Key Points
Question
Can rare genetic variants for Alzheimer disease be identified using nonstatistical approaches?
Findings
In this genetic association study, variants with high functional effect were observed in participants with Alzheimer disease but not in controls in NOTCH3, a gene previously associated with cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and TREM2 (Q33X) that in homozygous form causes Nasu-Hakola disease.
Meaning
Different mutations in the same gene or variable dose of a particular mutation may be associated with dissimilar types of dementia.
Abstract
Importance
Some of the unexplained heritability of Alzheimer disease (AD) may be due to rare variants whose effects are not captured in genome-wide association studies because very large samples are needed to observe statistically significant associations.
Objective
To identify genetic variants associated with AD risk using a nonstatistical approach.
Design, Setting, and Participants
Genetic association study in which rare variants were identified by whole-exome sequencing in unrelated individuals of European ancestry from the Alzheimer’s Disease Sequencing Project (ADSP). Data were analyzed between March 2017 and September 2018.
Main Outcomes and Measures
Minor alleles genome-wide and in 95 genes previously associated with AD, AD-related traits, or other dementias were tabulated and filtered for predicted functional impact and occurrence in participants with AD but not controls. Support for several findings was sought in a whole-exome sequencing data set comprising 19 affected relative pairs from Utah high-risk pedigrees and whole-genome sequencing data sets from the ADSP and Alzheimer’s Disease Neuroimaging Initiative.
Results
Among 5617 participants with AD (3202 [57.0%] women; mean [SD] age, 76.4 [9.3] years) and 4594 controls (2719 [59.0%] women; mean [SD] age, 86.5 [4.5] years), a total of 24 variants with moderate or high functional impact from 19 genes were observed in 10 or more participants with AD but not in controls. These variants included a missense mutation (rs149307620 [p.A284T], n = 10) in NOTCH3, a gene in which coding mutations are associated with cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), that was also identified in 1 participant with AD and 1 participant with mild cognitive impairment in the whole genome sequencing data sets. Four participants with AD carried the TREM2 rs104894002 (p.Q33X) high-impact mutation that, in homozygous form, causes Nasu-Hakola disease, a rare disorder characterized by early-onset dementia and multifocal bone cysts, suggesting an intermediate inheritance model for the mutation. Compared with controls, participants with AD had a significantly higher burden of deleterious rare coding variants in dementia-associated genes (2314 vs 3354 cumulative variants, respectively; P = .006).
Conclusions and Relevance
Different mutations in the same gene or variable dose of a mutation may be associated with result in distinct dementias. These findings suggest that minor differences in the structure or amount of protein may be associated with in different clinical outcomes. Understanding these genotype-phenotype associations may provide further insight into the pathogenic nature of the mutations, as well as offer clues for developing new therapeutic targets.
This genetic association study uses whole-exome sequencing to identify genetic variants associated with the risk of Alzheimer disease in Alzheimer’s Disease Sequencing Project participants of European ancestry.
Introduction
Alzheimer disease (AD) is the most common type of dementia and affects an estimated 5.7 million individuals in the United States, with the number projected to rise to 14 million by 2050.1 Susceptibility to AD is highly heritable (h2 = 58%-79%),2 but only about one-third of the genetic component is accounted for by common variants discovered through genome-wide association studies.2 Some of the unexplained heritability of AD may be due to rare variants, which remain challenging to discover in genomic studies because of statistical power limitations, despite large sample sizes.3 Genome-wide searches have identified AD associations with rare variants in relatively few genes, including TREM2, AKAP9, UNC5C, ZNF655, IGHG3, and CASP7,4,5,6,7,8 and methods to evaluate rare variants are still under development.3 We applied a strategy focused on rare variants occurring only in cases to identify and characterize additional high-penetrance risk variants in AD that would be otherwise undetected in analyses that do not render results when a variant is not observed in the control group.
Methods
Study Population and Data Pipeline
The Alzheimer’s Disease Sequencing Project (ADSP) performed whole-exome sequencing (WES) on DNA samples obtained from participants of non-Hispanic European ancestry (EA) and a group of Caribbean Hispanic individuals that was deemed too small for inclusion in this study. A total of 5617 participants with AD met National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria for possible, probable, or definite AD after clinical and/or neuropathologic examination9 and 4594 controls were cognitively normal. The ADSP participants were selected using a risk score based on age, sex, and APOE ε4 carrier status to maximize cases most likely to have AD risk variants and controls most likely to have AD protective variants. Sample characteristics are provided in eTable 1 in the Supplement.
Written informed consent was obtained from all participants who were 60 years or older or from their authorized legal representative. This study was approved by the Boston University Institutional Review Board. Data were analyzed between March 2017 and September 2018. This study followed the Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guideline.
Gene Selection and Variant-Filtering Pipeline
Rare variants were analyzed under 2 different schemes: one considering all genes in the genome and another focused on genes for which there was prior evidence linking them to AD, AD-related endophenotypes, or other disorders in which adult-onset dementia was the cardinal feature. Selection of genes for the latter analysis scheme (listed in eTable 2 in the Supplement) was based on a review of the literature and required either genome-wide significant association findings or generally accepted functional evidence. Details about DNA sequencing, data quality control, and variant selection and annotation are provided in the eMethods and eTable 3 in the Supplement. The study design is illustrated in eFigure 1 in the Supplement.
Rare Variant Analysis in an Independent Data Set
To extend and enhance the discovery of novel associations, we evaluated WES data obtained from 19 AD-affected first- or second-cousin pairs identified in the Utah Population Database belonging to a pedigree with a statistical excess of AD risk. These pedigrees are genealogically independent at least as far back as the early 1800s. Details of the Utah Population Database, case classification, and identification of high-risk pedigrees have been published elsewhere.18 A series of steps that included filters for sharing among affected relatives, frequency in several public next-generation sequencing databases, pathogenicity, and relevance to AD pathologic factors resulted in 130 variants exome wide for further evaluation (eMethods in the Supplement).
