Abstract
Background
As a member of short-chain fatty acids, acetate exhibits anti-inflammatory capacity. The present study aimed to investigate the effect of acetate on lipopolysaccharide (LPS)-induced acute lung injury (ALI) and explored its underlying mechanism.
Material/Methods
Acetate (250 mM, 400 μL) was given intraperitoneally 30 minutes after LPS (5 mg/kg) intratracheal injection. Lung tissues and bronchoalveolar lavage fluid (BALF) were collected 6 hours after the challenge of LPS. The histopathology scores, wet-to-dry weight ratios, protein content, and cytokine levels in BALF were assessed.
Results
The acetate treatment resulted in improved lung pathological score, alleviated LPS-induced microvascular permeability, and suppressed the production of reactive oxygen species. Furthermore, acetate decreased the level of pro-inflammatory cytokines and chemokines in the lungs and BALF, consistent with the declined immune cell counting found in BALF. In addition, phosphorylation levels of mitogen-activated protein kinase (MAPK) pathway in lung tissues were downregulated by acetate.
Conclusions
These results suggested that acetate exerts its protective effects via anti-inflammatory and anti-oxidant activities on LPS-induced ALI.
MeSH Keywords: Acute Lung Injury, Inflammation, Lipopolysaccharides, Mitogen-Activated Protein Kinase
Background
Acute respiratory distress syndrome or acute lung injury (ALI) is a progressive clinical syndrome defined by acute hypoxemic respiratory failure with bilateral pulmonary infiltrates consistent with edema [1]. Despite progress in the early diagnosis and supportive therapy, mortality rates for patients with ALI remains high [2], and there is an urgent need for extensive research on its pathogenesis and management. The pathogenesis of ALI is associated with excessive inflammatory response, inflammatory cell accumulation, cytotoxic mediators release, pulmonary vasculature permeability increase, alveolar capillary barrier dysfunction, and oxidative damage [3,4]. As an outer membrane component of gram-negative bacteria, lipopolysaccharide (LPS) is known to induce the influx of neutrophils and macrophages, and induce the release of pro-inflammatory cytokines in lungs [5,6]. Therefore, LPS-induced ALI is the most widely used animal model of ALI [7].
Acetate is a metabolite that anaerobic bacteria release into the lumen of the gut, which is then absorbed by intestinal epithelial cells and distributed to the peripheral capillaries [8]. In recent years, it was found that acetate can play a crucial role in regulating cell signaling pathways in the immune system [9–11]. In germ-free mouse models of diseases, aggravation of the inflammatory response was found to be partly associated with a lack of gut microbiotas that generated acetate [12]. In addition, the anti-inflammatory effects of acetate have been extensively studies in inflammatory bowel diseases [13]. In a rat model of neuroinflammation, acetate administration was found to attenuate LPS-induced neuroglia activation and inhibit immunoreactivity [14]. In a model of head trauma, acetate also showed neuroprotective effects [15]. However, whether acetate regulates LPS-induced ALI in mice remains unclear. Therefore, the aim of our study was to explore the regulatory effect of acetate on inflammation in LPS-induced ALI in mice.
In this study, we evaluated the effects of acetate on LPS-induced ALI and investigated the underlying mechanism. The histopathology change, pulmonary vasculature permeability, immune cells counting, and inflammatory cytokine levels in lungs and bronchoalveolar lavage fluid (BALF), as well as oxidative stress damage, were assessed.
Material and Methods
Mice
Healthy male C57BL/6 mice (8–10 weeks old, weight 20–25 g) were obtained from Shanghai SLAC Laboratory Animals Center (Shanghai, China). Mice were housed in a 12-hour light-dark cycle environment, with free access to water and food. The animal experiments were approved by the Scientific Investigation Board of Naval Medical University.
Animal model
To establish the ALI model, mice were randomly assigned to one of 4 groups (in each group, n=10). The groups were: 1) the control group, 2) the acetate group, 3) the LPS group, and 4) the LPS and acetate group. Mice were anesthetized with sevoflurane and the trachea was exposed by ophthalmic scissors and forceps. Then, LPS (Escherichia coli O111: B4; Sigma; 5 mg/kg) was injected into the trachea using 100 μL micro syringe to induce ALI [7]. Mice in the control group and the acetate group received equal volume of phosphate buffer saline (PBS). Then, all mice were placed in cages and kept breathing spontaneously. Thirty minutes after the challenge of LPS or PBS, mice were intraperitoneally injected with PBS or acetate 400 μL (250 mM). In previous research, rats were given intra-arterial infusions of acetate at 20 μmol/kg/minute for 2 hours (total dosage 2.4 mmol/kg) [16]. Therefore, we used the dosage of acetate at 4 mmol/kg based on the guidance of dose conversion between species [17]. Increased volume load could aggravate the severity of lung injury. To minimize this influence, we adjusted the volume to 400 μL by intraperitoneal injection. Six hours after LPS administration, the mice were sacrificed by CO2 inhalation, and then lung tissue and BALF were obtained.
