This systematic review and meta-analysis examines the association of cannabis use during adolescence with risk of developing subsequent major depression, anxiety, and suicidal behavior in young adulthood.
Key Points
Question
Is adolescent cannabis consumption associated with risk of depression, anxiety, and suicidality in young adulthood?
Findings
In this systematic review and meta-analysis of 11 studies and 23 317 individuals, adolescent cannabis consumption was associated with increased risk of developing depression and suicidal behavior later in life, even in the absence of a premorbid condition. There was no association with anxiety.
Meaning
Preadolescents and adolescents should avoid using cannabis as use is associated with a significant increased risk of developing depression or suicidality in young adulthood; these findings should inform public health policy and governments to apply preventive strategies to reduce the use of cannabis among youth.
Abstract
Importance
Cannabis is the most commonly used drug of abuse by adolescents in the world. While the impact of adolescent cannabis use on the development of psychosis has been investigated in depth, little is known about the impact of cannabis use on mood and suicidality in young adulthood.
Objective
To provide a summary estimate of the extent to which cannabis use during adolescence is associated with the risk of developing subsequent major depression, anxiety, and suicidal behavior.
Data Sources
Medline, Embase, CINAHL, PsycInfo, and Proquest Dissertations and Theses were searched from inception to January 2017.
Study Selection
Longitudinal and prospective studies, assessing cannabis use in adolescents younger than 18 years (at least 1 assessment point) and then ascertaining development of depression in young adulthood (age 18 to 32 years) were selected, and odds ratios (OR) adjusted for the presence of baseline depression and/or anxiety and/or suicidality were extracted.
Data Extraction and Synthesis
Study quality was assessed using the Research Triangle Institute item bank on risk of bias and precision of observational studies. Two reviewers conducted all review stages independently. Selected data were pooled using random-effects meta-analysis.
Main Outcomes and Measures
The studies assessing cannabis use and depression at different points from adolescence to young adulthood and reporting the corresponding OR were included. In the studies selected, depression was diagnosed according to the third or fourth editions of Diagnostic and Statistical Manual of Mental Disorders or by using scales with predetermined cutoff points.
Results
After screening 3142 articles, 269 articles were selected for full-text review, 35 were selected for further review, and 11 studies comprising 23 317 individuals were included in the quantitative analysis. The OR of developing depression for cannabis users in young adulthood compared with nonusers was 1.37 (95% CI, 1.16-1.62; I2 = 0%). The pooled OR for anxiety was not statistically significant: 1.18 (95% CI, 0.84-1.67; I2 = 42%). The pooled OR for suicidal ideation was 1.50 (95% CI, 1.11-2.03; I2 = 0%), and for suicidal attempt was 3.46 (95% CI, 1.53-7.84, I2 = 61.3%).
Conclusions and Relevance
Although individual-level risk remains moderate to low and results from this study should be confirmed in future adequately powered prospective studies, the high prevalence of adolescents consuming cannabis generates a large number of young people who could develop depression and suicidality attributable to cannabis. This is an important public health problem and concern, which should be properly addressed by health care policy.
Introduction
Cannabis is the world’s most widely used illicit drug, with 3.8% of the global population having used cannabis in the past year.1 Prevalence of use as a fraction of the world’s population has remained stable since the 1990s,1 although patterns in individual countries are changing, with the percentage of individuals aged 18 to 29 years in the United States who reported using cannabis in the past year roughly doubling between 2001 to 2002 and 2012 to 2013, from 10.5% to 21.2%.2 Among US adolescents from 1991 to 2011, the prevalence of cannabis was high, with 20.9% of adolescents reporting use in the past month3; additionally, about 7% of US high school seniors are daily or near-daily users of cannabis.4 In Canada, among youth aged 15 to 19 years, the rate of past-year cannabis use in 2015 was 20.6%.5 Similarly, in Australia, 4% of adolescents aged 14 to 19 years use cannabis weekly6; in England, 4% of adolescents aged 11 to 15 years used cannabis in the last month.7 It is estimated that 87.6 million adults in the European Union (aged 15 to 64 years), or 26.3% of this age group, have tried cannabis during their lives.8 Last year, prevalence rates among individuals aged 15 to 34 years range from 3.5% in Hungary to 21.5% in France.8
The main pharmacologically active cannabinoid in the cannabis plant (Cannabis sativa or indica) is Δ-9 tetrahydrocannabinal (THC), which mediates most of its psychoactive and mood-related effects and also has addictive properties. The regular use of cannabis during adolescence is of profound concern9 as use in this age group is associated with an increased likelihood of deleterious consequences, such as diminished scholastic achievement, lower degree attainment and school abandonment, liability to addiction,10 earlier onset of psychosis,11 and neuropsychological decline.12 Furthermore, in the general population, there is substantive evidence for statistical associations between cannabis use and increased risk of motor vehicle crashes; the development of psychoses with the highest risk among the most frequent and high potency cannabis users; increased cannabis use frequency and the progression to developing problem cannabis use; adverse birth outcome in the offspring of mothers with cannabis smoking habits; and worse respiratory symptoms and more frequent chronic bronchitis episodes with long-term cannabis smoking.9,13
Little attention has been specifically paid in the public health discourse as to the impact of adolescent cannabis use on the risk of developing depressive symptoms and mood disorders, even though researchers have published on this topic since the 1970s.14 Some clinical studies have found a larger effect in women,15 while the rate of depression in adulthood may remain elevated even when cannabis use is stopped after adolescence.16 Moreover, many preclinical studies in laboratory animals have also reported an association between pubertal exposure to cannabinoids and adult-onset depressive symptoms, in addition to elucidating the neurobiological mechanisms of this observed effect.17,18,19 The adolescent brain is indeed still under development and psychotropic drugs used at this time may thus alter the physiological neurodevelopment, especially of the frontal cortex and limbic system.20,21
To date and to our knowledge, there has been no systematic review or meta-analysis summarizing the association of cannabis use during adolescence on the risk of depression in young adulthood. A meta-analysis published in 2014 examined the association of cannabis use and depression,22 but this study included youth and adults and therefore did not estimate the specific risk of use during adolescence. The study also did not consider the risk of suicidal behavior and the comorbid anxiety often associated with depression. Two other systematic reviews and meta-analyses23,24 analyzed the association between cannabis and depression, but those studies likewise focused on use in the general population (adolescents and adults) and thus could not study the window of risk in adolescence.
