Key Points
Question
Does physical activity have a potential causal role in reducing risk for depression?
Findings
In this 2-sample mendelian randomization study using genetic instruments from large-scale genome-wide association studies to support potential causal inference, higher levels of physical activity (indexed by objective accelerometer data) were linked to reduced odds for major depression.
Meaning
Findings strengthen empirical support for physical activity as an effective prevention strategy for depression.
This 2-sample mendelian randomization study assesses bidirectional relationships between physical activity and depression among phenotype samples with self-reported and accelerometer-based physical activity and major depressive disorder from nonoverlapping genome-wide association studies.
Abstract
Importance
Increasing evidence shows that physical activity is associated with reduced risk for depression, pointing to a potential modifiable target for prevention. However, the causality and direction of this association are not clear; physical activity may protect against depression, and/or depression may result in decreased physical activity.
Objective
To examine bidirectional relationships between physical activity and depression using a genetically informed method for assessing potential causal inference.
Design, Setting, and Participants
This 2-sample mendelian randomization (MR) used independent top genetic variants associated with 2 physical activity phenotypes—self-reported (n = 377 234) and objective accelerometer-based (n = 91 084)—and with major depressive disorder (MDD) (n = 143 265) as genetic instruments from the largest available, nonoverlapping genome-wide association studies (GWAS). GWAS were previously conducted in diverse observational cohorts, including the UK Biobank (for physical activity) and participating studies in the Psychiatric Genomics Consortium (for MDD) among adults of European ancestry. Mendelian randomization estimates from each genetic instrument were combined using inverse variance weighted meta-analysis, with alternate methods (eg, weighted median, MR Egger, MR–Pleiotropy Residual Sum and Outlier [PRESSO]) and multiple sensitivity analyses to assess horizontal pleiotropy and remove outliers. Data were analyzed from May 10 through July 31, 2018.
Main Outcomes and Measures
MDD and physical activity.
Results
GWAS summary data were available for a combined sample size of 611 583 adult participants. Mendelian randomization evidence suggested a protective relationship between accelerometer-based activity and MDD (odds ratio [OR], 0.74 for MDD per 1-SD increase in mean acceleration; 95% CI, 0.59-0.92; P = .006). In contrast, there was no statistically significant relationship between MDD and accelerometer-based activity (β = −0.08 in mean acceleration per MDD vs control status; 95% CI, −0.47 to 0.32; P = .70). Furthermore, there was no significant relationship between self-reported activity and MDD (OR, 1.28 for MDD per 1-SD increase in metabolic-equivalent minutes of reported moderate-to-vigorous activity; 95% CI, 0.57-3.37; P = .48), or between MDD and self-reported activity (β = 0.02 per MDD in standardized metabolic-equivalent minutes of reported moderate-to-vigorous activity per MDD vs control status; 95% CI, −0.008 to 0.05; P = .15).
Conclusions and Relevance
Using genetic instruments identified from large-scale GWAS, robust evidence supports a protective relationship between objectively assessed—but not self-reported—physical activity and the risk for MDD. Findings point to the importance of objective measurement of physical activity in epidemiologic studies of mental health and support the hypothesis that enhancing physical activity may be an effective prevention strategy for depression.
Introduction
Depression is a common psychiatric condition that represents a leading cause of disability worldwide.1 Despite this, efforts to prevent depression have been challenging, with few established protective factors, particularly modifiable targets for prevention. One promising target is physical activity, defined broadly as musculoskeletal movement resulting in energy expenditure.2 The relationship between physical activity and depression has received much attention in recent years. For example, meta-analytic data from randomized clinical trials3 have suggested that physical activity is linked to reduced depressive symptoms in at-risk populations, and prospective studies4,5 have demonstrated associations between higher levels of physical activity and decreased risk for later depression.