Histopathology
Left lung samples were harvested 6 hours after LPS treatment, infused with 4% paraformaldehyde solution for at least 48 hours, then paraffin embedded. Sections of 4–5 μm thickness were stained with hematoxylin and eosin; the severity of lung injury was assessed as previously described [18]. The severity of lung injury was scored based on 4 items: alveolar congestion, hemorrhage, neutrophil infiltration in alveolar cavity, and thickness of alveolar wall. Each item was classified into 5 grades depending on the severity of damage: 0 for minimal damage, 1 for mild damage, 2 for moderate damage, 3 for severe damage, and 4 for maximal damage.
Lung wet-to-dry (W/D) weight ratio
Right lungs were excised 6 hours after treatment with LPS, and wet weights were recorded. Then the lung lobes were placed in an oven at 80°C for 72 hours to obtain dry weights. Lung wet/dry (W/D) weight ratios were calculated to assess lung edema.
Myeloperoxidase (MPO), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) measurements
The left lobe of the lung of each mouse was weighed and then homogenized to a 10% suspension. The homogenates were centrifuged at 12 000 rpm for 10 minutes at 4°C, and supernatants were collected for further analysis. Myeloperoxidase (MPO), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) measurements in lung tissue samples were measured with specific commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). MPO activity, MDA level, SOD activity, and GSH-Px activity were expressed as unit/g of wet tissue, nmol/mg protein, unit/mg, and unit, respectively.
Evans blue staining of lung samples
Evans blue accumulation in the lung tissue was evaluated as described previously [19]. In brief, mice were injected with 25 mg/kg Evans blue via the tail vein 6 hours after LPS administration; then 2 hours later, the lungs were perfused with PBS containing heparin. Then, the left lobe of the lung was excised, weighed, and homogenized. Evans blue in the lung tissue was extracted and quantitated by spectrophotometry.
Bronchoalveolar lavage fluid (BALF) analysis
Mice were sacrificed 6 hours after LPS treatment. BALF samples were obtained by intratracheal lavage with 1 mL ice-cold PBS. Then the fluid samples were centrifuged at 1500 rpm for 10 minutes. Cells in BALF samples were collected and stained with anti-F4/80-FITC (eBioscience, San Jose, CA, USA) to detect macrophages or incubated with anti-Ly6G-PE (eBioscience, San Jose, CA, USA) and anti-CD11b-APC (eBioscience, San Jose, CA, USA) for neutrophil detection by flow cytometry. Flow cytometry data was acquired on a FACS Canto II flow cytometer (BD Bioscience, San Jose, CA, USA) and analyzed with the FlowJo software, version 7.6.1 (Tree Star, Ashland, OR, USA). Tumor necrosis factor (TNF)-α, interleukin (IL)-1b, and IL-6 levels in BALF were measured by enzyme-linked immunosorbent assay (ELISA) (R&D system, Minneapolis, MN, USA) according to the manufacturer’s instructions. Total protein concentration in the supernatant was measured using the bicinchoninic acid (BCA) assay method (Thermo Scientific, IL, USA). Lactate dehydrogenase (LDH) activity in BALF was detected using the LDH Cytotoxicity Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Real-time polymerase chain reaction (PCR)
RNA in the right lower lobe was extracted with TRIzol reagent (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. Reverse-transcription was performed with a PrimeScript RT reagent kit (Takara Biotechnology, Dalian, China). Real-time polymerase chain reaction (PCR) was performed using SYBR green master mix (Takara Biotechnology, Dalian, China) on a 7900 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). The primers used for real-time PCR are shown in Table 1.
Table 1.
The primers for real-time PCR.
| Primer name | Forward | Reverse |
|---|---|---|
| TNF-α | AATGGCCTCCCTCTCATCAG | CCCTTGAAGAGAACCTGGGA |
| IL-6 | TACCACTCCCAACAGACCTG | GGTACTCCAGAAGACCAGAGG |
| IL-1β | CTTCAGGCAGGCAGTATCACTC | TTGTTGTTCATCTCGGAGCC |
| CXCL-1 | GCTGGGATTCACCTCAAGAA | TGGGGACACCTTTTAGCATC |
| CCL-2 | CTCTTCCTCCACCACCAT | CTCTCCAGCCTACTCATTG |
| COX-2 | GTGTAAGAGGCTGGGAGTGC | CTGACTCTGCGGAAGTCTCC |
| B2M | CGGCCTGTATGCTATCCAGA | GGGTGAATTCAGTGTGAGCC |
Western blot analysis
Six hours after the LPS challenge, the right upper lobe of the lung was homogenized in protein extract solution and protease inhibitors (Beyotime Biotechnology, Shanghai, China). Then, the protein concentration was evaluated by the BCA protein assay kit (Thermo Scientific, IL, USA). Equal amounts of protein were loaded on a 10% SDS-polyacrylamide gel and transferred into a polyvinylidene difluoride membrane. Then, the membranes were blocked with 5% BSA (bovine serum albumin) and incubated with antibodies. Protein bands were demonstrated by enhanced chemiluminescence western blot kit (Thermo Scientific, IL, USA).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Two-tailed student’s t-test and one-way analysis of variance (ANOVA) were used for comparisons. Data are presented as mean ± standard error measurement (SEM), and P<0.05 was considered statistically significant.
