Key Points
Question
Is use of amyloid positron emission tomography (PET) associated with subsequent change in the management of patients with mild cognitive impairment (MCI) or dementia of uncertain etiology?
Findings
In this longitudinal study that included 11 409 participants with MCI or dementia of uncertain cause, patient management 90 days after amyloid PET changed (compared with the pre-PET plan) in 60.2% of patients with MCI and 63.5% of patients with dementia.
Meaning
Amyloid PET was associated with changes in the subsequent management of diagnostically challenging patients with cognitive disorders.
Abstract
Importance
Amyloid positron emission tomography (PET) detects amyloid plaques in the brain, a core neuropathological feature of Alzheimer disease.
Objective
To determine if amyloid PET is associated with subsequent changes in the management of patients with mild cognitive impairment (MCI) or dementia of uncertain etiology.
Design, Setting, and Participants
The Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) study was a single-group, multisite longitudinal study that assessed the association between amyloid PET and subsequent changes in clinical management for Medicare beneficiaries with MCI or dementia. Participants were required to meet published appropriate use criteria stating that etiology of cognitive impairment was unknown, Alzheimer disease was a diagnostic consideration, and knowledge of PET results was expected to change diagnosis and management. A total of 946 dementia specialists at 595 US sites enrolled 16 008 patients between February 2016 and September 2017. Patients were followed up through January 2018. Dementia specialists documented their diagnosis and management plan before PET and again 90 (±30) days after PET.
Exposures
Participants underwent amyloid PET at 343 imaging centers.
Main Outcomes and Measures
The primary end point was change in management between the pre- and post-PET visits, as assessed by a composite outcome that included Alzheimer disease drug therapy, other drug therapy, and counseling about safety and future planning. The study was powered to detect a 30% or greater change in the MCI and dementia groups. One of 2 secondary end points is reported: the proportion of changes in diagnosis (from Alzheimer disease to non–Alzheimer disease and vice versa) between pre- and post-PET visits.
Results
Among 16 008 registered participants, 11 409 (71.3%) completed study procedures and were included in the analysis (median age, 75 years [interquartile range, 71-80]; 50.9% women; 60.5% with MCI). Amyloid PET results were positive in 3817 patients with MCI (55.3%) and 3154 patients with dementia (70.1%). The composite end point changed in 4159 of 6905 patients with MCI (60.2% [95% CI, 59.1%-61.4%]) and 2859 of 4504 patients with dementia (63.5% [95% CI, 62.1%-64.9%]), significantly exceeding the 30% threshold in each group (P < .001, 1-sided). The etiologic diagnosis changed from Alzheimer disease to non–Alzheimer disease in 2860 of 11 409 patients (25.1% [95% CI, 24.3%-25.9%]) and from non–Alzheimer disease to Alzheimer disease in 1201 of 11 409 (10.5% [95% CI, 10.0%-11.1%]).
Conclusions and Relevance
Among Medicare beneficiaries with MCI or dementia of uncertain etiology evaluated by dementia specialists, the use of amyloid PET was associated with changes in clinical management within 90 days. Further research is needed to determine whether amyloid PET is associated with improved clinical outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02420756
In an attempt to understand if amyloid PET imaging improves the care of patients with MCI or dementia, this cohort study investigates associations between information provided by amyloid PET scan and change in clinical management, defined as a composite of change in drug therapy or counseling about safety and future planning.
Introduction
The development of positron emission tomography (PET) ligands that detect amyloid-β (Aβ) plaques, a core neuropathological feature of Alzheimer disease,1 has had a major effect on Alzheimer disease clinical research and drug development.2,3 Amyloid PET could also be a useful clinical tool in the diagnostic assessment of patients with cognitive decline.4 The diagnosis of Alzheimer disease and related disorders based on clinical criteria has limited sensitivity and specificity compared with autopsy.5 The addition of amyloid PET to the clinical assessment could enhance diagnostic accuracy, although Aβ deposition also occurs in the setting of other neurodegenerative disorders and in cognitively normal older adults.6,7
Three Aβ tracers—fluorine 18 (18F)–labeled florbetapir, 18F-labeled flutemetamol, and 18F-labeled florbetaben—have been approved for clinical use in the United States and other countries. In PET-to-autopsy studies performed with these tracers in end-of-life populations, PET scans performed during life had 88% to 98% sensitivity and 80% to 95% specificity for detecting moderate-frequent (according to the Consortium to Establish a Registry for Alzheimer’s Disease scale8) neuritic amyloid plaques at autopsy.9,10,11 However, reimbursement of amyloid PET by third-party payers has been limited. In 2013 the US Centers for Medicare & Medicaid Services concluded that there was insufficient evidence to justify routine coverage of amyloid PET but agreed to provide coverage with evidence development in studies investigating whether amyloid PET improves health outcomes, including short-term outcomes related to changes in management as well as longer-term dementia outcomes.12
The Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) Study is a US-wide study assessing the utility of amyloid PET in Medicare beneficiaries who meet appropriate use criteria for amyloid PET.13,14 The first aim of the study, the results of which are reported in this article, was to evaluate the association between amyloid PET and subsequent change in clinical management.
Methods
Study Oversight and Design
The study was managed by the American College of Radiology (ACR) under a central institutional review board (IRB) (Advarra, formerly Schulman Associates). A number of sites required local IRB approval. Written informed consent from participating dementia and imaging specialists was obtained by the ACR. Written informed consent for patient participation was obtained by the dementia specialist, either directly from the patient or, in instances in which the specialist determined that the patient lacked capacity to consent, from a legally authorized representative, with patient assent.
