 8 h. After that, normal medium was added, and the cells were cultured 24 h in a normal incubator. Cells were randomly divided into 3 groups: a normal L-O2 cells group (control group), an ischemia-reperfusion group (IR group), an IR group treated with normal saline (10 ml, 0.9%) (NS group), and an IR group treated with different doses of sufentanil (5, 10, 20, and 40 μM) (5, 10, 20, and 40 μM groups). The morphology of L-O2 cells was then viewed under a microscope (OLYMPUS, Japan).
Cell viability assay
The CCK-8 kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to determine cell viability. Briefly, L-O2 cells were seeded into 96-well plates and replenished with medium containing CCK-8 solution (15 μL CCK-8 in 100 μL medium) and incubated at 37°C for 4 h. The absorbance was analyzed at 490 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The viability of L-O2 cells was detected. All experiments were performed in triplicate.
Flow cytometry
The cells were collected into 10-ml centrifuge tubes and centrifuged at 1000 rpm for 5 min, then we removed the supernatant and washed the cells with incubation buffer. After centrifugation of 1000 rpm for 5 min, the cells were resuspended with 100 ul label solution and incubated for 15 min with exposure to light. Then, we repeated the centrifugation and washed the cells with incubation buffer. The SA-FLOUS solution was added into centrifuge tubes and the cells were incubated without light at 4°C for 20 min. The excitation wavelength was 488 nm, and detection wavelength was 515 nm for FITC and over 560 nm for PI using flow cytometry (FACSCalibur) and CellQuest Pro software (both from BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s protocols. Experiments were repeated 3 times.
Hepatocyte functional measurement
AST activity was measured by use of an AST activity assay kit (MAK055-1KT, Sigma-Aldrich) at OD 450 nm. After incubation with a lactate dehydrogenase (LDH) assay kit (88953, Thermo Scientific™, USA) at room temperature for 30 min, reactions were stopped and LDH activity was determined by measuring the absorbance at 490 nm.
Measurement of cytokines
Levels of HIF-1α, TNF-α, IL1β, and IL-6 in the supernatant of the L-O2 cells culture obtained by centrifugation (15 min; 1000 g; 2–8°C) were measured using HIF-1α (DYC1935-5), TNF-α (DTA00C), IL-1β (DLB50), and IL-6 (S6050) ELISA kits (all from R&D Systems, Inc., Minneapolis, MN, USA) in accordance with the manufacturer’s protocol.
MDA and SOD assay
Cells were homogenized in 10 ml physiological saline and centrifuged at 12 000 g for 15 min at 4°C to obtain the supernatant, then the MDA and SOD contents were measured by ELISA kits (MDA kit, ml022446; SOD kit, ml022368) from Shanghai Enzyme-linked Biotechnology Co. (Shanghai, China) according to the manufacturer instructions.
Western blot
Cells were washed 3 times with phosphate-buffered saline, then they were homogenized in ice-cold lysis buffer and boiled for 10 min. The BCA Protein Assay Kit (P0010; Beyotime Institute of Biotechnology) was used to determine the protein concentration. Proteins were separated with sodium dodecyl sulfate polyacrylamide gels (SDS/PAGE), and transferred to nitrocellulose membranes. After 30-min incubation with 3% BSA at room temperature, they were incubated overnight at 4°C with primary antibody against p-p38 (#4511), ERK1/2 (#4695), p-ERK1/2 (#4376), JNK (#9252), and p-JNK (#9251) obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). p65 (ab32536), COX2 (ab52237), and p38 (ab178867) were purchased from Abcam (Cambridge, UK). Then, the membranes were washed with TBST and incubated with secondary antibody for 1 h. Protein signals were detected using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). GAPDH was used as the internal control.
