This registry-based cohort study compares adverse event rates up to 3 years after initiation of treatment among patients receiving combination warfarin and aspirin therapy (without a therapeutic indication for aspirin use) vs those receiving warfarin monotherapy.
Key Points
Question
Among patients receiving warfarin sodium for management of atrial fibrillation or venous thromboembolism, how often are patients receiving aspirin (acetylsalicylic acid) without a clear therapeutic indication, and what is the clinical impact?
Findings
In a registry-based cohort study of 3688 propensity score–matched patients followed up prospectively at anticoagulation clinics, without a heart valve replacement or recent acute coronary syndrome, 37.5% received aspirin. These patients had a statistically higher rate of bleeding, emergency department visits, and hospitalizations for bleeding; there was no observed difference in thrombosis rates.
Meaning
Some patients receiving anticoagulation treatment with warfarin may be receiving concomitant aspirin therapy that may increase bleeding risk with unclear therapeutic benefit.
Abstract
Importance
It is not clear how often patients receive aspirin (acetylsalicylic acid) while receiving oral anticoagulation with warfarin sodium without a clear therapeutic indication for aspirin, such as a mechanical heart valve replacement, recent percutaneous coronary intervention, or acute coronary syndrome. The clinical outcomes of such patients treated with warfarin and aspirin therapy compared with warfarin monotherapy are not well defined to date.
Objective
To evaluate the frequency and outcomes of adding aspirin to warfarin for patients without a clear therapeutic indication for combination therapy.
Design, Setting, and Participants
A registry-based cohort study of adults enrolled at 6 anticoagulation clinics in Michigan (January 1, 2010, to December 31, 2017) who were receiving warfarin therapy for atrial fibrillation or venous thromboembolism without documentation of a recent myocardial infarction or history of valve replacement.
Exposure
Aspirin use without therapeutic indication.
Main Outcomes and Measures
Rates of any bleeding, major bleeding events, emergency department visits, hospitalizations, and thrombotic events at 1, 2, and 3 years.
Results
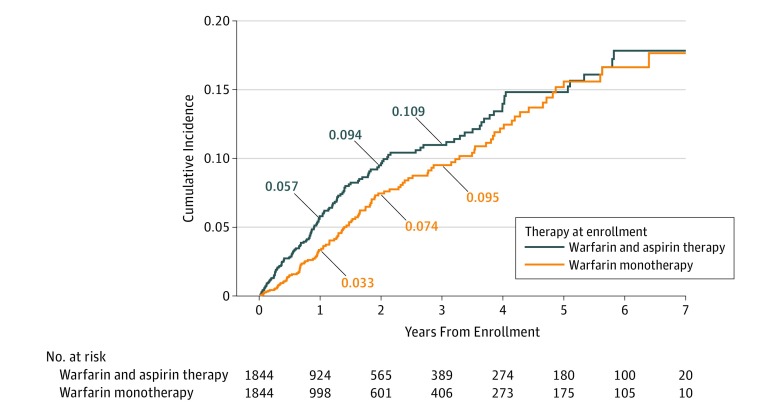
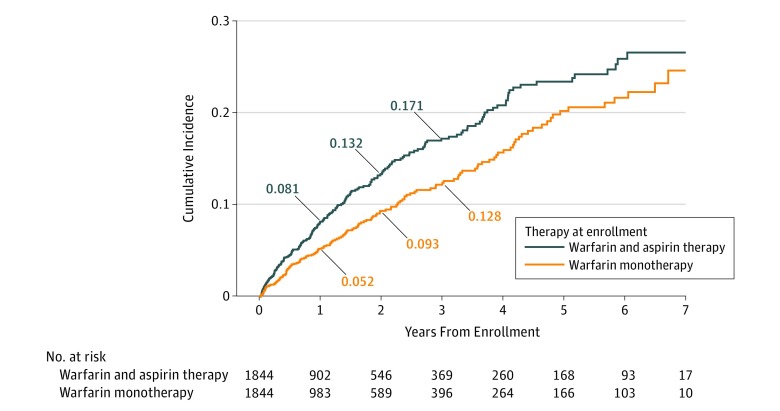
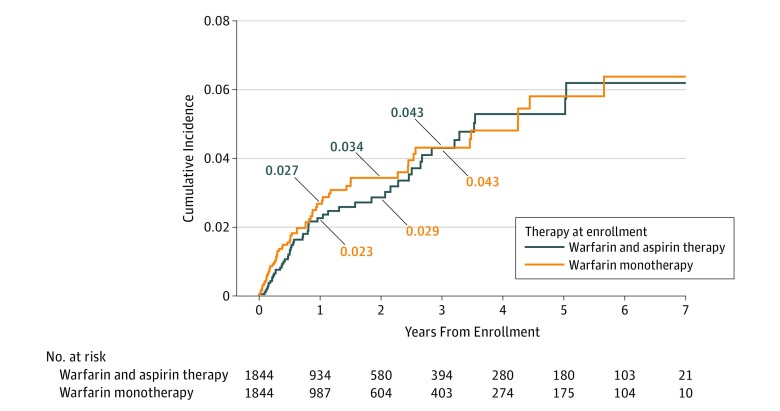
Of the study cohort of 6539 patients (3326 men [50.9%]; mean [SD] age, 66.1 [15.5] years), 2453 patients (37.5%) without a clear therapeutic indication for aspirin were receiving combination warfarin and aspirin therapy. Data from 2 propensity score–matched cohorts of 1844 patients were analyzed (warfarin and aspirin vs warfarin only). At 1 year, patients receiving combination warfarin and aspirin compared with those receiving warfarin only had higher rates of overall bleeding (cumulative incidence, 26.0%; 95% CI, 23.8%-28.3% vs 20.3%; 95% CI, 18.3%-22.3%; P < .001), major bleeding (5.7%; 95% CI, 4.6%-7.1% vs 3.3%; 95% CI, 2.4%-4.3%; P < .001), emergency department visits for bleeding (13.3%; 95% CI, 11.6%-15.1% vs 9.8%; 95% CI, 8.4%-11.4%; P = .001), and hospitalizations for bleeding (8.1%; 6.8%-9.6% vs 5.2%; 4.1%-6.4%; P = .001). Rates of thrombosis were similar, with a 1-year cumulative incidence of 2.3% (95% CI, 1.6%-3.1%) for those receiving combination warfarin and aspirin therapy compared with 2.7% (95% CI, 2.0%-3.6%) for those receiving warfarin alone (P = .40). Similar findings persisted during 3 years of follow-up as well as in sensitivity analyses.