Statistical Analysis
Haplotype Analysis
PLINK was used to find common single-nucleotide polymorphisms (SNPs) near the rare variant of interest within a specified kilobase (kb) window. The wildcard option was used to infer haplotypes and estimate haplotype frequencies.10 Haploview was used to visualize regional linkage disequilibrium and confirm haplotypes and frequencies among different SNP combinations using multimarker haplotype association tests.11
Protein Homologic Modeling and Pathway Analysis
Protein homologic modeling was performed for several high-impact variants in NOTCH3 with BLAST-P, version 2.7.1,12 SWISS-MODEL, SMTL version 2019-02-13 (PDB release 2019-02-08),13 and Maestro, version 11.2 software.14 Additional details of the modeling procedures are provided in the eMethods in the Supplement. A high-confidence (confidence score >0.7) human protein-protein interaction network was then created with version 10 of the STRING database for NOTCH3 and its ligand JAG1.15 The set of genes forming the protein network was tested for gene-set enrichment using Protein Analysis Through Evolutionary Relationships pathways and the Fisher exact test with false discovery rate multiple test correction.16
Estimation of Burden of Rare Variants
A gene-set test was performed to evaluate the burden of high- and moderate-impact mutations in the set of AD- or dementia-related genes among participants with AD compared with controls. Logistic regression models, including covariates for sex, age, sequencing center, and principal components of ancestry, were evaluated using the Combined and Multivariate Collapsing method17 in R, version 3.5.0 (R Foundation). Findings were considered significant at 2-tailed P < .05.
Results
After performing data-filtering steps, 5617 participants with AD (3202 [57.0%] women; mean [SD] age, 76.4 [9.3] years) and 4594 controls (2719 [59.0%] women; mean [SD] age, 86.5 [4.5] years) remained for analysis.
Rare Variants in NOTCH3
Evaluation of high- and moderate-impact rare variants in genes that were previously established as genetically or functionally associated with AD or dementia revealed a missense mutation in NOTCH3 (rs149307620; p.A284T) that was present in 10 AD cases, but no controls (eTable 4 in the Supplement). This variant is rare in EAs (minor allele frequency [MAF], 0.0005)19 and was verified by Sanger sequencing in 8 of these participants for whom DNA was available. Because several other high- or moderate-impact NOTCH3 mutations have been associated with cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a diagnostically distinct disorder marked by severe headaches in young adulthood followed by strokes and dementia later in life,20 we sought clinical and autopsy data from the participants with AD with the rs149307620 mutation to determine whether they are enriched for cerebrovascular risk factors. Neuropathologic information that was available for one of these participants revealed moderate atherosclerosis but no arteriosclerosis, lacunes, or microinfarcts, which are hallmarks of CADASIL (eTable 5 in the Supplement).
Autopsy also confirmed the presence of AD abnormalities (Consortium to Establish a Registry for Alzheimer’s Disease [CERAD] neuritic plaques, moderate; CERAD diffuse plaques, moderate; and Braak neurofibrillary degeneration, stage VI). The mean (SD) age at symptom onset for the 10 NOTCH3 mutation carriers was 80.5 (6.7) years, which was similar to that for the entire sample of ADSP EA cases (80.9 [9.1] years) and greater than age at onset of cognitive impairment among individuals with CADASIL (usually <50 years). None of the NOTCH3 mutation carriers had a history of clinical strokes (although 1 carrier had multiple infarcts shown on magnetic resonance imaging and a history of diabetes and cardiovascular disease) and all had prominent memory impairment as the initial presentation with a progressive course. One other NOTCH3 mutation (rs114447350; p.P2074Q) was observed in 4 participants with AD but not in controls (eTable 4 in the Supplement). Unlike rs149307620, this variant is not rare in EAs (MAF, 0.024) or in persons of African ancestry (MAF, 0.091),21 suggesting it is unlikely to be pathogenic.
Because rs149307620 is rare, we investigated the possibility that this mutation occurred once or only a few times by performing a haplotype analysis with common SNPs. This analysis revealed a 5-SNP haplotype with a frequency of 15% in the participants with AD and 14% in controls that is common to all 10 cases with the NOTCH3 mutation (eFigure 2 in the Supplement). The mean pairwise identical-by-descent (IBD) sharing for the 10 mutation carriers (mean [SD] π̂ = 0.028 [0.025]) is slightly larger than the mean pairwise IBD sharing within the rest of ADSP sample (mean [SD] π̂ = 0.013 [0.026]), indicating that the carriers are not more closely related to each other than to all participants. Taken together, these results suggest that the mutation in these participants originated in a common ancestor who lived many generations ago.