Statistical analysis
Data are expressed as the mean ± standard deviation. All statistical analyses were performed using SPSS version 14.0 statistical software (SPSS, Inc., Chicago, IL, USA). The t test was used to evaluate the differences between 2 groups, while differences between multiple groups were assessed by one-way analysis of variance followed by Dunnett’s post hoc test. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Sufentanil reduces damage to IR hepatocytes
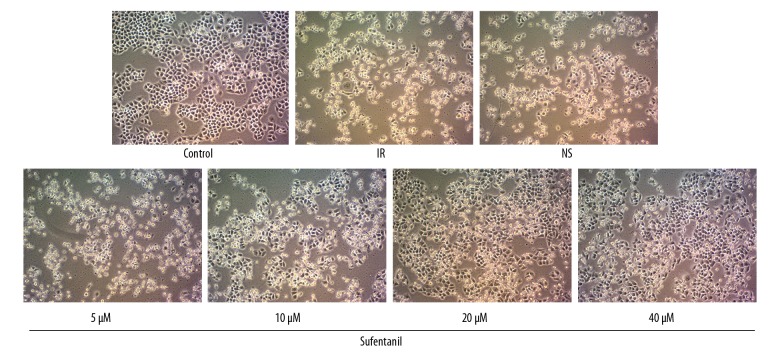
As shown in Figure 1, after IR, L-O2 cells became rounded and were floating in the nutrient medium, with lower adhesive rate than the normal cells in the control group. Surprisingly, incubation of damaged IR cells with sufentanil in various gradient concentrations (5, 10, 20, and 40 μM) reversed the abnormal cellular morphology and the increased attachment rate in a dose-dependent manner, while sterile saline solution treatment had no such effect in the NS group.
Figure 1.
Morphology of L-O2 cells after different treatments. Inverted microscopy showed that L-O2 cells were becoming round globular cell clusters with lower adhesive rate after IR treatment; however, Sufentanil restored the appearance of L-O2 cell organelles and increased cell adhesion, and higher concentration of sufentanil was associated with faster cell recovery (×200).
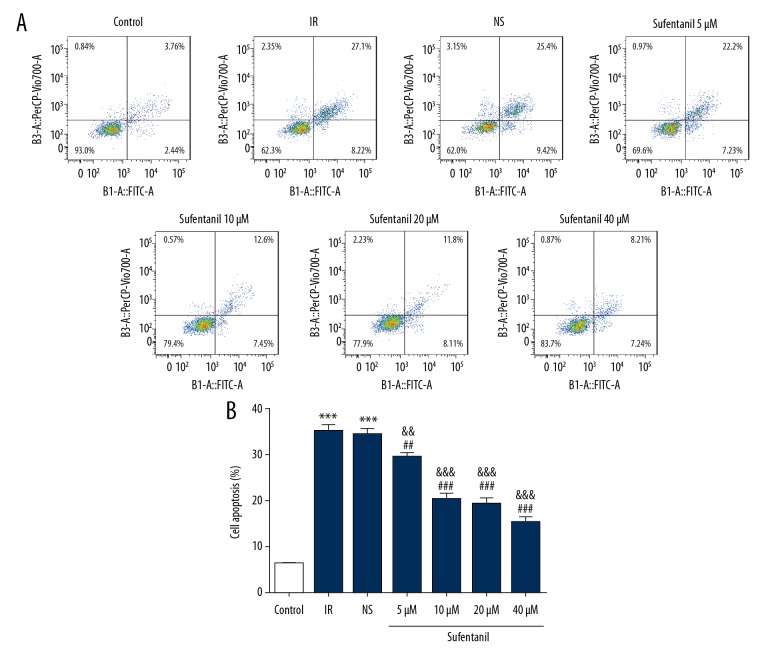
To further characterize the effect of sufentanil on cell activity, we used the Cell Counting Kit-8 (CCK-8) to confirm the effects of sufentanil treatment on cell viability of L-O2 cells with IR. After IR, L-O2 cells showed a substantial decrease in cell viability compared to the control group (Figure 2). The viability of L-O2 cells increased with the concentration of sufentanil, which had showed a brief increase followed by a gradual decrease in parallel with increased concentration, peaking at 10 μM sufentanil (Figure 2). Flow cytometry also suggested that sufentanil treatment gradually decreased the apoptosis induced by IR with increasing dosages (Figure 3A, 3B).