The study was designed as a single-group, multisite longitudinal study to assess the clinical utility of amyloid PET in cognitively impaired patients who met appropriate use criteria for clinical amyloid PET.13,14 The full protocol, including template consent forms, is available in Supplement 1; pre-PET and post-PET case report forms are available in Supplement 2 and Supplement 3, respectively.
Study Population
Participating dementia specialists were recruited through professional societies, the Alzheimer’s Association, industry partners, and media outreach. Per the appropriate use criteria, a dementia specialist was defined as a physician board certified in neurology, psychiatry, or geriatric medicine who devotes 25% or more of patient contact time to the evaluation and care of acquired cognitive impairment.14 The imaging specialists who interpreted scans were required to have board certification in diagnostic radiology or nuclear medicine and to have successfully completed vendor-provided training for interpreting amyloid PET scans.
Patients were recruited by dementia specialists from their clinical practices. Eligible patients were Medicare beneficiaries aged 65 or older, English or Spanish speaking, with a diagnosis of mild cognitive impairment (MCI) or dementia established by a dementia specialist within the past 24 months. All patients were required to have completed a comprehensive diagnostic assessment, including global cognition assessed via the Mini-Mental State Examination (range, 0 [worst] to 30 [best]) or Montreal Cognitive Assessment (range, 0 [worst] to 30 [best]) at the time of enrollment, laboratory testing within the past 12 months, and head computed tomography or magnetic resonance imaging within the past 24 months. Patients were further required to meet appropriate use criteria for amyloid PET: (1) the etiologic cause of cognitive impairment remained uncertain after a comprehensive evaluation by the dementia specialist; (2) Alzheimer disease was a diagnostic consideration; and (3) knowledge of amyloid PET status was expected to alter diagnosis and management.13 Patients were excluded if amyloid status was already known based on prior PET or cerebrospinal fluid (CSF) analysis or if learning amyloid status could, in the opinion of the specialist, cause significant psychological harm. The complete inclusion and exclusion criteria are listed in the study protocol in Supplement 1. To avoid disproportionate recruitment from any single referring specialist, the maximum enrollment for an individual clinician was limited to 160 patients for the patient management aim and 250 for the entire study.
To compare the diversity of the study cohort with that of the general population of Medicare beneficiaries, participant race and ethnicity were classified by the dementia specialists or their designees (ie, study coordinator or practice administrator) into at least 1 race category (American Indian, Alaskan Native, Asian, black or African American, Native Hawaiian or Pacific Islander, white, not reported, unknown) and at least 1 ethnicity category (not Hispanic or Latino, Hispanic or Latino, not reported, unknown). The protocol and registration form did not specify how race or ethnicity should be ascertained.
Study Procedures
Pre-PET Assessment
Dementia specialists completed a pre-PET case report form (Supplement 2) that described the patient’s demographics, primary etiologic diagnosis, physician confidence that Alzheimer disease pathology was contributing to cognitive impairment (scale range, 1 [definitely not] to 10 scale [certain]), and the physician’s intended management plan if he or she had no access to amyloid PET. Elements of the management plan recorded in the case report form included use of medications approved for symptomatic treatment of Alzheimer disease (ie, cholinesterase inhibitors or memantine); use of other pertinent drugs not specific to Alzheimer disease (drugs that affect cognition, mood, or behavior and drugs used to treat other neurologic conditions or address dementia risk factors); counseling about safety (eg, home safety, medication monitoring, driving) and future planning (eg, medical and financial decision making, advance directives). Plans for referrals to patient/caregiver support resources, other specialists, and additional diagnostic testing were also recorded.
PET Acquisition, Interpretation, and Disclosure
Amyloid PET was completed within 30 days of the pre-PET assessment at accredited imaging facilities with 1 of 3 US Food and Drug Administration (FDA)–approved Aβ ligands following published practice guidelines.15 Scans were interpreted by participating imaging specialists using approved reading methodologies for each tracer.9,10,11 Based on FDA guidelines, scans were interpreted dichotomously as “negative” (white matter retention only) or “positive” (cortical tracer retention). PET results were disclosed to patients by the dementia specialists, who could then recommend changes to the pre-PET management plan. Disclosure of PET results and immediate management changes occurred as part of clinical care (ie, outside of designated study visits).
Post-PET Assessment
An additional follow-up visit with the referring dementia specialist was required 90 (±30) days after the PET scan. At this visit, the referring specialist completed the post-PET case report form (Supplement 3), documenting the implemented patient management plan as well as changes in diagnosis and diagnostic confidence. Specialists were specifically asked whether PET results informed each post-PET management item. The post-PET period of 90 days was selected to allow sufficient time to implement care recommendations after the PET scan but to provide a short enough time window to minimize the influence of other events (unrelated to PET) on patient management.
Patient deaths that occurred between registration and the post-PET visit were reported by the referring specialist on the post-PET form. To monitor for any potential suicides related to learning amyloid status, each reported death prompted a call from a study team physician (G.D.R, B.E.H., B.A.S.) to the referring dementia specialist to determine the cause of death.
End Points
Primary End Point
The primary end point was change between pre-PET and 90-day post-PET patient management in 1 or more of the following: Alzheimer disease drug therapy; other drug therapy; or counseling about safety and future planning. These elements were selected because they collectively represent the foundation of a comprehensive treatment plan for cognitive disorders.16 For each category, change was defined as starting, stopping, or modifying (eg, adjusting dose of an existing medication) that element of the treatment plan when comparing the pre-PET and post-PET reported information.
Secondary End Point
The secondary end point reported in this article was change in diagnosis (from Alzheimer disease to non–Alzheimer disease and vice versa) between pre- and post-PET visits.