Conclusions and Relevance
Compared with warfarin monotherapy, receipt of combination warfarin and aspirin therapy was associated with increased bleeding and similar observed rates of thrombosis. Further research is needed to better stratify which patients may benefit from aspirin while anticoagulated with warfarin for atrial fibrillation or venous thromboembolism; clinicians should be judicious in selecting patients for combination therapy.
Introduction
Warfarin sodium and aspirin (acetylsalicylic acid) are widely used for the treatment or prevention of thromboembolic disease and atherosclerotic diseases. Patients often receive warfarin or a direct oral anticoagulant for stroke prevention in atrial fibrillation (AF) and for the treatment and secondary prevention of venous thromboembolic disease (deep vein thrombosis, pulmonary embolism, and venous thromboembolism [VTE]).1 Many patients initiating therapy with warfarin are already receiving aspirin, or they subsequently initiate aspirin therapy because of other comorbidities. Indications for aspirin include primary prevention in those individuals at risk for coronary artery disease (CAD),2,3 the prevention of colorectal cancer in high-risk individuals,2 the management of stable ischemic heart disease,4 management of peripheral arterial disease,5,6 and/or the secondary prevention of stroke after noncardioembolic stroke or transient ischemic attack.7,8 Use of combined warfarin and aspirin is recommended in only a few scenarios. These scenarios include acute coronary syndromes (ACSs), particularly with percutaneous coronary interventions9,10 and mechanical heart valves for some patients.11,12,13 Aside from these conditions, there are limited data to guide us on deciding which patients should receive warfarin and aspirin combination therapy.
The 2012 American College of Chest Physicians guidelines for AF recommend that, for stable CAD, defined as no ACS within the previous year, warfarin monotherapy be used instead of combination therapy with aspirin.10 Likewise, the 2016 European Society of Cardiology Guidelines for AF advise against combined platelet inhibitors and oral anticoagulant use without another medical indication. They also recommend that antiplatelet therapy be discontinued for patients with stable CAD with AF, except potentially for high-risk patients.14 These recommendations are supported by a 2007 meta-analysis showing no mortality benefit and a significant risk of harm associated with the combined use of warfarin and aspirin for patients without mechanical heart valves.15 Subsequent observational studies and prospective trials16,17 have reinforced these findings.
We sought to assess the frequency of combination therapy in a community-based setting of patients anticoagulated for AF or VTE. In addition, we sought to determine the characteristics and outcomes of patients receiving warfarin monotherapy for AF or VTE compared with those who were receiving combination warfarin and aspirin therapy without a strong medical indication. Our study was designed to overcome the following limitations of previous reports about this important topic: (1) the patient populations were restricted only to those with AF (not including VTE); (2) the amount of time that a patient’s international normalized ratio (INR) was in the therapeutic range (TTR) was not standardized between groups or was not available; (3) sicker patients were more likely to be receiving aspirin therapy, which may have led to confounding; (4) aspirin use was not routinely documented; (5) sample size was small; and (6) follow-up duration was short.
Methods
Study Design and Participants
We retrospectively collected and analyzed data from a cohort of patients treated with warfarin therapy followed up longitudinally between January 1, 2010, and December 31, 2017. Patients receiving direct oral anticoagulants were not included. Adult patients, newly initiated on warfarin therapy for AF or VTE between January 1, 2010, and December 31, 2016, were identified in the Blue Cross Blue Shield of Michigan–sponsored Michigan Anticoagulation Quality Improvement Initiative (MAQI2) registry, which is a collaborative of 6 outpatient anticoagulation clinics throughout the state of Michigan that includes both academic and community practices; all forms of health insurance are included.18 For our primary analysis, patients were excluded from our study if they had fewer than 3 months of warfarin therapy, experienced a myocardial infarction (MI) within 6 months before initiation of warfarin therapy, and/or had a history of heart valve replacement (mechanical or bioprosthetic). This study was approved by the institutional review board at the University of Michigan, Ann Arbor, and at Henry Ford Hospital, Beaumont Health, Huron Valley Sinai Hospital, Spectrum Health, and Memorial Healthcare before data collection. Because of the nature of the study, a waiver of informed consent was granted by the same institutions as a quality improvement project.
