Key Points
Question
Among patients with atrial fibrillation, what is the effect of catheter ablation, compared with medical therapy, on cardiovascular events and mortality?
Findings
In this randomized clinical trial involving 2204 patients with atrial fibrillation, catheter ablation, compared with medical therapy, did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest (8.0% vs 9.2%, respectively; hazard ratio, 0.86).
Meaning
Among patients with atrial fibrillation, catheter ablation, compared with medical therapy, did not significantly reduce the primary composite outcome.
Abstract
Importance
Catheter ablation is effective in restoring sinus rhythm in atrial fibrillation (AF), but its effects on long-term mortality and stroke risk are uncertain.
Objective
To determine whether catheter ablation is more effective than conventional medical therapy for improving outcomes in AF.
Design, Setting, and Participants
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation trial is an investigator-initiated, open-label, multicenter, randomized trial involving 126 centers in 10 countries. A total of 2204 symptomatic patients with AF aged 65 years and older or younger than 65 years with 1 or more risk factors for stroke were enrolled from November 2009 to April 2016, with follow-up through December 31, 2017.
Interventions
The catheter ablation group (n = 1108) underwent pulmonary vein isolation, with additional ablative procedures at the discretion of site investigators. The drug therapy group (n = 1096) received standard rhythm and/or rate control drugs guided by contemporaneous guidelines.
Main Outcomes and Measures
The primary end point was a composite of death, disabling stroke, serious bleeding, or cardiac arrest. Among 13 prespecified secondary end points, 3 are included in this report: all-cause mortality; total mortality or cardiovascular hospitalization; and AF recurrence.
Results
Of the 2204 patients randomized (median age, 68 years; 37.2% female; 42.9% had paroxysmal AF and 57.1% had persistent AF), 89.3% completed the trial. Of the patients assigned to catheter ablation, 1006 (90.8%) underwent the procedure. Of the patients assigned to drug therapy, 301 (27.5%) ultimately received catheter ablation. In the intention-to-treat analysis, over a median follow-up of 48.5 months, the primary end point occurred in 8.0% (n = 89) of patients in the ablation group vs 9.2% (n = 101) of patients in the drug therapy group (hazard ratio [HR], 0.86 [95% CI, 0.65-1.15]; P = .30). Among the secondary end points, outcomes in the ablation group vs the drug therapy group, respectively, were 5.2% vs 6.1% for all-cause mortality (HR, 0.85 [95% CI, 0.60-1.21]; P = .38), 51.7% vs 58.1% for death or cardiovascular hospitalization (HR, 0.83 [95% CI, 0.74-0.93]; P = .001), and 49.9% vs 69.5% for AF recurrence (HR, 0.52 [95% CI, 0.45-0.60]; P < .001).
Conclusions and Relevance
Among patients with AF, the strategy of catheter ablation, compared with medical therapy, did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest. However, the estimated treatment effect of catheter ablation was affected by lower-than-expected event rates and treatment crossovers, which should be considered in interpreting the results of the trial.
Trial Registration
ClinicalTrials.gov Identifier: NCT00911508
This randomized trial compares the effect of pulmonary vein isolation via catheter ablation vs rate and rhythm control using medical therapy on a composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest in patients with atrial fibrillation (AF).
Introduction
Atrial fibrillation (AF) is not only the most common cardiac tachyarrhythmia, but also the most perplexing from a clinical management perspective. Some patients with AF are symptomatic to the point of disabling decrements in quality of life, while others remain asymptomatic. In epidemiologic studies, AF also has significant adverse prognostic effects and has been associated with poor outcomes including reduced survival1,2,3 and an increased risk of major nonfatal cardiac morbidities, including stroke, congestive heart failure, and late cognitive impairment. How much this risk can be mitigated by restoring sinus rhythm remains uncertain.
Antiarrhythmic drug therapy has been the primary treatment for AF for decades, but limited effectiveness combined with incompletely assessed risks have led to the development of other strategies to maintain sinus rhythm. Starting in 1998, reports appeared suggesting that ablative intervention was more effective than antiarrhythmic drug therapy in reducing episodes of recurrent paroxysmal AF.4,5,6,7,8 Since those early reports, the use of ablation has been extended to more difficult and higher-risk patients9 despite the lack of large randomized comparative trial evidence of improved clinical outcomes. Recently, a small trial of ablation vs medical therapy in symptomatic patients with AF and class II or worse systolic heart failure provided evidence suggesting that successful ablation may extend survival.10
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, an investigator-initiated, multicenter, prospective, randomized, open-label clinical trial funded by the National Heart, Lung, and Blood Institute and industry partners, was designed to test the hypothesis that ablative therapy for AF is more effective than state-of-the-art drug therapy in a broad population of symptomatic and inadequately treated patients with AF.11
Methods
Trial Design and Setting
Each site’s institutional review board or ethics committee approved the study. Written informed consent was obtained from all patients. The trial protocol and statistical analysis plan are available in Supplement 1 and trial protocol amendments in Supplement 2. Details of the trial design have also been previously published.11 Race and ethnicity were classified by the patient and investigators as required by the National Institutes of Health (NIH) using NIH-specified categories.
Eligible patients were aged 65 years and older or younger than 65 years with 1 or more risk factors for stroke (hypertension, heart failure, history of stroke, diabetes, or other heart problems), had 2 or more episodes of paroxysmal AF or 1 episode of persistent AF in the prior 6 months, and were suitable for catheter-based treatment or rhythm and/or rate control drug therapy. Patients were excluded if they had a prior left atrial catheter ablation for AF or had failed 2 or more antiarrhythmic drugs. Full eligibility criteria are listed in eTable 1 in Supplement 3. Eligible patients were randomized in equal proportions to either catheter ablation or drug therapy using permuted block randomization with stratification by clinical site. The block size (concealed from investigators) was randomly selected with equal probability between 2 and 4. Randomization was accomplished using a centralized, interactive voice response and web-based randomization system (IXRS; Almac). By protocol, ablation procedures all included pulmonary vein isolation. The addition of ancillary ablation techniques, including linear, ganglion plexus, and electrogram-based approaches, were left to the discretion of the investigators.4,12 Physicians performing ablations were required to have a 100-case experience to participate in the trial. It was recommended that patients randomized to medical therapy receive rate control medications first. If the patient had previously failed rate control therapy, then rhythm control drug therapy could be initiated in an approach consistent with contemporaneous guidelines.