Figure 2.
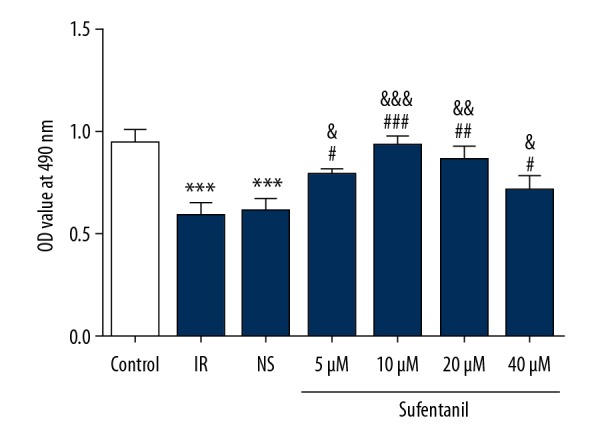
The viability of L-O2 cells after exposure to sufentanil (5, 10, 20, and 40 μM) for 24 h. *** p<0.001 vs. control group; # p<0.05, ## p<0.01, ### p<0.001 vs. IR group; & p<0.05, && p<0.01, &&& p<0.001 vs. NS group.
Figure 3.
(A) Apoptosis of L-O2 cells after exposure to sufentanil (5, 10, 20, and 40 μM) for 24 h was evaluated using flow cytometry. (B) Quantification of cell apoptosis in different groups. *** p<0.001 vs. control group; ## p<0.01, ### p<0.001 vs. IR group; && p<0.01, &&& p<0.001 vs. NS group.
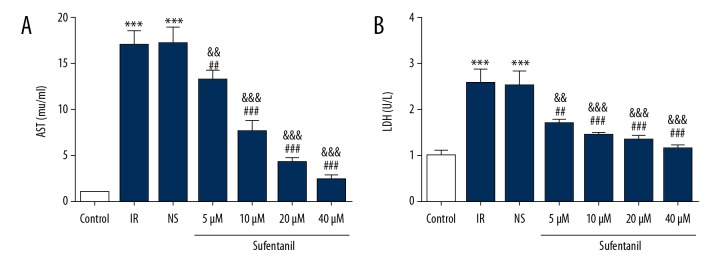
We used hepatocyte functional measurement kit to directly assess the expression of AST and LDH after sufentanil pre-incubation. The levels of AST and LDH were significantly higher in the IR group compared to the control group (Figure 4A, 4B), which shows that hepatocyte damage is organ-specific. We also found that after sufentanil treatment, cells had lower levels of AST and LDH than in the IR and NS groups, showing a linearly decreasing trend with increasing dosage (Figure 4). Our results reveal that sufentanil can reduce IR damage to hepatic cells.
Figure 4.
The levels of AST (A) and LDH (B) in different groups. *** p<0.001 vs. control group; ## p<0.01, ### p<0.001 vs. IR group; && p<0.01, &&& p<0.001 vs. NS group.
