Key Points
Question
What is the effectiveness of subsymptom threshold aerobic exercise vs a placebo-like stretching program prescribed to adolescents in the short term after sport-related concussion?
Findings
In this randomized clinical trial of 103 adolescents, those assigned to aerobic exercise recovered faster (13 days) than those assigned to placebo-like stretching (17 days), a significant difference.
Meaning
Early subthreshold aerobic exercise appears to be an effective treatment for adolescents after sport-related concussion.
This randomized clinical trial assesses the effectiveness of aerobic exercise vs a placebo-like stretching regimen for individuals age 13 to 18 years in the acute phrase of recovery from sport-related concussion.
Abstract
Importance
Sport-related concussion (SRC) is a significant public health problem without an effective treatment.
Objective
To assess the effectiveness of subsymptom threshold aerobic exercise vs a placebo-like stretching program prescribed to adolescents in the acute phase of recovery from SRC.
Design, Setting, and Participants
This multicenter prospective randomized clinical trial was conducted at university concussion centers. Male and female adolescent athletes (age 13-18 years) presenting within 10 days of SRC were randomly assigned to aerobic exercise or a placebo-like stretching regimen.
Interventions
After systematic determination of treadmill exercise tolerance on the first visit, participants were randomly assigned to a progressive subsymptom threshold aerobic exercise or a progressive placebo-like stretching program (that would not substantially elevate heart rate). Both forms of exercise were performed approximately 20 minutes per day, and participants reported daily symptoms and compliance with exercise prescription via a website.
Main Outcomes and Measures
Days from injury to recovery; recovery was defined as being asymptomatic, having recovery confirmed through an assessment by a physician blinded to treatment group, and returning to normal exercise tolerance on treadmill testing. Participants were also classified as having normal (<30 days) or delayed (≥30 days) recovery.
Results
A total of 103 participants were included (aerobic exercise: n = 52; 24 female [46%]; stretching, n = 51; 24 female [47%]). Participants in the aerobic exercise group were seen a mean (SD) of 4.9 (2.2) days after the SRC, and those in the stretching group were seen a mean (SD) of 4.8 (2.4) days after the SRC. There were no differences in age, sex, previous concussions, time from injury, initial symptom severity score, or initial exercise treadmill test and physical examination results. Aerobic exercise participants recovered in a median of 13 (interquartile range [IQR], 10-18.5) days, whereas stretching participants recovered in 17 (IQR, 13-23) days (P = .009 by Mann-Whitney test). There was a nonsignificant lower incidence of delayed recovery in the aerobic exercise group (2 participants [4%] in the aerobic group vs 7 [14%] in the placebo group; P = .08).
Conclusions and Relevance
This is, to our knowledge, the first RCT to show that individualized subsymptom threshold aerobic exercise treatment prescribed to adolescents with concussion symptoms during the first week after SRC speeds recovery and may reduce the incidence of delayed recovery.
Trial Registration
ClinicalTrials.gov identifier: NCT02710123
Introduction
Mild traumatic brain injury,1 which includes sport-related concussion (SRC),2 is a significant public health problem with no proven effective intervention. Up to 30% of children and adolescents remain symptomatic 1 month after injury.3 Exercise can exacerbate symptoms after concussion, so return of normal exercise tolerance (ie, the ability to exercise to the level of one’s physical fitness without concussion symptoms)4,5 is a primary determinant of physiological readiness to return to a sport after a concussion.6,7 The mechanism of exercise intolerance after SRC is unknown. Possible mechanisms include dysregulation of the autonomic nervous system,8 reduced cardiac stroke volume,9 and impaired control of cerebral blood flow.10 Since aerobic exercise training has beneficial effects on autonomic nervous system regulation,11 cerebral blood flow regulation,10 cardiovascular physiology,12 and brain neuroplasticity,13 it is reasonable to consider whether subsymptom threshold aerobic exercise training (ie, regular exercise performed at an intensity below that which exacerbates symptoms) could help patients with concussion recover more rapidly.