To investigate the possibility that the mutation carriers belong to a particular subpopulation, we plotted the first 2 principal components of ancestry that were derived previously for the entire sample7 and observed that 8 of the 10 mutation carriers were clustered in a distinct minor portion of the sample (eFigure 3 in the Supplement). Analysis of mitochondrial DNA variants revealed that most individuals in this cluster had mitochondrial haplogroups K1a1b1a or K1a9 that are common among Ashkenazi Jewish individuals.22 Moreover, NOTCH3 mutations carriers accounted for 4.0% of the participants with AD who have either the K1a1b1a or K1a9 haplogroup. The proportion of mutation carriers in this cluster was significantly greater among participants with AD (8 of 358 [2.2%]) than controls (0 of 337) (Z = 2.76, P = .006). The frequency of the rs149307620 mutation is about 25 times higher in Ashkenazi Jewish individuals (MAF, 0.0046) compared with other EA groups (MAF, 0.00019).19
Analysis of the 130 variants that met the filtering criteria in the 19 affected cousin pairs from the Utah high-risk pedigrees provided additional support for a role of NOTCH3 in AD. Both affected individuals in 1 family who are half-first cousins had 2 rare NOTCH3 missense mutations—rs141402160 (p.G248A) and rs140914494 (p.A198E)—each with a population frequency of 0.0002.20 Review of available clinical and family history information for this family did not indicate findings consistent with CADASIL in the probands or relatives. The pedigree of the carriers of the rs141402160 and rs140914494 mutations includes 7 additional members who had AD or dementia listed on death certificates (eFigure 4 in the Supplement). All but 1 of the individuals in the line of descent from the common ancestor of the pair died before age 60 years, prior to the age at onset of AD in the cases. An affected cousin pair in an independent family had the NOTCH3 missense variant rs112197217 (p.H1133Q), which has a frequency of 0.010 in EAs,21 but is rarer in other populations (eFigure 5 in the Supplement). The evidence of AD among other relatives in this family was inconclusive.
To further distinguish which of the 5 NOTCH3 variants identified in the ADSP WES sample and Utah families may be related to AD, we screened for these variants in whole-genome sequence (WGS) data obtained from a multiethnic sample of unrelated 1432 participants with AD and 1660 controls in the ADSP Extension Study, 550 participants with AD and 283 controls in the ADSP multiethnic Family Study,23 and 809 participants in the Alzheimer’s Disease Neuroimaging Initiative Study (239 participants with AD, 321 participants with mild cognitive impairment, 249 controls).24 Characteristics of participants in these data sets are provided in eTable 6 in the Supplement. The minor alleles for rs11219217 (n = 70) and rs114447350 (n = 286) were observed appreciably in participants with AD, participants with mild cognitive impairment, and controls of multiple ethnicities suggesting that they are not associated with AD risk. The rs149307620 variant was found in 1 AD case (age at onset, 89 years) and 1 mild cognitive impairment case (age at onset, 76 years), but not in controls. The rare rs141402160 and rs140914494 variants were not detected in any of the WGS samples.
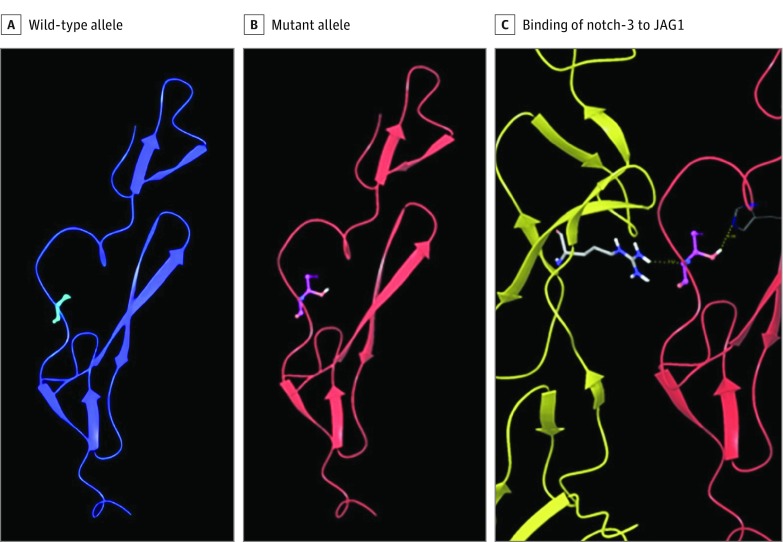
Protein modeling showed that the rs149307620 mutation is located in the EGF repeat region between EGF10 and EGF11 and more precisely in the EGF calcium binding (EGF_CA) domain, near the Jagged-1 (JAG1)-NOTCH3 binding site.25 Modeling predicted that the major allele for rs149307620 results in wild-type notch-3 with a corresponding amino acid alanine (Figure 1A). The alanine side chain is nonpolar and would not be predicted to have any intraprotein or interprotein interactions. The minor allele for rs149307620 results in mutant notch-3 with a corresponding amino acid threonine (Figure 1B). Threonine is polar and will form hydrogen bonds where possible with itself or with a polar histidine nearby in NOTCH3. This action then alters the backbone conformation in this region in the model and allows additional interactions with a polar arginine at the site of JAG1-NOTCH3 binding (Figure 1C). These results suggest that the mutant Notch-3 causes greater interaction with the ligand, possibly changing downstream processes. The other AD-associated NOTCH3 mutations, rs141402160 and rs140914494, also involve either the gain or loss of an alanine. In both instances, the mutation change leads to increased polarity and hydrogen bonding with possible increased interactions to a greater or lesser extent that observed with rs149307620.
Figure 1. Notch-3 Protein Model Highlighting Position of the Alzheimer Disease–Associated Single-Nucleotide Polymorphism rs149307620 (p.A284T).
Predicted model for wild-type allele with alanine at mutation site (A), mutant allele with threonine at mutation site (B), and binding of notch-3 (red) to JAG1 ligand (yellow) (C). Possible hydrogen bonding that would likely cause greater interaction with the ligand is displayed.