Although such findings point to a potential protective role of physical activity for depression, several questions remain. First, does physical activity causally influence risk for depression—or is this better explained by reverse causation? Some studies6,7 show that depression may also lead to reduced physical activity, but few studies have simultaneously tested both directional relationships. Second, does measurement of physical activity matter? Literature to date has relied mostly on self-reported measures of activity,5 which may be subject to confounding by participant mood, memory inaccuracy, and social desirability bias.8 Third, does the relationship between physical activity and depression persist when potential confounding is minimized? Although randomized clinical trials minimize confounding from unaccounted variables by design, they are intensive to conduct and have been of relatively limited size, with a mean of fewer than 60 participants per trial.3,9,10 More critically, randomized clinical trials have focused on treating symptoms in depressed individuals rather than testing preventive effects of physical activity on depression, which has population-wide implications but requires large samples unselected for depression. The most convincing evidence to date that physical activity is associated with a reduced risk for depression comes from meta-analyses of prospective studies,5 which are high quality yet still limited by the breadth of behavioral, social, and genetic confounders that cannot be fully ruled out in observational designs.
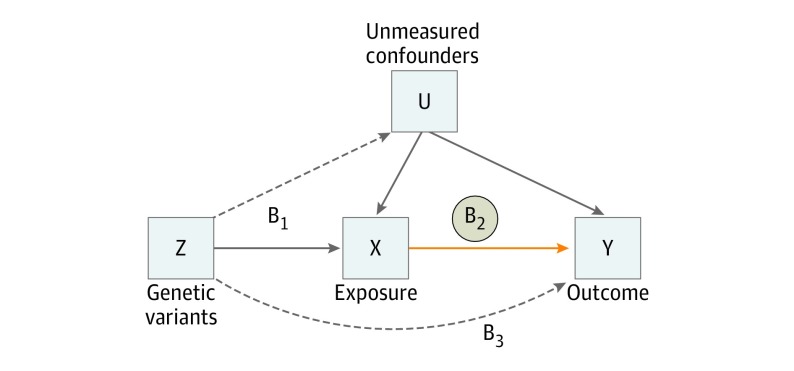
Mendelian randomization (MR) is an alternative method for potential causal inference that treats genetic variation as a natural experiment in which individuals are essentially assigned to higher vs lower mean levels of a nongenetic exposure during their lifetime.11 Because genetic variants are considered to be allocated randomly before birth, they are relatively independent of environmental factors and established well before onset of disease, thereby minimizing issues of residual confounding and reverse causation that limit typical observational studies. If an exposure such as physical activity causally influences an outcome such as depression, then a variant that affects physical activity should be expected to influence depression to a proportional degree, provided no separate pathway exists by which this variant can affect depression, a phenomenon known as horizontal pleiotropy. Under these conditions, variants strongly associated with an exposure of interest may serve as proxies, or instruments, for estimating potential causal relationship with an outcome (Figure 1). In a 2-sample MR design, instruments can be extracted from summary statistics of large-scale, nonoverlapping genome-wide association studies (GWAS), which have recently become available for physical activity12 and major depressive disorder (MDD).13 Herein, we apply bidirectional MR to assess the potential causal relationship of physical activity with the risk for depression, and vice versa. Furthermore, we examine genetic instruments for physical activity assessed subjectively via self-report and objectively using wearable accelerometers.
Figure 1. Mendelian Randomization (MR) Model.
B2 indicates the causal relationship of interest to be estimated, where B2 = B1/B3. B1 and B3 represent estimated direct effects of a genetic variant on the exposure (eg, physical activity) and outcome (eg, depression), respectively. Solid paths are theorized to exist; dashed paths are theorized to be nonsignificant according to MR assumptions.
Methods
This study relied on deidentified summary-level data that have been made publically available; ethical approval had been obtained in all original studies. Summary data were available for a combined sample of 611 583 adult participants, with corresponding GWAS sample sizes detailed below. Data were analyzed for this study from May 10 through July 31, 2018.