All patients were to receive anticoagulation based on contemporaneous guidelines.13,14 Patients who received a catheter ablation were treated with anticoagulation for at least 3 months after the ablation, with a recommendation that this be continued throughout the trial in patients with CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke/transient ischemic attack/thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age 65-75 years, sex category [female]) scores of 2 or more, following recommendations of the American Heart Association/American College of Cardiology/European Society of Cardiology guidelines13 and guidelines from the ablation consensus documents.12,15,16 Details of medical treatments used in the trial are provided in the trial design paper.11
The trial originally planned to enroll 3000 patients who would be followed up for approximately 3 years with a primary end point of all-cause mortality.11 Based on information available during the design phase of the trial, the mortality rate in the drug group was projected to be approximately 12% after 3 years of follow-up.11 We hypothesized that there would be a 30% relative risk reduction in patients treated with ablation based on synthesis of information from multiple published sources, as described in the trial design paper.11
Due to slow enrollment and lower than expected aggregated event rates, the data and safety monitoring board, after completing a scheduled review of trial progress in early 2013, recommended modifying the trial design. In response, the study leadership, blinded to treatment-specific outcomes, modified the design in February 2013 by (1) elevating the key secondary end point (composite of death, disabling stroke, serious bleeding, or cardiac arrest) to the primary end point, (2) changing all-cause mortality to the key secondary end point, and (3) extending study follow-up to an average of 4 years or longer, all of which supported a reduction in the sample size to 2200 patients. The new primary end point event rate was expected to be equal to or greater than the originally anticipated mortality rate, and the effect of ablation was again assumed to be a 30% relative reduction. These projections assumed up to 25% treatment crossover from drug therapy to ablation while maintaining the desired 90% level of statistical power.11
Scheduled patient follow-up occurred at 3, 6, and 12 months and then every 6 months thereafter. All events for each component of the primary end point were reviewed and adjudicated in a blinded fashion by an independent clinical events committee using prospectively determined event definitions. Death was defined as all-cause mortality, disabling stroke (including intracranial bleeding) as an irreversible physical limitation defined by a Rankin Stroke Scale score of 2 or greater, and serious bleeding as bleeding accompanied by hemodynamic compromise requiring surgical intervention or a transfusion of 3 or more units of blood.
Three of 13 prespecified secondary end points of the trial are reported in this article: overall mortality, overall mortality or cardiovascular (CV) hospitalization, and AF recurrence (based on the subset of patients with the CABANA electrocardiogram [ECG] event recording system). The reason for hospitalization was characterized by the site principal investigator and reported on the hospitalization case report form. To determine AF recurrence rates, patients were provided with an ECG event recorder for chronicling symptomatic events; for 24-hour autodetect, full-disclosure, real-time recordings on a quarterly basis; and to obtain 96-hour Holter recordings every 6 months regardless of symptoms. Monitoring algorithms provided beat-to-beat morphologic analyses as well as rate and rhythm information. In countries that prohibited the use of the trial event recording system for regulatory reasons, largely equivalent, standard ECG event recording systems were used. Over the course of follow-up, a 30-second episode of AF in either treatment group, confirmed through blinded review by an ECG Core Laboratory Committee, was used to define the end point of recurrent AF.
In the analysis of long-term AF recurrence, a conventional 3-month blanking period from therapy initiation was used in both treatment groups during which arrhythmia recurrences were not counted toward the recurrent AF end point. A repeat ablation for recurrent AF could be performed during this time if necessary. Similarly, patients randomized to drug therapy were allowed serial drug trials during the blanking period to find an effective, best-tolerated regimen. All other end points, including adverse events, were chronicled from the time of randomization.
Quality-of-life outcomes are reported in a companion article.17 Other secondary end points (eTable 2 in Supplement 3) will be reported in future publications.
Statistical Methods
Prespecified Primary, Secondary, and Subgroup Analyses by Intention to Treat
The primary treatment comparisons between the randomized groups were performed according to the intention-to-treat (ITT) principle based on a time-to-first-event analysis using the log-rank test.18 Kaplan-Meier cumulative event rates19 were calculated for each group, with event or censoring times measured from the time of randomization. Relative risks were expressed as hazard ratios [HRs] with associated 95% CIs derived using the Cox proportional hazards model.20 The proportional hazards assumption of the model was checked by examining a treatment by (log) time interaction term and by assessing Schoenfeld residuals,21 as detailed in eAppendix 1 in Supplement 3. The Cox model was also used to assess the consistency of treatment effects by testing for interactions between treatment strategy and prespecified baseline characteristics. Recurrent atrial arrhythmia incidence rates were calculated from the end of the blanking period by ITT, and adjusted statistical comparisons were performed with mortality as a competing risk.22
Two-sided significance testing was used with a conventional significance level of .05, unless otherwise specified. There was no adjustment for multiple comparisons among the secondary end points (eAppendix 1 in Supplement 3).
For patients who withdrew consent or were lost to follow-up during the study, all information collected to the point of consent withdrawal or final contact was analyzed. For patients who did not complete the study and did not experience an outcome event, their time-to-event measure was censored at the last contact date. Imputation of outcome events was not performed.
Prespecified Sensitivity Analyses
“Treatment received” comparisons were performed using the Cox model with catheter ablation included as a time-dependent covariate. “Per-protocol” comparisons were performed in which the drug group consisted of all patients randomized to drug therapy, with the follow-up of patients who received drug therapy and crossed over to catheter ablation censored at the time of ablation. The per-protocol catheter ablation group included patients randomized to catheter ablation who received an ablation within the 6-month time window following randomization. As prespecified, a shorter window (3 months) and a longer window (12 months) were also considered. The prespecified treatment received and per-protocol treatment comparisons were adjusted for a prespecified set of baseline patient characteristics (age, sex, race/ethnicity, AF type, years since onset of AF, history of heart failure, structural heart disease, CHA2DS2-VASc score, history of coronary artery disease, and hypertension). Additional details on these analyses are provided in eAppendix 1 in Supplement 3.
Post Hoc Sensitivity Analysis
To evaluate the possibility that treatment effect varied among the 126 enrolling sites, we performed a post hoc ITT mixed-model analysis of the primary end point comparison adjusted for enrolling site as a random effect.
Trial Monitoring
An independent data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute performed interim reviews of the study at regular intervals during the trial. One formal interim treatment comparison of primary end point data was performed and monitored with the use of 2-sided, symmetric O’Brien-Fleming23 boundaries generated with the Lan-DeMets α-spending function approach to group sequential testing.24 A significance level of .049 was required for the primary end point at the final analysis to adjust for the interim analysis.
All analyses were performed using SAS software version 9.4 or later versions (SAS Institute).