The goal of this investigation is to systematically review and analyze longitudinal prospective cohort studies that measured cannabis use during adolescence (18 years and younger) and evaluate the risk of depression, anxiety, and suicidality during young adulthood (aged 18 to 32 years). Given the high percentage of depression in adolescents smoking cannabis at the baseline, only longitudinal prospective cohort studies controlling for baseline depression were included in the study.
Methods
Search Strategy
The literature search was conducted by a health sciences librarian (J.B.) to identify all relevant clinical literature reporting on the association between cannabis use during adolescence and mood disorders. The search period covered the years from inception to January 23, 2017. Database search strategies complied with the Institute of Medicine standards25 and were not limited by language or by year of publication. Searches used MeSH headings26 and text words as suitable. The Medline strategy was developed with input from the research team and then peer reviewed by a second librarian using the PRESS standard.27 After the initial Medline strategy was finalized, it was adapted for the other databases (Medline search details appear in eMethods 1 in the Supplement). The vocabulary and syntax of the strategy were tailored to allow for optimal electronic searching of the following databases: Medline (Ovid), Embase (Ovid), CINAHL, PsycInfo (Ovid), and Proquest Dissertations and Theses. The results were compiled and duplicates removed using EndNote X7 (EndNote, Clarivate Analytics).
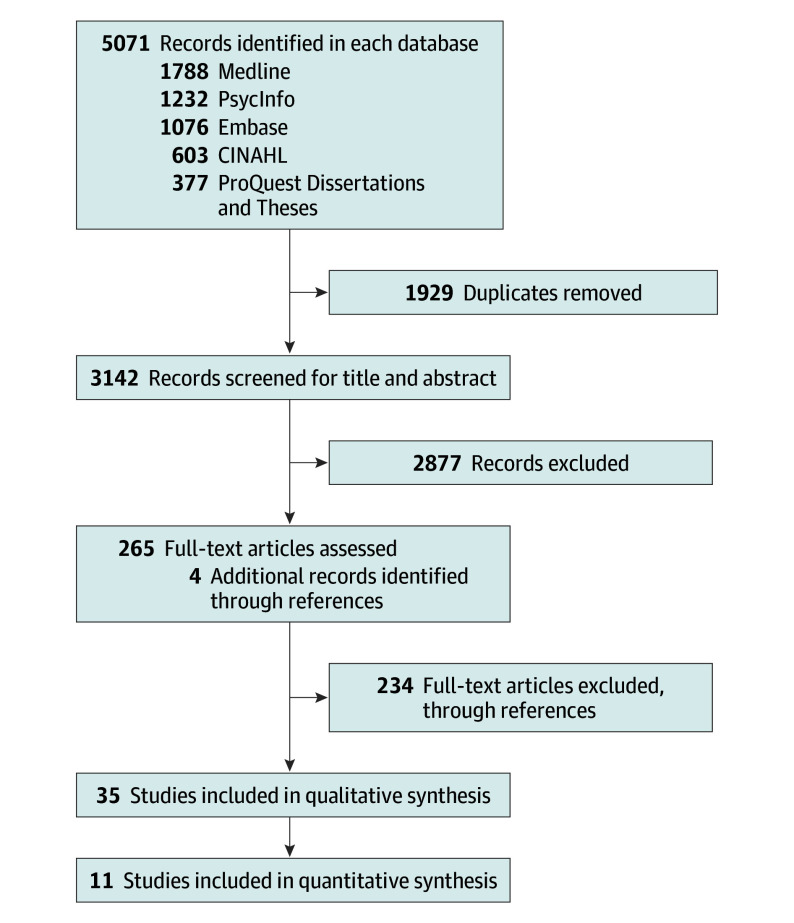
References of included studies and pertinent reviews were manually verified for further clinical studies. Grey literature was searched using Grey Matters, a practical search tool for evidence-based medicine available through the Canadian Association for Drugs and Technologies in Health.28 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for reporting of systematic reviews were followed and fulfilled.29 DistillerSR, an advanced systemic literature review software was used to manage the screening process (DistillerSR, Evidence Partners) (Figure 1).
Figure 1. Selection Process for Study Inclusion in the Systematic Review and Meta-analysis.