The standard of care for SRC has been prescribed rest until symptoms resolve, an approach that is diametrically opposed to aerobic exercise training.14 The rest-is-best approach was based on animal research15 and consensus guidelines.16 Studies are beginning to show, however, that there is no harm17 and there may even be benefit18 of self-selected moderate levels of physical activity or prescribed aerobic exercise19,20 for those with delayed recovery, which is defined as symptoms lasting more than 2 weeks in adults or more than 4 weeks in children and adolescents.6 In a recent large observational study,18 pediatric patients who reported engaging in moderate levels of physical activity within 7 days of injury had a significant reduction in the rate of delayed recovery compared with those who reported no physical activity. The prevention of delayed recovery is very important because of the negative influence of persistent symptoms on academic and social functioning in adolescents.21
The most recent International Concussion in Sport Group consensus statement says that interventions including closely monitored active rehabilitation programs involving subsymptom threshold exercise should be studied using high-quality designs with matched control participants and accounting for potential confounding factors, such as time from injury and number of prior concussions. The purpose of this study was to evaluate the effectiveness of individualized subsymptom threshold aerobic exercise vs a placebo-like stretching program prescribed to adolescents in the acute phase after SRC. We studied adolescents because they appear to take the longest to recover from concussion.22,23 We hypothesized that early subthreshold aerobic exercise would speed recovery from SRC.
Methods
Study Design
This study was a parallel randomized clinical trial (RCT) of subsymptom threshold aerobic exercise treatment vs a placebo-like program of stretching exercises (allocation ratio, 1:1) prescribed in the acute phase after SRC on time to recovery in adolescent athletes. After a first visit, participants were followed up weekly by their physician until recovery or for 30 days. After 30 days, the RCT ended, and participants who had delayed recovery were provided interdisciplinary treatment. All participants were followed up to determine the number of days to recovery. This trial was approved by the University at Buffalo institutional review board. The consent or assent of each participant, depending on legal age, was obtained at the initial clinic visit. All participants 17 years and younger provided assent, and their parents provided consent. The trial protocol is available in Supplement 1. No changes to study design were made after study commencement.
Study Locations
The study was conducted at 3 US university-based outpatient concussion management clinics in Western New York and 1 such clinic in Winnipeg, Manitoba, Canada. The clinics had treadmills for exercise testing and received referrals from athletic trainers, primary care physicians, and emergency departments.
Participants
Inclusion Criteria
Male and female adolescent athletes (age 13-18 years) presenting within 10 days of SRC were evaluated by an experienced sports medicine physician who diagnosed the concussion according to International Concussion in Sport Group criteria.6 Exclusion criteria were (1) evidence of focal neurological deficit; (2) an inability to exercise because of orthopedic injury, cervical spine injury, diabetes, or known heart disease; (3) an increased cardiac risk according to American College of Sports Medicine criteria24; (4) a history of moderate or severe traumatic brain injury, defined as a brain injury with an associated Glasgow Coma Scale score of 12 or less; (5) a current diagnosis of and treatment with medication for attention-deficit/hyperactivity disorder, learning disorder, depression, anxiety, or a history of more than 3 prior concussions (because these factors are associated with delayed recovery25); (6) sustaining another head injury during the research period before recovery; (7) a symptom severity score of less than 5 points on the postconcussion symptom scale during an initial clinical visit26; (8) an ability to exercise to exhaustion without symptom exacerbation on the first visit; and/or (9) limited English proficiency.
Baseline Assessment
Sports medicine physicians diagnosed concussions at the initial clinic visit. Diagnosis was based on a thorough history (including cognitive evaluation and concussion symptom questionnaire),27 a standardized physical examination, and an exercise tolerance assessment with the Buffalo Concussion Treadmill Test (BCTT).28
Randomization and Blinding
A statistician-created, computer-generated randomization scheme was used in blocks of 3, stratified by sex and clinic location, and premade folders were provided to all clinics to use sequentially. Research assistants reviewed patient appointment lists daily and enrolled and assigned the participants to the intervention or control group after a physician diagnosis of concussion. Given the nature of treatment, research assistants and participants were not blinded to exercise allocation, but treating physicians (who made the clinical recovery determination) were.
Treatment Interventions
No interventions were initiated prior to 48 hours from injury.6 During the consent process, all participants were told that they would be randomly assigned to 1 type of low-level exercise, but there was no indication of the superiority of one over the other.
Aerobic Exercise Group
Participants in this group were instructed to perform aerobic exercise each day on a stationary bike or a treadmill, at home or in a gym under supervision, at the prescribed target heart rate (HR) while wearing a provided Polar H7 Bluetooth Heart Rate Sensor and Fitness Tracker to monitor HR. Participants could walk or jog if they did not have access to exercise equipment. They were instructed not to stretch before or after aerobic exercise. The subsymptom threshold aerobic exercise prescription target HR was calculated as 80% of the HR achieved at symptom exacerbation on the BCTT at the first visit.29 Participants were instructed to stop their home exercise session if their symptoms increased by 2 or more points from their preexercise symptom level (on a 10-point visual analog scale) or at 20 minutes, whichever came first.30 They were told to rest apart from the prescribed exercise and not participate in contact sports, gym class, or team practice. Rest included advice on limiting activities that exacerbated symptoms, such as avoiding excessive use of mobile phones or computers. A new target HR was determined by weekly clinic BCTT performance for as long as the participant remained symptomatic.