Sufentanil reverses typical inflammatory and ROS-related molecular changes during hepatic IRI
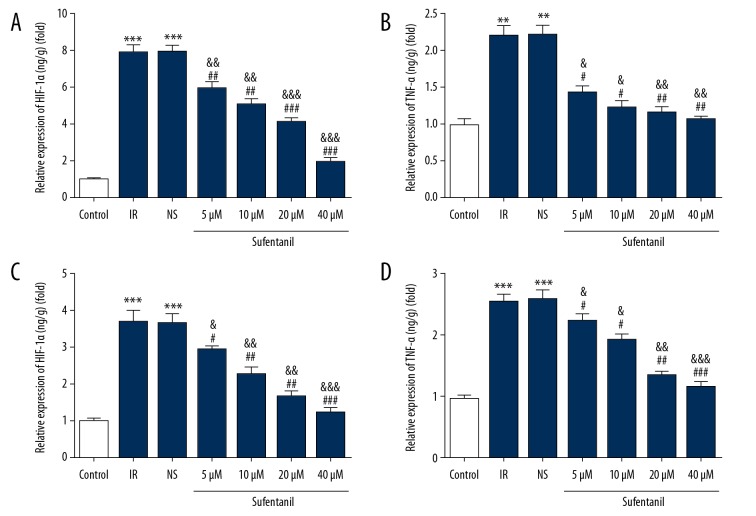
Hypoxia promotes progression of inflammation in multiple ways [22,23], in which hypoxia-inducible factor 1 alpha (HIF-1α) plays a critical role as a nuclear transcription activator [24,25]. Accumulation of initial cytokines such as tumor necrosis factor-α (TNF-α) [26], interleukins 1 beta (IL-1β) [27], and interleukins 6 (IL-6) [28] results in neutrophil acceleration and adhesion, leading to damage of tissues and organs during IRI. Based on these molecular changes during IR, we directly assessed their expressions after sufentanil pretreatment of IR L-O2 cells using ELISA. Figure 5 shows that the IR treatment of cells caused an increase in the levels of HIF-1α, TNF-α, IL-1β, and IL-6, which were all downregulated by sufentanil in a dose-dependent manner.
Figure 5.
Quantification of the levels of HIF-1α (A), TNF-α (B), IL-1β (C), and IL-6 (D) in different groups using ELISA. ** p<0.01, *** p<0.001 vs. control group; # p<0.05, ## p<0.01, ### p<0.001 vs. IR group; & p<0.05, && p<0.01, &&& p<0.001 vs. NS group.
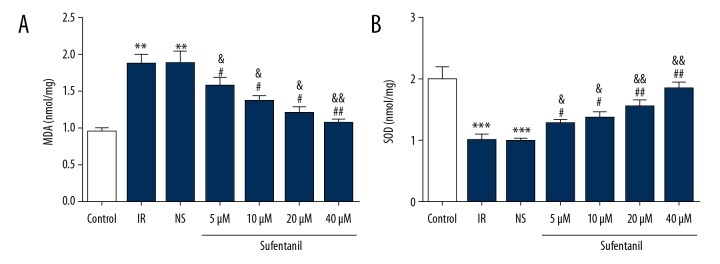
Generation of abundant reactive oxygen species (ROS) during hypoxia leads to damage through oxidative stress and lipid peroxidation [29,30], and inflammation-related neutrophil hyperactivation increases oxidative stress, thereby causing increased damage [31]. The changes in MDA generated by ROS-induced lipid peroxidation [32] and SOD, eliminated ROS [33]. Our results show that MDA in IR cells was significantly upregulated, but SOD was significantly reduced in the IR group compared to normal cells in the control group (Figure 6). Surprisingly, our analysis also revealed sufentanil increased SOD and decreased MDA compared to the IR and NS groups (Figure 6). In conclusion, sufentanil reverses deleterious changes of inflammatory and ROS-related molecules during hepatic IRI, showing its protective role in IRI.
Figure 6.
Expression level of MDA (A) and SOD (B) in different groups. ** p<0.01, *** p<0.001 vs. control group; # p<0.05, ## p<0.01 vs. IR group; & p<0.05, && p<0.01 vs. NS group.