Data were abstracted from the time of patient enrollment in the anticoagulation clinic through the earliest of either discontinuation of warfarin or last follow-up before June 30, 2017. The data collection was performed by trained abstractors using standardized data collection forms (eMethods in the Supplement). Participating clinic staff routinely review all medications (including over-the-counter medicines, such as aspirin) at the time of enrollment in the anticoagulation clinic through 2 complementary mechanisms: (1) a review of the patient’s medical record and (2) a review directly with the patient or the patient’s caregiver. These medications are documented in the anticoagulation clinic encounter notes and subsequently entered by the data abstractors into the MAQI2 registry.
The MAQI2 data registry is designed to require entry to key data elements, including demographic data, INR laboratory data, and details about any adverse event (eg, location of a bleeding event). Through combined use of wide-ranging validation rules during data entry and an automated program that identifies missing information and prompts for completion and correction, there were no missing data in the variables used in the analysis.
Data Collection and Outcome Measures
For each patient, collected data at enrollment included patient demographics, comorbidities, bleeding and thrombosis risk factors, histories of bleeding or thrombosis, and medications (Table). Outcomes for bleeding included any patient-reported bleeding, major bleeding as defined by the International Society on Thrombosis and Hemostasis,19 emergency department (ED) visits for bleeding, and hospitalizations related to bleeding. Thrombotic outcomes included ischemic strokes, transient ischemic attack, VTE, ACS/MI, ED visits, and hospitalizations for thrombosis. Mortality data were also collected. A HAS-BLED20 (hypertension abnormal renal/liver function stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score, modified to exclude aspirin use and labile INR, was calculated for each patient at the time of enrollment. Similarly, a Charlson Comorbidity Index21 value was also calculated. Patients were studied from the time of enrollment to the first event in each category or to the last follow-up if no event occurred.
Table. Characteristics of Patients Receiving Combination Warfarin and Aspirin Therapy vs Warfarin Monotherapy Before and After Propensity Score Matchinga.
| Characteristic | Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Before Matching | After Matching | |||||
| Warfarin and Aspirin (n = 2453) | Warfarin (n = 4086) | P Value | Warfarin and Aspirin (n = 1844) | Warfarin (n = 1844) | P Value | |
| Age, mean (SD), y | 70.3 (12.7) | 63.6 (16.5) | <.001 | 69.2 (13.2) | 69.6 (13.5) | .33 |
| Male sex, No. (%) | 1393 (56.8) | 1933 (47.3) | <.001 | 969 (52.5) | 969 (52.5) | >.99 |
| Weight <50 kg, No./total No. (%) | 42/2377 (1.8) | 90/3903 (2.3) | .15 | 37/1786 (2.1) | 36/1762 (2.0) | >.99 |
| Body mass index >30, No./total No. (%)b | 1093/2321 (47.1) | 1743/3750 (46.5) | .64 | 828/1744 (47.5) | 792/1687 (46.9) | .61 |
| Alcohol or drug use, No. (%) | 113 (4.6) | 190 (4.7) | .94 | 79 (4.3) | 78 (4.2) | >.99 |
| Tobacco use, No. (%) | ||||||
| Former | 888 (36.2) | 1021 (25.0) | <.001 | 583 (31.6) | 591 (32.0) | .80 |
| Current | 147 (6.0) | 358 (8.8) | <.001 | 119 (6.5) | 111 (6.0) | .64 |
| Indication, No. (%) | ||||||
| Atrial fibrillation/atrial flutter | 1629 (66.4) | 1727 (42.3) | <.001 | 1132 (61.4) | 1167 (63.3) | .20 |
| Deep vein thrombosis/pulmonary embolism | 799 (32.6) | 2321 (56.8) | <.001 | 694 (37.6) | 656 (35.6) | .16 |
| Both | 25 (1.0) | 38 (0.9) | .72 | 18 (1.0) | 21 (1.1) | .75 |
| Comorbidities, No. (%) | ||||||
| Antiphospholipid antibody syndrome | 6 (0.2) | 6 (0.1) | .38 | 4 (0.2) | 4 (0.2) | >.99 |
| Coronary artery disease | 976 (39.8) | 471 (11.5) | <.001 | 463 (25.1) | 434 (23.5) | .14 |
| Cancer | 522 (21.3) | 821 (20.1) | .25 | 399 (21.6) | 390 (21.1) | .75 |
| Congestive heart failure | 556 (22.7) | 485 (11.9) | <.001 | 336 (18.2) | 345 (18.7) | .73 |
| Chronic liver disease | 48 (2.0) | 70 (1.7) | .47 | 40 (2.2) | 39 (2.1) | >.99 |