Stretching Group
Participants were instructed to follow a prescribed stretching program and given the same instructions about resting as the aerobic group. Importantly, the attention provided to the adolescent by the treating physician and research staff was the same as the aerobic exercise group. They were provided a booklet containing a gentle, whole-body, progressive stretching program (with pictures and instructions) that would not considerably elevate HR31 to perform for 20 minutes per day. As with the aerobic exercise group, they were provided with Polar HR monitors to use during each stretching session, and they performed the BCTT weekly at each clinic visit. The stretching program was advanced each week much like the aerobic exercise prescription.
Data Collection
Participants reported symptoms each evening between 7 pm and 10 pm on a password-protected website using the postconcussion symptom scale26 until they were declared recovered by the study physician or for 30 days, whichever came first. Participants also reported on whether they carried out the prescribed exercise that day. To enhance compliance, participants received daily text-message reminders. Clinical data were retrieved from electronic medical records. Research visit data were hand recorded and then transferred to a database.
Outcomes
The primary outcome measure was days to recovery since date of injury. Recovery was defined as symptom resolution to normal, confirmed by a normal physical examination (ie, a normal neurological examination including normal vestibular and oculomotor systems)32 and further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms on the BCTT.7 The BCTT results were also considered independently. Symptom resolution was defined as reporting a symptom severity score of 7 points or fewer on the postconcussion symptom scale for at least 3 consecutive days.6,33 The first day of the 3 days of symptom resolution was considered to be the date of recovery (if confirmed by the independent assessments of the treating physician and the BCTT results). Treating physicians were also blinded to group assignment. For participants not recovering within 30 days, the date of recovery was determined (using the same criteria) via medical record review. Secondary outcome measures were the proportion of participants with delayed recovery, which was defined as recovery requiring more than 30 days,6 and daily symptom scores. There were no changes to outcome measures after study commencement.
Sample Size
We used data from a pilot study of exercise vs stretching in patients with concussion31 and estimated 4.2 days and 7.8 days as the SDs of the mean days to recovery for the aerobic exercise and stretching exercise groups, respectively. We used an underlying normal distribution to simulate time-to-recovery data with these SDs. Using a 2-sample, 2-sided t test, we calculated an 80% chance to detect a clinically significant mean difference of 3.7 days in recovery time between groups with 50 participants in each group. No interim analysis was planned. Adverse events and near misses were recorded and evaluated by the treating physician.
Data Analysis
Analyses were based on per-protocol analysis. Baseline characteristics were analyzed to assess cluster differences between aerobic and stretching groups. We assessed groupwise differences in normally distributed variables (ie, age, total physical examination findings, total symptom severity scores on initial visit, and days to initial visit) using analysis of variance testing. We used χ2 tests to assess groupwise differences in sex and prior concussions. As the main outcome measure (number of days to recovery) was not normally distributed, the Mann-Whitney test was used for the main outcome measure. Days to recovery was also demonstrated with Kaplan-Meier curves. The equality of the survivor function across treatment groups was evaluated by log-rank tests. To control for the effect of potential confounding variables identified a priori based on prior literature (ie, age, sex, prior concussions, and time since injury),3 a multivariable parametric survival model was constructed using Akaike information criteria to identify the best model. There were no significant differences between data categorized by treatment centers in age (via analysis of variance), sex (via a Fisher exact test), prior concussion history (via a Fisher exact test), time since injury (via a Kruskal-Wallis test), or initial symptom score (via a Kruskal-Wallis test), so the treatment site was not included in any of the models.
For secondary outcomes, we used a test of proportions to evaluate the proportion of participants with delayed recovery in each group. The effect of treatment was further evaluated by a mixed-effects linear regression model to account for repeated measures of symptom scores. The outcome was the daily symptom score, and the model included the same covariates as in the survival analyses. Missing values for symptoms were calculated as the mean of day-before and day-after scores. A P value less than .05 determined statistical significance, and all tests were 2-sided. Statistical analyses were performed using SPSS version 23 (IBM) or Stata version 14 (StataCorp). Additional details are available in the trial protocol in Supplement 1.