Data Sources and Instruments
Physical Activity
We drew on summary statistics from a recent GWAS of physical activity conducted among UK Biobank Study participants.12 This GWAS examined the following 2 continuous physical activity phenotypes: (1) self-reported moderate-to-vigorous physical activity (in standardized units of inverse-normalized metabolic-equivalent minutes per week) and (2) objective accelerometer-based activity, specifically overall mean acceleration (in milligravities across at least 72 hours of wrist-worn accelerometer wear). The GWAS for self-reported activity (n = 377 234) identified 9 independent genome-wide significant single-nucleotide polymorphisms (SNPs), although SNP-based heritability was modest at approximately 5%.12 The GWAS for accelerometer-based activity (n = 91 084) identified only 2 independent genome-wide significant SNPs, although SNP-based heritability was estimated much higher at 14%. These heritability estimates suggest that SNPs beyond those currently identified as genome-wide significant may contribute to variation in physical activity. Given this, we used the following 2 sets of genetic instruments: (1) only SNPs previously reported as genome-wide significant and (2) top SNPs meeting a more relaxed threshold (P < 1 × 10−7). This method of relaxing the statistical threshold for genetic instruments has been used in psychiatric MR research when few significant SNPs are available.14,15 When the more relaxed threshold was used, we clumped SNPs for independence (ie, when SNPs were correlated at r2 > 0.001, only 1 representative SNP was retained) based on European ancestry reference data from the 1000 Genomes Project. Where SNPs for the exposure phenotype were not available in the summary statistics of the outcome phenotype, we replaced them with overlapping proxy SNPs in high-linkage disequilibrium (r2 > 0.80) identified using the LDproxy search on the online platform LDlink (https://ldlink.nci.nih.gov/). Resulting lists of instrument SNPs for each phenotype are given in eTables 1 to 4 in the Supplement.
Depression
We drew on summary statistics from the largest and most recent GWAS for MDD, defined as a lifetime diagnosis of major depression based primarily on structured assessments by trained interviewers, clinician-administered checklists, or medical record review.13 Overall, this case-control GWAS identified 44 independent genome-wide significant SNPs for MDD. For the MR analysis, we used meta-analytic results for MDD that left out UK Biobank samples, because the physical activity GWAS was also conducted in the UK Biobank, and without 23andMe samples owing to general access constraints. This elimination resulted in a GWAS meta-analytic subsample of 143 265. As instruments, we used independent clumped SNPs meeting a relaxed threshold (P < 1 × 10−6) to account for the reduced meta-analytic subsample, with similar procedures for identifying proxy SNPs as needed. The resulting list of instrument SNPs is found in eTable 5 in the Supplement.
Statistical Analysis
Mendelian randomization analyses were conducted in the R computing environment using the TwoSampleMR package (R Project for Statistical Computing). This package harmonizes exposure and outcome data sets containing information on SNPs, alleles, effect sizes (odds ratios [ORs] converted to β statistics by log transformation), standard errors, P values, and effect allele frequencies for selected exposure instruments. Herein, we allowed the forward strand of ambiguous SNPs to be inferred where possible based on allele frequency information; however, strand-ambiguous SNPs with intermediate effect allele frequencies (>0.42) were considered unresolvable. We also conducted sensitivity analyses where strand-ambiguous SNPs were excluded from MR analysis, which did not change the pattern of findings; thus, results using the full set of SNPs were reported.
For each direction of potential influence, we combined MR estimates using inverse variance–weighted (IVW) meta-analysis, which essentially translates to a weighted regression of SNP-outcome effects on SNP-exposure effects where the intercept is constrained to zero. Again, results can be biased if instrument SNPs show horizontal pleiotropy, influencing the outcome through causal pathways other than the exposure, thereby violating instrumental variable assumptions.16 We therefore compared IVW results with other established MR methods whose estimates are known to be relatively robust to horizontal pleiotropy, although at the cost of reduced statistical power.17 These methods include the weighted median approach, which selects the median MR estimate as the causal estimate,18 and MR Egger regression, which allows the intercept to be freely estimated as an indicator of average pleiotropic bias.16 We also applied MR-PRESSO (Pleiotropy Residual Sum and Outlier)19 to detect and correct for any outliers reflecting likely pleiotropic biases for all reported results. Effect estimates are reported in β values where the outcome was continuous (ie, self-reported or objectively assessed physical activity levels) and converted to ORs where the outcome was dichotomous (ie, MDD status).
To assess robustness of significant results, we conducted further tests for horizontal pleiotropy using meta-analytic methods to detect heterogeneous outcomes, including leave-1-SNP-out analyses, the modified Cochran Q statistic, and the MR Egger intercept test of deviation from the null.20 These tests vary in their assumptions but essentially capture the extent to which the effect for 1 or more instrument SNP is exaggerated in magnitude, as would be the case if that SNP not only acted through the hypothesized pathway, but through other unaccounted causal pathways. Finally, we looked up each instrument SNP and their proxies (r2 > 0.80) in the PhenoScanner GWAS database (version 2; http://phenoscanner.medschl.cam.ac.uk) to assess any previous associations (P < 1 × 10−5) with potential confounding traits and assessed the effects of manually removing these SNPs from the MR analysis to rule out possible pleiotropic effects.