Results
Acetate ameliorated LPS-mediated histopathologic changes in the lung
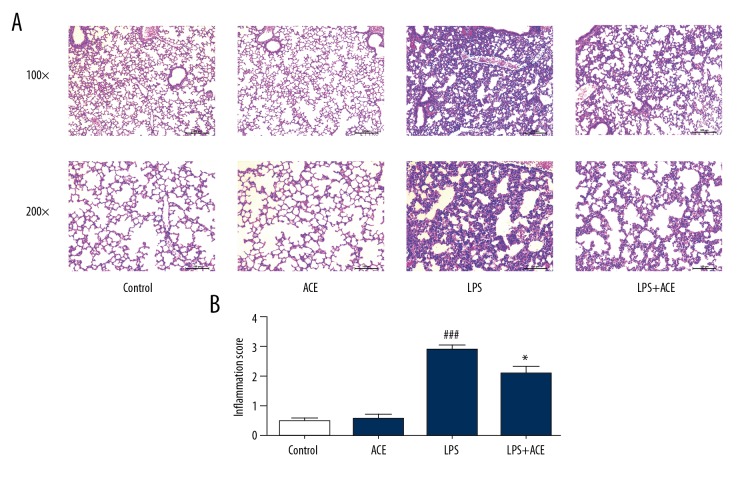
In the present study, we assessed whether acetate could alleviate pathological damage to the lung in LPS-induced ALI. Six hours after LPS challenge, mice were sacrificed by CO2 inhalation, and lung tissues were harvested for hematoxylin and eosin staining. In the LPS-treated group, inflammatory responses, including interstitial edema, thickened pulmonary septum, and inflammatory cell infiltration were observed microscopically. These pathological changes in acetate-treated mice were significantly attenuated (Figure 1). These findings indicated that acetate could ameliorate histopathological damage due to LPS-induced lung injury in mice.
Figure 1.
Acetate attenuates LPS-induced lung histopathologic changes. (A) Histological section of the lungs was stained by hematoxylin and eosin staining, magnification (100× and 200×). The severity of lung injury is manifested as alveolar congestion, hemorrhage, neutrophil infiltration in alveolar cavity, and thickness of alveolar wall. (B) The lung injury scores. The values presented are mean ±SEM (n=10), ### P<0.001 versus the control group; * P<0.05 versus the LPS-treated group. LPS – lipopolysaccharide; SEM – standard error measurement.
Acetate inhibited the permeability of the alveolar-capillary barrier in LPS-induced ALI
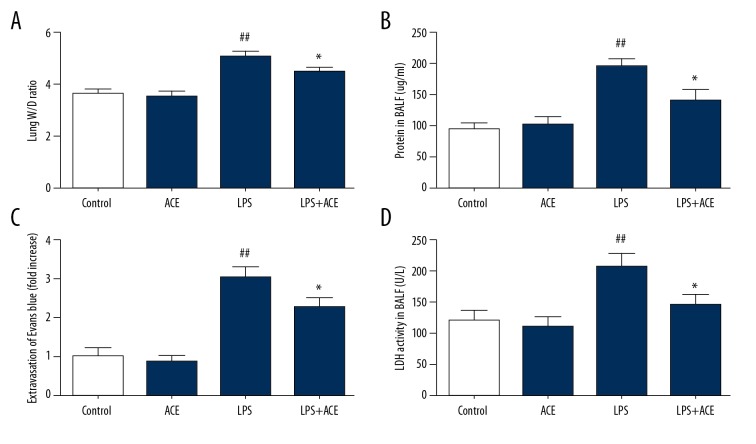
The lung W/D weight ratio, Evans blue leakage in lung tissues, and total protein concentration in BALF are used in studies to reflect pulmonary edema and the dysregulation of alveolar-capillary barrier function [20,21]. As shown in Figure 2A and 2B, in our study, acetate alleviated the increased lung W/D weight ratio and protein concentration in BALF. Evans blue dye was used to assess pulmonary vascular permeability. Compared with the LPS-treated group, quantitative analysis of Evans blue leakage from the vessels into lung tissues in our study showed lower dye accumulation in the acetate-treated animals (Figure 2C). LDH activity in BALF can reflect the severity of lung damage [7]. As shown in Figure 2D, in our study, LDH activity was remarkably increased after LPS treatment, and alleviated by acetate administration. Taken together, our study data suggested that acetate could ameliorate alveolar-capillary barrier damage in ALI.
Figure 2.