Study Selection
Two reviewers (T.A. and T.Z.) independently assessed all titles and abstracts along with full texts of potentially relevant articles. Studies were included if they met the following criteria: (1) reported in an original article in a peer-reviewed journal; (2) included population-based data that were collected longitudinally and prospectively; (3) the exposure variable referred specifically to cannabis; (4) outcome measures referred specifically to depression, suicidal behavior, anxiety (often comorbid to depression), or mixed anxiety-depressive symptoms; (5) the outcome variable (depression, anxiety, suicidality) was controlled for at baseline, (6) assessed cannabis use in adolescents younger than 18 years (at least 1 assessment point) and then again assessed them for depression in young adulthood (aged 18 to 32 years); (7) data were either presented as an odds ratio (OR); and (8) controlled and adjusted for the following confounding factors: age, sex, and depression and/or anxiety at baseline. Parental level of education, socioeconomic status, alcohol consumption, and cigarette smoking were also controlled for in almost all included studies. When multiple studies were found reporting on the same population cohort at different time points, only the study reporting the longest follow-up was chosen.
Data Extraction
A structured form in DistillerSR was used to extract the following data: the year of publication; the sample size at follow-up; the mean age of participants at baseline and at follow-up; the sex distribution; the methods to define cannabis use (in terms of frequency of use and age at use, if provided); and the methods to define depression, anxiety, and suicidal behaviors, as well as control variables for adjustment. The 35 longitudinal studies retained for the meta-analysis reporting the outcome of depression in young adulthood (reported as OR, β, intercept, frequency, or relative risk) are in eTable 1 in the Supplement.10,15,16,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61 A summary of the articles selected for full-text review (N = 269) by both authors and the reason for exclusion (n = 234) or retention (n = 35) appear in eTable 2 in the Supplement. Only studies reporting adjusted OR for basal depression or anxiety or suicidality were included in the meta-analysis. References cited in articles meeting inclusion criteria and references cited in previous systematic reviews on cannabis use and depression were manually explored for other relevant studies.
Parameters Definitions
Cannabis use in most studies was measured with a self-reported questionnaire. Most studies reported the frequency of use in the last year or the last 6 months, distinguishing weekly users, daily users, and occasional users (eTable 1 in the Supplement). However, when a single study reported the OR for several possible frequencies of cannabis use (eg, nonusers vs occasional users, weekly users, and daily users, among other classifications), only the OR for the highest frequency of use classification vs nonusers was included in our pooled analysis.
Depression or anxiety was considered in a dichotomous manner according to the third and fourth editions of the Diagnostic and Statistical Manual of Mental Disorders62 criteria or with scales (eg, Symptom Checklist-90, Composite International Diagnostic Interview, General Health Questionnaire-12, Clinical Interview Schedule-Revised) with a determined cutoff points (eTable 1 in the Supplement). Suicide attempts and/or ideations were evaluated with questionnaires and/or standardized interview assessment or scales (eTable 1 in the Supplement).
Quality Assessment
The risk of bias of the included articles was assessed using the Research Triangle Institute item bank on risk of bias and precision of observational studies.63 Unlike quality assessments, the Research Triangle Institute item bank does not create a score quantifying a degree of bias but uses a list of criteria to determine where in a study the bias may be located. The 29 items relate to precision (sample size and efficiency from stratification/matching to balance exposure groups on key confounders), bias (selection, performance, attrition, detection, reporting, and information), and overall believability.
Statistical Analysis
Only longitudinal prospective cohort studies reporting outcome data about cannabis use (baseline) and depression (follow-up) and adjusting for depression and/or anxiety and/or suicidality at baseline were included in the meta-analysis (eTable 1 in the Supplement). We recorded OR estimates for the association between cannabis use in adolescence and 4 different outcomes: depression, anxiety, suicidal ideation, and suicidal attempts in early adulthood. All studies reported ORs adjusted for prior history of depression and other confounding variables (eg, sex, tobacco smoking, alcohol). We pooled the ORs for each outcome using random-effects models64 since considerable heterogeneity was expected. The presence of between-study heterogeneity was tested using Cochran Q65 and quantified by I2 (ie, the percentage of the total variance that is due to between-study heterogeneity).
Exploration of Heterogeneity via Meta-regression
We fit mixed-effects meta-regression models to explore the heterogeneity attributable to 2 covariates: the method of defining depression (structured diagnosis vs questionnaire) and the country where the study was conducted (Australia, United States, or other countries). These analyses were carried out using the metafor package in the R software program, version 3.4.066 (The University of Auckland).
Sensitivity Analysis and Propensity Score Analysis
One set of sensitive analysis was done to rule out possible cohorts’ overlap. Moreover, the cohort with the highest OR for depression46 was entirely reanalyzed using both the classical methods for factors adjustment (multivariate regression analysis) and using the propensity score adjusted regression methods to better estimate the differences in the distribution of the confounding variables67 using SAS, version 9.4 (SAS Institute Inc). Details are in eMethods 2 in the Supplement.