Results
Accelerometer-Based Physical Activity and Depression
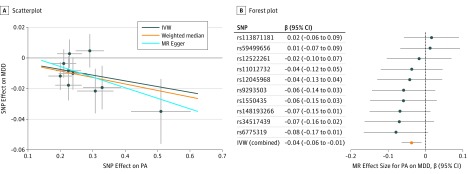
We found evidence of a protective causal relationship between accelerometer-based physical activity with MDD (IVW OR, 0.74 for MDD per 1-SD unit increase in mean acceleration; 95% CI, 0.59-0.92; P = .006); weighted median and MR Egger analysis yielded similar pattern of effects (Table 1), with 10 SNPs meeting the relaxed statistical threshold (Figure 2). The MR estimate was not statistically significant with only 2 genome-wide significant SNPs (IVW OR, 1.12; 95% CI, 0.72-1.75; P = .60) (eTable 6 and eFigure 1 in the Supplement), which provided insufficient data for alternative MR methods and sensitivity analyses. For the 10 SNPs, MR-PRESSO did not detect any potential outliers. Furthermore, analyses leaving out each SNP revealed that no single SNP drove these results but rather reflected an overall combined pattern of opposite relationships with physical activity vs MDD (eFigure 2 in the Supplement). Similarly, the modified Q statistic indicated no notable heterogeneity (Q = 6.01; P = .74) across instrument SNP effects. The MR Egger intercept test further suggested no horizontal pleiotropy (intercept, 0.008; standard error, 0.02; P = .60). In the PhenoScanner database, we identified 2 of the 10 SNPs for accelerometer-based activity nominally associated with depression-relevant traits (ie, rs59499656 with body mass index and rs9293503 with educational attainment). However, removing both SNPs did not change the pattern of results. When we further mapped SNPs to known genes in public databases and examined whether any genes have been implicated in GWAS of relevant traits, removing SNPs produced no substantive change in results (eMethods 1, which includes eFigure 3 and eTables 6 and 7, in the Supplement).
Table 1. MR Results for the Relationship Between Accelerometer-Based Activity Effect and MDD.
| Method | OR (95% CI)a | P Value | No. of SNPs |
|---|---|---|---|
| IVWb | 0.74 (0.59-0.92) | .006 | 10 |
| Weighted medianb | 0.71 (0.53-0.95) | .02 | 10 |
| MR Eggerb | 0.57 (0.22-1.48) | .28 | 10 |
Abbreviations: IVW, inverse variance–weighted; MDD, major depressive disorder; MR, mendelian randomization; OR, odds ratio; SNP, single-nucleotide polymorphism.
Indicates odds for MDD per 1-SD increase in mean acceleration.
No MR-PRESSO (Pleiotropy Residual Sum and Outlier) outliers were detected. P < 1 × 10−7 for top SNPs.
Figure 2. Mendelian Randomization (MR) Plots for Relationship of Accelerometer-Based Activity With Major Depressive Disorder (MDD).
A, Scatterplot of single-nucleotide polymorphism (SNP) potential effects on physical activity (PA) vs MDD, with the slope of each line corresponding to estimated MR effect per method. B, Forest plot of individual and combined SNP MR-estimated effects sizes. Data are expressed as raw β values with 95% CI. P < 1 × 10−7 for top SNPs. IVW indicates inverse variance–weighted method.
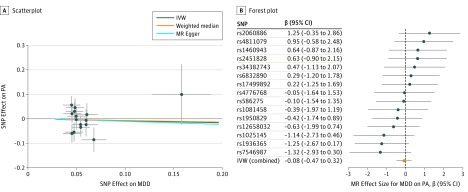
In the other direction, across all MR methods, we found no evidence of causal relationships of MDD with accelerometer-based activity (Table 2). MR-PRESSO detected 1 outlier, and MR estimates remained null after removal of this outlier (IVW β = −0.08 in mean acceleration per MDD vs control status; 95% CI, −0.47 to 0.32; P = .70). The weighted median and MR Egger yielded a similar pattern of effects (Table 2 and Figure 3).
