Key Points
Question
Does differential prescribing of opioids by race/ethnicity and income class explain opioid overdoses concentrated among low-income white communities?
Findings
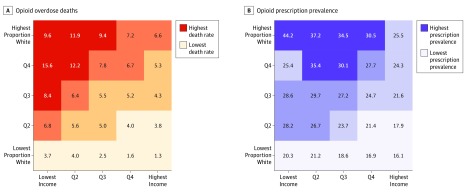
In prescription drug monitoring program data from 2011 through 2015, 44.2% of all adults in California in the regions with the lowest-income/highest proportion–white population received at least 1 opioid prescription annually compared with 16.1% in the regions with the highest-income/lowest proportion–white population and 23.6% across California. Opioid overdose deaths were concentrated in lower-income, mostly white regions, with a 10-fold difference in overdose rates across the race/ethnicity–income gradient.
Meaning
Race/ethnicity and income class disparities existing in access to opioids via the California health care system may have played a role in the race/ethnicity–income pattern of overdose deaths in the current opioid epidemic.
Abstract
Importance
Most drug epidemics in the United States have disproportionately affected nonwhite communities. Notably, the current opioid epidemic is heavily concentrated among low-income white communities, and the roots of this racial/ethnic phenomenon have not been adequately explained.
Objective
To examine the degree to which differential exposure to opioids via the health care system by race/ethnicity and income could be driving the observed social gradient of the current opioid epidemic, as well as to compare the trends in the prevalence of prescription opioids with those observed for stimulants and benzodiazepines.
Design, Setting, and Participants
This population-based study used 2011 through 2015 records from California’s prescription drug monitoring program (Controlled Substance Utilization Review and Evaluation System), which longitudinally tracks all patients receiving controlled substance prescriptions in the state and contained unique records for 29.7 million individuals who received such a prescription from 2011 to 2015. Data were analyzed between January and May 2018.
Exposures
A total of 1760 zip code tabulation areas (ZCTAs) in California, with associated racial/ethnic composition and per capita income.
Main Outcomes and Measures
The percentage of individuals receiving at least 1 prescription each year was calculated for opioids, benzodiazepines, and stimulants.
Results
A nearly 300% difference in opioid prescription prevalence across the race/ethnicity–income gradient was observed in California, with 44.2% of adults in the quintile of ZCTAs with the lowest-income/highest proportion–white population receiving at least 1 opioid prescription each year compared with 16.1% in the quintile with the highest-income/lowest proportion–white population and 23.6% of all individuals 15 years or older. Stimulant prescriptions were highly concentrated in mostly white high-income areas, with a prevalence of 3.8% among individuals in the quintile with the highest-income/highest proportion–white population and a prevalence of 0.6% in the quintile with the lowest-income/lowest proportion–white population. Benzodiazepine prescriptions did not have an income gradient but were concentrated in mostly white areas, with 15.7% of adults in the quintile of ZCTAs with the highest proportion–white population receiving at least 1 prescription each year compared with 7.0% among the quintile with the lowest proportion–white population.
Conclusions and Relevance
The race/ethnicity and income pattern of opioid overdoses mirrored prescription rates, suggesting that differential exposure to opioids via the health care system may have induced the large, observed racial/ethnic gradient in the opioid epidemic. Across drug categories, controlled medications were much more likely to be prescribed to individuals living in majority-white areas. These discrepancies may have shielded nonwhite communities from the brunt of the prescription opioid epidemic but also represent disparities in treatment and access to all medications.
This California population-based study assesses differential exposure to opioids via the health care system by race/ethnicity and income and describes the observed social gradient of the current opioid epidemic.
Introduction
A salient feature of the current opioid epidemic is that it is heavily concentrated among low-income white communities.1,2 This feature is unique because most previous drug epidemics in the United States have disproportionately affected nonwhite communities.3 Globally, most epidemics occur in social minority groups.4 Therefore, the concentration of this epidemic in a racial/ethnic majority group is epidemiologically noteworthy, and it speaks to the unique social context of the current opioid crisis. The roots of this atypical racial/ethnic phenomenon have not been adequately explained in the existing literature.