| Chronic kidney disease | 387 (15.8) | 395 (9.7) | <.001 | 249 (13.5) | 241 (13.1) | .73 |
| Diabetes | 759 (30.9) | 819 (20.0) | <.001 | 509 (27.6) | 522 (28.3) | .65 |
| History of falls | 79 (3.2) | 116 (2.8) | .38 | 57 (3.1) | 55 (3.0) | .92 |
| Hypercoagulable state | 54 (2.2) | 137 (3.4) | .01 | 45 (2.4) | 49 (2.7) | .75 |
| Hypertension | 1888 (77.0) | 2341 (57.3) | <.001 | 1347 (73.0) | 1354 (73.4) | .81 |
| Peripheral arterial disease | 221 (9.0) | 89 (2.2) | <.001 | 87 (4.7) | 80 (4.3) | .60 |
| Previous PCI/CABG | 474 (19.3) | 174 (4.3) | <.001 | 189 (10.2) | 167 (9.1) | .17 |
| Seizure disorder | 27 (1.1) | 51 (1.2) | .59 | 23 (1.2) | 20 (1.1) | .76 |
| History of Bleeding or Thrombosis, No. (%) | ||||||
| Duration of bleeding | ||||||
| ≤30 dc | 42 (1.7) | 71 (1.7) | .94 | 35 (1.9) | 33 (1.8) | .90 |
| >30 dc | 46 (1.9) | 81 (2.0) | .76 | 39 (2.1) | 36 (2.0) | .82 |
| Diathesis | 11 (0.4) | 31 (0.8) | .13 | 11 (0.6) | 11 (0.6) | >.99 |
| History of embolism, not DVT/PE | 23 (0.9) | 22 (0.5) | .06 | 14 (0.8) | 17 (0.9) | .72 |
| Previous event | ||||||
| Cerebrovascular accident or transient ischemic attack | 352 (14.3) | 335 (8.2) | <.001 | 243 (13.2) | 245 (13.3) | .96 |
| DVT/PE | 338 (13.8) | 715 (17.5) | <.001 | 277 (15.0) | 288 (15.6) | .65 |
| Gastrointestinal bleed | 116 (4.7) | 150 (3.7) | .04 | 82 (4.4) | 84 (4.6) | .94 |
| Remote myocardial infarction, >6 mo | 339 (13.8) | 154 (3.8) | <.001 | 154 (8.4) | 144 (7.8) | .55 |
| Medications, No. (%) | ||||||
| Angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker | 1134 (46.2) | 1198 (29.3) | <.001 | 765 (41.5) | 788 (42.7) | .44 |
| Aspirin, mg | ||||||
| ≤100 | 2060 (84.0) | NA | NA | 1549 (84.0) | NA | NA |
| >100 | 393 (16.0) | NA | NA | 295 (16.0) | NA | NA |
| β-Blocker | 1566 (63.8) | 1631 (39.9) | <.001 | 1054 (57.2) | 1089 (59.1) | .21 |
| Calcium channel blocker | 641 (26.1) | 825 (20.2) | <.001 | 469 (25.4) | 477 (25.9) | .79 |
| Estrogen or progesterone | 14 (0.6) | 53 (1.3) | .01 | 13 (0.7) | 12 (0.7) | >.99 |
| Heparin, low-molecular-weight heparin, or fondaparinux | 447 (18.2) | 1076 (26.3) | <.001 | 373 (20.2) | 354 (19.2) | .46 |
| Loop diuretic | 679 (27.7) | 680 (16.6) | <.001 | 441 (23.9) | 465 (25.2) | .37 |
| Nonaspirin antiplatelet | 215 (8.8) | 98 (2.4) | <.001 | 87 (4.7) | 87 (4.7) | >.99 |
| Nonsteroidal anti-inflammatory | 107 (4.4) | 155 (3.8) | .26 | 81 (4.4) | 74 (4.0) | .63 |
| Any statin | 1550 (63.2) | 1366 (33.4) | <.001 | 1000 (54.2) | 1005 (54.5) | .87 |
| Time in therapeutic range, mean (SD), [median], %d | 62.3 (18.1) [65.1] | 61.1 (19.3) [63.5] | .01 | 62.6 (18.3) [65.3] | 62.6 (18.6) [65.6] | .71 |
| Follow-up, mean (SD) [median], mo | 23.2 (22.5) [13.5] | 20.2 (21.0) [10.4] | <.001 | 22.7 (22.2) [12.8] | 23.2 (21.9) [14.0] | .27 |
| Modified HAS-BLED score, mean (SD) [median]e | 2.4 (1.2) [2.0] | 1.8 (1.2) [2.0] | <.001 | 2.2 (1.1) [2.0] | 2.2 (1.2) [2.0] | .95 |
| Charlson Comorbidity Index score, mean (SD) [median] | 4.8 (2.0) [5.0] | 3.6 (2.1) [4.0] | <.001 | 4.5 (1.9) [4.0] | 4.5 (1.9) [4.0] | .54 |
Abbreviations: CABG, coronary artery bypass graft; DVT, deep vein thrombosis; HAS-BLED, hypertension abnormal renal/liver function stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly; INR, international normalized ratio; NA, not applicable; PCI, percutaneous coronary intervention; PE, pulmonary embolism; TIA, transient ischemic attack.
If not otherwise specified, denominator is noted at the top of the column.
Calculated as weight in kilograms divided by height in meters squared.
Indicates bleeding history as assessed at the time of warfarin therapy initiation.
Therapeutic range was defined as an INR between 2 and 3.
HAS-BLED score modified to exclude aspirin use and labile INR.
Statistical Analysis
Patients receiving the combination of warfarin and aspirin therapy and patients who were receiving warfarin monotherapy were propensity score matched based on demographics, anticoagulation indications, history of bleeding or thrombosis, medications, TTR, and comorbidities. Outcomes were studied and compared between the 2 matched groups.