Results
Study Population
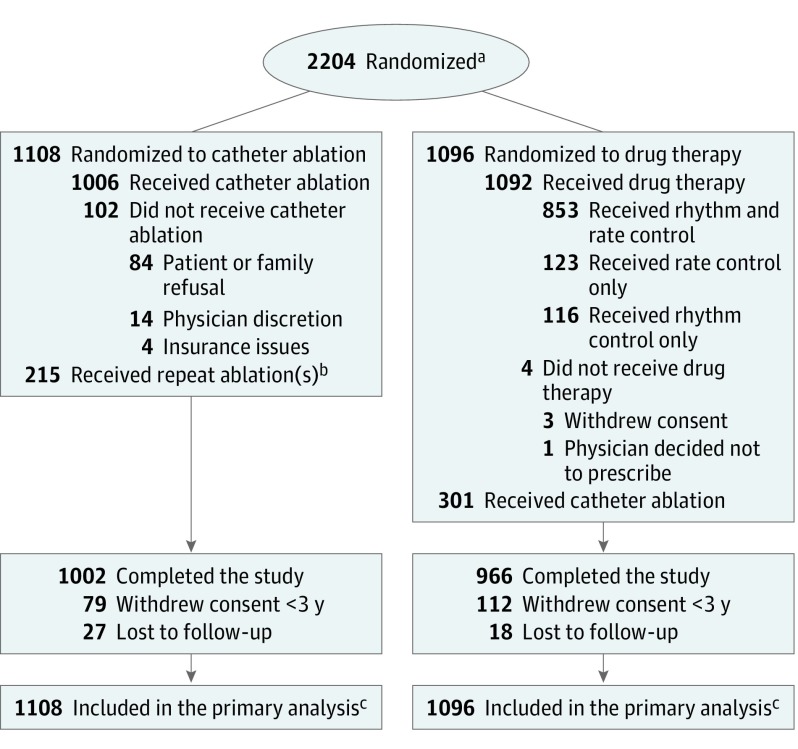
Between November 2009 and April 2016, 2204 patients from 126 sites across 10 countries were randomly assigned to receive catheter ablation (1108 patients) or drug therapy (1096 patients) (Figure 1). Follow-up continued through December 31, 2017, for a median duration of 48.5 months (25th percentile: 29.9 months, 75th percentile: 62.1 months).
Figure 1. Randomization and Patient Flow in the CABANA Trial.
aSites were not required to provide screening logs during the recruitment phase; thus, the number of patients assessed for eligibility is not available.
bTwenty five patients underwent repeat catheter ablation during the blanking period; 190 patients had at least 1 repeat catheter ablation during the postblanking period for a total of 215.
cOutcomes of patients who did not complete the study (ie, withdrew consent or were lost to follow-up) were included to the point of consent withdrawal or final contact. Primary and key secondary end points were analyzed using time-to-event methodology; thus, all available follow-up information was used. For patients who did not complete the study and did not experience an outcome event, their time-to-event measure was censored at the last contact date. There was no imputation of outcome events. At the end of the trial, a publicly available death registry search was performed for patients enrolled in North America who were lost or withdrew from the trial.
Characteristics at Baseline
The 2 treatment groups showed the balance in baseline factors expected from randomization (Table 1). The patients randomized to catheter ablation had a median age of 68 years, 37.3% were women, and 10.2% belonged to racial or ethnic minorities. The patients randomized to drug therapy had a median age of 67 years, 37.0% were women, and 10.2% belonged to racial or ethnic minorities. Paroxysmal AF was present in 42.4% of patients in the catheter ablation group and 43.5% in the drug therapy group, with the remainder having persistent or long-standing persistent AF (Table 1). The study population had a substantial burden of CV risk factors (Table 1): 80.6% with hypertension, 25.5% with diabetes, 19.2% with coronary artery disease, 10.0% with a prior stroke or transient ischemic attack, 15.3% with history of congestive heart failure, and 56.5% with a CHA2DS2-VASc score greater than 2 (median, 3).
Table 1. Baseline Demographics and Clinical Characteristics.
| Baseline Characteristic | No. (%) | |
|---|---|---|
| Catheter Ablation (n = 1108) | Drug Therapy (n = 1096) | |
| Patients | ||
| Age, median (Q1, Q3), y | 68 (62, 72) | 67 (62, 72) |
| <65 | 375 (33.8) | 391 (35.7) |
| 65-<75 | 577 (52.1) | 553 (50.5) |
| ≥75 | 156 (14.1) | 152 (13.9) |
| Sex | ||
| Male | 695 (62.7) | 690 (63.0) |
| Female | 413 (37.3) | 406 (37.0) |
| Racea | ||
| White | 1018 (92.0) | 1007 (92.1) |
| Black or African American | 39 (3.5) | 38 (3.5) |
| Otherb | 50 (4.5) | 48 (4.4) |
| Ethnicity | ||
| Hispanic or Latino | 30 (2.7) | 32 (2.9) |
| Not Hispanic or Latino | 1074 (97.3) | 1062 (97.1) |
| Body mass index, median (Q1, Q3)c | 30 (27, 34) | 30 (26, 35) |
| AF severity (CCS class)d | ||
| 0 (Least severe) | 105 (9.5) | 118 (10.8) |
| 1 | 166 (15.1) | 173 (15.9) |
| 2 | 350 (31.8) | 353 (32.4) |
| 3 | 401 (36.5) | 382 (35.0) |
| 4 (Most severe) | 78 (7.1) | 65 (6.0) |
| Heart function severity (NYHA class)e | ||
| I (Least severe) | 153 (13.9) | 126 (11.6) |
| II/III (Most severe) | 376 (34.3) | 400 (36.7) |
| Medical history | ||
| Hypertension or LVH | 924 (83.4) | 927 (84.7) |
| Hypertension | 876 (79.1) | 900 (82.2) |
| LVH | 334 (38.7) | 328 (42.1) |
| Diabetes | 280 (25.3) | 281 (25.7) |
| Sleep apnea | 262 (23.6) | 246 (22.5) |
| Coronary artery disease | 208 (18.8) | 216 (19.7) |
| Heart failure | 174 (15.7) | 163 (14.9) |
| Family history of AF | 130 (11.8) | 122 (11.2) |
| Prior CVA or TIA | 117 (10.6) | 103 (9.4) |
| Prior CVA | 68 (6.1) | 58 (5.3) |
| Thromboembolic events | 41 (3.7) | 49 (4.5) |
| Ejection fraction ≤35% | 38/790 (4.8) | 31/740 (4.2) |
| Comorbidities | ||
| CHA2DS2-VAScf | ||
| Median (Q1, Q3) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) |
| 0-1 (Lowest risk) | 208 (18.8) | 187 (17.1) |
| 2 | 273 (24.6) | 291 (26.6) |
| 3 | 308 (27.8) | 329 (30.0) |
| 4 | 178 (16.1) | 151 (13.8) |
| ≥5 (Highest risk) | 141 (12.7) | 138 (12.6) |
| Arrhythmia History | ||
| Time since onset of AF, y | ||
| Median (Q1, Q3) | 1.1 (0.3, 4.1) | 1.1 (0.3, 3.7) |
| Type of AF at enrollmentg | ||
| Persistent | 524 (47.3) | 518 (47.3) |
| Paroxysmal | 470 (42.4) | 476 (43.5) |
| Long-standing persistent | 114 (10.3) | 101 (9.2) |
| Prior hospitalization for AF | 449 (40.6) | 425 (38.8) |
| Prior direct cardioversion | 398 (36.0) | 411 (37.5) |
| History of atrial flutter | 140 (12.9) | 158 (14.6) |
| Prior ablation for atrial flutter | 48 (4.3) | 60 (5.5) |
| Rhythm control therapyh | ||
| 1 Rhythm control drug | 398 (81.6) | 452 (82.2) |
| ≥2 Rhythm control drugs | 90 (18.4) | 98 (17.8) |
Abbreviations: AF, atrial fibrillation; CCS, Canadian Cardiovascular Society; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65-75 years, sex category (female); CVA, cerebral vascular accident; LVH, left ventricular hypertrophy; NYHA, New York Heart Association; Q1, Q3, quartiles (25th and 75th percentiles); TIA, transient ischemic attack.