Acetate alleviates the damage of lungs induced by LPS. (A) Lung tissues were weighed and calculated the W/D weight ratio. (B) Protein concentration in BALF was measured. (C) Spectrophotometric analysis of Evans blue dye in lung tissues was quantified. (D) LDH activity in BALF was determined to assess lung damage. The values presented are mean ±SEM (n=10), ## P<0.01 versus the control group; * P<0.05 versus the LPS-treated group. LPS – lipopolysaccharide; W/D – wet/dry; BALF – bronchoalveolar lavage fluid; LDH – lactate dehydrogenase; SEM – standard error measurement.
Acetate reduced inflammatory cell accumulation in LPS-induced ALI
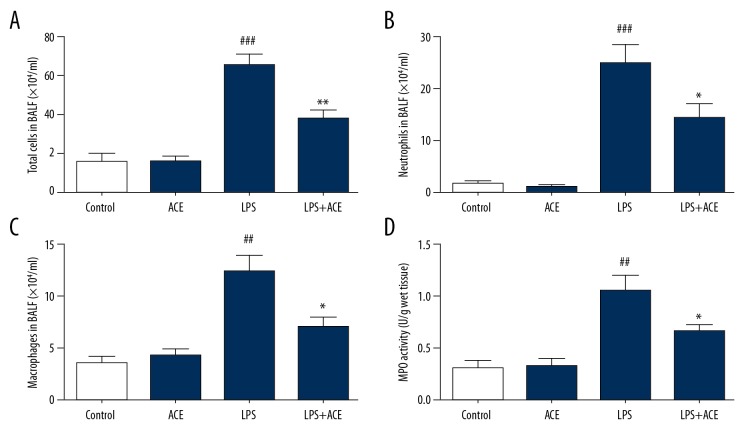
LPS instilled intratracheally and alveolar-capillary barrier disruption could induce the recruitment of inflammatory cells, especially neutrophils. The migration of neutrophil is one of the core mechanism in ALI. These cells release pro-inflammatory cytokines which also damage the alveolar-capillary barrier and aggravate lung injury [22]. Therefore, in our study, we assessed whether acetate played a role in LPS-induced inflammatory cell infiltration. As shown in Figure 3A–3C, LPS increased the count of total cells, neutrophils, and macrophages in BALF, and these cell counts were reduced dramatically in mice administered with acetate. To further explore the effect of acetate on cell infiltration, we assessed MPO activity; the results showed that administration of acetate reduced MPO activity in LPS-induced ALI (Figure 3D). These results indicated that acetate inhibited inflammatory cell recruitment in ALI.
Figure 3.
Acetate affects inflammatory cells and MPO activity in LPS-induced ALI: (A) total cells, (B) neutrophils, and (C) alveolar macrophages were detected by flow cytometry in BALF. (D) MPO activity was measured in lung homogenates. The values presented are mean ±SEM (n=10), ## P<0.01, ### P<0.001 versus the control group; * P<0.05, ** P<0.01 versus LPS-treated group. MPO – myeloperoxidase; LPS – lipopolysaccharide; ALI – acute lung injury; BALF – bronchoalveolar lavage fluid; SEM – standard error measurement.
Acetate reduced LPS-induced inflammatory cytokines
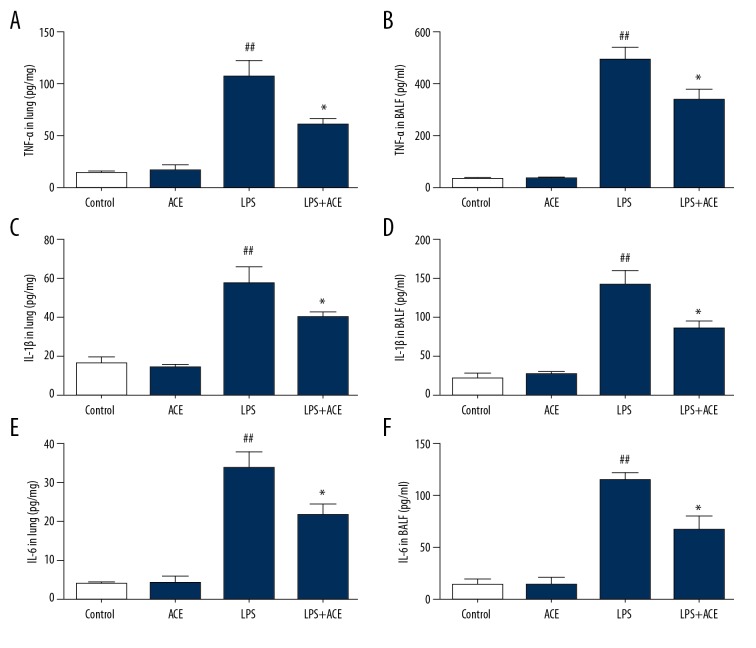
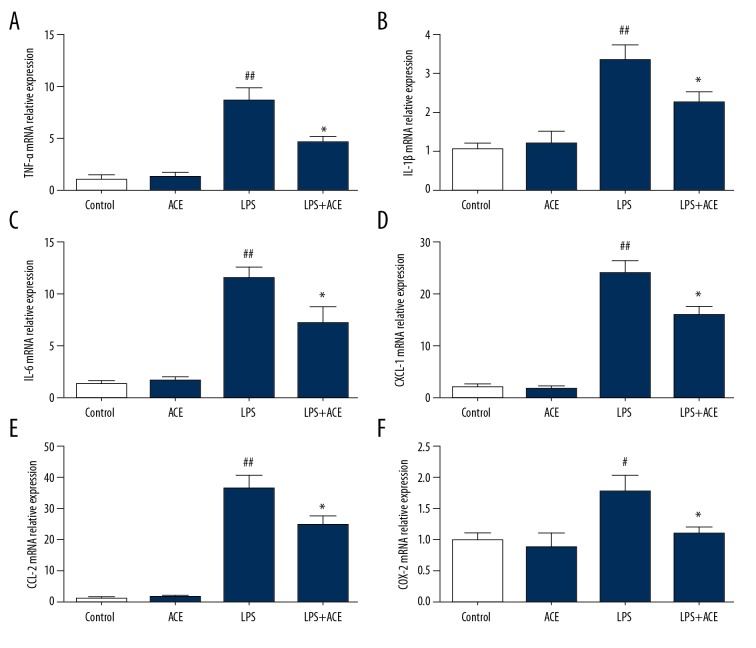