The first sensitivity analysis excluded patients who did not maintain their original aspirin use assignments during follow-up. In the second sensitivity analysis, we excluded patients with CAD, peripheral arterial disease, and a history of percutaneous coronary interventions or coronary artery bypass grafting. Our study only collected ACS/MI event data between January 1, 2010, and November 2015. Our third sensitivity analysis only included patients enrolled and followed up for at least 3 months during this nearly 5-year period (eFigure 1 in the Supplement).
The data of patients who met the inclusion criteria were analyzed based on their aspirin use at the time of warfarin initiation (warfarin monotherapy or combination warfarin and aspirin therapy). Demographics, anticoagulation indications, concomitant use of other medications, duration of follow-up, comorbidities, TTR, and history of bleeding or thrombosis were compared between the 2 groups using χ2 tests and Fisher exact tests for binary variables and unpaired, 2-tailed t tests and Wilcoxon rank-sum tests for continuous variables.
Based on all of the above characteristics, propensity scores were generated as a probability of receiving warfarin and aspirin combined therapy through a logistic regression model. The propensity score was used to produce matched warfarin and aspirin combined therapy and warfarin monotherapy groups using a greedy matching technique22 (1:1). A standardized difference of less than 0.1 was used to indicate a negligible difference in the covariates between the groups.23 The complete list of propensity-matched variables before and after matching is provided in eFigure 2 in the Supplement. The individual HAS-BLED and Charlson Comorbidity Index score components were used in the logistic regression model for propensity matching.
Differences between the groups were evaluated using the McNemar tests for binary variables and paired, 2-tailed t tests and Wilcoxon signed rank tests for continuous variables. We then examined the differences using survival analysis between the 2 matched groups for each of several endpoints: any bleeding, major bleeding, ED visits and hospitalizations for bleeding, and thrombosis. To account for competing risk brought by unrelated death, cumulative incidences were used to represent event probabilities and compared with Gray tests. A 2-tailed P < .05 was used to indicate statistical significance. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Of the study cohort of 6539 patients, 3326 (50.9%) were men and the patients had a mean (SD) age of 66.1 (15.5) years. During the study period, 2453 patients (37.5%) were receiving combination warfarin and aspirin therapy without a clear therapeutic indication for receiving aspirin compared with 4086 (62.5%) receiving warfarin monotherapy. Use of warfarin and aspirin therapy did not change significantly during the study period (range, 33.1% to 40.7% of patients) (eFigure 3 in the Supplement). Low-dose aspirin (≤100 mg daily) was used by 2060 patients (84.0%) receiving combination warfarin and aspirin therapy. The 2 groups differed in their demographics, indication for anticoagulation, comorbidities, concomitant medications, and TTR (Table). After propensity score matching, there were no significant differences between the 2 matched groups (Table and eFigure 2 in the Supplement).
In the propensity score–matched groups, patients treated with combination warfarin and aspirin therapy experienced significantly more overall bleeding events compared with those receiving warfarin monotherapy (cumulative incidence at 1 year: 26.0% [95% CI, 23.8%-28.3%] vs 20.3% [95% CI, 18.3%-22.3%]; P < .001) (eFigure 4 in the Supplement). The increased risk of bleeding persisted for patients throughout most of the follow-up period. The number of major bleeding events was also higher for the combination warfarin and aspirin therapy group, with a cumulative incidence of 5.7% (95% CI, 4.6%-7.1%) at 1 year compared with 3.3% (95% CI, 2.4%-4.3%) at 1 year for patients receiving warfarin alone (P < .001) (Figure 1). At 1 year, ED visits related to bleeding were higher for the combination warfarin and aspirin group (13.3%; 95% CI, 11.6%-15.1%) compared with the warfarin monotherapy group (9.8%; 95% CI, 8.4%-11.4%) (eFigure 5 in the Supplement). Similarly, hospital admissions related to bleeding were higher for the combination warfarin and aspirin group compared with the warfarin monotherapy group (8.1%; 95% CI, 6.8%-9.6% vs 5.2%; 95% CI, 4.1%-6.4%) (P = .001 for each comparison) (Figure 2). These findings also persisted during most of the follow-up period.
Figure 1. Cumulative Incidence of Major Bleeding by Treatment.
Figure 2. Cumulative Incidence of Hospital Admissions or Bleeding by Treatment.
While receiving combination warfarin and aspirin therapy, 2.3% of patients (95% CI, 1.6%-3.1%) had a thrombotic event at 1 year compared with 2.7% of patients (95% CI, 2.0%-3.6%) receiving warfarin alone (P = .40) (Figure 3). At 1 year, 1.8% (95% CI, 1.2%-2.6%) of combination warfarin and aspirin–treated patients had an ED visit related to thrombosis compared with 1.9% (95% CI, 1.3%-2.7%) of patients receiving warfarin alone (P = .84). The cumulative incidence of thrombosis-related admissions for patients receiving combination warfarin and aspirin therapy was 2.1% (95% CI, 1.4%-2.9%) vs 2.1% (95% CI, 1.5%-3.0%) for patients receiving warfarin therapy alone (P = .96).
Figure 3. Cumulative Incidence of Thrombosis by Treatment.