Race was determined by the site investigator in conjunction with the patient based on predefined categories as required by the National Institutes of Health (NIH) using NIH-specified categories.
Asian, American Indian/Alaskan Native, Hawaiian, or other Pacific Islander and multiracial.
Calculated as weight in kilograms divided by height in meters squared.
On a scale of 0 to 4, with 0 indicating the least severe and 4, the most severe symptoms of AF.
On a scale of I to IV, with I indicating the least severe and IV, the most severe symptoms of heart failure.
On a scale of 0 to 9, with 0 indicating the lowest risk of stroke and 9, the highest risk of stroke.
Persistent = AF episode sustained for ≥7 days or cardioversion is performed more than 48 hours after AF onset. Paroxysmal = AF episodes lasting ≥1 hour in duration that terminate spontaneously within 7 days or cardioversion is performed within 48 hours of AF onset. Long-standing persistent = continuous AF of >1 year duration.
Current or past use of rhythm control therapy reported at the time of enrollment.
Treatments
Among the 1108 patients randomly assigned to the catheter ablation group, 1006 (90.8%) underwent ablation at a median of 29 days following randomization, and 102 patients did not receive ablation due to patient or family refusal (82.4%) or physician decision (13.7%) (see eTable 3 in Supplement 3 for a comparison of patients who did vs did not undergo ablation). Among the catheter ablation patients, 25 underwent repeat ablation during the blanking period, with 190 patients undergoing at least 1 repeat ablation during the postblanking period, for a total of 215 patients (19.4%) with repeat procedures (Figure 1). Among the catheter ablation patients, 44.6% also received antiarrhythmic drugs at some point during the postblanking period (eTable 4 in Supplement 3), but only 26.5% of the catheter ablation patients were still taking an antiarrhythmic drug at last follow-up.
Among the 1096 patients assigned to the drug therapy group, 1092 (99.6%) received drug therapy, with 545 receiving 1 antiarrhythmic drug, 296 receiving 2, 106 receiving 3, and 22 receiving 4 or more different antiarrhythmic drugs over the course of the trial (eTable 4 in Supplement 3). Most patients (n = 969 [88.4%]) in the drug therapy group received rhythm control drugs during the trial. A total of 301 drug therapy patients (27.5%) crossed over to catheter ablation during the follow-up period (Figure 1). A comparison of these patients and other patients exclusively treated with drugs is provided in eTable 5 in Supplement 3.
ITT (As Randomized) Treatment Comparisons
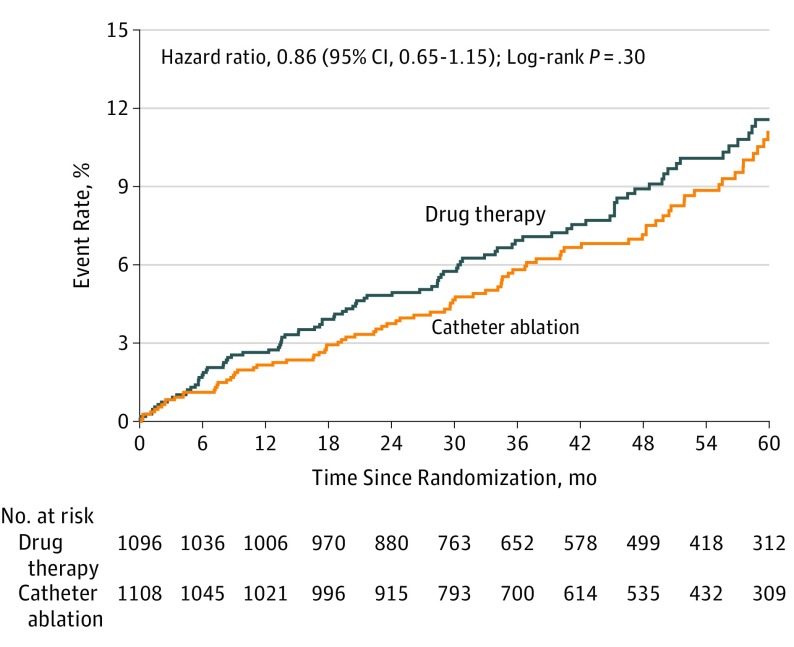
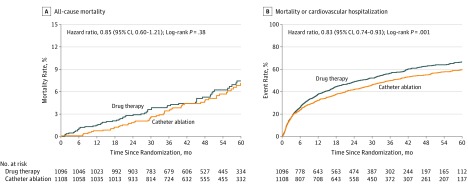
A primary outcome event occurred in 89 patients (8.0%) in the catheter ablation group and in 101 patients (9.2%) in the drug therapy group (HR for ablation vs drug therapy, 0.86 [95% CI, 0.65-1.15]; log-rank P = .30) (Table 2 and Figure 2). Four-year Kaplan-Meier event rates were 7.2% for catheter ablation and 8.9% for drug therapy patients (absolute difference, 1.7%; Table 2). For the key secondary end point of all-cause mortality, a total of 58 patients (5.2%) in the catheter ablation group and 67 patients (6.1%) in the drug therapy group died during follow-up (HR, 0.85 [95% CI, 0.60-1.21]; log-rank P = .38) (Table 2 and Figure 3A). Four-year mortality rates were 4.7% for catheter ablation and 5.3% for drug therapy patients (absolute difference, 0.6%; Table 2). The composite secondary end point of death from any cause or CV hospitalization occurred in 573 patients (51.7%) in the catheter ablation group and 637 patients (58.1%) in the drug therapy group (HR, 0.83 [95% CI, 0.74-0.93]; log-rank P = .001), with 4-year rates of 54.9% for catheter ablation and 62.7% for drug therapy patients (absolute difference, 7.8%; Table 2 and Figure 3B). Details regarding the causes of CV hospitalizations are provided in eTable 6 in Supplement 3.