The opioid epidemic has been described as a “disease of despair” in public health literature, linked to poverty and lack of economic opportunity among increasingly downwardly mobile sectors of working class America.5,6,7,8 This theory may explain the income gradient observed in the opioid epidemic; across the racial/ethnic spectrum, higher overdose rates are observed in lower-income communities.6 However, given continued racial/ethnic disparities in income and employment status, this theory alone cannot account for the preponderance of the epidemic among white communities.9,10,11
The opioid epidemic has also been attributed to changes within the health care system that increased the availability of prescription opioids.5,6 Opioid consumption has risen steadily in the United States during the past 3 decades, and this period has also seen the invention of powerful synthetic opioid analogues, such as fentanyl and the extended-release oxycodone, which were rapidly marketed and widely adopted.6,12,13,14,15 Renewed attention was placed on pain management, and pain became known as the “fifth vital sign.”16 Exposure to an opioid prescription has been identified as a risk factor for long-term use, with 1% to 15% of patients continuing opioid therapy at 90 days depending on setting.17,18,19 The majority of individuals who are dependent on heroin now report using a prescription medication as their first opioid of abuse,20 suggesting that health care professionals played an important role in the recent surge of addiction and related overdoses. This link between the health care system and opioid addiction may provide an explanation for the observed racial/ethnic differences in the outcomes of the opioid epidemic. Racial/ethnic disparities in access to health care, as well as to pain management and opioid medications specifically,21,22,23,24,25,26 are well documented and long-standing. It is therefore possible that the inductive effect of the health care system on the opioid epidemic has impacted various racial/ethnic groups differently, effectively concentrating the epidemic among lower-income white communities.
To test this theory, we quantify the race/ethnicity–income gradient in exposure to opioids by the California health care system using Prescription Drug Monitoring Program data. We use a metric that up to this point has not been widely used to characterize the crisis: the percentage of individuals receiving an opioid prescription each year. Most previous efforts to quantify the population-level consumption of opioids have focused on total volume. For example, an analysis published by the Centers for Disease Control and Prevention (CDC) in 2013 reported a consumption of 1021.7 prescriptions for opioids per 1000 residents in Louisiana.27 It is hard to gauge the human impact of this figure because it could represent 10% of the population receiving an average of about 10 prescriptions per year, 50% of the population receiving an average of about 2 prescriptions per year, or many other distributions. Thus, in the present study, we use the simple percentage of individuals who received at least 1 prescription for an opioid each year as a clear metric of the exposure of the population to opioids by the health care system. We also calculate the same metric for stimulants and benzodiazepines to compare the race/ethnicity–income gradients observed in prescription opioids to those of other controlled substances. We use the above metrics to compare the race/ethnicity–income gradient in opioid prescription with that observed for opioid-related overdose mortality.
Methods
We calculated the percentage of the population of California receiving a prescription for an opioid, benzodiazepine, or stimulant—the prescription prevalence rate. The numerator for each rate was the number of people receiving at least 1 prescription, and the denominator was the number of people in the population. These rates were calculated separately by age group, sex, and zip code tabulation area (ZCTA) for each year from 2011 through 2015. We obtained institutional review board exemption from UCLA (University of California, Los Angeles), which also waived the need for obtaining informed patient consent.
We obtained counts of patients receiving prescriptions using deidentified data from California’s Controlled Substance Utilization Review and Evaluation System (CURES) database, which tracks all prescriptions for the Drug Enforcement Administration–scheduled medication in California.28 Anonymized, patient-specific indicators allow for following up unique patients in a longitudinal fashion. Using National Drug Codes,29 we classified each medication as an opioid, benzodiazepine, stimulant, or other based on mapping available from the CDC,30 supplemented when necessary by physician expert knowledge. Opioid-based medications used to treat opioid dependence, such as methadone and buprenorphine, were not included in counts of opioid prescriptions. Patient zip code of residence was mapped onto ZCTAs31 by using a previously described crosswalk.32
Counts of patients receiving prescriptions were combined with population counts from the American Community Survey33 to create estimates of prevalence. We also used American Community Survey data to obtain sociodemographic information for each ZCTA, including the percentage of individuals identifying as non-Hispanic white and the mean per-person income. Shapefiles were obtained from the US Census Bureau34,35 and used for mapping. We obtained zip code–level data describing the rate of age-standardized opioid overdose mortality per 100 000 persons for 2011 through 2015 by using publicly available files from the California Department of Public Health.36
We evaluated trends in racial/ethnic and income patterns at the state level by classifying all ZCTAs in quintiles of the percentage of residents identifying as non-Hispanic white and of per-person income and by calculating the prevalence of each quintile as well as all 25 race/ethnicity–income quintile combinations. We decided to use a dichotomous index of racial/ethnic composition after observing that the main racial/ethnic differences in prescription prevalence were captured using a white/nonwhite scale (eFigure 1 in the Supplement). Geographic trends were explored with maps at the ZCTA level for the southern portion of Los Angeles County. We chose this area as the site for a more detailed case study analysis because it represents a large, heavily populated city with substantial socioeconomic and racial/ethnic diversities.