Results
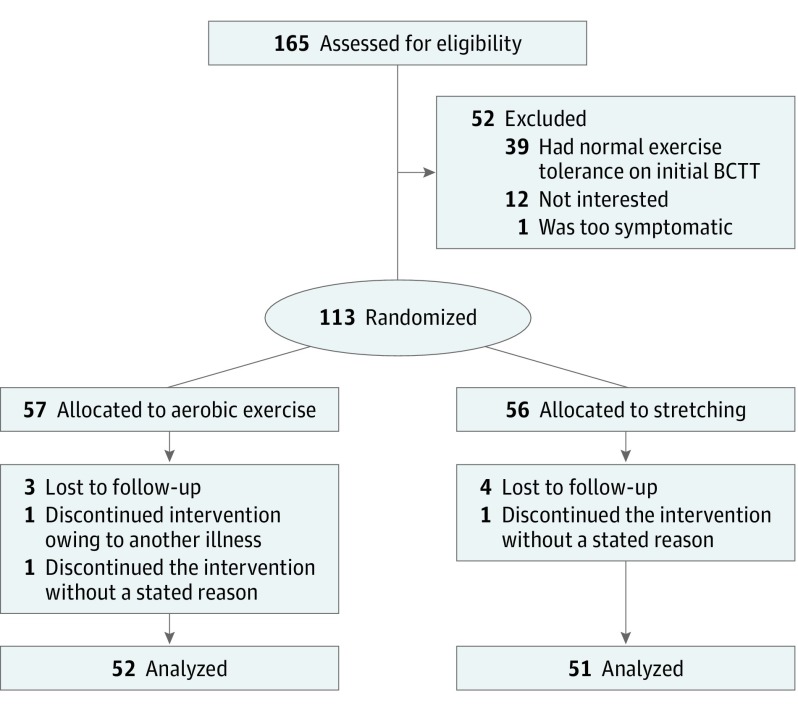
A total of 165 individuals met inclusion criteria. Twelve individuals were either not interested or did not have time to participate, 39 individuals were excluded before randomization because they did not have exercise intolerance on the BCTT, and 1 individual became severely symptomatic on the BCTT and decided to withdraw from the study. We considered this participant a near miss (Figure 1). After randomization, 10 of the 113 remaining participants were removed from the study during follow-up (Figure 1) for either not completing at least 75% of daily symptom reports or having missed 3 or more days in a row (n = 7), having another illness during intervention (n = 1), or not returning to the clinic (n = 2). Hence, 103 participants completed the study, with 52 participants making up the aerobic exercise group and 51 participants the stretching group. There were no demographic differences (ie, age, sex, time since injury) or clinical differences (eg, physical examination findings, exercise tolerance) between those who refused participation or those who were excluded when compared with the final study population. Analysis was by original assigned groups. The recruitment period was from September 2015 to June 2018, and the study ended when the target sample size was met. There were no adverse events, but 1 near miss was reported during the trial. There were no reported breaks in the blinding of physicians.
Figure 1. Consolidated Standards of Reporting Trials Flowchart.
Lost to follow-up was defined as not completing at least 75% of reports and/or missing more than 3 days of reporting in a row. BCTT indicates the Buffalo Concussion Treadmill Test.
Participants in the aerobic exercise and stretching groups did not significantly differ in age, sex, previous concussions, time since injury, initial symptom severity score, or initial BCTT and physical examination results (Table). No participants experienced seizures. Four participants (8%) from the stretching group and 2 (4%) from the exercise group had brief losses of consciousness at the time of injury (ie, <1 minute; P = .36). Four participants (8%) in the stretching group had asthma, and 1 (2%) had hypothyroidism. Four participants (8%) in the aerobic exercise group had asthma, 2 (4%) had hypothyroidism, and 1 (2%) had a repaired atrial septal defect.