Mortality rates for the 2 groups were similar at 1 year as well (4.4%; 95% CI, 3.4%-5.6% for combination warfarin and aspirin therapy vs 3.7%; 95% CI, 2.8%-4.8% for warfarin monotherapy; P = .34). The type of thrombotic event (ischemic stroke, transient ischemic attack, deep vein thrombosis, pulmonary embolism, and other including left atrial thrombus, valvular thrombus, or thromboembolism of unknown origin) or the rate of central nervous system bleeding did not significantly differ between the 2 groups.
Sensitivity Analysis
In sensitivity analyses, the above findings were largely unchanged. In the first sensitivity analysis (eliminating patients who initiated or discontinued aspirin therapy during follow-up), all of the above findings were unchanged, except the category of major bleeding no longer maintained statistical significance (eTable 1 in the Supplement). In the second sensitivity analysis (eliminating patients with any history of MI, CAD, peripheral arterial disease, percutaneous coronary interventions, or coronary artery bypass grafting) and the third sensitivity analysis (only including patients from 2010-2015, when ACS/MI data were collected), there was no change in our study findings (eTables 2 and 3 in the Supplement).
In further sensitivity analyses, we excluded patients with a history of stroke, transient ischemic attack, heart valve replacement, or recent MI. The results were largely unchanged from the primary analysis. Although the incidence of major bleeding events was higher for patients receiving combination warfarin and aspirin therapy compared with those receiving warfarin monotherapy at 1 year (5.1% vs 3.2%, P = .04), the incidence did not remain significantly increased for the duration of the study period. The sample size was smaller for this analysis, with 1392 patients in each group.
Discussion
In this large, registry-based cohort study of patients followed up at anticoagulation clinics while receiving warfarin therapy for atrial fibrillation and/or VTE without a clear therapeutic indication for receiving aspirin, 37.5% of patients received aspirin therapy. Treatment with combination warfarin and aspirin therapy compared with warfarin monotherapy was associated with a significant increase in bleeding, major bleeding, ED visits, and hospitalizations. These results persisted for at least 3 years of follow-up, without any observed added benefit regarding thrombotic events.
The rates of combination warfarin and aspirin use did not significantly change over time, reflecting the limited data available to guide clinical decisions for this patient population. The publication of the 2012 American College of Chest Physicians guidelines10 about the management of atrial fibrillation did not seem to have any influence regarding aspirin use in this population. The observed rate of aspirin use among patients without clear therapeutic indication is consistent with what has been reported previously.16,17,24 Often, when patients are receiving aspirin for primary prevention of CAD, the medication is not discontinued when patients initiate warfarin therapy for newly diagnosed AF or VTE. This regimen could be, in part, explained by the number of clinicians from the different medical specialists involved in antithrombotic medication management (primary care, general cardiology, interventional cardiology, electrophysiology, hematology, and/or vascular surgery). Patients with substantial vascular or thrombotic risk factors may be intentionally maintained on combination warfarin and aspirin therapy. Prospective studies are needed, especially for high-risk patients, to determine the optimal patient selection for and duration of combination therapy.
Potential need of patients to require multiple antithrombotic medications is a common clinical scenario. Registry data in a US population suggest that, through 2011, approximately 35% of patients with AF are receiving combination therapy with oral anticoagulation and aspirin; 39% of those patients have no known atherosclerotic disease.24 In more recent clinical trials, 21% to 35% of patients with AF were receiving aspirin with oral anticoagulation.25 A recently published, retrospective medical record review of 219 patients receiving oral anticoagulation with aspirin, without recent ACS or revascularization, similarly found that 22.8% of patients had no apparent therapeutic indication for combination therapy.16 Approximately 30% of this group reported CAD or diabetes for which they were receiving aspirin therapy that was not discontinued when warfarin was prescribed for AF. It is estimated that there are more than 800 000 patients in North America receiving combination warfarin and aspirin therapy for AF17; considering the additional number of patients treated for VTE, this is a substantial number of patients currently receiving combination therapy. Our nonrandomized data suggested that the number needed to harm26,27 may only have been 36 persons for major bleeding and 29 persons for hospital admission among patients receiving combination warfarin and aspirin therapy compared with those receiving warfarin monotherapy. An intervention targeted at reducing aspirin use in this population could have profound public health impact if these estimates are confirmed in prospective studies.
Antiplatelet therapy use is dynamic for many patients. The clinicians at the anticoagulation clinics in this study routinely assessed the use of antiplatelet medications, which was a notable strength of this study. Removing matched patients who initiated or discontinued aspirin therapy during the study period in our first sensitivity analysis gave very similar results (eTable 1 in the Supplement). Major bleeding was significantly different only for the first year but not for the duration of follow-up. Differences in bleeding after the first year may have been affected by bleeding events during the first year or by the lower power of the study to detect a difference. Our second sensitivity analysis focused on patients without atherosclerotic comorbidity found higher bleeding complications with combination warfarin and aspirin therapy compared with warfarin monotherapy.