Table 2. MR Results for the Relationship Between MDD and Accelerometer-Based Activity.
| Method | β (95% CI)a | P Value | SNPs |
|---|---|---|---|
| Main modelb | |||
| IVW | −0.08 (−0.47 to 0.32) | .70 | 15 |
| Weighted median | −0.07 (−0.62 to 0.48) | .82 | 15 |
| MR Egger | −0.13 (−2.11 to 1.86) | .90 | 15 |
| With outlier | |||
| IVW | 0.05 (−0.41 to 0.51) | .83 | 16 |
| Weighted median | −0.04 (−0.59 to 0.51) | .98 | 16 |
| MR Egger | 1.05 (−0.96 to 3.06) | .33 | 16 |
Abbreviations: IVW, inverse variance–weighted; MDD, major depressive disorder; MR, mendelian randomization; OR, odds ratio; SNP, single-nucleotide polymorphism.
Indicates change in mean acceleration per MDD vs control status.
Indicates model with MR-PRESSO (Pleiotropy Residual Sum and Outlier) outlier (rs78676209) removed. P < 1 × 10−6 for top SNPs.
Figure 3. Mendelian Randomization (MR) Plots for Relationship of Major Depressive Disorder (MDD) With Accelerometer-Based Activity.
A, Scatterplot of single-nucleotide polymorphism (SNP) effects on MDD vs their effects on physical activity (PA), with slope of each line corresponding to estimated MR effect per method. B, Forest plot of individual and combined SNP MR-estimated effects sizes. Data are expressed as raw β values with 95% CI. IVW indicates inverse variance–weighted method.
Self-reported Physical Activity and Depression
In contrast, we found no statistically significant evidence of a relationship between self-reported activity and MDD, regardless of instrument SNP threshold (outlier-adjusted IVW OR, 1.28 for MDD per 1-SD increase in metabolic-equivalent minutes of moderate-to-vigorous activity [95% CI, 0.87-1.90; P = .21] for 24 top SNPs; IVW OR, 1.45 for MDD per 1-SD increase in metabolic-equivalent minutes of moderate-to-vigorous activity [95% CI, 0.57-3.37; P = .48] for 6 genome-wide significant SNPs) (eTables 9-11 and eFigures 4 and 5 in the Supplement), or between MDD and self-reported activity (for 14 top SNPs, outlier-adjusted IVW β = 0.02 in standardized metabolic-equivalent minutes of moderate-to-vigorous activity for MDD vs control status; 95% CI, −0.008 to 0.05; P = .15) (eTable 12 and eFigure 6 in the Supplement).
Discussion
Depression is a common and debilitating condition, with a high societal burden of morbidity and mortality.21 As such, identification of effective strategies for preventing depression has substantial implications for improving population health. Recent evidence has suggested that physical activity may protect against the risk for depression.3,4,5 However, if the relationship between physical activity and depression is not causal, recommendations to promote physical activity, while beneficial for other health outcomes, would yield limited results for depression. To strengthen causal inference, we apply a genetically informed method. Using MR with genetic instruments selected from large-scale GWAS, we find evidence supporting a potential causal relationship between physical activity and a reduced risk for depression.
Our results extend current literature in a number of ways. First, we examined self-reported and objectively measured (ie, accelerometer-based) physical activity and discovered that findings on the relationship with depression are specific to objectively measured—but not self-reported—activity. Meta-analytic data have shown that self-report and objective measures can yield discrepant estimates of physical activity.8,22,23 Self-report measures of activity may be affected by mood states and cognitive biases that also affect mental health, making it difficult to ascertain whether observed associations are true or simply artifacts of a common liability. For example, individuals vulnerable to depression may perceive themselves as more inactive and disengaged than their peers or compensate by overreporting activity. Although this does not invalidate the utility of self-reported measures, verifying their conclusions with objective measures is essential. Prior work has indicated that objectively measured physical activity is more heritable12 and hence may be closer to biological processes that could directly affect depression, as well as more powerfully instrumented by SNPs in the MR context.24 Only 1 prior study,25 to our knowledge, has incorporated genetic information, using a twin-based design, to assess the relationship between physical activity and depression. Contrary to our study, it did not yield evidence of such a relationship, perhaps owing to self-report measures and restricted definition of physical activity as leisure exercise (ie, intentionally performed to improve or maintain fitness) vs physical activity more broadly.2
We estimated a moderate but significant reduction of MDD risk per 1-SD increase in objectively measured physical activity. One SD of objectively measured physical activity in the UK Biobank Study has been reported to be approximately 8 milligravities (or 0.08 m/s2) of acceleration in a mean 5-second window of analyzed accelerometer data.12,26 Although no straightforward translation of these values into energy expenditure or step-based metrics is available, an 8-milligravity increase in mean acceleration is roughly what we might observe in a 24-hour period if—for example—a person replaced sedentary behavior with 15 minutes of vigorous activity (eg, running); just more than 1 hour of moderate physical activity (eg, fast walking); or some combination of light activity (eg, standing, stretching, easy chores) and more vigorous activity (eFigure 7 and eTable 13 in the Supplement).