Table. Demographics and Buffalo Concussion Treadmill Test Results.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Aerobic Exercise Group (n = 52) | Stretching Group (n = 51) | |
| Age, mean (SD), y | 15.3 (1.6) | 15.4 (1.7) |
| Female | 24 (46) | 24 (47) |
| Previous concussions | ||
| 0 | 26 (50) | 29 (57) |
| 1 | 16 (31) | 12 (24) |
| 2 | 9 (17) | 8 (16) |
| 3 | 1 (2) | 2 (4) |
| Time since injury, mean (SD), d | 4.9 (2.2) | 4.8 (2.4) |
| Quantitative findings at first visit, mean (SD) | ||
| Postconcussion symptom scale scorea | 30.8 (16.5) | 33.3 (19.7) |
| Abnormal physical examination findings, No. | 2.2 (1.9) | 2.8 (1.7) |
| Resting heart rate, bpm | 74.5 (12.7) | 75.2 (12.3) |
| Buffalo Concussion Treadmill Test findings | ||
| Heart rate at symptom exacerbation, bpm | 136.9 (26.2) | 136.6 (21.2) |
| Time to symptom exacerbation on first-visit test, min | 8.7 (4.9) | 8.6 (4.3) |
| Visual analog scale scores, mean (SD)b | ||
| Pretreadmill test score | 2.5 (1.8) | 2.8 (1.8) |
| Score at symptom exacerbation | 4.7 (2.2) | 5.1 (1.9) |
Maximum score, 132.
Maximum score, 10.
Recovery Time
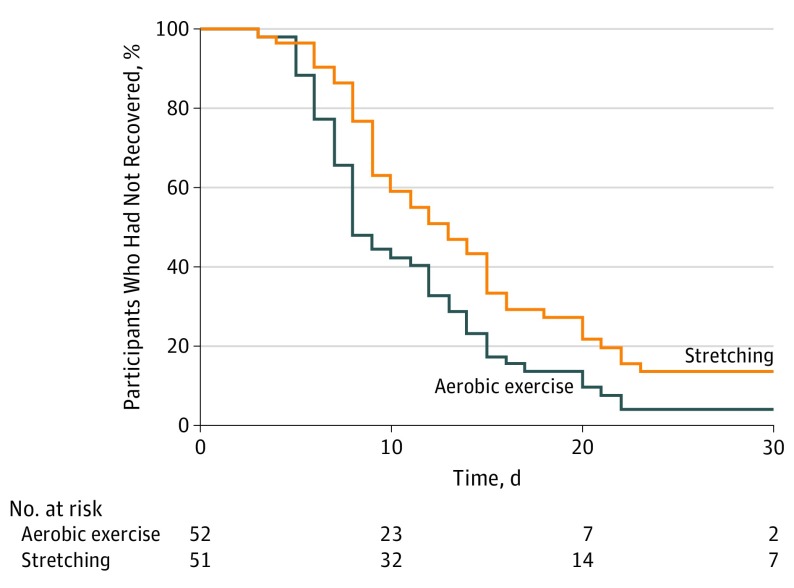
Aerobic exercise participants recovered in a median of 13 (interquartile range [IQR], 10-18.5) days, whereas stretching participants recovered in 17 (IQR, 13-23) days (P = .009). Figure 2 presents the Kaplan-Meier estimates of time to recovery since the start of the intervention (P = .01 per a log-rank test).
Figure 2. Kaplan-Meier Estimates of Time to Recovery.
Survival analysis comparing groups; the aerobic exercise group recovered significantly faster than the stretching group after adjusting for age, sex, time from injury to first clinical visit, and concussion history (z = 2.82; P = .005).
The logistic parametric survival model resulted in the best fit for the multivariable survival model (Akaike information criteria value, 191) after adjusting for age, sex, time from injury to first clinical visit, and concussion history, which demonstrated that the aerobic exercise group recovered significantly faster than the stretching group (z = 2.82; P = .005). The incidence of participants with delayed recovery since injury (>30 days) was higher in the stretching group (n = 7; median [IQR], 58 [36-62] days) compared with the aerobic exercise group (n = 2; median [IQR], 50 [46-54] days), but this did not reach significance (P = .08).