Table 2. Primary and Secondary Outcomes by Intention-to-Treat Analysis.
| Events, No. (%) | Kaplan-Meier 4-Year Event Rate, % | Hazard Ratio (95% CI)a | P Value | ||||
|---|---|---|---|---|---|---|---|
| Catheter Ablation Group (n = 1108) | Drug Therapy Group (n = 1096) | Catheter Ablation Group (n = 1108) | Drug Therapy Group (n = 1096) | Absolute Reduction | |||
| Primary end point (death, disabling stroke, serious bleeding, or cardiac arrest)b | 89 (8.0) | 101 (9.2) | 7.2 | 8.9 | 1.7 | 0.86 (0.65-1.15)c | .30 |
| Components of primary end point | |||||||
| Death | 58 (5.2) | 67 (6.1) | 4.7 | 5.3 | 0.6 | 0.85 (0.60-1.21) | .38 |
| Disabling stroke | 3 (0.3) | 7 (0.6) | 0.1 | 0.7 | 0.6 | 0.42 (0.11-1.62) | .19 |
| Serious bleeding | 36 (3.2) | 36 (3.3) | 3.0 | 3.7 | 0.7 | 0.98 (0.62-1.56) | .93 |
| Cardiac arrest | 7 (0.6) | 11 (1.0) | 0.7 | 1.1 | 0.4 | 0.62 (0.24-1.61) | .33 |
| Secondary end point | |||||||
| Death or cardiovascular hospitalization | 573 (51.7) | 637 (58.1) | 54.9 | 62.7 | 7.8 | 0.83 (0.74-0.93) | .001 |
Hazard ratio for comparing catheter ablation group vs drug therapy group.
Patients who experienced more than 1 of the component events are counted only once for the primary end point comparison based on the time until the first event. The numbers listed for the individual component events sum to more than the number of patients with a primary event because some patients experienced more than 1 of the component events.
The hazard ratios and 95% CIs are based on 2 995 989 patient-days of follow-up.
Figure 2. Kaplan-Meier Estimates of the Incidence of the Primary End Point.
Kaplan-Meier estimates of the cumulative risk of death, disabling stroke, serious bleeding, or cardiac arrest (primary end point by intention-to-treat analysis). The median (25th, 75th percentile) length of patient follow-up was 4.1 years (2.5, 5.1) in the catheter ablation group and 4.0 years (2.5, 5.2) in the drug therapy group.
Figure 3. Kaplan-Meier Estimates of All-Cause Mortality and Mortality or Cardiovascular Hospitalization by Intention-to-Treat Analysis.
A, The median (25th, 75th percentiles) length of patient follow-up was 4.1 years (2.5, 5.1) in the catheter ablation group and 4.0 years (2.5, 5.2) in the drug therapy group. B, The median (25th, 75th percentiles) length of patient follow-up was 4.1 years (2.5, 5.1) in the catheter ablation group and 4.0 years (2.5, 5.2) in the drug therapy group.
Adjustment for site as a random effect (post hoc analysis) did not change the estimated treatment effect (HR, 0.86 [95% CI, 0.64-1.15]; Wald test P = .29).
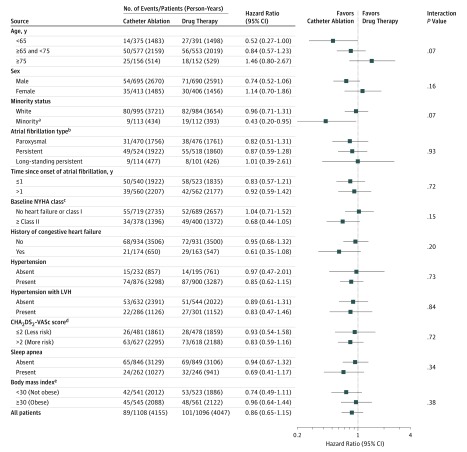
Subgroup Analysis
Examination of prespecified subgroups based on clinical and demographic characteristics did not identify relative variations in the treatment effect of ablation large enough to be clinically significant while also possessing sufficient precision to exclude the null effect (ie, HR, 1) (Figure 4).
Figure 4. Primary End Point Subgroup Analysis (Intention to Treat).
The squares represent the hazard ratios and the bars indicate the 95% CIs. AF indicates atrial fibrillation; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65-75 years, sex category (female); LVH, left ventricular hypertrophy; NYHA, New York Heart Association.
aMinority = Hispanic or Latino or nonwhite race. Minority status was determined by the site investigator in conjunction with the patient based on predefined categories as required by the National Institutes of Health (NIH) using NIH-specified categories.
bParoxysmal = AF episodes lasting ≥1 hour in duration that terminate spontaneously within 7 days or cardioversion is performed within 48 hours of AF onset. Persistent = AF episode sustained for ≥7 days or cardioversion is performed more than 48 hours after AF onset. Long-standing persistent = continuous AF >1 year in duration.
cOn a scale of I to IV, with I indicating the least severe and IV, the most severe symptoms of heart failure.
dOn a scale of 0 to 9, with 0 indicating the lowest risk of stroke and 9, the highest risk of stroke.
eCalculated as weight in kilograms divided by height in meters squared.
Treatment Received Analyses
In the prespecified treatment received analyses, the HR for catheter ablation vs drug therapy with respect to the primary end point was 0.67 (95% CI, 0.50-0.89; P = .006). For all-cause mortality, the corresponding HR was 0.60 (95% CI, 0.42-0.86; P = .005) and for death or CV hospitalization, the HR was 0.83 (95% CI, 0.74-0.94; P = .002). No deaths occurred in the first 30 days after initiation of drug therapy or catheter ablation. One disabling stroke occurred in the drug therapy group within the first 30 days of treatment (eTable 7 in Supplement 3).
Per-Protocol Treatment Comparisons
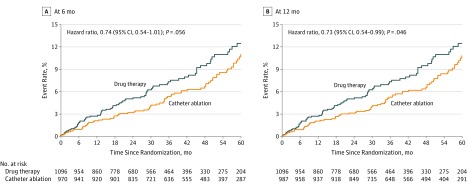
In per-protocol treatment comparisons, patients randomized to catheter ablation who received ablation within a designated window following randomization were compared with patients randomized to the drug therapy group. For the 6-month protocol window, the HR for catheter ablation vs drug therapy for the primary end point was 0.74 (95% CI, 0.54-1.01) (Figure 5A). For the 12-month per-protocol window, the corresponding HR was 0.73 (95% CI, 0.54-0.99) (Figure 5B). The per-protocol HRs for the key secondary end point of all-cause mortality were 0.69 (95% CI, 0.47-1.01) and 0.68 (95% CI, 0.47-0.99) for the 6-month and 12-month definitions of a protocol ablation procedure, respectively (eFigure 1 and eTable 8 in Supplement 3). The subgroup assessment for the primary end point by per-protocol analysis is shown in eFigure 2 in Supplement 3.