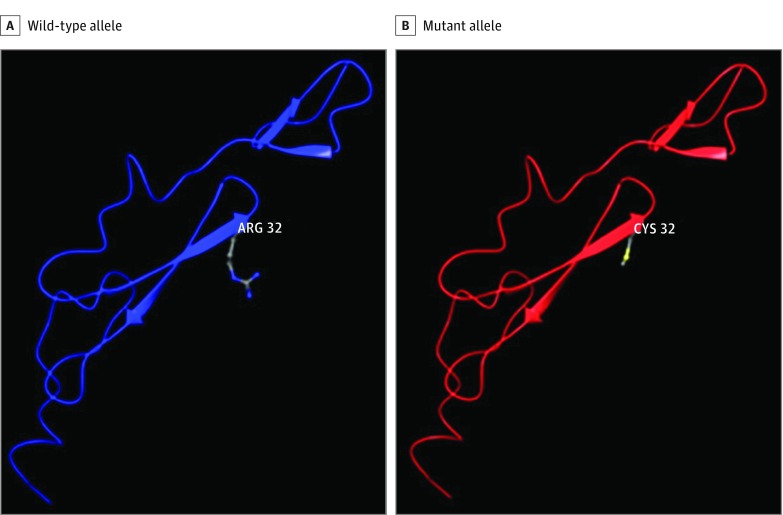
Unlike the rs149307620 and rs141402160 mutations, but similar to the rs140914494 mutation, most of the more than 25 reported distinct NOTCH3 mutations causing CADASIL are located in exons 3 and 4 (Table 1).26 However, 1 CADASIL-associated variant, rs137852641, is a missense mutation in codon 332 in exon 6, resulting in the replacement of an arginine residue with a cysteine27 that is proximate to rs149307620 (codon 284 in exon 6) (Figure 2).
Table 1. NOTCH3 Mutations With Predicted Functional Impact on AD Risk.
| SNP | Position (chr 19)a | Exon | GnomAD Frequency | Protein Position | Residue Change | Observed Mutation Carriers |
|---|---|---|---|---|---|---|
| rs140914494 | 15 192 046 | 4 | 0.00003 | 198 | Ala>Glu | AD-affected relative pair |
| rs141402160 | 15 191 804 | 5 | 0.00005 | 248 | Gly>Ala | AD-affected relative pair |
| rs149307620 | 15 191 610 | 6 | 0.00029 | 284 | Ala>Thr | 11 Participants with AD, 1 participant with MCI |
Abbreviations: AD, Alzheimer disease; GnomAD, Genome Aggregation Database; MCI, mild cognitive impairment; SNP, single-nucleotide polymorphism.
Chromosome position according to GRCh38.p12 assembly.
Figure 2. Homologous Protein Modeling of Cerebral Autosomal-Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy (CADASIL) NOTCH3 rs137852641 (p.R332C) Mutation.
Predicted model for wild-type allele with arginine at the mutation site (A) and mutant allele with cysteine at the mutation site (B). Gain of a cysteine residue disrupts disulfide bonding within the protein, affecting overall protein structure.
To further explore the biological functions and pathways for NOTCH3 in AD, a high-confidence protein-protein interaction network was constructed including NOTCH3 and JAG1. The resulting 30-gene interaction network contains several AD-related genes, including BACE1, PSEN1, PSEN2, and APP (eFigure 6 in the Supplement). Gene-set enrichment analysis revealed that the network genes were significantly enriched in the Notch signaling pathway (P = 6.48 × 10−49), angiogenesis (P = 1.61 × 10−12), and 2 AD-related pathways involving secretase-mediated amyloid precursor protein cleavage (P = 3.50 × 10−16) and presenilin γ-secretase complex (P = 5.78 × 10−26) (Table 2).
Table 2. Gene-Set Enrichment Analysis of NOTCH3/JAG1 Protein-Protein Interaction Network.
| PANTHER Pathway | No. of Genes Annotated to Pathway | No. of Genes in Network | Expected P Valuea | Fold Enrichment | Unadjusted P Value | FDR |
|---|---|---|---|---|---|---|
| Notch signaling | 42 | 22 | .06 | >100 | 3.98 × 10−51 | 6.48 × 10−49 |
| Presenilin | 123 | 14 | .18 | >100 | 7.09 × 10−28 | 5.78 × 10−26 |
| Amyloid secretase | 69 | 7 | .10 | >100 | 8.59 × 10−18 | 3.50 × 10−16 |
| Angiogenesis | 173 | 10 | .25 | 40.54 | 4.92 × 10−14 | 1.61 × 10−12 |
Abbreviations: FDR, false discovery rate; PANTHER, Protein Analysis Through Evolutionary Relationships classification system.
Expected probability of observing at least x number of genes out of the total number of genes in the PANTHER list annotated to a particular pathway, given the proportion of genes in the reference Homo sapiens whole genome that are annotated to that pathway.
TREM2 Q33X
We also identified the high-impact TREM2 rs104894002 (p.Q33X) mutation in 4 of 5617 (0.071%) participants with AD (eTable 4 in the Supplement), a frequency that is slightly lower than that observed in a TREM2 sequencing study of participants with AD and controls in 2013 (2 of 1084 [0.17%] participants with AD).4 Because this mutation in a homozygous state causes Nasu-Hakola disease, a rare autosomal-recessive disorder characterized by early-onset dementia and multifocal bone cysts,28 we evaluated clinical data obtained from the 4 TREM2 Q33X mutation carriers to assess potential pleiotropic effects (eTable 7 in the Supplement). All of these participants met the criteria for probable AD and none had reported bone cysts or unusual behavioral symptoms.