Additional prespecified end points related to the study’s first aim and not reported here include frequency of reduction in unnecessary diagnostic tests and Alzheimer disease drug therapy at the individual patient level (secondary outcome) and identification of specific scenarios in which use of amyloid PET is associated with the greatest rate of change in patient management (exploratory outcome). The second aim of the study, which is ongoing, uses Medicare claims to assess 1-year hospitalization rates and emergency department visits (as well as additional health outcomes) in study participants and compare them with a matched control group of Medicare beneficiaries who have not undergone amyloid PET.
Statistical Analyses
Full details of the statistical plan are available in the eMethods in Supplement 4. In brief, for the primary end point, binomial estimates of rates (proportions) of change were calculated with Wilson confidence intervals for the overall composite and for each composite category. The primary objective was to assess whether the primary composite end point changed in 30% or more of participants, assessed separately in the MCI and dementia subgroups. The 30% threshold was selected to reflect change in a clinically meaningful proportion of participants, consistent with previous studies of coverage with evidence development examining the association between diagnostic imaging and changes in management17 and supported by previous work assessing the clinical utility of amyloid PET.18 The primary null hypothesis (overall rate of change <30%) vs the alternative hypothesis (overall rate of change ≥30%) was tested separately for the MCI and dementia subgroups using a Wald test with α = .025 (1-sided). A 1-sided P value is reported because the null and alternative hypotheses were 1-sided and a 1-sided test was used.
A total sample size of 11 050 cases was chosen to provide 80% power for testing the primary hypothesis within the MCI and dementia subgroups, assuming an alternative value of 32% for the overall rate of change in each group.
Binomial estimates with Wilson intervals were derived for all rates (proportions) reported in the article. With the exception of the test of the primary hypothesis, all other P values reported in the article are 2-sided. Comparisons of correlated proportions were made using the McNemar test.
Three prespecified exploratory analyses are reported in this article. First, the relationship between amyloid PET results and the probability of change in the primary end point was examined using mixed-effects logistic regression. The model included a random effect for site and fixed effects for age (in years), sex (male/female), education (dichotomized as college graduate or advanced degree vs other), pre-PET use of Alzheimer disease drugs (yes/no), pre-PET primary etiologic diagnosis (Alzheimer disease vs other), level of impairment (MCI vs dementia) and amyloid PET result (positive/negative). The model also included all 2-way and the 3-way interaction between PET result, primary etiologic diagnosis, and level of impairment. The significance of fixed-effect coefficients (including interactions) was assessed using t tests. Multiple imputation for the set of participants with PET scans was used to account for missing data in the regression analysis, with 25 complete data sets generated and analyzed.
Second, agreement between pre-PET suspected etiology and PET results was examined by reporting proportions of participants with concordant diagnoses and PET results (ie, pre-PET diagnosis of Alzheimer disease and positive PET result; or pre-PET diagnosis of non–Alzheimer disease and negative PET result) with 95% confidence intervals.
Third, rates of referrals to therapeutic trials at the pre-PET and post-PET visit are reported as proportions with 95% confidence intervals.
In addition to the prespecified end points, post hoc analyses assessed rates of change in individual components of the management end point in the MCI and dementia subgroups, overall and stratified by scan results; changes in diagnosis, stratified by PET results; changes between pre- and post-PET diagnostic confidence; changes between pre- and post-PET overall use of Alzheimer disease drugs and additional diagnostic tests (at the population level); and changes between pre- and post-PET rates of amyloid positivity in patients referred to Alzheimer disease clinical trials.
A Bonferroni correction was used to control for multiple comparisons in exploratory and post hoc analyses reporting formal tests of significance. Specifically, a P value less than .0036 would be required for significance, after applying this correction to a total of 14 comparisons, including tests for 10 coefficients of effects in the regression analysis (including 8 in the reported final model and 2 additional interactions) and tests for the 4 comparisons involved in the analysis of change in the use of Alzheimer disease drugs by PET result and impairment status. However, this correction was post hoc, and results of the reported analyses should be interpreted as exploratory.
Statistical computations were performed using SAS/STAT version 9.4 (SAS Institute Inc) and R version 3.5.1 (R Foundation for Statistical Computing).
Results
Study Participants
Physicians
Nine hundred forty-six dementia specialists from 595 unique practices across the United States participated in the study (eFigure 1 in Supplement 4). Participants were scanned at 343 PET facilities, and scans were interpreted by 733 imaging specialists.
Patients
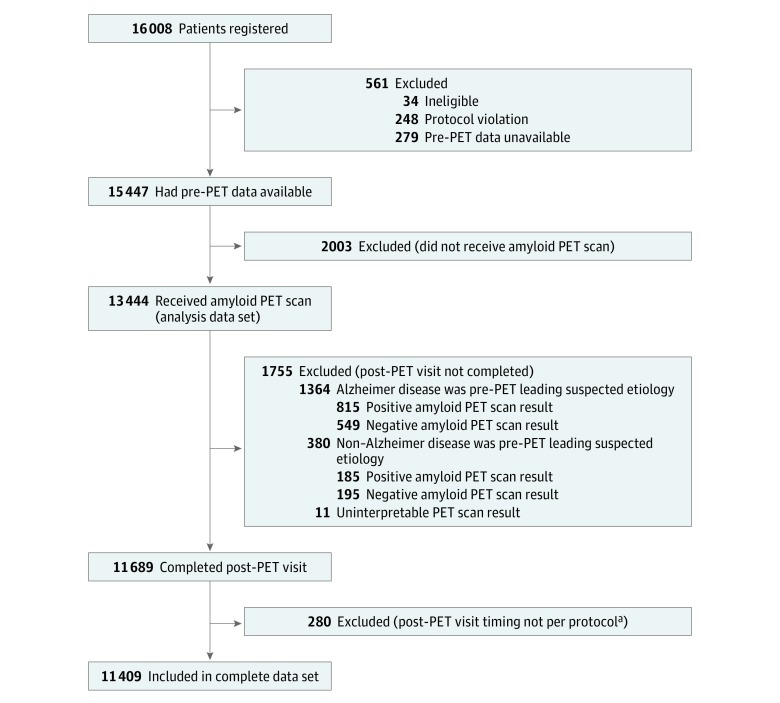
Medicare beneficiaries (n = 16 008) were registered for the study aim reported in this article between February 8, 2016, and September 20, 2017; of these, 11 409 (71.3%) had complete information and were included in the final analysis data set (Figure 1). Reasons for exclusion included PET not performed (n = 2003), no documentation of a post-PET visit (n = 1755), and other protocol violations (n = 841). Only 34 of the 16 008 patients registered to the study were deemed ineligible. The study did not track patients screened for the study but not registered.