Results
Results of the Meta-analyses
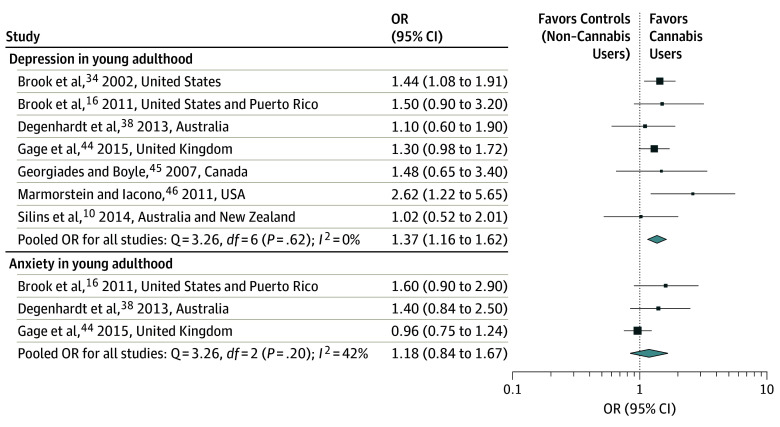
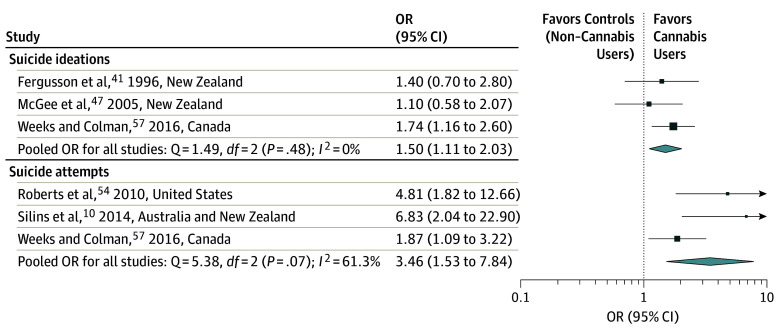
A total of 11 articles met the inclusion criteria for the meta-analysis (Figure 1): 7 for depression10,16,34,38,44,45,46; 3 for anxiety,16,38,44 3 for suicidal ideation,41,47,57 and 3 for suicide attempts.10,54,57 To estimate the extent to which cannabis use during adolescence was associated with increased odds of developing depression in young adulthood, we pooled results from 7 studies.10,16,34,38,44,45,46 The pooled OR for depression during young adulthood among cannabis users compared with nonusers was 1.37 (95% CI, 1.16-1.62; I2 = 0%; Figure 2). Cannabis use during adolescence was associated with increased odds of developing anxiety in young adulthood when pooling 3 studies16,38,44 (OR, 1.18; 95% CI, 0.84-1.67; I2 = 42.0%; Figure 2). Suicidal ideation is a symptom of depression, while suicide represents one of the most severe sequelae of mental illness. Results from 3 studies that had measured the association of cannabis use during adolescence with subsequent suicidal ideation within adolescence41,57 and in young adulthood47 were pooled with a resultant OR of 1.50 (95% CI, 1.11-2.03; I2: 0%; Figure 3). For the number of suicide attempt outcomes within adolescence54,57 or during young adulthood,10 the results were pooled with a resultant OR of 3.46 (95% CI, 1.53-7.84; I2 = 61.3%; Figure 3).
Figure 2. Forest Plot Showing Adjusted Odds Ratio (OR) and 95% CIs for Depression and Anxiety in Young Adulthood According to Cannabis Use in Individual Studies.
Figure 3. Forest Plot Showing Adjusted Odds Ratio (OR) and 95% CIs for Suicidal Ideations and Attempts According to Cannabis Use in Individual Studies.
Exploration of Heterogeneity via Meta-regression
There was no evidence that the use of structured diagnostic tools such as the DSM, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, or scales to measure depression had an impact on the pooled OR. The pooled OR among studies using structured diagnostic tools was 1.38 (95% CI, 1.15-1.66), while it was 1.28 (95% CI, 0.83-1.97; P = .74) among studies using scales.
No association was found between the country in which the study was conducted and the reported OR. The pooled OR among studies carried out in the United States was not significantly different from those of other countries. The pooled OR among studies from the United States was 1.54 (95% CI, 1.20-1.98); the pooled OR among studies from other countries was 1.24 (95% CI, 0.99-1.56; P = .21).
Sensitivity Analysis and Propensity Score Analysis
Since there were concerns about the potential overlapping of cohorts between the study by Degenhardt et al38 (Victorian Adolescent Health Cohort Study) and Silins et al10 (including a section of the Victoria cohort plus the Australian Temperament Project, the Christchurch Health and Development Study), we did a sensitivity analysis by removing the study by Degenhardt et al38 when assessing the association of cannabis with depression. The new OR was 1.40 (95% CI, 1.17-1.67) and remained significant.
Since the OR for depression was modestly elevated and even if error was random, if depression is more frequent in cannabis users,37 adjusting for depression would not eliminate it as a confounder and an apparent association could be observed in the range of the results seen here. As reported by Harder and colleagues68 the OR risk for depression after adolescent cannabis use becomes nonsignificant when the propensity score–adjusted regression methods is used.
To overcome this impact, the data set by Marmorstein and Iacono46 (reporting the highest OR for depression; Figure 2) was reanalyzed with multivariable logistic regression method and the propensity score method. The replication of the previously published work using the classical methods (multivariable logistic regression) resulted in a OR of 2.5 (95% CI, 1.1-5.5) (major depressive disorder by age 24 years as predicted by cannabis use disorder by age 17 years, controlling for sex, parental major depressive disorder and major depressive disorder, alcohol use disorders and nicotine dependence by age 17 years), while using a propensity score method the OR was 2.6 (95% CI, 1.2-5.5).