Other Rare Mutations
A total of 32 moderate- or high-impact variants in 24 previously established genes for AD or other dementias were each observed in 4 or more participants with AD and no controls (eTable 4 in the Supplement). Five of these variants were previously reported as associated with AD and include missense mutations in PSEN1 (rs63749824/p.A75V [n = 7]29; rs63750592/p.R35Q [n = 4]),30 SORL1 (rs139710266/p.Y391C [n = 5]),31 and MAPT (rs63750424/p.R741W [n = 4]),32 and a stop-gain mutation in ABCA7 (rs145987355/ p.E1679X [n = 4]).33 Genome-wide, 24 variants in 19 genes with moderate to high functional impact were observed in 10 or more participants with AD but absent in controls (eTable 8 in the Supplement). Further examination of the genes represented by multiple variants revealed that 10 participants had 3 ABCD4 missense variants (rs57773157/p.G248, rs34992370/p.V172I, rs58272575/p.G59R) that co-occur in a rare 8-SNP haplotype spanning 12.9 kb with a frequency of 0.3% in cases and 0% in controls (eFigure 7A in the Supplement). Another 10 participants had 2 CELSR1 missense variants (rs61741871/p.2983A and rs75983687/p.2703M), and 8 of these participants also had 2 GTSE1 missense variants (rs34404175/p.A219V and rs35503220/p.A293A). One participant was homozygous for all 4 variants. The participants who had these CELSR1 and GTSE1 variants share a rare 12-SNP haplotype spanning 77.6 kb with a frequency of 0.1% in cases and 0.1% in controls (eFigure 7B in the Supplement). Estimates of IBD sharing for the 10 participants with the ABCD4 variants were only slightly higher (mean [SD] π̂ = 0.015 [0.028]) and for the 8 participants with AD and the CELSR1 and GTSE1 variants (mean [SD] π̂ = 0.008 [0.015]) were lower than genome-wide IBD sharing, suggesting that they are not more closely related to each other than to all participants. There were few common SNPs in the 500-kb region including the rare ABCD4 variants, suggesting high-sequence conservation in this region.
To identify additional genes that may have overrepresentation of deleterious AD-related variants, we filtered genes that contained at least 3 distinct variants, each occurring in at least 5 participants with AD but absent in controls (eTable 9 in the Supplement). The ABCD4 rs61744947/p.P2983A variant appears on the same haplotype containing the other 3 ABCD4 variants. Three LAMC3 variants were observed in the same 7 participants. TTN had the greatest number of distinct variants (n = 6) that were observed in participants with AD only. Genome-wide, 9 genes not previously associated with AD contained a high functional impact variant that was present in at least 7 participants with AD but absent in controls (eTable 10 in the Supplement).
Rare Variant Burden
To test if AD is associated with greater burden of rare deleterious variants, gene burden tests were performed for models including high-impact variants and high- and moderate-impact variants for MAF of 0.01 or lower and MAF of 0.5 or lower in genes previously associated with AD risk, AD-related traits, or other dementias (Table 3). These analyses showed that participants with AD had a significantly higher burden of moderate- and high-impact rare deleterious variants in this group of genes compared with controls (2314 vs 3354 cumulative variants, respectively; P = .006).
Table 3. Rare Variant Burden for Established Alzheimer Disease Genes.
| Model | β (SE) | P Value |
|---|---|---|
| High impact, MAF ≤ 0.01 | 0.005 (0.166) | .98 |
| High/moderate impact, MAF ≤ 0.01 | 0.062 (0.023) | .006 |
| High impact, MAF ≤ 0.05a | 0.005 (0.166) | .98 |
| High/moderate impact, MAF ≤ 0.05a | 0.061 (0.022) | .006 |
Abbreviation: MAF, minor allele frequency.
No deleterious variants with MAF between 0.01 and 0.05 were observed in this group of genes.
Discussion
We identified several rare variants that have a high probability of damage to protein structure and may increase AD risk. These variants were not detected in previous analyses of the same ADSP WES data set that were agnostic with respect to functional impact of the variants and conducted using current statistical testing approaches.7 Our focus on variants observed in participants with AD but not controls yielded results that are often undetected by traditional genetic association methods that cannot evaluate empty cells, regardless of sample size or frequency of variants among cases. Several of our top-ranked results confirm previously identified AD associations with rare variants, including PSEN1 rs6374982429 and rs63750592,30 SORL1 rs139710266,31 MAPT rs63750424,32 and ABCA7 rs145987355,33 which suggest that novel findings identified by our approach may be robust. Two of our novel findings offer additional evidence of shared genetic mechanisms between AD and other rare dementia syndromes, namely CADASIL and Nasu-Hakola disease. Our study also suggests that participants with AD have a significantly higher burden of deleterious rare coding variants in known AD, AD-related, or other dementia genes compared with controls. This observation generalizes previous findings in SORL1,31,34,35 MAPT,32,36 TREM2,4,37,38 and ABCA733,39,40,41 that both common and rare variants in the same gene may independently contribute to AD risk.