Figure 1. Study Flow: Imaging Dementia–Evidence for Amyloid Scanning (IDEAS).
PET indicates positron emission tomography.
aVisit less than 60 days or more than 120 days after PET scan.
Characteristics of the final analysis cohort are shown in Table 1 and did not differ meaningfully from those of all patients with pre-PET information (eTable 1 in Supplement 4). At baseline, 60.5% of patients were diagnosed with MCI (median age, 75 years; 49.6% female) and 39.5% with dementia (median age, 77 years; 52.8% female). Median scores on the Mini-Mental State Examination (MCI, 27; dementia, 22) and Montreal Cognitive Assessment (MCI, 23; dementia, 18) were comparable to values previously reported in patients with MCI and dementia.6,7,19 At the pre-PET visit, Alzheimer disease was the leading suspected etiology of cognitive impairment in 76.9% of all patients. Alzheimer disease drugs were prescribed at baseline in 34.5% of patients with MCI and 59.3% of patients with dementia. Amyloid PET scans were read as positive in 3817 (55.3%) of patients with MCI and 3154 (70.1%) of patients with dementia. Nine of 11 409 scans were considered uninterpretable.
Table 1. Patient Characteristics.
| Characteristic | Level of Impairment | |
|---|---|---|
| Mild Cognitive Impairment (n = 6905) | Dementia (n = 4504) | |
| Age, median (IQR), y | 75 (70-79) | 77 (72-81) |
| Sex, No. (%) | ||
| Women | 3425 (49.6) | 2379 (52.8) |
| Men | 3480 (50.4) | 2125 (47.2) |
| Race, No. (%)a | ||
| Black or African American | 206 (3.0) | 225 (5.0) |
| White | 6212 (90.0) | 3828 (85.0) |
| Other race | 487 (7.1) | 451 (10.0) |
| Hispanic ethnicity, No. (%)b | 209 (3.0) | 244 (5.4) |
| Highest level of education, No. (%) | ||
| High school graduate (including equivalency) or less | 1824 (26.4) | 1917 (42.6) |
| Some college or associate degree | 1763 (25.5) | 933 (20.7) |
| Bachelor’s degree | 1777 (25.7) | 927 (20.6) |
| Postgraduate degree | 1541 (22.3) | 727 (16.1) |
| MMSE, median (IQR)c | 27 (25-29) | 22 (18-25) |
| MoCA, median (IQR)c | 23 (21-25) | 18 (14-21) |
| Alzheimer disease leading suspected pre-PET etiology, No. (%) | 5043 (73.0) | 3727 (82.7) |
| Taking Alzheimer disease drugs at enrollment, No. (%) | 2384 (34.5) | 2671 (59.3) |
| Amyloid PET results, No. (%)d | ||
| Positive | 3817 (55.3) | 3154 (70.1) |
| Negative | 3082 (44.7) | 1347 (29.9) |
Abbreviations: IQR, interquartile range; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; PET, positron emission tomography.
Race as recorded by dementia specialist, study coordinator, or practice administrator. “Other race” indicates American Indian, Asian, Native Hawaiian or Pacific Islander, unknown, or not reported.
Ethnicity as recorded by dementia specialist, study coordinator, or practice administrator; grouped as Hispanic and other (not Hispanic, unknown, or not reported).
Range, 0-30; lower scores indicate worse global cognition.
Nine cases with uninterpretable test results not included (6 in MCI group, 3 in dementia group).
Primary End Point
Changes between the pre-PET and post-PET composite management end point occurred in 60.2% (95% CI, 59.1%-61.4%) of patients with MCI and 63.5% (95% CI, 62.1%-64.9%) of patients with dementia (Table 2), significantly exceeding the target of 30.0% composite change in each group (P < .001, 1-sided). Physicians reported that PET results contributed substantially to the post-PET management plan in 85.2% of instances in which a change was made (eTable 2 in Supplement 4).
Table 2. Changes in Management Composite.
| Mild Cognitive Impairment (n = 6905) | Dementia (n = 4504) | |||
|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | |
| Primary Outcome | ||||
| Overall change | 4159 | 60.2 (59.1-61.4)a | 2859 | 63.5 (62.1-64.9)a |
| Changes by Componentb | ||||
| Alzheimer disease drugs | 3014 | 43.6 (42.5-44.8) | 2022 | 44.9 (43.4-46.3) |
| Non–Alzheimer disease drugsc | 1582 | 22.9 (21.9-23.9) | 1144 | 25.4 (24.1-26.7) |
| Counseling | 1681 | 24.3 (23.3-25.4) | 934 | 20.7 (19.6-21.9) |
P < .001 for testing the primary hypothesis (≥30% overall change in management in the MCI and dementia cohorts).