Systematic Review of Nonpoolable Studies and Trajectory-Based Studies
Twenty-four longitudinal studies did not report OR poolable data or reported the same cohorts and were thus not included in the meta-analysis; most of these studies reported findings as regression coefficients (β) or percentages rather than ORs or based their results on trajectories of cannabis use over time (eTable 1 in the Supplement). Interestingly, most of these studies reported a positive correlation between adolescent cannabis use and later depression,15,30,31,32,33,34,35,36,37,40,42,43,49,50,51,52,53,61 reinforcing the results of the meta-analysis. A few studies analyzed the different trajectories of cannabis use patterns from adolescence through young adulthood. For example, 1 study of 1232 first-year college students established 6 cannabis use trajectories varying from nonuse, to late-increase use, to college-peak use, to chronic use.35 The 6 cannabis trajectory groups were not significantly different on year-1 health-related variables but differed on all 10-year mental health outcomes tested, including depression and anxiety. Chronic and late increased users of cannabis fared the worst for the depression score after controlling for covariates. Interestingly, the higher risk for depression and anxiety in adolescents that continue to smoke until age 29 years was also confirmed by Degenhardt et al,38 suggesting that even the chronic use in young age is detrimental for mental health.
There is evidence that younger users of cannabis (aged 14 to 15 years) were at significantly higher risk of suicidal behaviors, although, overall, the association between cannabis use and depression did not vary with age42; moreover, girls seem more susceptible than boys to develop adult depression if they smoke cannabis in adolescence.15 Two trajectory studies demonstrated that quitting cannabis by the end of adolescence did not protect people from some of the serious effects of the drug.16,39 Other studies likewise found significant positive associations between cannabis use during adolescence and later anxiety and depression paralleled by academic unpreparedness, delinquency, and poorer academic performance.36
In contrast, a few studies failed to find a statistical correlation between cannabis and depressive symptoms.39,48,55,56,59,60 One study found no evidence of increased depression among nonusers vs other groups, although the chronic group of users reported significantly more anxiety symptoms at age 33 years than the other groups, after controlling for baseline anxiety.39 Another study supported a self-medication hypothesis in male individuals, in which adolescents with higher depressive symptoms at baseline used more cannabis.59 This study failed to support a link between using cannabis as an adolescent and later depression in male or female individuals but found instead a positive association between tobacco and depression.
Another study69 found an increased risk of depression among adolescents who possessed the short allele of the serotonin transporter gene (5-HTTLPR genotype) (S/S), evincing an interaction between genetics and an environmental exposure (cannabis).
Discussion
This meta-analysis shows that cannabis consumption in adolescence is associated with increased risk of developing major depression in young adulthood and suicidality, especially suicidal ideation. The association between cannabis and suicidal behavior was previously reported by Borges et al.70
Major depressive disorder is a debilitating mental illness associated with increased morbidity, mortality, and disability among those affected. During 2013 to 2016, 8.1% of individuals in the United States older than 20 years experienced depression in a given 2-week period.71 Although the causes of major depressive disorder are multifactorial and complex, this meta-analysis suggests that the cannabis exposure could be 1 factor contributing to depression in young adulthood. These data indicate that cannabis use during adolescence is associated with a moderately increased risk of depression in young adulthood. The effect size is modest, but considering that 20.9% of adolescents in the United States report monthly use of cannabis3 and 7% of US high school seniors are daily or near-daily users,4 the consequences of cannabis use during adolescence are magnified in young adulthood. In this study, the estimated population attributable risk is 7.2%. This translates to some 413 326 young adult cases of depression potentially attributable to cannabis exposure, considering that the population of young people between 18 and 34 years in the United States is 70 872 11872 and the incidence of depression is 8.1%.71 This study also is consistent with the greater part of research on brain imaging literature demonstrating the negative influence of cannabis in brain plasticity during the development. The brain indeed remains in a state of active, experience-guided development from the prenatal period through childhood and adolescence until the age of approximately 21 years.20,21 During this period, it is intrinsically more vulnerable to the adverse long-term effects of environmental insults, such as exposure to the THC.
Indeed, a recent review from more than 30 human magnetic resonance imaging studies from earlier cannabis users compared with controls have pointed out that neuroanatomic alterations emerged across regions notably with high cannabinoid receptor type 1. These alterations were more pronounced in people consuming higher doses at earlier age at onset73 and are mostly characterized by a decrease in volume (hippocampus, amygdala, prefrontal cortex) or greater gray matter density in cannabis users than in controls in the left nucleus accumbens extending to subcallosal cortex, hypothalamus, sublenticular extended amygdala, and left amygdala.74 Animal studies have demonstrated that cannabis consumption during adolescence produces an increase in anhedonia and anxiety in adulthood, paralleled by a decrease in serotonin (a neurotransmitter linked to depression) and an increase in norepinephrine (a neurotransmitter linked to anxiety).17 Studies have also demonstrated that, following long-term adolescent cannabinoid exposure, dopamine neurons become less responsive to the stimulating action of cannabinoids and a long-lasting cross-tolerance for morphine, cocaine, and amphetamine occur.18 Adolescent rats treated with THC also have fewer synaptic contacts during adulthood and reduced efficiency of hippocampal networks75; these differences may constitute the neurobiological underpinning of the cognitive and behavioral deficits that are observed in humans.9 Some of the detrimental effects of cannabis on behavior seem more pronounced in females in both animals19 and humans,15 although this finding remains controversial. Additional studies further demonstrate the neurobiological and the pathophysiological depressionlike behavior of adolescent rats when exposed to cannabinoids.19
Limitations
The limitations of this meta-analysis are intrinsic to the methodology itself. First, even if only longitudinal studies adjusted for premorbid depression were included, since the individual patient data from the majority of studies were not reanalyzed using causal inference methods, strong causal association cannot be made with respect to the relationship between cannabis and later depression, suicide, or anxiety. Moreover, not all studies have adjusted for other drugs of abuse and cigarettes, or psychosocial factors (ie, school abandonment, drug abuse in peers) that may be linked to depression and early cannabis consumption.46,49 Second, the longitudinal studies included in our analysis used heterogeneous methods of detecting major depressive disorder: some studies34,38,44,46 in the depression analysis used Diagnostic and Statistical Manual of Mental Disorders or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, criteria, including a structured interview45 to detect major depressive episodes, 1 study used a symptoms-based checklist,16 another study used a dichotomous measure derived from different diagnostic interviews and the Depression Anxiety Stress scale.10 It was also not possible to evaluate the exact quantity of cannabis consumed among adolescents in individual studies since they used measures of frequency of use and rather than the precise quantity. Furthermore, the potency of cannabis has generally increased since 1980,76 and potency varies across geographic location.8 Thus, the content of THC in “one-weekly joint” likely shifted from 1980 to the present day and across locales.