We observed the rare NOTCH3 rs149307620 allele in 11 participants with AD and 1 participant with mild cognitive impairment, but not in controls, in the combined ADSP WES, ADSP WGS, and Alzheimer’s Disease Neuroimaging Initiative WGS data sets. The most remarkable finding from analysis of the Utah high-risk pedigree WES data set was rare NOTCH3 rs140914494 and rs141402160 alleles in a pair of affected half first-cousins. These mutations in exons 4 (rs140914494), 5 (rs141402160), and 6 (rs149307620) are located in the JAG1 binding site and involve the gain or loss of an alanine residue (Table 1). Based on this evidence alone, it is unclear whether one or both of the rs140914494 and rs141402160 mutations, which are likely in cis given their probable inheritance from a single common ancestor, have a role in AD. In contrast, the rs114447350 and rs112197217 variants are located near the end of the coding sequence (exons 33 and 21, respectively) and may be clinically benign42 and thus unlikely to be causally related to AD. Many other NOTCH3 variants have been associated with CADASIL that typically replace the wild-type amino acid with a cysteine residue or replace a highly conserved cysteine residue with another amino acid,20,26,27 although there are several exceptions.43 Available clinical and autopsy data for the individuals with NOTCH3 mutations were consistent with the diagnosis of AD and not CADASIL. Our protein modeling demonstrated that the AD-associated NOTCH3 mutations in exons 4 to 6 result in quantitative changes in hydrogen bonding causing increased ligand interaction, whereas CADASIL NOTCH3 mutations lead to qualitative changes involving disrupted disulfide bonding that affect overall protein structure and receptor maturation and differ with respect to their consequences on both ligand binding and ligand-induced signaling.26,27
Our protein-protein interaction network and gene-set enrichment analyses demonstrated that NOTCH3 is associated with AD pathways and biological processes. Notch-3 signaling can be triggered by both delta-JAG-type ligands and requires ADAM10 and presenilin-1 or -2, making it part of the AD-related presenilin pathway.44 Previous studies showed that JAG1-Notch signaling and subsequent hippocampal neurogenesis and astrogenesis are regulated by cleavage by BACE1, a promising AD drug target.44 This process, which is more active during early development and decreases in adulthood, affects normal neuronal development and alters neurogenesis and thus can have long-term effects.44 In addition, Notch-3 is a substrate for γ-secretase (presenilin) inhibition, which, when dysregulated, can cause misprocessing of the amyloid precursor protein resulting in accumulation of the toxic amyloid-β peptide.45
To our knowledge, these collective genetic and bioinformatics findings provide the strongest possible pathogenic link to date between NOTCH3 and AD. A previous study reported an association of AD with a distinct NOTCH3 mutation (p.R1231C) in a Turkish family46; however, this variant was detected in only 1 affected member and there is conflicting information about its pathogenicity.47 Sassi et al48 tested the hypothesis that genes associated with mendelian adult-onset leukodystrophy are also associated with AD in a sample including 332 sporadic participants with AD and 676 controls and found a significant gene-based association with NOTCH3, a result driven primarily by a common synonymous coding variant.
The TREM2 Q33X mutation that was observed in 4 participants with AD in our sample and in 4 participants with AD and 1 unaffected relative with an unspecified age in gene-resequencing studies targeting TREM24,49,50 is rarer than the well-documented R47H variant that has been associated with increased risk of AD in several studies,4,51 including the ADSP cohort.7,8 Homozygosity of this mutation causes Nasu-Hakola disease, a rare disorder characterized by early-onset dementia and multifocal bone cysts,28 and has also been observed in a member of a Turkish family with frontotemporal dementia–like syndrome, including the appearance of aggressive behavior and generalized tonic-clonic seizures before age 30 years but without bone involvement.52 Because persons with Nasu-Hakola disease and the frontotemporal dementia syndrome case have a more severe phenotype overall and much earlier onset of dementia symptoms than participants with late-onset AD who are heterozygous for Q33X, the behavior of this mutation may more resemble an intermediate inheritance than an autosomal-dominant model. This idea is consistent with the observation that both living parents of a patient with Nasu-Hakola disease who were obligate Q33X heterozygotes had evidence of β-amyloid deposition by cerebrospinal fluid analysis and florbetapir positron emission tomographic imaging.52
Furthermore, unlike the TREM2 R47H mutation and rare coding variants at other loci that have been associated with AD,4,5,6,7,8 the NOTCH3 rs149307620 and TREM2 Q33X mutations appear to be fully penetrant among persons surviving to late age, which perhaps would be the first examples of causative mutations for late-onset AD. This assertion is somewhat speculative given the small number of participants with AD documented to have these mutations.
Our study also implicated multiple functional variants in several novel genes as risk factors for AD. Mutations in ABCD4 cause an inborn error of vitamin B12 metabolism.53 Vitamin B12 deficiency is associated with cognitive impairment, and the level of circulating vitamin B12 has been associated with AD risk.54 ABCD4 encodes an adenosine triphosphate–binding cassette transporter that is in the same family as well-established AD gene ABCA7.39,40 The AD-associated CELSR1 rs61741871 (P2983A) missense variant has also been associated with craniorachischisis, which is a severe neural tube defect,55 and other CELSR1 variants have been identified as ischemic stroke risk factors in Japanese individuals.56 The CELSR1-3 family of genes has multiple functions in the nervous system and distinct roles in brain development and maintenance.57 GTSE1 regulates G1/S cell cycle transition and microtubule stability and is involved in pivotal neurodegeneration pathways.58 It is not clear which of these CELSR1 and GTSE1 mutations may directly influence AD risk. LAMC3 encodes laminin subunit γ 3, and multiple experimental studies have linked laminins to AD.59,60 LAMC3 has been significantly associated with age at onset of AD.61
Limitations
Our study has several limitations. Because we focused on rare variants, our sample of more than 10 000 participants was inadequate to establish statistical significance. Thus, our findings require replication in independent samples. We were unable to replicate these findings in the Alzheimer’s Disease Genetics Consortium genome-wide association study data set because of low and inconsistent imputation quality for these rare variants, despite the use of the large Haplotype Reference Consortium reference panel.62 In addition, our genome-wide MAC cutoffs for focusing on particular variants were arbitrary and, therefore, some important findings may have been overlooked.