We conducted all data preparation and analyses between January and May 2018 using R, version 3.4.1 (The R Project for Statistical Computing).37 More information regarding data preparation and completeness is provided in the eTable and eAppendix in the Supplement.
Results
The CURES database contains unique records for 29.7 million individuals who received a prescription for a Drug Enforcement Administration–scheduled substance from 2011 to 2015. Among these individuals, the mean (SD) age was 46.5 (20.6) years, and 57.0% were female.
Opioid Overdose Mortality and Prescriptions
From 2011 through 2015, there were 9534 reported opioid overdose deaths in California. These deaths included overdoses attributed to any opioid, including prescription medication, heroin, and synthetic opioids, such as fentanyl. Overdose deaths were highly concentrated in lower-income and mostly white areas. We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California (Figure 1A). Per 100 000 people in age-standardized rates, there were 9.6 opioid overdose deaths each year in the highest proportion–white/lowest-income quintile of ZCTAs compared with 1.3 in the lowest proportion–white/highest-income quintile. This racial/ethnic and income gradient is consistent with prior research describing the unique concentration of the opioid epidemic in low-income and majority-white areas.2,3
Figure 1. Opioid Overdose Deaths and Prescription Prevalence.
A, Annual age-standardized opioid overdose death rates per 100 000 people. B, Annual prevalence of receiving at least 1 prescription for an opioid among individuals 15 years or older. Both figures represent the entire state of California, showing quartiles of mean annual rates during the 2011 through 2015 study period. The y-axis represents the percentage of individuals in each zip code tabulation area identifying as non-Hispanic white, by quintiles (Qs). The x-axis represents quintiles of the mean per capita annual income at the zip code tabulation area level. Values for all 25 quintile-quintile pairs are shown with color and text.
In California, 23.6% of all individuals 15 years or older received a prescription for an opioid medication each year during the study period. Opioid prescriptions were concentrated among ZCTAs with a higher proportion–white/lower-income population, with a mean annual prevalence of 44.2% among individuals 15 years or older in the highest proportion–white/lowest-income quintile of ZCTAs compared with 16.1% for the lowest proportion–white/highest-income quintile (Figure 1B). We examined the race/ethnicity–income gradient for prescription prevalence rates using age standardization and found little differences in trends compared with crude rates.
Benzodiazepine and Stimulant Prescriptions
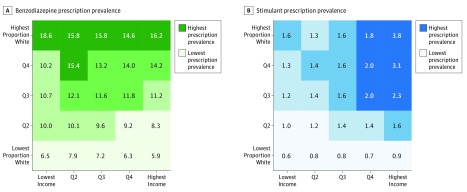
Benzodiazepine prescription prevalence was 10.2% per year for individuals who were 15 years or older during the study period. In contrast to the marked racial/ethnic and income pattern observed for opioids, benzodiazepine rates varied strongly along lines of race/ethnicity only; little variation was observed by income status (Figure 2A). Among individuals older than 15 years, prevalence among the highest proportion–white quintile of ZCTAs was 15.7%, whereas it was 7.0% among the lowest proportion–white quintile.
Figure 2. Benzodiazepine and Stimulant Prescription Prevalences.
Annual prevalence of receiving at least 1 prescription for a benzodiazepine (A) or a stimulant (B). Both figures represent the entire state of California, showing quartiles (Qs) of mean annual rates during the 2011 through 2015 study period. A, Values for all individuals 15 years or older. B, Data for all individuals of any age because stimulant prescription receipt is highest in the age group of 10 to 14 years.
Stimulant prescriptions were highly concentrated among higher proportion–white/higher-income ZCTAs (Figure 2B). We observed a mean annual prescription prevalence of 3.8% among the highest proportion–white/highest-income quintile vs 0.6% in the lowest-income/lowest proportion–white quintile of ZCTAs.