It is unlikely that publication bias is a concern in the context of our research as we included studies that had identified cannabis as 1 of the primary predictors of interest. It is more likely that we may have missed studies that examined the use of cannabis as a secondary interest.
Conclusions
These findings highlight the importance of initiatives aiming to educate teenagers on the risks associated with using cannabis and teach them skills to resist peer pressure. Given the likelihood of a window of risk during adolescence when the deleterious effects of cannabis are most pronounced,10 the findings in this meta-analysis suggest that cannabis is a serious public health concern and there is an urgent need to implement better drug use prevention programs targeting the use of cannabis among adolescents and interventions aimed at educating adolescents to develop the skills to resist peer pressure on drug consumption.77
eMethods 1. Search strategy used in the current systematic review and meta-analysis
eMethods 2. Multivariate Regression analyses and Propensity Score adjustment analyses in the cohort reported by Marmorstein and Iacono (2017)
eTable 1. Characteristics of all included studies in the systematic review and meta-analysis
eTable 2. Summary of full-articles reviewed, included or excluded in this systematic review and meta-analysis
References
- 1.World Drug Report. Vienna, Austria: United National Office on Drugs and Crime; 2017. [Google Scholar]
- 2.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72(12):1235-1242. doi: 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choo EK, Benz M, Zaller N, Warren O, Rising KL, McConnell KJ. The impact of state medical marijuana legislation on adolescent marijuana use. J Adolesc Health. 2014;55(2):160-166. doi: 10.1016/j.jadohealth.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 4.Johnston LD. OMP, Bachman J, Schulenberg J. Monitoring the Future National Results on Drug Use: 2012 Overview. Ann Arbor, Michigan: Institute for Social Research, University of Michigan; 2013. [Google Scholar]
- 5.Canadian Tobacco Alcohol and Drugs (CTADS): 2015 summary. https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2015-summary.html. Accessed December 28, 2018.
- 6.Australian Institute of Health and Welfare (AIHW) . 2010 National Drug Strategy Household Survey Report. Canberra, Australia: Australian Institute of Health and Welfare; 2011. [Google Scholar]
- 7.Fuller E, Hawkins V. Smoking, drinking and drug use among young people in England in 2011. London, UK: Health and Social Care Information Centre; 2012. [Google Scholar]
- 8.European Drug Report 2018: Trends and Developments. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2018. [Google Scholar]
- 9.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227. doi: 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silins E, Horwood LJ, Patton GC, et al. ; Cannabis Cohorts Research Consortium . Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293. doi: 10.1016/S2215-0366(14)70307-4 [DOI] [PubMed] [Google Scholar]
- 11.Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40(6):1509-1517. doi: 10.1093/schbul/sbt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657-E2664. doi: 10.1073/pnas.1206820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Academies of Sciences . The Health Effects of Cannabis and Cannabinoids: the Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 14.Paton S, Kessler R, Kandel D. Depressive mood and adolescent illicit drug use: a longitudinal analysis. J Genet Psychol. 1977;131(2d Half):267-289. [DOI] [PubMed] [Google Scholar]
- 15.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325(7374):1195-1198. doi: 10.1136/bmj.325.7374.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook JS, Lee JY, Brown EN, Finch SJ, Brook DW. Developmental trajectories of marijuana use from adolescence to adulthood: personality and social role outcomes. Psychol Rep. 2011;108(2):339-357. doi: 10.2466/10.18.PR0.108.2.339-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bambico FR, Nguyen N-T, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37(3):641-655. doi: 10.1016/j.nbd.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 18.Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56(2):86-94. doi: 10.1016/j.biopsych.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry. 2016;79(7):578-585. doi: 10.1016/j.biopsych.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 20.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861-863. doi: 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- 21.Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19(10):558-566. doi: 10.1016/j.tics.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 22.Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44(4):797-810. doi: 10.1017/S0033291713001438 [DOI] [PubMed] [Google Scholar]
- 23.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328. doi: 10.1016/S0140-6736(07)61162-3 [DOI] [PubMed] [Google Scholar]
- 24.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493-1504. doi: 10.1046/j.1360-0443.2003.00437.x [DOI] [PubMed] [Google Scholar]
- 25.Eden J, Levit L, Berg A, Morton S.. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 26.Lowe HJ, Barnett GO. Understanding and using the medical subject headings (MeSH) vocabulary to perform literature searches. JAMA. 1994;271(14):1103-1108. doi: 10.1001/jama.1994.03510380059038 [DOI] [PubMed] [Google Scholar]