Post hoc analysis.
Include drugs that affect cognition, mood, or behavior and drugs used to treat other neurologic conditions or address dementia risk factors.
Secondary End Point
The etiologic diagnosis changed from Alzheimer disease to non–Alzheimer disease in 2860 of 11 409 patients (25.1% [95% CI, 24.3%-25.9%]) and from non–Alzheimer disease to Alzheimer disease in 1201 of 11 409 (10.5% [95% CI, 10.0%-11.1%]).
Exploratory Analyses
Multivariable Analysis of Factors Associated With Change in the Primary End Point
After controlling for multiple comparisons, there were no significant associations between age, sex, education, or pre-PET Alzheimer disease drug use and change in composite management (Table 3). There were significant interactions between PET scan result and pre-PET primary etiologic diagnosis and between scan result and level of impairment (P < .001 for both). Using the estimates reported in Table 3 and combining all 3 factors, the odds ratio (OR) of a change in management by PET scan result (positive vs negative) was highest among participants with MCI and a pre-PET non–Alzheimer disease etiologic diagnosis (OR, 3.54 [95% CI, 2.96-4.23]) and lowest in participants with dementia and pre-PET etiologic diagnosis of Alzheimer disease (OR, 1.14 [95% CI, 0.99-1.32]). Results of the imputation analysis (performed in all patients with completed scans) were similar to results of the final analysis data set and did not alter the conclusions (eTable 3 in Supplement 4).
Table 3. Logistic Regression Analysis Assessing Factors Associated With Change in the Composite Management End Point.
| Effect | OR (95% CI) | P Valuea |
|---|---|---|
| Interceptb | 0.48 (0.28-0.80) | .006 |
| Main effects | ||
| Age (10 y) | 1.09 (1.02-1.17) | .008 |
| Sex (1 = female, 0 = male) | 1.04 (0.95-1.13) | .40 |
| Education (1 = college or advanced degree, 0 = other) | 0.92 (0.84-1.01) | .08 |
| Pre-PET Alzheimer disease drugs (1 = yes, 0 = no) | 0.93 (0.85-1.02) | .12 |
| Amyloid PET result (1 = positive, 0 = negative) | 3.54 (2.96-4.23) | <.001 |
| Primary pre-PET etiologic diagnosis (1 = Alzheimer disease, 0 = other) | 1.30 (1.12-1.51) | <.001 |
| Level of impairment (1 = Dementia, 0 = MCI) | 1.57 (1.36-1.82) | <.001 |
| Interactionsc | ||
| PET result × primary pre-PET etiological diagnosis Alzheimer disease | 0.60 (0.49-0.73) | <.001 |
| PET result × level of impairment | 0.54 (0.45-0.64) | <.001 |
Abbreviations: MCI, mild cognitive impairment; OR, odds ratio; PET, positron emission tomography.
Results considered significant at P < .0036 after Bonferroni correction for multiple comparisons.
Analysis set (n = 11 400) did not include 9 cases with uninterpretable amyloid PET results.
The 3-way interaction of amyloid PET result × primary pre-PET etiologic diagnosis × level of impairment and the 2-way interaction of primary pre-PET etiologic diagnosis × level of impairment were not significant (P = .91 and P = .89, respectively). The estimated Akaike Information Criterion for the full model, including these 2 interactions, was 50 474.5 and for the reduced, final model was 50 469.5.
Concordance Between Pre-PET Diagnosis and PET Results
Results of amyloid PET were positive in 5595 of 8770 (63.8% [95% CI, 62.8%-64.8%]) patients with a pre-PET diagnosis of Alzheimer disease and negative in 1261 of 2639 (47.8% [95% CI, 45.9%-49.7%]) patients with a pre-PET diagnosis of non–Alzheimer disease.
Referrals to Therapeutic Trials
There was an overall reduction in clinical trial referrals in the population, from 17.7% (95% CI, 17.0%-18.4%) pre-PET to 13.2% (95% CI, 12.6%-13.8%) post-PET (eTable 4 in Supplement 4).
Post Hoc Analyses
Change in Individual Components of the Management End Point
The most common change in management involved Alzheimer disease drug use, which changed in 43.6% (95% CI, 42.5%-44.8%) of patients with MCI and 44.9% (95% CI, 43.4%-46.3%) of patients with dementia (Table 2). Changes in non–Alzheimer disease drugs were reported in 22.9% (95% CI, 21.9%-23.9%) of patients with MCI and 25.4% (95% CI, 24.1%-26.7%) of patients with dementia, while changes in counseling were reported in 24.3% (95% CI, 23.3%-25.4%) of patients with MCI and 20.7% (95% CI, 19.6%-21.9%) of patients with dementia. Specialists often changed more than 1 element of the management composite (eTable 5 in Supplement 4). Changes in all management aspects were more common in patients with positive vs negative amyloid PET results (eTable 6 in Supplement 4).
Changes in Diagnosis Stratified by PET Results
The proportion of Alzheimer disease diagnosis increased from 80.3% (95% CI, 79.3%-81.2%) pre-PET to 95.5% (95% CI, 94.9%-95.9%) post-PET in patients with a positive scan result, while in patients with negative scan results the rate of Alzheimer disease diagnosis decreased from 71.5% (95% CI, 70.2%-72.8%) pre-PET to 10.2% (95% CI, 9.3%-11.1%) post-PET.