Next, we assessed the production of cytokines in lung tissue and BALF. The results showed that LPS significantly enhanced TNF-α, IL-1b, and IL-6 levels in lungs and BALF. These cytokines were significantly reduced by treatment with acetate (Figure 4); however, the production of IL-10 was not affected by acetate (Supplementary Figure 1). Subsequently, the mRNA levels of pro-inflammatory cytokines in lung tissues were detected to further evaluate the anti-inflammatory effects of acetate. The mRNA levels of TNF-α, IL-1b, and IL-6 were significantly increased in the LPS group, and this effect was inhibited by acetate administration (Figure 5A–5C). We also found that acetate could reduce the mRNA expression of chemokines (CXCL-1, CCL-2, and COX-2) induced by LPS (Figure 5D–5F). These findings indicated that acetate suppressed the production of inflammatory mediators in LPS-induced ALI.
Figure 4.
Acetate suppresses inflammatory cytokines in lung tissues and BALF; (A, B) TNF-α, (C, D) IL-1b, and (E, F) IL-6 were measured in lung tissues and BALF. The values presented are mean ±SEM (n=10), ## P<0.01 versus the control group; * P<0.05 versus the LPS-treated group. BALF – bronchoalveolar lavage fluid; TNF – tumor necrosis factor; IL – interleukin; SEM – standard error measurement; LPS – lipopolysaccharide.
Figure 5.
Acetate reduces cytokine mRNA expressions in lung tissues in LPS-induced ALI: (A) TNF-α, (B) IL-1b, (C) IL-6, (D) CXCL-1, (E) CCL-2, and (F) COX-2. The values presented are mean ±SEM (n=10), # P<0.05, ## P<0.01 versus the control group; *P < 0.05 versus the LPS-treated group. LPS – lipopolysaccharide, ALI – acute lung injury; TNF – tumor necrosis factor; IL – interleukin; SEM – standard error measurement.
Acetate inhibited oxidative stress in LPS-induced lung injury
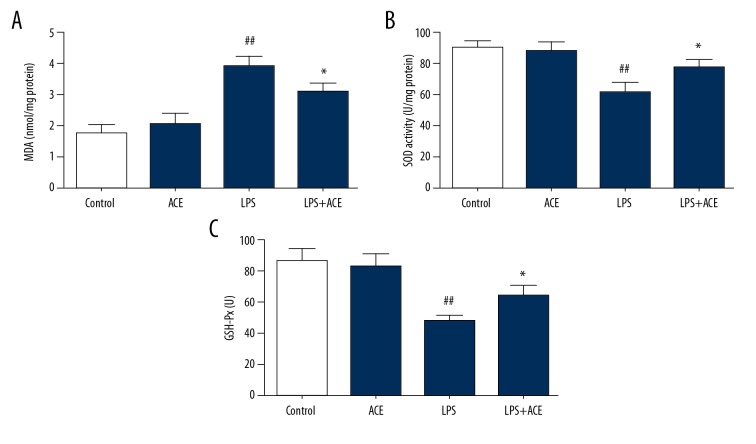
MDA expression, SOD activity, and GSH-Px levels were measured in lung tissues to assess oxidative damage. Compared with the control group, MDA levels were higher in the LPS-treated animals, and acetate could alleviate MDA expression (Figure 6A). The results also showed that SOD activity and GSH-Px levels in lung tissues were inhibited by LPS challenge, while acetate could reverse these changes (Figure 6B, 6C). These results indicated that acetate alleviated oxidative damage in LPS-induced lung injury.
Figure 6.