Second, it has remained unclear to date whether inverse associations between physical activity and depression are owing potentially to a protective relationship between physical activity and depression and/or a relationship between depression and reduced physical activity. Using bidirectional MR, we found evidence of only 1 direction of this relationship, where physical activity demonstrated a potential causal relationship with depression, while depression does not appear to have a such a relationship with physical activity. Other factors may better explain the observed depression-activity relationship7 rather than depression directly compromising physical activity. For example, underlying conditions such as chronic pain could interfere with activity and lead to depression. However, our MR analyses may not be currently powered to detect small effects (for calculations, see eMethods 2 in the Supplement) that may become apparent when future discovery GWAS are expanded.
Limitations
This study has several limitations. First, although we drew on the largest available GWAS, some identified few genome-wide significant SNPs, which could result in relatively weak genetic instruments. To address this, we applied statistical criteria to include additional SNPs as instruments. This approach has been used in other MR studies where currently known genome-wide significant SNPs are limited.14,15 Second, despite selecting strongly associated SNPs, common SNPs do not yet explain much total variance in complex traits27 and so cannot be considered exact proxies of the exposure. In addition, because we do not yet know the biological action of these SNPs, it is impossible to fully rule out pleiotropic mechanisms without detailed functional follow-up of these loci, although we conducted the most up-to-date array of sensitivity analyses to rule out horizontal pleiotropy. Although horizontal pleiotropy is a concern for MR inference, vertical pleiotropy—in which an exposure acts on an outcome via other variables along the same causal pathway—is acceptable.17 For example, if physical activity causally reduces body mass index, and then body mass index causally affects MDD, this represents vertical pleiotropy for which we should not unnecessarily penalize the MR estimate.24 However, it is reassuring that our observed MR estimate was robust across sensitivity analyses, suggesting negligible bias from evident sources of pleiotropy. Third, we used summary GWAS data for MDD and not for depressive symptoms in individuals with or without MDD. Although meta-analyses have shown that physical activity is associated with improved symptoms in individuals with depression,9,10,28 our study was not designed to address this issue. Also, we only considered overall levels of physical activity in relation to depression, whereas recent work has revealed complicated associations between the type, duration, frequency, and intensity of physical activity and mental health29 that could affect the size and direction of observed MR estimates but could not be assessed in the present study. Fourth, SNPs associated with physical activity were identified in the UK Biobank Study, which consists of individuals aged 40 to 70 years, whereas samples in the MDD GWAS included a wider range of age groups. Physical activity in younger individuals may be influenced by other variants that share different associations with MDD, although such GWAS data are not yet available. Moreover, we do not have demographic data on all of the GWAS participants, such as age and sex, which limits clinical generalizability of these findings to other populations. Finally, we cannot interpret effect sizes in the same way as a clinical trial in which individuals are assigned to a discrete program of physical activity of defined length, because MR estimates reflect lifelong effects of assignment to genetic variants. However, our MR estimate is notably similar in magnitude to those of recent meta-analytic observational data.5
Despite these limitations, our application of MR represents a test of whether genetic instruments provide independent support for potentially protective relationships between physical activity and depression risk. Our novel triangulation of genetic variants as instruments for causal inference30 obviates typical challenges for observational research while strengthening evidence from such studies.3,4,5 Stronger evidence of causal relationships is of great importance because few modifiable factors for preventing depression are known. If physical activity truly reduces risk for depression, it would be useful to promote physical activity not only in the population at large, where this can yield public health returns at the level of human productivity and reduced health care burden, but also for individuals at risk for developing new depression, such as adolescents or those facing depressogenic exposures, such as violence-exposed individuals or workers in high-stress environments.