Change in Diagnostic Confidence
Prior to PET, clinicians reported diagnostic confidence in the uncertain range (4-7 on the Likert scale) in 72.4% (95% CI, 71.6%-73.3%) of patients, and this proportion was reduced to 16.2% (95% CI, 15.5%-16.9%) at the post-PET visit (eFigure 2 in Supplement 4).
Changes Between Pre- and Post-PET Overall Use of Alzheimer Disease Drugs and Diagnostic Tests
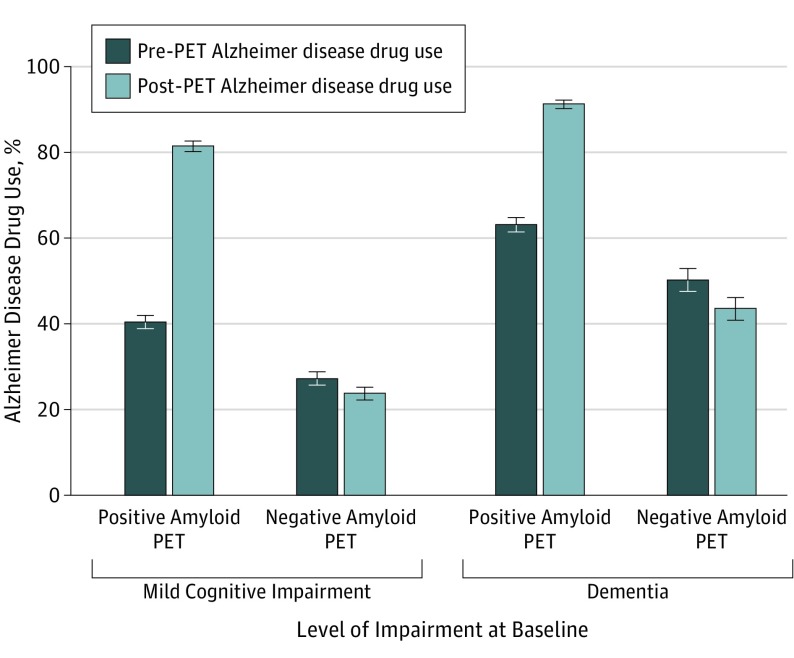
In patients with positive PET results, the overall use of Alzheimer disease drugs in the population increased significantly, from 40.4% (95% CI, 38.9%-42.0%) to 81.5% (95% CI, 80.2%-82.7%) in patients with MCI (P < .001) and from 63.2% (95% CI, 61.5%-64.8%) to 91.2% (95% CI, 90.2%-92.2%) in patients with dementia (P < .001) (Figure 2; eTable 7 in Supplement 4). There were modest but significant reductions in the use of Alzheimer disease drugs in patients with a negative scan result, from 27.2% (95% CI, 25.7%-28.8%) to 23.7% (95% CI, 22.2%-25.2%) in patients with MCI (P < .001) and from 50.3% (95% CI, 47.6%-52.9%) to 43.5% (95% CI, 40.9%-46.2%) in patients with dementia (P < .001). There were reductions between the pre-PET and post-PET plans in the overall use of some diagnostic procedures, including neuropsychological testing, other imaging tests, and CSF studies (eTable 4 in Supplement 4).
Figure 2. Changes in Overall Use of Alzheimer Disease Medications.
All contrasts between pre–positron emission tomography (PET) and post-PET use were significant (P < .001). Error bars indicate 95% CIs.
Pre- and Post-PET Rates of Amyloid Positivity in Patients Referred to Alzheimer Disease Clinical Trials
The rate of amyloid PET positivity (required for inclusion in many Alzheimer disease drug trials) increased from 65.5% (95% CI, 63.4%-67.6%) in patients intended for trial referral at the pre-PET visit to 92.9% (95% CI, 91.5%-94.2%) in patients referred to Alzheimer disease trials post-PET.
Deaths Between Pre-PET and Post-PET Visits
Seventy-five deaths were reported on post-PET forms, of which 17 occurred after enrollment but before the PET scan. Study physicians were able to reach the referring specialists in 41 of 58 remaining cases. The most common reported causes of death were cardiac (12), unknown (9), infection (5), and pulmonary (4). There were no reported suicides.
Discussion
In this large, multisite, practice-based study, core elements of the patient management plan changed after amyloid PET in a majority of patients with MCI and patients with dementia meeting appropriate use criteria. The changes captured in the composite management end point reflect the core elements of a comprehensive treatment plan for cognitive disorders, including the use of Alzheimer disease drugs, use of other drugs that affect cognition or address dementia risk factors, and counseling about safety and future planning.16 Rates of change in both groups were significantly higher than the a priori target threshold of 30% and were comparable to results reported in smaller studies and recent meta-analyses.20,21,22
The most frequent change in management involved the use of Alzheimer disease drugs, the aspect of management most directly tied to etiologic diagnosis. Cholinesterase inhibitors and memantine are approved for the symptomatic treatment of Alzheimer disease,23 and cholinesterase inhibitors also show some efficacy in the treatment of dementia with Lewy bodies and Parkinson disease dementia,24 conditions that often involve amyloid deposition.7 Conversely, Alzheimer disease drugs are associated with worse outcomes in some amyloid-negative dementias, such as frontotemporal dementia.25,26 Change in the use of Alzheimer disease drugs after PET was linked to amyloid status (Figure 2), although in some instances clinicians elected to continue the use of Alzheimer disease drugs in patients with a negative scan result, perhaps reflecting the favorable safety profile of these medications27 as well as the lack of alternative therapies for other disorders.