Los Angeles Case Study
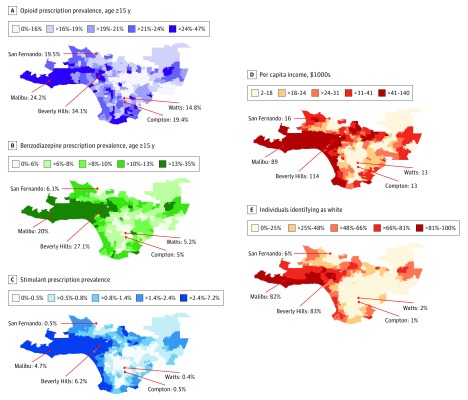
Figure 3 shows a ZCTA-level map for Los Angeles County of the prescription prevalence of opioids (Figure 3A), benzodiazepines (Figure 3B), and stimulants (Figure 3C), as well as per capita income (Figure 3D) and percentage of residents self-reporting as non-Hispanic white (Figure 3E). This map provides a more granular illustration of many of the trends observed at the state level in previous figures.
Figure 3. Prescription Prevalence, Race/Ethnicity Distribution, and Income Level for the Los Angeles Case Study.
Data are mapped at the zip code tabulation area level. Prescription prevalence of opioids (A) and benzodiazepines (B) are shown for individuals 15 years or older and stimulants (C) for individuals of all ages. Mean per capita income (×$1000) (D) and the percentage of individuals identifying as non-Hispanic white (E) are also shown. All values are shown in quintiles and represent means of annual rates from 2011 through 2015.
Noteworthy areas of majority-white and high-income ZCTAs stretch east to west from Malibu through Beverly Hills and also along the coast. These areas show markedly elevated rates of stimulant prescription prevalence relative to the rest of the county and generally high levels of benzodiazepine and opioid prescription prevalence. South Central Los Angeles is composed of mainly lower-income, mostly nonwhite ZCTAs. This area shows low levels of prescription prevalence rates across all drug classes and markedly low levels of stimulant prescriptions. The geographic variations in prescription prevalence of all drug classes reflect similar degrees of social, economic, racial/ethnic, and residential segregation across Los Angeles.
Discussion
Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, the current opioid crisis is largely found among lower-income and majority-white communities. Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system. Across the income spectrum of the state of California, and especially within the lowest-income quintile of ZCTAs, we observed much higher rates of opioid prescription in the areas with the highest proportion–white population.
The vast majority of individuals receiving opioid prescriptions in California are not dependent opioid users. As shown in previously published work,38 the bulk of those individuals are receiving sporadic prescriptions for small quantities of opioids, representing short-term treatment courses for acute conditions. Only a tiny fraction of opioid users show behavior consistent with dangerous patterns even though they do consume a large share of the total volume of prescribed opioids. The race/ethnicity–income pattern observed in the present study therefore represents both a difference in the negative consequences of opioid addiction and also a noteworthy social trend in the general use patterns of opioids for acute, medically appropriate purposes. The nearly 300% gradient observed across the state also reflects the nature of the health care system and its distribution of prescription opioids in California.
Important clues that help explain the observed racial/ethnic gradient in opioid prescription rates can be found in the large body of literature documenting minority disparities stemming from implicit biases and reduced access to the health care system. For instance, clinicians are more likely to prescribe opioids for pain management to white patients than to racial/ethnic minority patients presenting with the same symptoms across numerous clinical settings and geographies.21,22,23,24,25,26,39 One foundational study showed that Hispanic patients were 2 times less likely to receive analgesics following long bone fractures than white patients, after accounting for other factors.40 Similar discrepancies in pain medication prescribing were found for black patients relative to white patients.26
Recent studies have found that health care professionals often underestimate the pain of black patients when compared with white patients and that such racial/ethnic biases in the detection of pain are seen among health care professionals who report no explicit racial/ethnic biases.25 These gaps, coupled with decreased access to the health care system for many racial/ethnic minority groups,41,42 have led several authors to suggest that there is a national crisis of insufficiently medicated pain among minority communities in the United States.3,21,23,39 In light of a similar gradient in opioid overdose deaths, these disparities in opioid prescription may have played an accidental protective role in minimizing the opioid epidemic among minority communities. Nevertheless, they also represent the undertreatment of the legitimate medical needs of patients of color and remain an important inequity to be ameliorated.