Conclusions
This study leverages MR to support causal inference regarding putative protective factors in mental health. Our findings validate a potential protective relationship between physical activity and depression and point to the importance of objective measurement of physical activity in epidemiologic studies of mental health. Overall, this study supports the hypothesis that enhancing physical activity is an effective prevention strategy for depression.
eTable 1. Top SNPs (n = 10) for Accelerometer-Based Activity (P < 1 × 10−7)
eTable 2. Genome-Wide Reported SNPs (n = 2) for Accelerometer-Based Activity
eTable 3. Top SNPs (n = 25) for Self-Reported Activity (P < 1 × 10−7)
eTable 4. Genome-Wide Reported SNPs (n = 8) for Self-Reported Physical Activity
eTable 5. Top SNPs (n = 17) for Depression (P < 1 × 10−6)
eTable 6. Mendelian Randomization Results of Accelerometer-Based Activity (Genome-Wide SNPs Only) → Depression
eTable 7. Instruments SNPs, dbSNP Genes, and GWAS-Linked Traits
eTable 8. Mendelian Randomization Results for Sensitivity Analysis
eTable 9. Mendelian Randomization Results of Self-Reported Activity (Top SNPs P < 1 × 10−7) → Depression
eTable 10. Mendelian Randomization Results of Self-Reported Activity (Top SNPs P < 1 × 10−7) → Depression, Further Excluding APOE SNP
eTable 11. Mendelian Randomization Results of Self-Reported Activity (Genome-Wide SNPs Only) → Depression
eTable 12. Mendelian Randomization Results of Depression (Top SNPs P < 1 × 10−6) → Self-Reported Activity
eTable 13. Contextualizing 1-SD Increase in Objectively Measured Physical Activity
eFigure 1. Mendelian Randomization Plots for Accelerometer-Based Activity (Genome-Wide SNPs Only) → Depression
eFigure 2. Leave-One-Out Analyses for SNPs Associated With Accelerometer-Based Activity (PA, Top SNPs P < 1 × 10−7) → Depression
eFigure 3. Mendelian Randomization Plots for Accelerometer-Based Activity (Sensitivity Analysis) → Depression
eFigure 4. Mendelian Randomization Plots for Self-Reported Activity (Top SNPs P < 1 × 10−7) → Depression
eFigure 5. Mendelian Randomization Plots for Self-Reported Activity (Genome-Wide SNPs Only) → Depression
eFigure 6. Mendelian Randomization Plots for Depression (Top SNPs P < 1 × 10−6) → Self-Reported Activity
eFigure 7. Contextualizing 1-SD Increase in Objectively Measured Physical Activity
eMethods 1. Further Sensitivity Analyses for Main Result With 10 Accelerometer-Based SNPs
eMethods 2. MR Power Calculations
References
- 1.Lépine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):3-7. doi: 10.2147/NDT.S19617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131. [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry. 2018;75(6):566-576. doi: 10.1001/jamapsychiatry.2018.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45(5):649-657. doi: 10.1016/j.amepre.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175(7):631-648. doi: 10.1176/appi.ajp.2018.17111194 [DOI] [PubMed] [Google Scholar]
- 6.Schuch F, Vancampfort D, Firth J, et al. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2017;210:139-150. doi: 10.1016/j.jad.2016.10.050 [DOI] [PubMed] [Google Scholar]
- 7.Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31(4):306-315. doi: 10.1016/j.genhosppsych.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 8.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(1):56. doi: 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51. doi: 10.1016/j.jpsychires.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 10.Cooney G, Dwan K, Mead G. Exercise for depression. JAMA. 2014;311(23):2432-2433. doi: 10.1001/jama.2014.4930 [DOI] [PubMed] [Google Scholar]
- 11.Byrne EM, Yang J, Wray NR. Inference in psychiatry via 2-sample mendelian randomization: from association to causal pathway? JAMA Psychiatry. 2017;74(12):1191-1192. doi: 10.1001/jamapsychiatry.2017.3162 [DOI] [PubMed] [Google Scholar]