Acetate inhibits oxidative stress in LPS-induced mice: (A) MDA, (B) SOD activity, and (C) GSH-Px were determined to measure the oxidative stress. The values presented are mean ±SEM (n=10), ## P<0.01 versus the control group; * P<0.05 versus the LPS-treated group. LPS – lipopolysaccharide; MDA – malondialdehyde; SOD – superoxide dismutase; GSH-Px – glutathione peroxidase; SEM – standard error measurement.
Acetate inhibited LPS-induced mitogen-activated protein kinase (MAPK) pathway activation
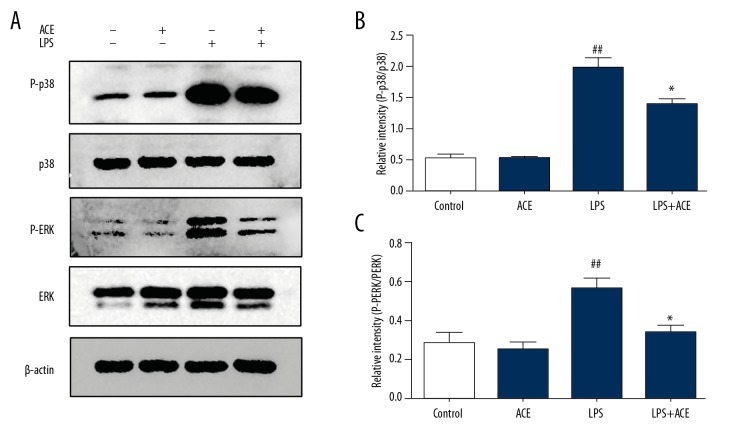
Finally, we assessed the phosphorylation levels of mitogen-activated protein kinase (MAPK) pathway in lung tissues to explore the molecular evidence of the anti-inflammatory effects of acetate. The results showed that the phosphorylation levels of ERK and p38 increased significantly after LPS challenge, and acetate administration alleviated these effects (Figure 7).
Figure 7.
Acetate inhibits the phosphorylation of MAPK signaling in lungs. (A) Protein levels of p-p38, p38, p-ERK, and ERK in lung homogenates. (B, C) Densitometric analysis of the relevant bands was performed. The values presented are mean ±SEM (n=3, the data was representative of 3 independent experiments), ## P<0.01 versus the control group; * P<0.05 versus the LPS-treated group. MAPK – mitogen-activated protein kinase; SEM – standard error measurement; LPS – lipopolysaccharide.
Discussion
ALI is a common syndrome characterized by extensive interstitial and alveolar edema. Along with other disease progresses, ALI could progress to acute respiratory disease syndrome, which is associated with a high mortality [23]. Administration of LPS intratracheally has been utilized to explore preventive or therapeutic strategies in ALI [24]. Moreover, Kolomaznik et al. [25] reported that LPS could interact with surfactant specific proteins directly or effect alveolar type II cells, which could lead to serious clinic issues. Acetate participates in many biological processes such as cell proliferation, immune response, and even tumorigenesis [26].
We found that acetate protected mice against LPS-induced ALI through its anti-inflammatory effect. First, acetate remarkably improved histological manifestations, lowering the pathological score. Consistently, LDH activity, which is an index of cell damage, was inhibited by acetate. Second, our study results indicated that acetate alleviated exudation in the alveoli. Pulmonary edema, featured by increased permeability of the pulmonary capillary and neutrophil infiltration, is common in ALI [27]. To evaluate edema severity, the W/D weight ratio and total protein in BALF were assessed in our study. Compared with the LPS group, protein concentration and the W/D weight ratio in the acetate-treated group were remarkably decreased. Similarly, BALF from acetate-treated mice showed noticeably less inflammatory cell infiltration. MPO is an index reflecting neutrophil margination and adhesion [28,29]. As expected, LPS-induced ALI in our study was accompanied by elevated MPO activity in lung tissues. Interestingly, MPO activity in lung tissues decreased after acetate treatment, which is consistent with the reduction of neutrophil infiltration. Finally, acetate suppressed the production of pro-inflammatory cytokines in LPS-induced ALI. Pro-inflammatory cytokines, including IL-6, IL-1b, and TNF-α, have been shown to play crucial roles in LPS-induced ALI [30]. Excessive pro-inflammatory cytokines are usually linked with organ dysfunction [31]. In our study, acetate suppressed the production of pro-inflammatory cytokines in lung tissues and BALF, but did not regulate the production of anti-inflammatory cytokine like IL-10, which indicated the anti-inflammatory effect of acetate was not intermediated by IL-10 signaling. Besides, the gene expression of these inflammatory cytokines in lungs were similar to the protein level. The MAPK pathway has been shown to be an essential part of signal transduction in LPS-induced ALI [32]. To explore the underlying mechanism, we assessed the phosphorylation levels of the MAPK pathway. We found that acetate markedly decreased the phosphorylation levels of several members of the MAPK pathway like ERK and p38. Taken together, these results confirmed the protective effects of acetate on LPS-induced ALI.