Effects of sufentanil on L-O2 cells through suppressing p38/ERK/JNK/NF-κB-p65/COX2 pathways
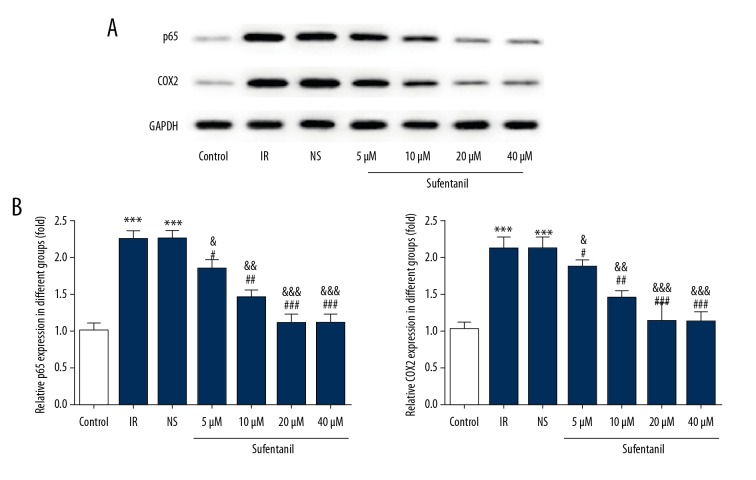
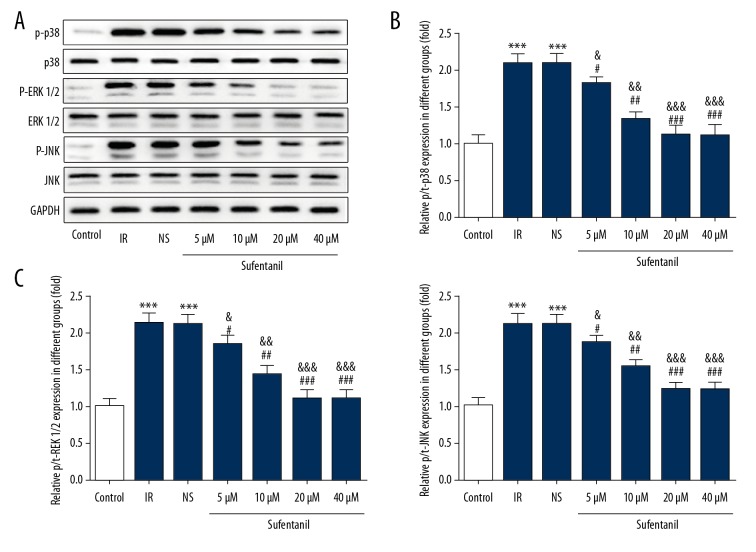
Sufentanil-induced downregulation of HIF-1α, TNF-α, IL-1β, and IL-6 raises a question about whether sufentanil functions through the classical p38/ERK/JNK/NF-κB-p65/COX2 signaling pathway. We demonstrated that as sufentanil dose increases, protein expression of p65 (a subunit composing NF-κB) and COX-2 is significantly reduced compared with the IR and NS groups (Figure 7). To assess the activity of the ERK1/2 signal pathway in L-O2 cells after IR treatment, the levels of total and phosphorylated ERK1/2, JNK, and p38 were measured by Western blot analysis. In parallel with COX2 and p65, phosphorylation levels of p38, ERK1/2, and JNK were all downregulated in a dose-dependent manner by sufentanil, whereas there was no significant difference in expression of total p38, ERK1/2, and JNK (Figure 8), suggesting that the protective role of sufentanil against hepatic IRI may function through suppressing the p38/ERK/JNK/NF-κB-p65/COX2 pathways.
Figure 7.
Effects of sufentanil on p65 and COX2 expression in L-O2 cells. (A) Representative Western blots showing the levels of p65 and COX2. (B) Histograms summarizing the results shown in A. Results are expressed as mean ±S.D (n=6). *** p<0.001 vs. control group; # p<0.05, ## p<0.01, ### p<0.001 vs. IR group; & p<0.05, && p<0.01, &&& p<0.001 vs. NS group.
Figure 8.
Effects of sufentanil on MAPK (p38/ERK/JNK) signaling in L-O2 cells. (A) Representative Western blots showing the levels of total and phosphorylated ERK1/2, JNK, and p38. (B, C) Histograms summarizing the results shown in A. Results are expressed as mean ±S.D (n=6). *** p<0.001 vs. control group; # p<0.05, ## p<0.01, ### p<0.001 vs. IR group; & p<0.05, && p<0.01, &&& p<0.001 vs. NS group.