Daily Symptom Reporting
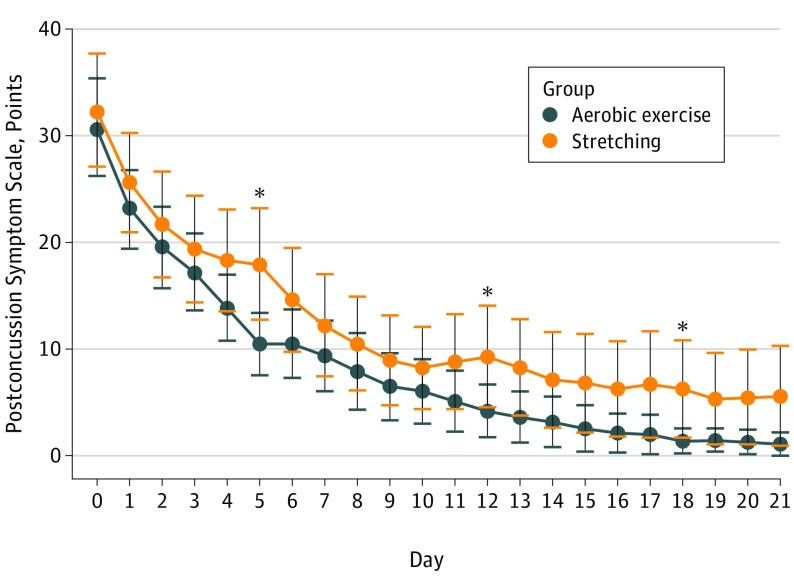
Figure 3 shows daily symptom reports. There was no significant difference in compliance in daily symptom reporting between the groups: aerobic exercise group participants completed 522 of 623 daily symptom reports (83.8%) and stretching group participants completed 587 of 678 daily symptom reports (86.6%; P = .16). In 987 of the 1109 daily reports (89%), participants stated they performed their prescribed exercise. Total symptom scores appeared to decrease more rapidly in the exercise group, although this did not reach significance.
Figure 3. Daily Symptom Severity Score per the Postconcussion Symptom Scale.
Bars indicate 95% CIs; asterisks, a significant difference on analysis of variance.
Discussion
This is, to our knowledge, the first RCT to evaluate individualized, progressive subsymptom threshold aerobic exercise prescribed within 1 week of concussion. We found that aerobic exercise safely improved recovery from SRC in adolescents with concussion symptoms compared with a placebo-like stretching intervention. There was a tendency for aerobic exercise to also prevent some adolescents from having a delayed recovery; this was a potentially crucial outcome, given the burden of social and academic problems during prolonged recovery in this age group. The results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery.
There were no adverse effects of early subsymptom threshold exercise, although we had 1 near miss. In an RCT of early prescribed exercise in collegiate athletes a mean of 2 days after SRC, Maerlender at al17 found no significant difference in recovery time vs relative rest but also reported no lasting effects of temporary symptom exacerbations during exercise. This study17 was limited by a small sample size and lacked a placebo-like control group. The present study confirms the safety of prescribed early exercise after SRC, provided that the individual does not exceed his or her symptom-exacerbation threshold. This is important because nearly one-third of children and adolescents engage in no physical activity in the first week after concussion,18 with associated increased risk of delayed recovery. Sustained rest until symptom resolution does not have solid scientific support for improving outcome after SRC,6,34 which the most recent (2017) statement from the International Concussion in Sport Group reflects.6
These results suggest that early prescribed aerobic exercise may prevent patients from experiencing delayed recovery. Several posttraumatic pathologies appear to be responsible for delayed recovery, including cervical injury, vestibular and oculomotor dysfunction, posttraumatic headache syndromes, and affective disorders, or (often) a combination of these.25,35,36 Patients with delayed recovery may therefore no longer have abnormal global metabolic and/or cerebrovascular function37 as the source of ongoing symptoms38 and could benefit from targeted therapies such as cervical physical therapy, vestibular rehabilitation, vision therapy, and/or cognitive behavioral therapy.36,39,40 During the study period, participants had access to usual care interventions, such as advice on sleep hygiene, academic accommodations, and judicious use of acetaminophen or ibuprofen for headache, consistent with current guidelines.6 Fifty of 52 participants in the aerobic exercise group had recovered by 30 days and therefore did not require additional medical therapies, which often involve substantial time and resources. If early subthreshold exercise reduces the need for additional therapies, it could improve quality of life for patients with delayed recovery.41,42
Limitations
This study did not address the mechanisms responsible for the beneficial effect of exercise after concussion. Other limitations include that participants were not observed during prescribed exercise, and some participants may have been exercising unbeknownst to the researchers, although this would have reduced rather than enhanced the comparative efficacy of aerobic exercise we observed.
Participants were not blinded to treatment; thus, intervention bias is possible. We think this unlikely, since all participants received an active intervention without being told which might be better, and they received equal attention from the investigators. We did not assess expectation of benefit (ie, perceived credibility of each intervention prior to initiation) to estimate the potential magnitude of expectancy bias.
The results should not be generalized to younger children, adults with risk factors for heart disease, or patients with concussions incurred by other mechanisms of injury (eg, car crashes, work injuries). Larger samples from multiple centers using an intent-to-treat design are needed to enhance generalizability, allow for studying mechanisms of action of exercise, and provide definitive evidence of the potential to prevent delayed recovery. Furthermore, the efficacy of exercise intervention may be diminished in health care centers without familiarity in the nuances of exercise testing and exercise-based treatment of patients with concussions.