Clinicians modified the use of Alzheimer disease drugs frequently in patients with MCI (Table 2), despite lack of evidence for efficacy of these drugs at the MCI stage.28 Pivotal trials of cholinesterase inhibitors and memantine in MCI and Alzheimer disease dementia predated the availability of Alzheimer disease biomarkers. This likely resulted in considerable biological heterogeneity in clinical trial cohorts, particularly for MCI, for which rates of amyloid negativity approach 50% to 60% in patients who meet clinical criteria.6,29 Patients with MCI who are carriers of the apolipoprotein E ε4 allele, which is associated with positive amyloid PET results,30 have been shown to benefit from cholinesterase inhibitors in secondary trial analyses.31 Nevertheless, adjustments in Alzheimer disease drug use based on amyloid PET results (in patients with MCI or dementia) reflect physician behavior rather than evidence-based practice.
Lower proportions of change were observed in use of non–Alzheimer disease drugs and counseling. Best practice dictates that use of non–Alzheimer disease drugs, including drugs with psychoactive properties and medications used to treat dementia risk factors, should be optimized regardless of the primary cause of cognitive impairment.32 Counseling about safety and future planning is informed by disease stage, level of impairment, and perhaps less by etiologic diagnosis.
The presence of amyloid is required, but not sufficient, for a neuropathological diagnosis of Alzheimer disease.8 Accordingly, an important role of amyloid PET is to exclude Alzheimer disease as a possible cause of cognitive impairment. In this study, amyloid PET results were negative in a significant minority of patients with a pre-PET diagnosis of Alzheimer disease, and conversely most patients with negative scan results had a pre-PET diagnosis of Alzheimer disease. This diagnosis and subsequent treatment may have persisted if these patients had no access to amyloid PET. As reported in previous studies,20,21 use of amyloid PET was associated with frequent changes in diagnosis, improved diagnostic confidence, and reduced use of other diagnostic tests. There was also an increase in the proportion of amyloid positivity in patients referred to Alzheimer disease clinical trials, which would considerably improve the efficiency of screening for trials requiring positive amyloid PET results as an inclusion criterion.33
While refinement of diagnosis and core elements of the management plan are valuable for the care of patients with cognitive impairment,16 an important future goal is to test whether amyloid PET is associated with changes in additional patient-oriented outcomes. The second aim of this study is to use Medicare claims data to compare health outcomes and overall resource utilization in study participants with outcomes and utilization in a matched control group of Medicare beneficiaries who have not undergone amyloid PET.
This study has several strengths. The size of patient cohorts and number of participating physicians are considerably larger than in previous investigations. While previous studies were primarily conducted at academic centers, the network of sites in this study included mostly private practices. PET scans were interpreted by local imaging specialists, in contrast to the centralized interpretations used in most previous studies.20,21 Measured outcomes reflected implemented rather than intended management.
Limitations
This study has several limitations. First, the nonrandomized design and lack of a control group limit the direct attribution of changes in management to PET. However, the rates of changes in management were similar to those reported in randomized studies,20,21 and physicians ascribed a large majority of all changes to the scan results. Second, patients were included in the study based on criteria that “knowledge of PET results is expected to change diagnosis and management.” Therefore, the a priori threshold for the rate of changes in management could have been set higher than 30%, although the observed rates of change in the primary end point were substantially higher than the prespecified threshold. Third, observed changes in diagnosis and management represent the behavior of specialized physicians rather than evidence-based standard of care. Fourth, this study did not directly compare the association between amyloid PET and changes in clinical management with management changes associated with other diagnostic tools, such as 18F-labeled fludeoxyglucose PET or CSF Alzheimer disease biomarkers. Fifth, based on third-party report (which may be inaccurate), participants in the study were primarily non-Hispanic white and do not adequately reflect the racial and ethnic diversity of Medicare beneficiaries or the US population. Sixth, there were relatively high rates of protocol noncompliance, which likely reflect the practice-based setting of the study.
Conclusions
Among Medicare beneficiaries with MCI or dementia of uncertain etiology evaluated by dementia specialists, the use of amyloid PET was associated with changes in clinical management within 90 days. Further research is needed to determine whether amyloid PET is associated with improved clinical outcomes.
Study Protocol
Pre-PET Case Report Form
Post-Pet Case Report Form
eMethods
eTable 1. Comparison of Final Analysis Cohort to All Participants With Pre-PET Data
eTable 2. PET Result Contribution to Post-PET Management Plan
eTable 3. Multiple Imputation Results for the Logistic Regression Model Shown in Table 3, Assessing Factors Associated With Change in the Composite Management Endpoint
eTable 4. Changes in Diagnostic Testing and Referrals
eTable 5. Combinations of Changes on Components of the Composite Management Endpoint
eTable 6. Changes in Management by Scan Result
eTable 7. Pre-PET and Post-PET Alzheimer Disease Drug Use by Level of Impairment and Amyloid PET Result
eFigure 1. Location of Dementia Clinics, PET Facilities, and Radiopharmaceutical Suppliers for IDEAS Study
eFigure 2. Changes in Diagnostic Confidence of Underlying Alzheimer Disease Pathology
eReferences
References
- 1.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55(3):306-319. doi: 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- 2.Jagust W. Is amyloid-β harmful to the brain? insights from human imaging studies. Brain. 2016;139(pt 1):23-30. doi: 10.1093/brain/awv326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattsson N, Carrillo MC, Dean RA, et al. Revolutionizing Alzheimer’s disease and clinical trials through biomarkers. Alzheimers Dement (Amst). 2015;1(4):412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laforce R Jr, Rabinovici GD. Amyloid imaging in the differential diagnosis of dementia. Alzheimers Res Ther. 2011;3(6):31. doi: 10.1186/alzrt93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. ; Amyloid PET Study Group . Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1-13. doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CM, Pontecorvo MJ, Beach TG, et al. ; AV-45-A16 Study Group . Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669-678. doi: 10.1016/S1474-4422(12)70142-4 [DOI] [PubMed] [Google Scholar]
- 10.Curtis C, Gamez JE, Singh U, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72(3):287-294. doi: 10.1001/jamaneurol.2014.4144 [DOI] [PubMed] [Google Scholar]
- 11.Sabri O, Sabbagh MN, Seibyl J, et al. ; Florbetaben Phase 3 Study Group . Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 2015;11(8):964-974. doi: 10.1016/j.jalz.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services (CMS) Decision Memo for Beta Amyloid Positron Emission Tomography in Dementia and Neurodegenerative Disease (CAG-00431N). CMS website. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=265. Published 2013. Accessed August 10, 2018.