Figure 5. Kaplan-Meier Estimates of the Primary End Point by Per-Protocol Analysis.
Kaplan-Meier estimates of the cumulative risk of death, disabling stroke, serious bleeding, or cardiac arrest (primary end point) by 6-month (A) and 12-month (B) per-protocol analysis. Figure includes patients randomized to catheter ablation who were ablated within 6 months (A) or 12 months (B) after randomization. It also includes all patients randomized to drug therapy, with follow-up censored at crossover to ablation. A, The median (25th, 75th percentiles) length of patient follow-up was 4.1 years (2.6, 5.2) in the catheter ablation group and 4.0 years (2.5, 5.2) in the drug therapy group. B, The median (25th, 75th percentiles) length of patient follow-up was 4.2 years (2.6, 5.2) in the catheter ablation group and 4.0 years (2.5, 5.2) in the drug therapy group.
AF Recurrence
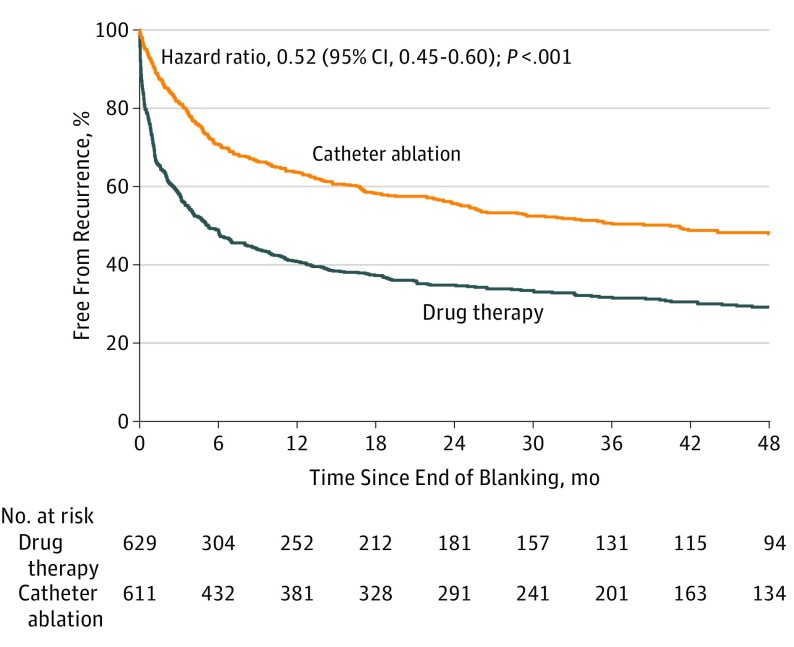
In 1240 patients using the study ECG event recording system, the secondary end point of postblanking AF (time to first recurrence) analyzed by ITT with death as a competing risk was reduced by 48% with catheter ablation compared with drug therapy (adjusted HR, 0.52 [95% CI, 0.45-0.60]; P < .001) (Figure 6). The adjusted HR for the postblanking incidence of either AF, atrial flutter, or atrial tachycardia was 0.53 (95% CI, 0.46-0.62; P < .001) (eFigure 3 in Supplement 3). Fifty seven percent of patients had persistent or long-standing persistent AF at the beginning of the trial, which was reduced to 26% in drug therapy and 16% in catheter ablation patients at trial completion. The benefits of catheter ablation on recurrent AF were consistent across prespecified subgroups (eFigure 4 in Supplement 3).
Figure 6. Recurrent Atrial Fibrillation After Blanking by Intention-to-Treat Analysis.
Freedom from recurrence of atrial fibrillation following the blanking period in 1240 patients who used the study electrocardiogram event recorders (intention-to-treat analysis with death as a competing risk). The median (25th, 75th percentiles) length of patient follow-up was 4.3 years (2.8, 5.0) in the catheter ablation group and 4.3 years (2.7, 5.3) in the drug therapy group.
Adverse Events
Non–end point adverse events are enumerated in eTables 9 and 10 in Supplement 3. The most common serious adverse event in the catheter ablation group was cardiac tamponade (0.8%). Other adverse events in the catheter ablation group included minor hematomas (2.3%) and pseudoaneurysms (1.1%). In the drug therapy group, thyroid disorders were reported in 1.6% and proarrhythmia in 0.8% of patients.
Discussion
Among patients with AF, catheter ablation, compared with medical therapy, did not significantly reduce the primary composite outcome. Relative to medical management, randomization to a strategy of AF ablation in this trial was associated with a 14% relative reduction in the primary composite end point, with a 95% CI for the effect that extended from a 35% lower to a 15% higher risk for catheter ablation. Because the confidence interval for the primary effect estimate lacks the precision to exclude a null effect (HR, 1.0), the trial primary ITT statistical comparison is inconclusive.25 The 4-year Kaplan-Meier event rates for the primary end point were 7.2% for catheter ablation and 8.9% for drug therapy, with an absolute treatment difference of 1.7% (HR, 0.86 [95% CI, 0.65-1.15]).
In addition to the primary outcome results, this article describes results for 3 of 13 prespecified secondary end point comparisons. Quality-of-life outcomes are reported separately.17 For the secondary end point of all-cause mortality, catheter ablation, compared with drug therapy, in the ITT analysis had an HR of 0.85 (95% CI, 0.60-1.21). The 4-year Kaplan-Meier mortality rates were 4.7% for the catheter ablation group and 5.3% for the drug therapy group. The secondary end point of mortality or CV hospitalization showed a significant 17% relative lower event rate for the catheter ablation group. There were no differences in serious bleeding between treatment groups, and disabling strokes were infrequent, although directionally favoring the catheter ablation group, as seen in other studies.10 The small number of strokes may be due to background therapy or a high level of adherence to ongoing anticoagulation (eTable 11 in Supplement 3).
The trial was originally projected to be able to detect a 30% mortality reduction with catheter ablation, assuming a 4% per year drug therapy mortality rate (approximately 12% at 3 years), based on previous AF trial data.11 Lower precision of the effect size estimate and an inconclusive statistical result for the primary end point by ITT is a predictable consequence of the lower-than-expected drug therapy group mortality rates (4.1% at 3 years).