Discussion
Hepatic IRI in liver surgery triggers a spectrum of postoperative complications. However, effective prevention and treatment are still lacking. Anesthetic preconditioning is a promising strategy that has been widely studied in IRI of various organs [34,35]. Anesthesia has been found to significantly attenuate acute liver inflammation induced by lipopolysaccharide/D-galactosamine [16], which raises the intriguing question of whether sufentanil contributes to alleviating hepatic IRI, since inflammation functions as an important initiator and progresses through the entire process.
Some clinical trials showed that volatile anesthetic preconditioning in liver surgery had a protective effect [36,37], and recent work suggested that one of the most important protective mechanisms of anesthetic preconditioning is inhibition of inflammation. It was reported that desflurane inhalation reduced endotoxin-induced cytokine responses, decreasing expression of TNF-α and IL-1β [38]. Similarly, according to Joseph et al., isoflurane preconditioning improved survival of hypoxic mice and decreased inflammatory reaction, with significant downregulation of NF-κB-p65, TNF-α, IL-6, and IL-10 [39]. In 2008, Hofstetter found that isoflurane inhalation after endotoxemia induction in rats attenuated systemic release of proinflammatory cytokines [40]. In addition, sevoflurane preconditioning in a rat model of liver IRI decreased inflammation by suppressing ICAM1 (intercellular adhesion molecule 1) expression and improved liver function parameters [41]. It was also found that sevoflurane anesthesia might share the same protective function with isoflurane in pig hepatic ischemia-reperfusion injury based on measurements of a number of liver parameters [42]. These results are similar to our experimental results.
We confirmed the protective effects of sufentanil in IR LO-2 cells by assessing morphology, cell activity, apoptosis, and the levels of AST and LDH. In the present study, the levels of AST and LDH were upregulated in the IR group. In the sufentanil-treated groups, there was a decrease in the release of AST and LDH from IR injury, revealing that cells were protected from IR-caused damage. In addition, our experiments provide 2 lines of evidence suggesting that sufentanil can help reduce inflammatory injury: 1) Sufentanil is sufficient to reduce expression of inflammation-related HIF-1α, TNF-α, IL-1β, and IL-6; 2) Phosphorylation levels of p38, ERK1/2, and JNK and expression of p65 and COX2 after sufentanil treatment are downregulated, indicating the possible pathways underlying the protective role of sufentanil. Our results suggest that sufentanil recovers the viability of L-O2 cells through the p38/ERK/JNK signaling pathway. The level of SOD was significantly higher but MDA was lower in the sufentanil treatment groups than in the IR and NS groups, indicating that sufentanil alleviated oxidative injury to L-O2 cells in the IR group.
There are certain limitations to the present study. First, the properties of sufentanil are complex and the exact functions of this compound have not been fully elucidated. Second, the present study only focused on the association between sufentanil and the changes of the main molecules participating in the p38/ERK/JNK signaling pathway and inflammatory responses, and gene silencing and overexpression of p38/ERK/JNK must be performed to study the underlying mechanism. Third, more research is required to determine the exact mechanism of action of sufentanil, and the anti-inflammatory effect also needs to be evaluated in vivo.
In our study, we also demonstrate the protective role of sufentanil preconditioning in inhibiting inflammation at the cellular level. At present our group is further studying the precise mechanism by which reduction of phosphorylation of p38, ERK1/2, and JNK results in downregulation of NF-κB-p65, then leading to decrease of inflammatory factors in a linear relationship, along with conducting experiments on sufentanil in animal hepatic IRI models. Sufentanil is widely used as an anesthetic drug, but recent research shows it also has some adverse effects. In view of the complex role of sufentanil and the fact that recent research on sufentanil is limited to cell experiments, further animal experiments and clinical trials are needed to determine whether sufentanil has a therapeutic anti-inflammatory effect. Therefore, in future work, we will pay more attention to the potential adverse effects of sufentanil and try to balance it with the positive influence of protecting against hepatic IRI.