Besides the anti-inflammatory effect, acetate also exerted a protective effect through its anti-oxidant ability. Oxidative stress is a common pathogenic mechanism in LPS-induced ALI [29]. It is known that reactive oxygen species (ROS) exhibits a dual function in ALI. On the one hand, ROS promotes pathogen clearance, but on the other hand, it induces cell apoptosis and alveolar-capillary barrier damage [33]. Liu et al. [34] demonstrated that acetate protected mice against ethanol-induced acute gastric mucosal lesion through its anti-oxidant ability. As shown in our study, acetate decreased MDA levels and increased SOD activity and GSH-Px in lung tissue.
As a member of short-chain fatty acids (SCFAs), acetate is an important type of metabolite. Although intestinal microbes are the main source of acetate, the biological effects of acetate are not limited to the intestine [35]. Previous studies revealed the crucial regulatory role of acetate in metabolism and inflammation. The anti-inflammatory effects of acetate have been previously reported [34]. The level of acetate in the blood is not sufficient to exert its biological activities, however, it has been reported that these inflammatory responses could enhance the production of SCFAs [36]. Therefore, we inferred that increased acetate is a part of the inherent reactions when the immune system responds to pathogenic stimuli. Our study results showed that acetate exerted a protective effect on ALI, including alleviating pulmonary edema, decreasing oxidative damage, and downregulating pro-inflammatory cytokines.
It is well-known that acetate activates the cell-surface receptor GPCRs to modify biological processes in cells. GPR43 and GPR41 are regarded as the receptors of acetate [37]. Acetate could activate NLRP3 inflammasome via GPR43 [38]. Smith et al. [11] reported that SCFAs bind to GPR43 and modulate the proliferation and function of regulatory T cells in the colon. Therefore, GPR43 might be involve in the protective effect of acetate; further research is needed to address this.
Except for activating GPCRs, acetate is known as a histone deacetylase complex (HDAC) inhibitor, which could promote histone acetylation and contribute to its anti-inflammatory effects [39]. Soliman et al. [40] found that acetate inhibits histone deacetylase activity and possibly effects neuro-inflammatory cascade. Tao et al. [41] reported that acetate promoted the function of FOXP3+Treg by inhibiting HDAC9 activity to alleviate ulcerative colitis. Zhang et al. [42] discovered that HDAC inhibitor could be beneficial during sepsis-induced ALI. Similarly, the interaction between acetate and HDAC6 has provided new therapeutic strategies for clostridium difficile infection [43]. In contrast, acetate increases the susceptibility of human CD4+T cells to human immunodeficiency virus (HIV)-1 infection [44]. Further study is needed to determine if acetate exerts its protective effects by promoting histone acetylation.
There were some limitations in our study. First, although we revealed the protective effects of acetate on LPS-induced ALI, the underlying molecular mechanisms are still poorly understood, which will be one of the focuses in our future research. Second, we only detected inflammation-related indicators at 6 hours after ALI, and we only used one dose of acetate (250 mM). If we had further evaluated other dose of acetate or other time points, the study results would have been more scientific and persuasive.
Conclusions
This study demonstrated that acetate protects mice against LPS-induced ALI in vivo by alleviating inflammatory responses. Acetate exerts protective effects via its anti-inflammatory and anti-oxidative ability. These findings may provide novel insights into the biological functions of organic acids such as acetate.
Supplementary Figure
Acetate did not affect the concentration of IL-10 in BALF. IL-10 was measured in BALF 6 hours after LPS challenge. The values presented are mean±SEM (n=10).
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81471845; 81772105)
Conflict of interests
None.