Treatment Assignment Sensitivity Analyses
ITT-based analyses preserve the benefit of randomization in protecting from treatment selection biases, but the results may be seriously biased by postrandomization crossovers and deviations from protocol-specified care.26 For example, a catheter ablation patient who does not get an ablation remains assigned to the ablation arm in an ITT analysis, but cannot provide any information about the prognostic benefits of ablation. No completely satisfactory solution exists for such complexities. Useful insights can be obtained by examining ITT analysis results in combination with sensitivity analyses on the ITT estimates using the treatment actually received and also by comparing treatment outcomes of the patient groups who followed the treatment-assignment protocol. In this trial, the smaller ITT relative treatment effect size (15% reduction in mortality rather than the 30% predicted) may be at least partially due to postrandomization biases created by patients crossing over from their assigned treatment group (9.2% of catheter ablation patients declined their assigned procedure and 27.5% of drug therapy patients crossed over to ablation) (Figure 1). The treatment received and per-protocol analyses resulted in HR estimates ranging from 0.60 to 0.69, respectively, for catheter ablation, compared with drug therapy, with respect to mortality and HRs ranging from 0.67 to 0.74, respectively, for the primary end point. The treatment received and per-protocol analyses potentially mitigate different forms of bias present in the ITT estimate of the treatment effect size but may add biases if compliance with treatment assignment is correlated with outcome independent of treatment effects.26
Decisions about use of catheter ablation in individual patients need to consider both relative and absolute treatment differences as well as procedural risks. Given the 4-year Kaplan-Meier event rates, for many patients meeting the eligibility criteria of this trial, expected treatment differences on an absolute scale will likely not be of sufficient magnitude to support a recommendation for catheter ablation on that basis alone.
Prior Randomized and Observational Comparisons of Catheter Ablation and Drug Therapy
The improvement in mortality or CV hospitalization outcomes, a secondary end point in CABANA, complements prior reports from the CASTLE-AF trial,10 as well as observational3 and randomized27,28 studies and a recent large, multiyear registry.29 A recent analysis of a US administrative database with 186 760 patients with AF treated with either ablation or drug therapy during the same years CABANA was conducted found a robust 25% relative reduction in the same composite end point used for this trial.30 These results are concordant with the results of treatment assignment sensitivity analyses in this trial showing that ablation was associated with improved primary end point and mortality outcomes, providing that the ablation group patients actually received ablative therapy.
AF Recurrence
For most patients with AF, the primary reason to consider catheter ablation is to mitigate the disruption that AF creates in their daily lives and consequent reductions in quality of life. This trial shows that catheter ablation is associated with a lower AF recurrence rate than drug therapy (50% vs 69% at 3 years postblanking follow-up). These results are generally concordant with the findings of earlier smaller trials on AF recurrence such as CASTLE-AF,10 RAAFT-2,31 STOP-AF,7 ThermoCool AF,8 and to a lesser degree MANTRA-PAF.32 The long-term follow-up from this trial also shows that for many patients with AF, ablation is not curative. One hundred and ninety patients (17.1%) required a repeat ablation during the postblanking follow-up period. The underlying pathophysiology leading to the initial onset of AF may increase the propensity for its recurrence even with initially successful ablation. Work is ongoing to understand whether risk factor management, with or without ablation, can reduce recurrence rates.
Adverse Events
Importantly, CABANA shows that prognostically adverse procedural complications associated with the catheter ablation strategy relative to medical management options were infrequent when the procedure was performed by experienced operators (eTables 9 and 10 in Supplement 3). Pericardial effusion with tamponade, while infrequent, was the most common adverse event in catheter ablation patients. Pulmonary vein stenosis was rare and atrial esophageal fistula formation was not observed. The adverse event rates observed in the trial are comparable with data from the First and Second International Ablation Registries,4,33,34 and are similar to those seen in the RAAFT-231 and MANTRA-PAF trials,32 but lower than those seen in the STOP-AF trial.7 Whether the rates of catheter ablation–related procedural complications would be the same outside of a clinical trial is unknown and would be an important consideration in discussing treatment options with patients.
Limitations
This study has several limitations. First, patient withdrawals from the trial, which occurred at a slightly higher rate in the drug therapy group, may have affected estimates of the treatment effect. Second, comparisons of the ITT results with the treatment received and per-protocol analyses suggest that the combined effect of crossovers and withdrawals reduced the estimated treatment effect and the precision of the effect size estimates as assessed by ITT. Third, catheter ablation and drug therapies may have changed over the course of a long trial in ways that might have affected outcome, although ablation techniques were largely consistent over the course of the trial, and crossovers were limited by the trial center to the extent possible.
Fourth, a small number of patients (11%) received only rate control drugs, which could have affected the results. Fifth, the AF recurrence data presented here come from the subset of patients who used the trial’s recording system, but findings were consistent when trial-wide recording systems were compared. Sixth, unblinded site adjudication of cause of hospitalization may have introduced bias into this end point relative to the centrally adjudicated components of the primary end point. Seventh, the significance threshold was not adjusted for the secondary end point comparisons. Performing multiple independent significance tests increases the probability that at least 1 test may achieve nominal statistical significance on a chance basis alone. Therefore, findings from the secondary and other analyses that are unique to CABANA may be reasonably viewed as more provisional or exploratory.
Conclusions
Among patients with AF, the strategy of catheter ablation, compared with medical therapy, did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest. However, the estimated treatment effect of catheter ablation was affected by lower-than-expected event rates and treatment crossovers, which should be considered in interpreting the results of the trial.