Conclusions
Overall, our findings suggest that sufentanil can provide new insights into a mechanism of anti-inflammatory reaction, highlighting its potential to prevent hepatic IRI. In addition, this study provides evidence that sufentanil reduces injury to L-O2 cells via the ERK1/2-dependent signaling pathway. Sufentanil appears to have a therapeutic effect for hepatic injury induced by IRI. Further studies are necessary to confirm the effects of sufentanil on other clinical diseases.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Miyashita T, Nakanuma S, Ahmed AK, et al. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur Surg. 2016;48:92–98. doi: 10.1007/s10353-015-0363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation – from bench to bedside. Nat Rev Gastroenterol Hepatol. 2012;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lango R, Mrozinski P. Clinical importance of anaesthetic preconditioning. Anestezjol Intens Ter. 2010;42:206–12. [PubMed] [Google Scholar]
- 4.Swyers T, Redford D, Larson DF. Volatile anesthetic-induced preconditioning. Perfusion. 2014;29:10–15. doi: 10.1177/0267659113503975. [DOI] [PubMed] [Google Scholar]
- 5.Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5:230–41. doi: 10.1007/BF03178274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston KD. The potential for μ-opioid receptor agonists to be anti-emetic in humans: A review of clinical data. Acta Anaesthesiol Scand. 2010;54:132–40. doi: 10.1111/j.1399-6576.2009.02115.x. [DOI] [PubMed] [Google Scholar]
- 7.Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1998;88:903–13. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Sarton MDE, Teppema PL, Dahan MDPA. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop anesthesiology. Anesthesiology. 1999;90(5):1329–38. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Stein C, Clark JD, Oh U, et al. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahonen J, Olkkola KT, Hynynen M, et al. Comparison of alfentanil, fentanyl and sufentanil for total intravenous anaesthesia with propofol in patients undergoing coronary artery bypass surgery. Br J Anaesth. 2000;85:533–40. doi: 10.1093/bja/85.4.533. [DOI] [PubMed] [Google Scholar]
- 11.Vora KS, Shah VR, Patel B, et al. Postoperative analgesia with epidural opioids after cesarean section: Comparison of sufentanil, morphine and sufentanil-morphine combination. J Anaesthesiol Clin Pharmacol. 2012;28:491–95. doi: 10.4103/0970-9185.101935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 13.McNally L, Bhagwagar Z, Fau-Hannestad J, Hannestad J. Inflammation, glutamate, and glia in depression: A literature review. CNS Spectr. 2008;13:501–10. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 14.Pan Q, Liu Y, Zheng J, et al. Protective effect of chloral hydrate against lipopolysaccharide/D-galactosamine-induced acute lethal liver injury and zymosan-induced peritonitis in mice. Int Immunopharmacol. 2010;10:967–77. [PubMed] [Google Scholar]
- 15.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver Ischemia and reperfusion injury: New insights into mechanisms of innate – adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–69. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solhjou Z, Athar H, Xu Q, Abdi R. Emerging therapies targeting intra-organ inflammation in transplantation. Am J Transplant. 2015;15:305–11. doi: 10.1111/ajt.13073. [DOI] [PubMed] [Google Scholar]
- 17.Singh RK, Diwan M, Dastidar SG, Najmi AK. Differential effect of p38 and MK2 kinase inhibitors on the inflammatory and toxicity biomarkers in vitro. Hum Exp Toxicol. 2018;37:521–31. doi: 10.1177/0960327117715901. [DOI] [PubMed] [Google Scholar]