We also observed that the concentration of prescription medications in mostly white communities is not unique to opioids. Although the trend by income level varies among drug classes, across the board, prescription medications were overwhelmingly prescribed at higher rates to patients who lived in areas with a higher proportion–white population. Prescriptions for stimulants were remarkably concentrated in ZCTAs with a higher-income/higher proportion–white population. Rates were highest among male adolescents aged 10 to 14 years (eFigure 2 in the Supplement), suggesting that the largest contribution of stimulant prescriptions is for the treatment of attention-deficit/hyperactivity disorder (ADHD). Substantial racial and ethnic disparities in the detection and subsequent treatment of ADHD have been noted in the literature, which may be reflected in the population-level pattern we observed.43,44,45 Benzodiazepine prescription was concentrated in the quintile of ZCTAs with the highest proportion–white population at more than double the rate observed in the quintile of ZCTAs with the lowest proportion–white population. However, little variation in benzodiazepine prescription prevalence was observed across income categories. Racial/ethnic disparities in the diagnosis and treatment of epilepsy, as well as anxiety and other mental health conditions treated with benzodiazepines, have also been previously noted and may play an important role in the overall trends observed.46,47
Granular maps of prescription prevalence rates in Los Angeles County revealed how stark social differences in the use of controlled substances are within the same city. In the high-income and mostly white neighborhoods around Beverly Hills, more than 1 of every 4 adults received a benzodiazepine prescription during the study period. In the low-income inner-city neighborhoods of Compton and Watts, that number was only 1 of every 20 adults. This 5-fold difference in the rate of a basic medical treatment between neighborhoods in the same city is unlikely to be explained by variation in the underlying need for these medications.
Limitations
This study was limited in its use of ecological-level sociodemographic information. We linked prescription prevalence to income and racial/ethnic composition at the ZCTA level. Although the 1760 ZCTAs included in the study provided a granular picture, we were unable to establish associations at the individual level. The ecological nature of the association we described limited our ability to identify mechanisms that may be at play. For example, we had no ability to determine if receiving a prescription for opioids made an individual more likely to become addicted and subsequently use heroin, or if the individuals overdosing on opioids were the same individuals receiving prescriptions for opioids. In the present analysis, we simply highlighted a similar social gradient observed between the outcomes of the opioid crisis and the level of opioid prescription by the health care system, which may represent an important driver of the epidemic.
The generalizability of the present study to the rest of the country may be affected by California’s generally low rates of controlled substance prescription relative to other states.27 The results, therefore, may not fully represent the magnitude of prescribing that we would observe in other states, although the race/ethnicity and income trends may be widely generalizable. However, California represented an apt location to study this topic because its wide range of racial/ethnic and socioeconomic diversity enabled us to study a more complete picture of the social dynamics of opioid prescription. Furthermore, given its large population size, California represents a sizable share of total prescribing in the United States.
Potential factors affecting the accuracy of our prevalence estimates included the possibility that the algorithm used by the Controlled Substance Utilization Review and Evaluation System database to track individuals over time is imperfect, leading to the same individual being listed more than once, which could inflate prevalence. The 3.7% of observations that we had to exclude due to missing data could have also resulted in a slight underestimate of prevalence (eTable in the Supplement). Our metric of opioid prescription prevalence included all opioids except those medications used to treat opioid dependence, such as methadone or buprenorphine, which may represent a limitation for interpretation. Although a small fraction of these medications may be abused, the vast majority are used for treatment of opioid dependence and were excluded from our measure of exposure to potentially addicting opioid medications.48
Conclusions
Although the opioid epidemic receives considerable attention in the medical and public health communities, the mainstream media, and even in the national political arena, race/ethnicity is seldom included in discussions about the epidemic. Previous work by the CDC illustrates substantial population-level geographic variations in prescription prevalence rates,27 but relatively few efforts have been made to explain this variation with sociodemographic data. Our results suggest that race/ethnicity and income are key factors by which variations in prescription prevalence may be understood. This study represents one of the first population-level efforts, to our knowledge, to quantify the social patterns of prescription drug use. The results provide important insights when trying to understand the prescription drug epidemic that is occurring in mostly white communities and the reported disparities in untreated pain, anxiety, and ADHD that are simultaneously found in minority communities.
eTable. Completeness for Each Step of Data Preparation Process
eAppendix. Data Preparation Process
eFigure 1. Racial Composition Variable Selection
eFigure 2. Age, Sex, Time Trends in Prescription Prevalence of Opioids, Benzodiazepines, and Stimulants
References
- 1.Henry J Kaiser Family Foundation. Opioid overdose deaths by race/ethnicity. https://www.kff.org/other/state-indicator/opioid-overdose-deaths-by-raceethnicity/. Published February 2018. Accessed December 29, 2018.