It is possible that cryptic relatedness in the sample may have exaggerated some of our results; however, among the highlighted findings, the largest pairwise IBD score of 0.11 (indicating an association slightly more distant than first-cousins) was observed for 1 pair of NOTCH3 mutation carriers. Our scheme for selecting genes previously associated with AD, AD-related traits, or other dementias omitted important loci that were reported after we completed most of our analyses (eg, ADAM1763), ascertained through a connection to a nondementing illness (eg, TBK164), or do not have variants linked to late-onset AD (eg, TYROBP65). In addition, because we were unable to validate all rare variants reported in this study owing, in part, to availability of specimens containing these variants, some of the highlighted associations may be false-positives due to variant calling errors. However, most of these variants, including TREM2 Q33X and all of those in ABCD4, CELSR1, and GTSE1, have been reported previously.21
Although one of the explicit goals of the ADSP is to identify variants that protect against AD,23 the design corresponding to the one we used to identify risk variants (ie, a controls-only analysis) is less rigorous because, in the absence of statistically significant tests, it is difficult to demonstrate a protective effect if the variant has reduced penetrance.
Conclusions
We observed associations with novel variants in previously established AD genes and with several novel potential AD genes that did not emerge in previous analyses of a large WES data set using conventional statistical thresholds.7 Several of the results implicating novel AD genes—in particular, ABCD4, CELSR1, GTSE1—merit further epidemiologic and experimental studies. Our findings with the NOTCH3 and TREM2 variants suggest that mutations in the same gene can result in dissimilar types of dementia. Moreover, a variable dose of a particular mutation (ie, TREM2 Q33X) can cause different types of dementia. These findings suggest that minor differences in protein structure or amount of wild-type protein can result in different clinical outcomes. Understanding these genotype-phenotype associations may provide further insight into the pathogenic nature of the mutations, as well as offer clues for developing new therapeutic targets.
eMethods. Detailed Methodology
eReferences
eTable 1. Characteristics of Subjects in the ADSP WES Case-Control Dataset
eTable 2. Previously Established Genes for AD, AD-Related Traits and Other Dementias
eTable 3. Filtering Pipeline of Rare Variants
eTable 4. High and Moderate Impact Rare Variants in Previously Established AD Genes Occurring In ≥ 4 Participants With AD and No Controls
eTable 5. Characteristics of AD Subjects in the ADSP WES Dataset With the NOTCH3 rs149307620 Mutation
eTable 6. Characteristics of Subjects in the WGS Replication Datasets
eTable 7. Characteristics of AD Subjects With the TREM2 rs104894002 Mutation (Q33X)
eTable 8. High and Moderate Impact Rare Variants Genome-Wide Occurring in > 10 Participants With AD and No Controls
eTable 9. Genes With ≥ 3 Distinct High/Moderate Disease Impact Rare Variants Each With a MAC ≥ 5 and Occurring Only in Cases
eTable 10. High Impact Rare Variants Genome-Wide With a MAC ≥ 7 and Occurring Only in AD Cases
eFigure 1. Study Design
eFigure 2. Haplotype Analysis of the Rare NOTCH3 rs149307620 Variant
eFigure 3. Population Substructure of the ADSP Discovery Sample
eFigure 4. Utah Pedigree Segregating rs141402160 and rs140914494 Mutations
eFigure 5. Utah Pedigree Segregating rs112197217 Mutation
eFigure 6. Protein-Protein Interaction Network Including NOTCH3 and JAG1
eFigure 7. Haplotype Analysis of ABCD4 and CELSR1/GTSE1
References
- 1.Alzheimer’s Association 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14:-. doi: 10.1016/j.jalz.2018.02.001 [DOI] [Google Scholar]
- 2.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168-174. doi: 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- 3.Sims R, van der Lee SJ, Naj AC, et al. ; ARUK Consortium; GERAD/PERADES, CHARGE, ADGC, EADI . Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49(9):1373-1384. doi: 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerreiro R, Wojtas A, Bras J, et al. ; Alzheimer Genetic Analysis Group . TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117-127. doi: 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logue MW, Schu M, Vardarajan BN, et al. ; Alzheimer’s Disease Genetics Consortium . Two rare AKAP9 variants are associated with Alzheimer’s disease in African Americans. Alzheimers Dement. 2014;10(6):609-618.e11. doi: 10.1016/j.jalz.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzel-Smith MK, Hunkapiller J, Bhangale TR, et al. ; Alzheimer’s Disease Genetics Consortium . A rare mutation in UNC5C predisposes to late-onset Alzheimer’s disease and increases neuronal cell death. Nat Med. 2014;20(12):1452-1457. doi: 10.1038/nm.3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bis JC, Jian X, Kunkle BW, et al. ; Alzheimer’s Disease Sequencing Project . Whole exome sequencing study identifies novel rare and common Alzheimer’s-associated variants involved in immune response and transcriptional regulation [published online August 14, 2018]. Mol Psychiatry. doi: 10.1038/s41380-018-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Zhu C, Beecham G, et al. A rare missense variant in CASP7 is associated with familial late-onset Alzheimer disease [published online January 3, 2019]. Alzheimers Dement. doi: 10.1016/j.jalz.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263-265. doi: 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- 12.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403-410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 13.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195-201. doi: 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- 14.Release S. 2018-3: Maestro. New York, NY: Schrödinger LLC; 2018. [Google Scholar]
- 15.Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362-D368. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mi H, Huang X, Muruganujan A, et al. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45(D1):D183-D189. doi: 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83(3):311-321. doi: 10.1016/j.ajhg.2008.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauwe JSK, Ridge PG, Foster NL, Cannon-Albright LA. Strong evidence for a genetic contribution to late-onset Alzheimer’s disease mortality: a population-based study. PLoS One. 2013;8(10):e77087. doi: 10.1371/journal.pone.0077087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi: 10.1038/383707a0 [DOI] [PubMed] [Google Scholar]