- 27.McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the peer review of electronic search strategies (PRESS EBC). Evid Based Libr Inf Pract. 2010;5(1):149-154. doi: 10.18438/B8SG8R [DOI] [Google Scholar]
- 28.Canadian Agency for Drugs and Technologies in Health . Grey Matters: a Practical Search Tool for Evidence-Based Medicine. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; 2013. [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arria AM, Caldeira KM, Bugbee BA, Vincent KB, O’Grady KE. Marijuana use trajectories during college predict health outcomes nine years post-matriculation. Drug Alcohol Depend. 2016;159:158-165. doi: 10.1016/j.drugalcdep.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212-1213. doi: 10.1136/bmj.325.7374.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baggio S, N’Goran AA, Deline S, et al. Patterns of cannabis use and prospective associations with health issues among young males. Addiction. 2014;109(6):937-945. doi: 10.1111/add.12490 [DOI] [PubMed] [Google Scholar]
- 33.Brook JS, Cohen P, Brook DW. Longitudinal study of co-occurring psychiatric disorders and substance use. J Am Acad Child Adolesc Psychiatry. 1998;37(3):322-330. doi: 10.1097/00004583-199803000-00018 [DOI] [PubMed] [Google Scholar]
- 34.Brook DW, Brook JS, Zhang C, Cohen P, Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry. 2002;59(11):1039-1044. doi: 10.1001/archpsyc.59.11.1039 [DOI] [PubMed] [Google Scholar]
- 35.Caldeira KM, O’Grady KE, Vincent KB, Arria AM. Marijuana use trajectories during the post-college transition: health outcomes in young adulthood. Drug Alcohol Depend. 2012;125(3):267-275. doi: 10.1016/j.drugalcdep.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Amico EJ, Tucker JS, Miles JN, Ewing BA, Shih RA, Pedersen ER. Alcohol and marijuana use trajectories in a diverse longitudinal sample of adolescents: examining use patterns from age 11 to 17 years. Addiction. 2016;111(10):1825-1835. doi: 10.1111/add.13442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degenhardt L, Coffey C, Carlin JB, Swift W, Moore E, Patton GC. Outcomes of occasional cannabis use in adolescence: 10-year follow-up study in Victoria, Australia. Br J Psychiatry. 2010;196(4):290-295. doi: 10.1192/bjp.bp.108.056952 [DOI] [PubMed] [Google Scholar]
- 38.Degenhardt L, Coffey C, Romaniuk H, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108(1):124-133. doi: 10.1111/j.1360-0443.2012.04015.x [DOI] [PubMed] [Google Scholar]
- 39.Epstein M, Hill KG, Nevell AM, et al. Trajectories of marijuana use from adolescence into adulthood: environmental and individual correlates. Dev Psychol. 2015;51(11):1650-1663. doi: 10.1037/dev0000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction. 1997;92(3):279-296. doi: 10.1111/j.1360-0443.1997.tb03198.x [DOI] [PubMed] [Google Scholar]
- 41.Fergusson DM, Lynskey MT, Horwood LJ. The short-term consequences of early onset cannabis use. J Abnorm Child Psychol. 1996;24(4):499-512. doi: 10.1007/BF01441571 [DOI] [PubMed] [Google Scholar]
- 42.Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97(9):1123-1135. doi: 10.1046/j.1360-0443.2002.00103.x [DOI] [PubMed] [Google Scholar]
- 43.Fleming CB, Mason WA, Mazza JJ, Abbott RD, Catalano RF. Latent growth modeling of the relationship between depressive symptoms and substance use during adolescence. Psychol Addict Behav. 2008;22(2):186-197. doi: 10.1037/0893-164X.22.2.186 [DOI] [PubMed] [Google Scholar]
- 44.Gage SH, Hickman M, Heron J, et al. Associations of cannabis and cigarette use with depression and anxiety at age 18: findings from the Avon Longitudinal Study of Parents and Children. PLoS One. 2015;10(4):e0122896. doi: 10.1371/journal.pone.0122896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgiades K, Boyle MH. Adolescent tobacco and cannabis use: young adult outcomes from the Ontario Child Health Study. J Child Psychol Psychiatry. 2007;48(7):724-731. doi: 10.1111/j.1469-7610.2007.01740.x [DOI] [PubMed] [Google Scholar]
- 46.Marmorstein NR, Iacono WG. Explaining associations between cannabis use disorders in adolescence and later major depression: a test of the psychosocial failure model. Addict Behav. 2011;36(7):773-776. doi: 10.1016/j.addbeh.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGee R, Williams S, Nada-Raja S. Is cigarette smoking associated with suicidal ideation among young people? Am J Psychiatry. 2005;162(3):619-620. doi: 10.1176/appi.ajp.162.3.619 [DOI] [PubMed] [Google Scholar]
- 48.Meier MH, Hill ML, Small PJ, Luthar SS. Associations of adolescent cannabis use with academic performance and mental health: a longitudinal study of upper middle class youth. Drug Alcohol Depend. 2015;156:207-212. doi: 10.1016/j.drugalcdep.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briere FN. Association Entre Consommation de Drogues Illicites et Symptomatologie Dépressive à l’Adolescence. Une Étude Longitudinale Auprès de Jeunes Québécois Fréquentant l’École Secondaire en Milieu Défavorisé [PhD thesis]. Montreal, Canada: Université de Montréal; 2011. [Google Scholar]
- 50.Newcomb AF, Bukowski WM, Pattee L. Children’s peer relations: a meta-analytic review of popular, rejected, neglected, controversial, and average sociometric status. Psychol Bull. 1993;113(1):99-128. doi: 10.1037/0033-2909.113.1.99 [DOI] [PubMed] [Google Scholar]
- 51.Pahl K, Brook JS, Koppel J. Trajectories of marijuana use and psychological adjustment among urban African American and Puerto Rican women. Psychol Med. 2011;41(8):1775-1783. doi: 10.1017/S0033291710002345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen W. Does cannabis use lead to depression and suicidal behaviours? a population-based longitudinal study. Acta Psychiatr Scand. 2008;118(5):395-403. doi: 10.1111/j.1600-0447.2008.01259.x [DOI] [PubMed] [Google Scholar]