- 2.Song Z. Mortality quadrupled among opioid-driven hospitalizations, notably within lower-income and disabled white populations. Health Aff (Millwood). 2017;36(12):2054-2061. doi: 10.1377/hlthaff.2017.0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netherland J, Hansen H. White opioids: pharmaceutical race and the war on drugs that wasn’t. Biosocieties. 2017;12(2):217-238. doi: 10.1057/biosoc.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn SC, Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur Bioterror. 2014;12(5):263-273. doi: 10.1089/bsp.2014.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruhm CJ. Deaths of Despair or Drug Problems? National Bureau of Economic Research; 2018. doi: 10.3386/w24188 [DOI] [Google Scholar]
- 6.Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182-186. doi: 10.2105/AJPH.2017.304187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollingsworth A, Ruhm CJ, Simon K. Macroeconomic Conditions and Opioid Abuse. National Bureau of Economic Research; 2017. doi: 10.3386/w23192 [DOI] [PubMed] [Google Scholar]
- 8.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112(49):15078-15083. doi: 10.1073/pnas.1518393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alliance for American Manufacturing. Unmade in America: industrial flight and the decline of black communities. http://s3-us-west-2.amazonaws.com/aamweb/uploads/research-pdf/UnmadeInAmerica.pdf. Published October 2016. Accessed December 29, 2018.
- 10.Pfeffer FT, Danziger S, Schoeni RF. Wealth disparities before and after the great recession. Ann Am Acad Pol Soc Sci. 2013;650(1):98-123. doi: 10.1177/0002716213497452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Census Bureau. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed December 29, 2018.
- 12.Han H, Kass PH, Wilsey BL, Li C-S. Increasing trends in schedule II opioid use and doctor shopping during 1999-2007 in California. Pharmacoepidemiol Drug Saf. 2014;23(1):26-35. doi: 10.1002/pds.3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227. doi: 10.2105/AJPH.2007.131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadland SE, Krieger MS, Marshall BDL. Industry payments to physicians for opioid products, 2013-2015. Am J Public Health. 2017;107(9):1493-1495. doi: 10.2105/AJPH.2017.303982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker WC, Fiellin DA. Abuse-deterrent opioid formulations—putting the potential benefits into perspective. N Engl J Med. 2017;376(22):2103-2105. doi: 10.1056/NEJMp1701553 [DOI] [PubMed] [Google Scholar]
- 16.Mandell BF. The fifth vital sign: a complex story of politics and patient care. Cleve Clin J Med. 2016;83(6):400-401. doi: 10.3949/ccjm.83b.06016 [DOI] [PubMed] [Google Scholar]
- 17.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi: 10.1056/NEJMsa1610524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbaugh CM, Lee JS, Hu HM, et al. Persistent opioid use among pediatric patients after surgery. Pediatrics. 2018;141(1):e20172439. doi: 10.1542/peds.2017-2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826. doi: 10.1001/jamapsychiatry.2014.366 [DOI] [PubMed] [Google Scholar]
- 21.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187-1204. doi: 10.1016/j.jpain.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 22.Chen I, Kurz J, Pasanen M, et al. Racial differences in opioid use for chronic nonmalignant pain. J Gen Intern Med. 2005;20(7):593-598. doi: 10.1007/s11606-005-0105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green CR, Baker TA, Sato Y, Washington TL, Smith EM. Race and chronic pain: a comparative study of young black and white Americans presenting for management. J Pain. 2003;4(4):176-183. doi: 10.1016/S1526-5900(02)65013-8 [DOI] [PubMed] [Google Scholar]
- 24.Hirsh AT, George SZ, Robinson ME. Pain assessment and treatment disparities: a virtual human technology investigation. Pain. 2009;143(1-2):106-113. doi: 10.1016/j.pain.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. doi: 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todd KH, Deaton C, D’Adamo AP, Goe L. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35(1):11-16. doi: 10.1016/S0196-0644(00)70099-0 [DOI] [PubMed] [Google Scholar]
- 27.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM; Centers for Disease Control and Prevention . Controlled substance prescribing patterns—prescription behavior surveillance system, eight states, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(9):1-14. doi: 10.15585/mmwr.ss6409a1 [DOI] [PubMed] [Google Scholar]
- 28.State of California Department of Justice. Controlled Substance Utilization Review and Evaluation System. https://oag.ca.gov/cures. Accessed February 10, 2018.