- 12.Klimentidis YC, Raichlen DA, Bea J, et al. Genome-wide association study of habitual physical activity in over 377 000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes (Lond). 2018;42(6):1161-1176. doi: 10.1038/s41366-018-0120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wray NR, Ripke S, Mattheisen M, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. doi: 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage SH, Jones HJ, Burgess S, et al. Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample mendelian randomization study. Psychol Med. 2017;47(5):971-980. doi: 10.1017/S0033291716003172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry. 2017;74(12):1226-1233. doi: 10.1001/jamapsychiatry.2017.3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195-R208. doi: 10.1093/hmg/ddy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. doi: 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt LA, Druss BG, Manderscheid RW, Walker ER. Excess mortality due to depression and anxiety in the United States: results from a nationally representative survey. Gen Hosp Psychiatry. 2016;39:39-45. doi: 10.1016/j.genhosppsych.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vancampfort D, Firth J, Schuch FB, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry. 2017;16(3):308-315. doi: 10.1002/wps.20458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46(1):99-106. doi: 10.1249/MSS.0b013e3182a0595f [DOI] [PubMed] [Google Scholar]
- 24.Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362(July):k601. doi: 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Moor MHM, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJC. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897-905. doi: 10.1001/archpsyc.65.8.897 [DOI] [PubMed] [Google Scholar]
- 26.Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One. 2017;12(2):e0169649. doi: 10.1371/journal.pone.0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14(7):507-515. doi: 10.1038/nrg3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86. doi: 10.1016/j.jad.2016.03.063 [DOI] [PubMed] [Google Scholar]
- 29.Chekroud SR, Gueorguieva R, Zheutlin AB, et al. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry. 2018;5(9):739-746. doi: 10.1016/S2215-0366(18)30227-X [DOI] [PubMed] [Google Scholar]
- 30.Munafò MR, Davey Smith G. Robust research needs many lines of evidence. Nature. 2018;553(7689):399-401. doi: 10.1038/d41586-018-01023-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Top SNPs (n = 10) for Accelerometer-Based Activity (P < 1 × 10−7)
eTable 2. Genome-Wide Reported SNPs (n = 2) for Accelerometer-Based Activity
eTable 3. Top SNPs (n = 25) for Self-Reported Activity (P < 1 × 10−7)
eTable 4. Genome-Wide Reported SNPs (n = 8) for Self-Reported Physical Activity
eTable 5. Top SNPs (n = 17) for Depression (P < 1 × 10−6)
eTable 6. Mendelian Randomization Results of Accelerometer-Based Activity (Genome-Wide SNPs Only) → Depression
eTable 7. Instruments SNPs, dbSNP Genes, and GWAS-Linked Traits
eTable 8. Mendelian Randomization Results for Sensitivity Analysis
eTable 9. Mendelian Randomization Results of Self-Reported Activity (Top SNPs P < 1 × 10−7) → Depression
eTable 10. Mendelian Randomization Results of Self-Reported Activity (Top SNPs P < 1 × 10−7) → Depression, Further Excluding APOE SNP
eTable 11. Mendelian Randomization Results of Self-Reported Activity (Genome-Wide SNPs Only) → Depression
eTable 12. Mendelian Randomization Results of Depression (Top SNPs P < 1 × 10−6) → Self-Reported Activity
eTable 13. Contextualizing 1-SD Increase in Objectively Measured Physical Activity
eFigure 1. Mendelian Randomization Plots for Accelerometer-Based Activity (Genome-Wide SNPs Only) → Depression
eFigure 2. Leave-One-Out Analyses for SNPs Associated With Accelerometer-Based Activity (PA, Top SNPs P < 1 × 10−7) → Depression
eFigure 3. Mendelian Randomization Plots for Accelerometer-Based Activity (Sensitivity Analysis) → Depression
eFigure 4. Mendelian Randomization Plots for Self-Reported Activity (Top SNPs P < 1 × 10−7) → Depression
eFigure 5. Mendelian Randomization Plots for Self-Reported Activity (Genome-Wide SNPs Only) → Depression
eFigure 6. Mendelian Randomization Plots for Depression (Top SNPs P < 1 × 10−6) → Self-Reported Activity
eFigure 7. Contextualizing 1-SD Increase in Objectively Measured Physical Activity
eMethods 1. Further Sensitivity Analyses for Main Result With 10 Accelerometer-Based SNPs
eMethods 2. MR Power Calculations