- 13.Johnson KA, Minoshima S, Bohnen NI, et al. ; Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce . Appropriate use criteria for amyloid PET. Alzheimers Dement. 2013;9(1):e-1-e-16. doi: 10.1016/j.jalz.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging. J Nucl Med. 2013;54(7):1011-1013. doi: 10.2967/jnumed.113.127068 [DOI] [PubMed] [Google Scholar]
- 15.Minoshima S, Drzezga AE, Barthel H, et al. SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0. J Nucl Med. 2016;57(8):1316-1322. doi: 10.2967/jnumed.116.174615 [DOI] [PubMed] [Google Scholar]
- 16.Naylor MD, Karlawish JH, Arnold SE, et al. Advancing Alzheimer’s disease diagnosis, treatment, and care. Alzheimers Dement. 2012;8(5):445-452. doi: 10.1016/j.jalz.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillner BE, Liu D, Coleman RE, et al. The National Oncologic PET Registry (NOPR): design and analysis plan. J Nucl Med. 2007;48(11):1901-1908. doi: 10.2967/jnumed.107.043687 [DOI] [PubMed] [Google Scholar]
- 18.Grundman M, Pontecorvo MJ, Salloway SP, et al. ; 45-A17 Study Group . Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27(1):4-15. doi: 10.1097/WAD.0b013e318279d02a [DOI] [PubMed] [Google Scholar]
- 19.Milani SA, Marsiske M, Cottler LB, Chen X, Striley CW. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimers Dement (Amst). 2018;10:773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantoni ER, Chalkidou A, O’ Brien JT, Farrar G, Hammers A. A systematic review and aggregated analysis on the impact of amyloid PET brain imaging on the diagnosis, diagnostic confidence, and management of patients being evaluated for Alzheimer’s disease. J Alzheimers Dis. 2018;63(2):783-796. doi: 10.3233/JAD-171093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea YF, Barker W, Greig-Gusto MT, Loewenstein DA, Duara R, DeKosky ST. Impact of amyloid PET imaging in the memory clinic: a systematic review and meta-analysis. J Alzheimers Dis. 2018;64(1):323-335. doi: 10.3233/JAD-180239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wilde A, van der Flier WM, Pelkmans W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 2018;75(9):1062-1070. doi: 10.1001/jamaneurol.2018.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider LS. Alzheimer disease pharmacologic treatment and treatment research. Continuum (Minneap Minn). 2013;19(2 Dementia):339-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2015;86(2):135-143. doi: 10.1136/jnnp-2014-307659 [DOI] [PubMed] [Google Scholar]
- 25.Boxer AL, Knopman DS, Kaufer DI, et al. Memantine in patients with frontotemporal lobar degeneration. Lancet Neurol. 2013;12(2):149-156. doi: 10.1016/S1474-4422(12)70320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84-87. doi: 10.1097/01.JGP.0000231744.69631.33 [DOI] [PubMed] [Google Scholar]
- 27.Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau-based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment. Neurology. 2018;90(3):126-135. doi: 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolk DA, Sadowsky C, Safirstein B, et al. Use of flutemetamol F 18-labeled positron emission tomography and other biomarkers to assess risk of clinical progression in patients with amnestic mild cognitive impairment. JAMA Neurol. 2018;75(9):1114-1123. doi: 10.1001/jamaneurol.2018.0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landau SM, Horng A, Fero A, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative . Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86(15):1377-1385. doi: 10.1212/WNL.0000000000002576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen RC, Thomas RG, Grundman M, et al. ; Alzheimer’s Disease Cooperative Study Group . Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379-2388. doi: 10.1056/NEJMoa050151 [DOI] [PubMed] [Google Scholar]
- 32.Austrom MG, Boustani M, LaMantia MA. Ongoing medical management to maximize health and well-being for persons living with dementia. Gerontologist. 2018;58(suppl 1):S48-S57. doi: 10.1093/geront/gnx147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. doi: 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Pre-PET Case Report Form
Post-Pet Case Report Form
eMethods
eTable 1. Comparison of Final Analysis Cohort to All Participants With Pre-PET Data
eTable 2. PET Result Contribution to Post-PET Management Plan
eTable 3. Multiple Imputation Results for the Logistic Regression Model Shown in Table 3, Assessing Factors Associated With Change in the Composite Management Endpoint
eTable 4. Changes in Diagnostic Testing and Referrals
eTable 5. Combinations of Changes on Components of the Composite Management Endpoint
eTable 6. Changes in Management by Scan Result
eTable 7. Pre-PET and Post-PET Alzheimer Disease Drug Use by Level of Impairment and Amyloid PET Result
eFigure 1. Location of Dementia Clinics, PET Facilities, and Radiopharmaceutical Suppliers for IDEAS Study
eFigure 2. Changes in Diagnostic Confidence of Underlying Alzheimer Disease Pathology
eReferences