References
- 1.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–27. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 3.Chopra M, Reuben JS, Sharma AC. Acute lung injury: Apoptosis and signaling mechanisms. Exp Biol Med (Maywood) 2009;234(4):361–71. doi: 10.3181/0811-MR-318. [DOI] [PubMed] [Google Scholar]
- 4.de la Vega MR, Dodson M, Gross C, et al. Role of Nrf2 and autophagy in acute lung injury. Curr Pharmacol Rep. 2016;2(2):91–101. doi: 10.1007/s40495-016-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanelli V, Ranieri VM. Mechanisms and clinical consequences of acute lung injury. Ann Am Thorac Soc. 2015;12(Suppl 1):S3–8. doi: 10.1513/AnnalsATS.201407-340MG. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Zhou Y, Li P, et al. Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Sci Rep. 2016;6:39473. doi: 10.1038/srep39473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 9.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–55. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111(6):2247–52. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst T, Sichelstiel A, Schär C, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 13.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13(20):2826–32. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisenauer CJ, Bhatt DP, Mitteness DJ, et al. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J Neurochem. 2011;117(2):264–74. doi: 10.1111/j.1471-4159.2011.07198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arun P, Ariyannur PS, Moffett JR, et al. Metabolic acetate therapy for the treatment of traumatic brain injury. J Neurotrauma. 2010;27(1):293–98. doi: 10.1089/neu.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-b-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–17. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishina K, Mikawa K, Takao Y, et al. Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology. 1998;88(5):1300–9. doi: 10.1097/00000542-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Dagvadorj J, Shimada K, Chen S, et al. Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2X7 receptor leading to interleukin-1α release. Immunity. 2015;42(4):640–53. doi: 10.1016/j.immuni.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Liu Y, Peng X, et al. NMDA receptor antagonist attenuates bleomycin-induced acute lung injury. PLoS One. 2015;10(5):e0125873. doi: 10.1371/journal.pone.0125873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Liu T, Duan JX, et al. Soluble epoxide hydrolase inhibitor attenuates lipopolysaccharide-induced acute lung injury and improves survival in mice. Shock. 2017;47(5):638–45. doi: 10.1097/SHK.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol. 2015;209:52–58. doi: 10.1016/j.resp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Butt Y, Kurdowska A, Allen TC. Acute lung injury: A clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–50. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 24.Konrad FM, Reutershan J. CXCR2 in acute lung injury. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/740987. 740987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolomaznik M, Nova Z, Calkovska A. Pulmonary surfactant and bacterial lipopolysaccharide: The interaction and its functional consequences. Physiol Res. 2017;66(Supplementum 2):S147–57. doi: 10.33549/physiolres.933672. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Lin SH, Ren F, et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun. 2016;7:11960. doi: 10.1038/ncomms11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu KC, Huang SS, Kuo YH, et al. Ugonin M, a Helminthostachys zeylanica constituent, prevents LPS-induced acute lung injury through TLR4-mediated MAPK and NF-kB signaling pathways. Molecules. 2017;22(4) doi: 10.3390/molecules22040573. pii: E573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: A new player in autoimmunity. Cell Immunol. 2017;317:1–8. doi: 10.1016/j.cellimm.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremserova S, Perecko T, Soucek K, et al. Lung Neutrophilia in myeloperoxidase deficient mice during the course of acute pulmonary inflammation. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/5219056. 5219056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Do-Umehara HC, Chen C, Urich D, et al. Suppression of inflammation and acute lung injury by Miz1 via repression of C/EBP-d. Nat Immunol. 2013;14(5):461–69. doi: 10.1038/ni.2566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Bhargava M, Wendt CH. Biomarkers in acute lung injury. Transl Res. 2012;159(4):205–17. doi: 10.1016/j.trsl.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X, Pu Y, Kong W, et al. Antidesmone, a unique tetrahydroquinoline alkaloid, prevents acute lung injury via regulating MAPK and NF-kB activities. Int Immunopharmacol. 2017;45:34–42. doi: 10.1016/j.intimp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Xiang M, Fan J, Fan J. Association of Toll-like receptor signaling and reactive oxygen species: A potential therapeutic target for post-trauma acute lung injury. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/916425. pii: 916425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Wang J, Shi Y, et al. Short chain fatty acid acetate protects against ethanol-induced acute gastric mucosal lesion in mice. Biol Pharm Bull. 2017;40(9):1439–46. doi: 10.1248/bpb.b17-00240. [DOI] [PubMed] [Google Scholar]
- 35.Moriyama M, Kurebayashi R, Kawabe K, et al. Acetate attenuates lipopolysaccharide-induced nitric oxide production through an anti-oxidative mechanism in cultured primary rat astrocytes. Neurochem Res. 2016;41(11):3138–46. doi: 10.1007/s11064-016-2038-2. [DOI] [PubMed] [Google Scholar]
- 36.Garland SH. Short chain fatty acids may elicit an innate immune response from preadipocytes: A potential link between bacterial infection and inflammatory diseases. Med Hypotheses. 2011;76(6):881–83. doi: 10.1016/j.mehy.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 37.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40(6):833–42. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 39.Sanford JA, Zhang LJ, Williams MR, et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016;1(4) doi: 10.1126/sciimmunol.aah4609. pii: eaah4609. [DOI] [PubMed] [Google Scholar]
- 40.Soliman ML, Rosenberger TA. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem. 2011;352(1–2):173–80. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- 41.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13(11):1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Jin S, Wang C, et al. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34(7):1676–83. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- 43.Lu LF, Kim DH, Lee IH, et al. Potassium acetate blocks clostridium difficile toxin A-induced microtubule disassembly by directly inhibiting histone deacetylase 6, thereby ameliorating inflammatory responses in the gut. J Microbiol Biotechnol. 2016;26(4):693–99. doi: 10.4014/jmb.1511.11063. [DOI] [PubMed] [Google Scholar]
- 44.Bolduc JF, Hany L, Barat C, et al. Epigenetic metabolite acetate inhibits class I/II histone deacetylases, promotes histone acetylation, and increases HIV-1 integration in CD4+ T Cells. J Virol. 2017;91(16) doi: 10.1128/JVI.01943-16. pii: e01943–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acetate did not affect the concentration of IL-10 in BALF. IL-10 was measured in BALF 6 hours after LPS challenge. The values presented are mean±SEM (n=10).