Conclusions
This study is, to our knowledge, the first to show that individualized subsymptom threshold aerobic exercise treatment prescribed during the first week after SRC safely speeds recovery in adolescents with concussion symptoms. This is not equivalent to a return to sport-specific play; rather, it is an early active intervention intended to improve recovery to the point where it is safe for the athlete to begin the graduated process of returning to his or her sport. The data provide preliminary evidence that a primary benefit of early subthreshold exercise treatment is a reduced incidence of delayed recovery (>30 days), which is potentially a very important result. Larger prospective studies should investigate mechanisms of action of aerobic exercise on the concussed brain and determine if prescribed early subthreshold exercise prevents some patients from having delayed recovery after concussion.
Trial Protocol.
Data Sharing Statement.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378. doi: 10.1097/00001199-200609000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Laker SR. Epidemiology of concussion and mild traumatic brain injury. PM R. 2011;3(10)(suppl 2):S354-S358. doi: 10.1016/j.pmrj.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 3.Zemek R, Barrowman N, Freedman SB, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Clinical Risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. doi: 10.1001/jama.2016.1203 [DOI] [PubMed] [Google Scholar]
- 4.Kozlowski KF, Graham J, Leddy JJ, Devinney-Boymel L, Willer BS. Exercise intolerance in individuals with postconcussion syndrome. J Athl Train. 2013;48(5):627-635. doi: 10.4085/1062-6050-48.5.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leddy JJ, Hinds AL, Miecznikowski J, et al. Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clin J Sport Med. 2018;28(1):13-20. doi: 10.1097/JSM.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 7.Darling SR, Leddy JJ, Baker JG, et al. Evaluation of the Zurich guidelines and exercise testing for return to play in adolescents following concussion. Clin J Sport Med. 2014;24(2):128-133. doi: 10.1097/JSM.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 8.Johnson BD, O’Leary MC, McBryde M, Sackett JR, Schlader ZJ, Leddy JJ. Face cooling exposes cardiac parasympathetic and sympathetic dysfunction in recently concussed college athletes. Physiol Rep. 2018;6(9):e13694. doi: 10.14814/phy2.13694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Fountaine MF, Toda M, Testa AJ, Hill-Lombardi V. Autonomic nervous system responses to concussion: arterial pulse contour analysis. Front Neurol. 2016;7:13. doi: 10.3389/fneur.2016.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral blood flow during treadmill exercise is a marker of physiological postconcussion syndrome in female athletes. J Head Trauma Rehabil. 2016;31(3):215-224. doi: 10.1097/HTR.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 11.Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003;33(1):33-46. doi: 10.2165/00007256-200333010-00003 [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki T, Sullivan CV, Ozoe N, Higaki H, Kawasaki J. A long-term, comprehensive exercise program that incorporates a variety of physical activities improved the blood pressure, lipid and glucose metabolism, arterial stiffness, and balance of middle-aged and elderly Japanese. Hypertens Res. 2011;34(9):1059-1066. doi: 10.1038/hr.2011.81 [DOI] [PubMed] [Google Scholar]
- 13.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017-3022. doi: 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg ND, Iverson GL. Is rest after concussion “the best medicine?”: recommendations for activity resumption following concussion in athletes, civilians, and military service members. J Head Trauma Rehabil. 2013;28(4):250-259. doi: 10.1097/HTR.0b013e31825ad658 [DOI] [PubMed] [Google Scholar]
- 15.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235. [PMC free article] [PubMed] [Google Scholar]
- 16.McCrory P, Meeuwisse W, Aubry M, et al. ; Kathryn Schneider, PT, PhD, Charles H. Tator, MD, PHD . Consensus statement on concussion in sport—the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Clin J Sport Med. 2013;23(2):89-117. doi: 10.1097/JSM.0b013e31828b67cf [DOI] [PubMed] [Google Scholar]
- 17.Maerlender A, Rieman W, Lichtenstein J, Condiracci C. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40(5):273-278. doi: 10.1080/87565641.2015.1067706 [DOI] [PubMed] [Google Scholar]
- 18.Grool AM, Aglipay M, Momoli F, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316(23):2504-2514. doi: 10.1001/jama.2016.17396 [DOI] [PubMed] [Google Scholar]