The observed rate of any bleeding reported in our study was high compared with other studies, resulting from the thoroughness of our manual data collection. Any bleeding in this study was by patient report only. The WOEST (What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting) trial28 showed comparable bleeding outcomes for oral anticoagulation with clopidogrel. The rate of major bleeding events for unmatched patients (eTable 4 in the Supplement) is consistent with the published literature.15,16,24,28,29,30,31,32
Our study results may advance the field, with a long duration of follow-up in a real-world setting. The detailed propensity score match of numerous variables significantly limited potential confounders. Furthermore, sensitivity analyses showing similar results increased confidence in our results. Our results were supported by the findings of similar studies about this topic. For example, Steinberg et al24 found similar results in a population of patients with AF using the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. That study differed because it included dabigatran (6.9% of patients) but excluded patients with atrial flutter and VTE. In addition, their results excluded 11% of patients lost to follow-up and had a shorter duration of follow-up (3 months). In 2008, Johnson et al33 published a similar study through Kaiser Permanente Colorado Anticoagulation Service with 6 months of follow-up. In that study of more than 4000 patients, combination therapy was similarly associated with increased bleeding events but not with coronary events after adjustment for confounding factors. Our study results, a decade later, were striking because excess aspirin use among patients treated with warfarin continued at high rates.
Strengths and Limitations
Whereas this study did not randomize aspirin use between the 2 groups, strong propensity score matching using numerous covariates enabled us to reduce selection biases and allowed for the comparison of 2 well-matched groups that would mimic a randomized clinical trial, as closely as a nonrandomized study design will allow. While our propensity score matching was comprehensive for potential confounding variables, it is always possible that some unknown confounders were present that could have influenced the results (eFigure 2 in the Supplement). In addition, our analysis did not adjust for the severity of the comorbid conditions, and it was conceivable that a patient with, for example, uncontrolled diabetes or hypertension, may have benefited more from the addition of aspirin therapy than a patient with well-managed comorbid conditions.
Strengths of the study were that patients were closely followed up while enrolled in the anticoagulation clinics, with data collected using predefined forms that included relevant clinical outcomes. Random medical record audits validated the accuracy of the data. The population-based cohort may have been more likely to reflect real-world practice compared with patients in a clinical trial. Despite being part of a quality improvement collaborative, all patients received standard anticoagulation care, and their findings likely are generalizable to other settings (including anticoagulation clinics and high-quality care outside of anticoagulation clinics). By controlling for warfarin management through the use of the TTR variable, we tried to limit variation between groups to the receiving of aspirin alone. We caution against extrapolating these findings to patients receiving direct oral anticoagulants with aspirin because the outcomes may be significantly different.
Potential limitations to the study included issues surrounding generalizability because of the geographically limited patient population followed up at the anticoagulation clinics. In addition, any medication changes, especially nonprescription aspirin use, may not have been well captured. However, given that data in this registry were abstracted from the medical record and that nurses and pharmacists in the anticoagulation clinic routinely reviewed any new or changed medications (especially antithrombotic medications, such as aspirin), we believe our findings are reliable and robust. This belief is supported by the results of the sensitivity analysis, in which patients with a known change in aspirin-use status were excluded (eTable 1 in the Supplement).
Recent MI, an exclusion criterion for the study, was defined on the MAQI2 data collection form as an event that occurred fewer than 6 months before warfarin therapy initiation. Therefore, a low number of included patients may have been within a year of having a MI and had a stronger indication for concomitant aspirin use. We anticipated this circumstance to represent less than 5% of our study population based on the number of patients receiving triple therapy with another nonaspirin antiplatelet agent. A total of 250 patients (3.5%) from our registry were excluded for having a recent MI (within 6 months). Excluding any patient with an MI or CAD (sensitivity analysis reported in eTable 2 in the Supplement) did not change our study findings.
The outcome of ACS/MI was assessed by evaluating which patients had an ED visit or a hospital admission for ACS/MI (eTable 3 in the Supplement). Given that such patients would likely receive inpatient care, this measure seemed appropriate. However, data for this outcome were only available for a portion of the study period and may not have been as well captured. Abstraction into the registry was limited to the data collected in the medical record at each anticoagulation clinic. Emergency department visits at outside hospitals that were not documented by the anticoagulation clinic would not be captured in our registry. Therefore, the observed event rates may have underestimated true event rates if patients received care outside of our included facilities. Finally, there were few thrombotic events (stroke, transient ischemic attack, or VTE) during the study period, limiting our power to detect differences for this outcome.
Conclusions
This study suggests that concurrent use of warfarin and aspirin therapy in patients without a heart valve replacement or recent ACS is associated with an increased risk of bleeding and related hospitalization. Similar to other studies published for more than a decade, we did not observe any clinical benefit of aspirin being prescribed with warfarin therapy. Unfortunately, the rate of combined warfarin and aspirin use has not declined as a result of those findings, emphasizing the need for greater awareness of this issue and efforts to discontinue aspirin therapy in these patients, especially low-risk patients. Further research is needed to help clinicians better stratify which patients should receive combination warfarin and aspirin therapy instead of warfarin monotherapy for VTE or AF.