Trial Protocol
Trial Protocol Amendments
eTable 1. CABANA Inclusion/Exclusion Criteria
eTable 2. CABANA Secondary End Points
eTable 3. Baseline Characteristics of Patients Randomized to Ablation Who Did vs Did Not Receive the Ablation
eTable 4. Rate and Rhythm Control Drug Use in the CABANA Treatment Groups (As Randomized) (A) At Enrollment and (B) During Follow-up After the Blanking Period
eTable 5. Baseline Characteristics of Patients Randomized to the Drug Group Who Did vs Did Not Cross Over to Ablation
eTable 6. Reasons for CABANA Cardiovascular Hospitalizations
eTable 7. Deaths and Strokes in CABANA Patients (As Randomized) With An Event < 30 Days Following Treatment vs Later During Follow-up
eTable 8. Results of Treatment Received and Per-Protocol Analyses
eTable 9. Adverse Events in Patients Randomized to Drug Therapy
eTable 10. Adverse Events in Patients Randomized to Ablation
eTable 11. Anticoagulant and Antiplatelet Use in the CABANA Treatment Groups (As Randomized) (A) At Enrollment and (B) During Follow-up After Randomization
eFigure 1. Kaplan-Meier Estimates of Mortality (Per Protocol)
eFigure 2. Subgroup analysis with hazard ratios and 95% confidence intervals for the primary endpoint by Per-Protocol analysis
eFigure 3. Recurrent Atrial Arrhythmias Post-Blanking (ITT)
eFigure 4. Forest Plot of Recurrent AF
eFigure 5. Modified CONSORT Diagram for ITT Analyses
eFigure 6. Modified CONSORT Diagram for Treatment Received Analyses
eFigure 7. Modified CONSORT Diagram for Per-Protocol Analyses
eAppendix 1. Commentary on Statistical Analyses
eAppendix 2. Trial Organization
Data Sharing Statement
References
- 1.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-952. doi: 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119-125. doi: 10.1161/CIRCULATIONAHA.105.595140 [DOI] [PubMed] [Google Scholar]
- 3.Bunch TJ, Crandall BG, Weiss JP, et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22(8):839-845. doi: 10.1111/j.1540-8167.2011.02035.x [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2(4):349-361. doi: 10.1161/CIRCEP.108.824789 [DOI] [PubMed] [Google Scholar]
- 5.Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118(24):2498-2505. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48(11):2340-2347. doi: 10.1016/j.jacc.2006.08.037 [DOI] [PubMed] [Google Scholar]
- 7.Packer DL, Kowal RC, Wheelan KR, et al. ; STOP AF Cryoablation Investigators . Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713-1723. doi: 10.1016/j.jacc.2012.11.064 [DOI] [PubMed] [Google Scholar]
- 8.Wilber DJ, Pappone C, Neuzil P, et al. ; ThermoCool AF Trial Investigators . Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333-340. doi: 10.1001/jama.2009.2029 [DOI] [PubMed] [Google Scholar]
- 9.Mont L, Bisbal F, Hernandez-Madrid A, et al. ; SARA investigators . Catheter ablation vs antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J. 2014;35:505-507. doi: 10.1093/eurheartj/eht457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrouche NF, Brachmann J, Andresen D, et al. ; CASTLE-AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 11.Packer DL, Mark DB, Robb RA, et al. ; CABANA Investigators . Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: study rationale and design. Am Heart J. 2018;199:192-199. doi: 10.1016/j.ahj.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuster V, Rydén LE, Cannom DS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society . ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257-e354. [DOI] [PubMed] [Google Scholar]
- 14.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101-e198. doi: 10.1016/j.jacc.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 15.Calkins H, Brugada J, Packer DL, et al. ; European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) . HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up: a report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4(6):816-861. doi: 10.1016/j.hrthm.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Kuck K, Cappato R, et al. ; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation . 2012 HRS/EHRA/ECAS/ACC/AHA/APHRS/STS/ESC expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendation for patient selection, procedural techniques, patient management, and follow up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9(4):632-696.e21. doi: 10.1016/j.hrthm.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Mark DB, Anstrom KJ, Sheng S, et al. ; CABANA Investigators . Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial [published online March 15, 2019]. JAMA. doi: 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalbfleisch J, Prentice R. The Statistical Analysis of Failure Time Data. 2nd ed Hoboken, NJ: John Wiley & Sons; 2002. doi: 10.1002/9781118032985 [DOI] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 20.Cox D. Regression models and life-tables (with discussion). J R Stat Soc B. 1972;34:187-220. [Google Scholar]
- 21.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239-241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 22.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 24.Lan K, DeMets L. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659-663. doi: 10.2307/2336502 [DOI] [Google Scholar]
- 25.Liao JM, Stack CB, Goodman S. Annals understanding clinical research: interpreting results with large P values. Ann Intern Med. 2018;169(7):485-486. doi: 10.7326/M18-2003 [DOI] [PubMed] [Google Scholar]
- 26.Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharmacol Ther. 1995;57(1):6-15. doi: 10.1016/0009-9236(95)90260-0 [DOI] [PubMed] [Google Scholar]
- 27.Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7(1):31-38. doi: 10.1161/CIRCEP.113.000806 [DOI] [PubMed] [Google Scholar]
- 28.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1644. doi: 10.1161/CIRCULATIONAHA.115.019406 [DOI] [PubMed] [Google Scholar]
- 29.Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. 2016;37(31):2478-2487. doi: 10.1093/eurheartj/ehw087 [DOI] [PubMed] [Google Scholar]
- 30.Noseworthy P, Gersh B, Kent D, et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability [published March 15, 2019]. Eur Heart J. doi: 10.1093/eurheartj/ehz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morillo CA, Verma A, Connolly SJ, et al. ; RAAFT-2 Investigators . Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692-700. doi: 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- 32.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367(17):1587-1595. doi: 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- 33.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3(1):32-38. doi: 10.1161/CIRCEP.109.859116 [DOI] [PubMed] [Google Scholar]
- 34.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111(9):1100-1105. doi: 10.1161/01.CIR.0000157153.30978.67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Trial Protocol Amendments
eTable 1. CABANA Inclusion/Exclusion Criteria
eTable 2. CABANA Secondary End Points
eTable 3. Baseline Characteristics of Patients Randomized to Ablation Who Did vs Did Not Receive the Ablation
eTable 4. Rate and Rhythm Control Drug Use in the CABANA Treatment Groups (As Randomized) (A) At Enrollment and (B) During Follow-up After the Blanking Period
eTable 5. Baseline Characteristics of Patients Randomized to the Drug Group Who Did vs Did Not Cross Over to Ablation
eTable 6. Reasons for CABANA Cardiovascular Hospitalizations
eTable 7. Deaths and Strokes in CABANA Patients (As Randomized) With An Event < 30 Days Following Treatment vs Later During Follow-up
eTable 8. Results of Treatment Received and Per-Protocol Analyses
eTable 9. Adverse Events in Patients Randomized to Drug Therapy
eTable 10. Adverse Events in Patients Randomized to Ablation
eTable 11. Anticoagulant and Antiplatelet Use in the CABANA Treatment Groups (As Randomized) (A) At Enrollment and (B) During Follow-up After Randomization
eFigure 1. Kaplan-Meier Estimates of Mortality (Per Protocol)
eFigure 2. Subgroup analysis with hazard ratios and 95% confidence intervals for the primary endpoint by Per-Protocol analysis
eFigure 3. Recurrent Atrial Arrhythmias Post-Blanking (ITT)
eFigure 4. Forest Plot of Recurrent AF
eFigure 5. Modified CONSORT Diagram for ITT Analyses
eFigure 6. Modified CONSORT Diagram for Treatment Received Analyses
eFigure 7. Modified CONSORT Diagram for Per-Protocol Analyses
eAppendix 1. Commentary on Statistical Analyses
eAppendix 2. Trial Organization
Data Sharing Statement