- 19.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med. 2010;20(1):21-27. doi: 10.1097/JSM.0b013e3181c6c22c [DOI] [PubMed] [Google Scholar]
- 20.Kurowski BG, Hugentobler J, Quatman-Yates C, et al. Aerobic exercise for adolescents with prolonged symptoms after mild traumatic brain injury: an exploratory randomized clinical trial. J Head Trauma Rehabil. 2017;32(2):79-89. doi: 10.1097/HTR.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iverson GL, Gioia GA. Returning to school following sport-related concussion. Phys Med Rehabil Clin N Am. 2016;27(2):429-436. doi: 10.1016/j.pmr.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Baker JG, Leddy JJ, Darling SR, et al. Factors associated with problems for adolescents returning to the classroom after sport-related concussion. Clin Pediatr (Phila). 2015;54(10):961-968. doi: 10.1177/0009922815588820 [DOI] [PubMed] [Google Scholar]
- 23.Carson JD, Lawrence DW, Kraft SA, et al. Premature return to play and return to learn after a sport-related concussion: physician’s chart review. Can Fam Physician. 2014;60(6):e310–, e312-e315.. [PMC free article] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine, Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 25.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health. 2012;4(2):147-154. doi: 10.1177/1941738111433673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293. doi: 10.1136/bjsports-2013-092225 [DOI] [PubMed] [Google Scholar]
- 27.Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5). Br J Sports Med. 2017;51(11):848-850. doi: 10.1136/bjsports-2017-09750 [DOI] [PubMed] [Google Scholar]
- 28.Leddy JJ, Baker JG, Kozlowski K, Bisson L, Willer B. Reliability of a graded exercise test for assessing recovery from concussion. Clin J Sport Med. 2011;21(2):89-94. doi: 10.1097/JSM.0b013e3181fdc721 [DOI] [PubMed] [Google Scholar]
- 29.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Curr Sports Med Rep. 2013;12(6):370-376. doi: 10.1249/JSR.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 30.Leddy JJ, Haider MN, Ellis M, Willer BS. Exercise is medicine for concussion. Curr Sports Med Rep. 2018;17(8):262-270. doi: 10.1249/JSR.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leddy JJ, Cox JL, Baker JG, et al. Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J Head Trauma Rehabil. 2013;28(4):241-249. doi: 10.1097/HTR.0b013e31826da964 [DOI] [PubMed] [Google Scholar]
- 32.Matuszak JM, McVige J, McPherson J, Willer B, Leddy J. A practical concussion physical examination toolbox: evidence-based physical examination for concussion. Sports Health. 2016;8(3):260-269. doi: 10.1177/1941738116641394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166-174. doi: 10.1207/s15324826an1303_4 [DOI] [PubMed] [Google Scholar]
- 34.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223. doi: 10.1542/peds.2014-0966 [DOI] [PubMed] [Google Scholar]
- 35.Baker JG, Freitas MS, Leddy JJ, Kozlowski KF, Willer BS. Return to full functioning after graded exercise assessment and progressive exercise treatment of postconcussion syndrome. Rehabil Res Pract. 2012;2012:705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis MJ, Leddy J, Willer B. Multi-disciplinary management of athletes with post-concussion syndrome: an evolving pathophysiological approach. Front Neurol. 2016;7:136. doi: 10.3389/fneur.2016.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(suppl 4):S24-S33. doi: 10.1227/NEU.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leddy J, Hinds A, Sirica D, Willer B. The role of controlled exercise in concussion management. PM R. 2016;8(3)(suppl):S91-S100. doi: 10.1016/j.pmrj.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 39.Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87-93. doi: 10.1097/NPT.0b013e3181dde568 [DOI] [PubMed] [Google Scholar]
- 40.Schneider KJ, Leddy JJ, Guskiewicz KM, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. 2017;51(12):930-934. doi: 10.1136/bjsports-2016-097475 [DOI] [PubMed] [Google Scholar]
- 41.Houston MN, Bay RC, Valovich McLeod TC. The relationship between post-injury measures of cognition, balance, symptom reports and health-related quality-of-life in adolescent athletes with concussion. Brain Inj. 2016;30(7):891-898. doi: 10.3109/02699052.2016.1146960 [DOI] [PubMed] [Google Scholar]
- 42.Fineblit S, Selci E, Loewen H, Ellis M, Russell K. Health-related quality of life after pediatric mild traumatic brain injury/concussion: a systematic review. J Neurotrauma. 2016;33(17):1561-1568. doi: 10.1089/neu.2015.4292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Data Sharing Statement.