eMethods. Supplemental Methods
eTable 1. Sensitivity Analysis Limiting Cohort to Patients Continuously on ASA or Not on ASA Throughout Follow-up
eTable 2. Sensitivity Analysis Limiting Cohort to Patients Without a History of CAD, CABG, PAD, or PCI, With ASA Use Defined at Enrollment
eTable 3. Sensitivity Analysis Limiting Cohort to Patients With Data on ACS/MI
eTable 4. Outcomes of Unmatched Patient Cohorts
eFigure 1. Study Schema
eFigure 2. Standardized Difference Plot
eFigure 3. Percent of Patients Without Recent Myocardial Infarction or Valve Replacement on Aspirin and Warfarin by Year
eFigure 4. Cumulative Incidence of Any Bleeding Over Time by Treatment
eFigure 5. Cumulative Incidence of ER Visits for Bleeding Over Time by Treatment
References
- 1.Marcy TR, Truong T, Rai A. Comparing direct oral anticoagulants and warfarin for atrial fibrillation, venous thromboembolism, and mechanical heart valves. Consult Pharm. 2015;30(11):644-656. doi: 10.4140/TCP.n.2015.644 [DOI] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K; US Preventive Services Task Force . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 3.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e637S-e668S. doi: 10.1378/chest.11-2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):3097-3137. doi: 10.1161/CIR.0b013e3182776f83 [DOI] [PubMed] [Google Scholar]
- 5.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Circulation. 2017;135(12):e686-e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Coello P, Bellmunt S, McGorrian C, et al. Antithrombotic therapy in peripheral artery disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e669S-e690S. doi: 10.1378/chest.11-2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 8.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e601S-e636S. doi: 10.1378/chest.11-2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. Circulation. 2013;127(4):e362-e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 10.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e531S-e575S. doi: 10.1378/chest.11-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura RA, Otto CM, Bonow RO, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease [published correction appears in Circulation. 2014;130(13):e120]. Circulation. 2014;129(23):e521-e643. doi: 10.1161/CIR.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 12.Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e576S-e600S. doi: 10.1378/chest.11-2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients With valvular heart disease. Circulation. 2017;135(25):e1159-e1195. doi: 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 14.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 15.Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med. 2007;167(2):117-124. doi: 10.1001/archinte.167.2.117 [DOI] [PubMed] [Google Scholar]
- 16.So CH, Eckman MH. Combined aspirin and anticoagulant therapy in patients with atrial fibrillation. J Thromb Thrombolysis. 2017;43(1):7-17. doi: 10.1007/s11239-016-1425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douketis JD. Combination warfarin-ASA therapy: which patients should receive it, which patients should not, and why? Thromb Res. 2011;127(6):513-517. doi: 10.1016/j.thromres.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 18.Barnes GD, Kline-Rogers E. Engaging with quality improvement in anticoagulation management. J Thromb Thrombolysis. 2015;39(3):403-409. doi: 10.1007/s11239-015-1184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 20.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile inr, elderly, drugs/alcohol concomitantly) score. J Am Coll Cardiol. 2011;57(2):173-180. doi: 10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg BA, Kim S, Piccini JP, et al. ; ORBIT-AF Investigators and Patients . Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation. Circulation. 2013;128(7):721-728. doi: 10.1161/CIRCULATIONAHA.113.002927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer JK, McBane RD, Wysokinski WE. How to choose appropriate direct oral anticoagulant for patient with nonvalvular atrial fibrillation. Ann Hematol. 2016;95(3):437-449. doi: 10.1007/s00277-015-2566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Laupacis A. A tutorial on methods to estimating clinically and policy-meaningful measures of treatment effects in prospective observational studies: a review. Int J Biostat. 2011;7(1):6. doi: 10.2202/1557-4679.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender R, Blettner M. Calculating the “number needed to be exposed” with adjustment for confounding variables in epidemiological studies. J Clin Epidemiol. 2002;55(5):525-530. doi: 10.1016/S0895-4356(01)00510-8 [DOI] [PubMed] [Google Scholar]
- 28.Dewilde WJ, Oirbans T, Verheugt FW, et al. ; WOEST Study Investigators . Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention. Lancet. 2013;381(9872):1107-1115. doi: 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 29.Lamberts M, Gislason GH, Lip GY, et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant. Circulation. 2014;129(15):1577-1585. doi: 10.1161/CIRCULATIONAHA.113.004834 [DOI] [PubMed] [Google Scholar]
- 30.Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170(16):1433-1441. doi: 10.1001/archinternmed.2010.271 [DOI] [PubMed] [Google Scholar]
- 31.Hamon M, Lemesle G, Tricot O, et al. Incidence, source, determinants, and prognostic impact of major bleeding in outpatients with stable coronary artery disease. J Am Coll Cardiol. 2014;64(14):1430-1436. doi: 10.1016/j.jacc.2014.07.957 [DOI] [PubMed] [Google Scholar]
- 32.Flaker GC, Gruber M, Connolly SJ, et al. ; SPORTIF Investigators . Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J. 2006;152(5):967-973. doi: 10.1016/j.ahj.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 33.Johnson SG, Rogers K, Delate T, Witt DM. Outcomes associated with combined antiplatelet and anticoagulant therapy. Chest. 2008;133(4):948-954. doi: 10.1378/chest.07-2627 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Sensitivity Analysis Limiting Cohort to Patients Continuously on ASA or Not on ASA Throughout Follow-up
eTable 2. Sensitivity Analysis Limiting Cohort to Patients Without a History of CAD, CABG, PAD, or PCI, With ASA Use Defined at Enrollment
eTable 3. Sensitivity Analysis Limiting Cohort to Patients With Data on ACS/MI
eTable 4. Outcomes of Unmatched Patient Cohorts
eFigure 1. Study Schema
eFigure 2. Standardized Difference Plot
eFigure 3. Percent of Patients Without Recent Myocardial Infarction or Valve Replacement on Aspirin and Warfarin by Year
eFigure 4. Cumulative Incidence of Any Bleeding Over Time by Treatment
eFigure 5. Cumulative Incidence of ER Visits for Bleeding Over Time by Treatment