- 29.US Food and Drug Administration. National drug code directory. https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm. Accessed February 10, 2018.
- 30.Prescription Drug Monitoring Program Training and Technical Assistance Center. Technical assistance guide No. 01-13: calculating daily morphine milligram equivalents. http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Published February 28, 2013. Accessed December 29, 2018.
- 31.US Census Bureau. ZIP code tabulation areas (ZCTAs). https://www.census.gov/geo/reference/zctas.html. Accessed February 10, 2018.
- 32.Dartmouth Atlas Project PCSA downloads: ZIP code to ZCTA 2009. http://www.dartmouthatlas.org/tools/downloads.aspx?tab=37. Published 2013. No longer available as of June 30, 2014. Accessed January 2014.
- 33.US Census Bureau. American FactFinder. https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed February 11, 2018.
- 34.US Census Bureau. Geography: cartographic boundary shapefiles—zip code tabulation areas (ZCTAs). https://www.census.gov/geo/maps-data/data/cbf/cbf_zcta.html. Accessed February 11, 2018.
- 35.US Census Bureau. Geography: cartographic boundary shapefiles—counties. https://www.census.gov/geo/maps-data/data/cbf/cbf_counties.html. Accessed February 11, 2018.
- 36.California Department of Public Health. California opioid overdose surveillance dashboard. https://discovery.cdph.ca.gov/CDIC/ODdash/. Accessed May 14, 2018.
- 37.R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed February 11, 2018.
- 38.Schneberk T, Raffetto B, Kim D, Schriger DL. The supply of prescription opioids: contributions of episodic-care prescribers and high-quantity prescribers. Ann Emerg Med. 2018;71(6):668-673. doi: 10.1016/j.annemergmed.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 39.Prunuske JP, St Hill CA, Hager KD, et al. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: a population-based study using 2010 NAMCS data. BMC Health Serv Res. 2014;14:563. doi: 10.1186/s12913-014-0563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269(12):1537-1539. doi: 10.1001/jama.1993.03500120075029 [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care. 2016;54(2):140-146. doi: 10.1097/MLR.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 43.Coker TR, Elliott MN, Toomey SL, et al. Racial and ethnic disparities in ADHD diagnosis and treatment. Pediatrics. 2016;138(3):e20160407. doi: 10.1542/peds.2016-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan PL, Staff J, Hillemeier MM, Farkas G, Maczuga S. Racial and ethnic disparities in ADHD diagnosis from kindergarten to eighth grade. Pediatrics. 2013;132(1):85-93. doi: 10.1542/peds.2012-2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan PL, Hillemeier MM, Farkas G, Maczuga S. Racial/ethnic disparities in ADHD diagnosis by kindergarten entry. J Child Psychol Psychiatry. 2014;55(8):905-913. doi: 10.1111/jcpp.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stockdale SE, Lagomasino IT, Siddique J, McGuire T, Miranda J. Racial and ethnic disparities in detection and treatment of depression and anxiety among psychiatric and primary health care visits, 1995-2005. Med Care. 2008;46(7):668-677. doi: 10.1097/MLR.0b013e3181789496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szaflarski M, Szaflarski JP, Privitera MD, Ficker DM, Horner RD. Racial/ethnic disparities in the treatment of epilepsy: what do we know? what do we need to know? Epilepsy Behav. 2006;9(2):243-264. doi: 10.1016/j.yebeh.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 48.Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev. 2011;4(1):28-41. doi: 10.2174/1874473711104010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Completeness for Each Step of Data Preparation Process
eAppendix. Data Preparation Process
eFigure 1. Racial Composition Variable Selection
eFigure 2. Age, Sex, Time Trends in Prescription Prevalence of Opioids, Benzodiazepines, and Stimulants