- 18.Sun H, Cai W, Wang X, et al. Vaccaria hypaphorine alleviates lipopolysaccharide-induced inflammation via inactivation of NFκB and ERK pathways in Raw 264.7 cells. BMC Complement Altern Med. 2017;17:120. doi: 10.1186/s12906-017-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki E, Brenner DA, Karin M. A liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–20. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Min JS, Kim B, et al. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett. 2015;584:191–96. doi: 10.1016/j.neulet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Delhanty PJ, van der Eerden BC, van der Velde M, et al. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol. 2006;188(1):37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- 22.Eltzschig HK, Eckle T. Ischemia and reperfusion – from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–85. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology. 2012;55:622–33. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug discov. 2014;13:852–69. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry BC, Soltys D, Toledo AH, Toledo-Pereyra LH. Tumor necrosis factor-α in liver ischemia/reperfusion injury. J Invest Surg. 2011;24:178–88. doi: 10.3109/08941939.2011.568594. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 28.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–77. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farmer EE, Mueller MJ. ROS-mediated lipid peroxidation and RES-activated signaling. Annu Rev Plant Biol. 2013;64:429–50. doi: 10.1146/annurev-arplant-050312-120132. [DOI] [PubMed] [Google Scholar]
- 31.McGovern TK, Chen M, Allard B, et al. Neutrophilic oxidative stress mediates organic dust-induced pulmonary inflammation and airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2016;310:L155–65. doi: 10.1152/ajplung.00172.2015. [DOI] [PubMed] [Google Scholar]
- 32.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Woith E, Stintzing F, Melzig MF. SOD activity and extremophilicity: A screening of various plant species. Pharmazie. 2017;72(8):490–96. doi: 10.1691/ph.2017.7493. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Lotz C, Roewer N, Broscheit JA. Comparison of volatile anesthetic-induced preconditioning in cardiac and cerebral system: Molecular mechanisms and clinical aspects. Eur J Med Res. 2018;23:10. doi: 10.1186/s40001-018-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez A, Taura P, Garcia Domingo MI, et al. Hepatic cytoprotective effect of ischemic and anesthetic preconditioning before liver resection when using intermittent vascular inflow occlusion: A randomized clinical trial. Surgery. 2015;157(2):249–59. doi: 10.1016/j.surg.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Beck-Schimmer B, Breitenstein S, Urech S, et al. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2006;248(6):909–18. doi: 10.1097/SLA.0b013e31818f3dda. [DOI] [PubMed] [Google Scholar]
- 37.Eichler K, Urner M, Twerenbold C, et al. Economic evaluation of pharmacologic pre- and postconditioning with sevoflurane compared with total intravenous anesthesia in liver surgery: A cost analysis. Anesth Analg. 2017;124:925–33. doi: 10.1213/ANE.0000000000001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boost KA, Hofstetter C, Flondor M, et al. Desflurane differentially affects the release of proinflammatory cytokines in plasma and bronchoalveolar fluid of endotoxemic rats. Int J Mol Med. 2006;17:1139–44. [PubMed] [Google Scholar]
- 39.McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia. Anesth Analg. 2007;104:1066–77. doi: 10.1213/01.ane.0000260321.62377.74. tables of contents. [DOI] [PubMed] [Google Scholar]
- 40.Flondor M, Hofstetter C, Boost KA, et al. Isoflurane inhalation after induction of endotoxemia in rats attenuates the systemic cytokine response. Eur Surg Res. 2008;40:1–6. doi: 10.1159/000107614. [DOI] [PubMed] [Google Scholar]
- 41.Mikrou A, Kalimeris KA, Lilis I, et al. Molecular studies of the immunological effects of the sevoflurane preconditioning in the liver and lung in a rat model of liver ischemia/reperfusion injury. Mol Immunol. 2016;72:1–8. doi: 10.1016/j.molimm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Ishida H, Kadota Y, Sameshima T, et al. Comparison between sevoflurane and isoflurane anesthesia in pig hepatic ischemia-reperfusion injury. J Anesth. 2002;16:44–50. doi: 10.1007/s540-002-8093-x. [DOI] [PubMed] [Google Scholar]