- 53.Repetto PB, Caldwell CH, Zimmerman MA. A longitudinal study of the relationship between depressive symptoms and cigarette use among African American adolescents. Health Psychol. 2005;24(2):209-219. doi: 10.1037/0278-6133.24.2.209 [DOI] [PubMed] [Google Scholar]
- 54.Roberts RE, Roberts CR, Xing Y. One-year incidence of suicide attempts and associated risk and protective factors among adolescents. Arch Suicide Res. 2010;14(1):66-78. doi: 10.1080/13811110903479078 [DOI] [PubMed] [Google Scholar]
- 55.Scholes-Balog KE, Hemphill SA, Evans-Whipp TJ, Toumbourou JW, Patton GC. Developmental trajectories of adolescent cannabis use and their relationship to young adult social and behavioural adjustment: a longitudinal study of Australian youth. Addict Behav. 2016;53:11-18. doi: 10.1016/j.addbeh.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 56.van Gastel WA, Vreeker A, Schubart CD, MacCabe JH, Kahn RS, Boks MPM. Change in cannabis use in the general population: a longitudinal study on the impact on psychotic experiences. Schizophr Res. 2014;157(1-3):266-270. doi: 10.1016/j.schres.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 57.Weeks M, Colman I. Predictors of suicidal behaviors in Canadian adolescents with no recent history of depression. Arch Suicide Res. 2017;21(2):354-364. doi: 10.1080/13811118.2016.1193076 [DOI] [PubMed] [Google Scholar]
- 58.Wilcox HC, Anthony JC. The development of suicide ideation and attempts: an epidemiologic study of first graders followed into young adulthood. Drug Alcohol Depend. 2004;76(suppl):S53-S67. doi: 10.1016/j.drugalcdep.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 59.Wilkinson AL, Halpern CT, Herring AH. Directions of the relationship between substance use and depressive symptoms from adolescence to young adulthood. Addict Behav. 2016;60:64-70. doi: 10.1016/j.addbeh.2016.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: predictors and outcomes. Dev Psychopathol. 2004;16(4):1007-1027. doi: 10.1017/S0954579404040118 [DOI] [PubMed] [Google Scholar]
- 61.Womack SR, Shaw DS, Weaver CM, Forbes EE. Bidirectional associations between cannabis use and depressive symptoms from adolescence through early adulthood among at-risk young men. J Stud Alcohol Drugs. 2016;77(2):287-297. doi: 10.15288/jsad.2016.77.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 63.Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65(2):163-178. doi: 10.1016/j.jclinepi.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 64.Nikolakopoulou A, Mavridis D, Salanti G. How to interpret meta-analysis models: fixed effect and random effects meta-analyses. Evid Based Ment Health. 2014;17(2):64. doi: 10.1136/eb-2014-101794 [DOI] [PubMed] [Google Scholar]
- 65.Lipsey MW, Wilson DB. Practical Meta-analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 66.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 67.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512-522. doi: 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harder VS, Stuart EA, Anthony JC. Adolescent cannabis problems and young adult depression: male-female stratified propensity score analyses. Am J Epidemiol. 2008;168(6):592-601. doi: 10.1093/aje/kwn184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otten R, Engels RC. Testing bidirectional effects between cannabis use and depressive symptoms: moderation by the serotonin transporter gene. Addict Biol. 2013;18(5):826-835. doi: 10.1111/j.1369-1600.2011.00380.x [DOI] [PubMed] [Google Scholar]
- 70.Borges G, Bagge CL, Orozco R. A literature review and meta-analyses of cannabis use and suicidality. J Affect Disord. 2016;195:63-74. doi: 10.1016/j.jad.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 71.Brody DJ, Pratt LA. J H. Prevalence of depression among adults aged 20 and over: United States, 2013–2016. Hyattsville, MD: National Center for Health Statistics; 2018. [Google Scholar]
- 72.Vespa J. The Changing Economics and Demographics of Young Adulthood: 1975-2016. Suitland, MD: US Department of Commerce Economics and Statistics Administration, US Census Bureau; 2017. [Google Scholar]
- 73.Lorenzetti V, Solowij N, Yücel M. The Role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry. 2016;79(7):e17-e31. doi: 10.1016/j.biopsych.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 74.Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34(16):5529-5538. doi: 10.1523/JNEUROSCI.4745-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubino T, Realini N, Braida D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763-772. doi: 10.1002/hipo.20554 [DOI] [PubMed] [Google Scholar]
- 76.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613-619. doi: 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faggiano F, Vigna-Taglianti FD, Versino E, Zambon A, Borraccino A, Lemma P. School-based prevention for illicit drugs’ use. Cochrane Database Syst Rev. 2005;(2):CD003020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search strategy used in the current systematic review and meta-analysis
eMethods 2. Multivariate Regression analyses and Propensity Score adjustment analyses in the cohort reported by Marmorstein and Iacono (2017)
eTable 1. Characteristics of all included studies in the systematic review and meta-analysis
eTable 2. Summary of full-articles reviewed, included or excluded in this systematic review and meta-analysis