- 21.Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium . A global reference for human genetic variation. Nature. 2015;526(7571):68-74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa MD, Pereira JB, Pala M, et al. A substantial prehistoric European ancestry amongst Ashkenazi maternal lineages. Nat Commun. 2013;4:2543. doi: 10.1038/ncomms3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beecham GW, Bis JC, Martin ER, et al. The Alzheimer’s Disease Sequencing Project: study design and sample selection. Neurol Genet. 2017;3(5):e194. doi: 10.1212/NXG.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nho K, Horgusluoglu E, Kim S, et al. ; ADNI . Integration of bioinformatics and imaging informatics for identifying rare PSEN1 variants in Alzheimer’s disease. BMC Med Genomics. 2016;9(suppl 1):30. doi: 10.1186/s12920-016-0190-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luca VC, Kim BC, Ge C, et al. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;355(6331):1320-1324. doi: 10.1126/science.aaf9739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joutel A, Vahedi K, Corpechot C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350(9090):1511-1515. doi: 10.1016/S0140-6736(97)08083-5 [DOI] [PubMed] [Google Scholar]
- 27.Oliveri RL, Muglia M, De Stefano N, et al. A novel mutation in the Notch3 gene in an Italian family with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: genetic and magnetic resonance spectroscopic findings. Arch Neurol. 2001;58(9):1418-1422. doi: 10.1001/archneur.58.9.1418 [DOI] [PubMed] [Google Scholar]
- 28.Ghezzi L, Carandini T, Arighi A, et al. Evidence of CNS β-amyloid deposition in Nasu-Hakola disease due to the TREM2 Q33X mutation. Neurology. 2017;89(24):2503-2505. doi: 10.1212/WNL.0000000000004747 [DOI] [PubMed] [Google Scholar]
- 29.Kauwe JSK, Jacquart S, Chakraverty S, et al. Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer’s disease presenilin 1 mutation. Ann Neurol. 2007;61(5):446-453. doi: 10.1002/ana.21099 [DOI] [PubMed] [Google Scholar]
- 30.Guerreiro RJ, Baquero M, Blesa R, et al. Genetic screening of Alzheimer’s disease genes in Iberian and African samples yields novel mutations in presenilins and APP. Neurobiol Aging. 2010;31(5):725-731. doi: 10.1016/j.neurobiolaging.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández MV, Black K, Carrell D, et al. ; NIA-LOAD family study group, NCRAD . SORL1 variants across Alzheimer’s disease European American cohorts. Eur J Hum Genet. 2016;24(12):1828-1830. doi: 10.1038/ejhg.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindquist SG, Holm IE, Schwartz M, et al. Alzheimer disease-like clinical phenotype in a family with FTDP-17 caused by a MAPT R406W mutation. Eur J Neurol. 2008;15(4):377-385. doi: 10.1111/j.1468-1331.2008.02069.x [DOI] [PubMed] [Google Scholar]
- 33.Vardarajan BN, Ghani M, Kahn A, et al. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann Neurol. 2015;78(3):487-498. doi: 10.1002/ana.24466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168-177. doi: 10.1038/ng1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jun G, Ibrahim-Verbaas CA, Vronskaya M, et al. ; IGAP Consortium . A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016;21(1):108-117. doi: 10.1038/mp.2015.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghavan NS, Brickman AM, Andrews H, et al. ; Alzheimer’s Disease Sequencing Project . Whole-exome sequencing in 20,197 persons for rare variants in Alzheimer’s disease. Ann Clin Transl Neurol. 2018;5(7):832-842. doi: 10.1002/acn3.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107-116. doi: 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruchaga C, Kauwe JSK, Harari O, et al. ; GERAD Consortium; Alzheimer’s Disease Neuroimaging Initiative (ADNI); Alzheimer Disease Genetic Consortium (ADGC) . GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013;78(2):256-268. doi: 10.1016/j.neuron.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. ; European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452-1458. doi: 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reitz C, Jun G, Naj A, et al. ; Alzheimer Disease Genetics Consortium . Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4,and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483-1492. doi: 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrer LA. Expanding the genomic roadmap of Alzheimer’s disease. Lancet Neurol. 2015;14(8):783-785. doi: 10.1016/S1474-4422(15)00146-5 [DOI] [PubMed] [Google Scholar]
- 42.Wheeler DL, Barrett T, Benson DA, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2007;35(database issue):D5-D12. doi: 10.1093/nar/gkl1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muiño E, Gallego-Fabrega C, Cullell N, et al. Systematic review of cysteine-sparing NOTCH3 missense mutations in patients with clinical suspicion of CADASIL. Int J Mol Sci. 2017;18(9):E1964. doi: 10.3390/ijms18091964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu X, He W, Luo X, Tsubota KE, Yan R. BACE1 regulates hippocampal astrogenesis via the Jagged1-Notch pathway. Cell Rep. 2013;4(1):40-49. doi: 10.1016/j.celrep.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konishi J, Kawaguchi KS, Vo H, et al. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67(17):8051-8057. doi: 10.1158/0008-5472.CAN-07-1022 [DOI] [PubMed] [Google Scholar]
- 46.Guerreiro RJ, Lohmann E, Kinsella E, et al. Exome sequencing reveals an unexpected genetic cause of disease: NOTCH3 mutation in a Turkish family with Alzheimer’s disease. Neurobiol Aging. 2012;33(5):1008.e17-1008.e23. doi: 10.1016/j.neurobiolaging.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landrum MJ, Lee JM, Benson M, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44(D1):D862-D868. doi: 10.1093/nar/gkv1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sassi C, Nalls MA, Ridge PG, et al. ; ARUK Consortium . Mendelian adult-onset leukodystrophy genes in Alzheimer’s disease: critical influence of CSF1R and NOTCH3. Neurobiol Aging. 2018;66:179.e17-179.e29. doi: 10.1016/j.neurobiolaging.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuyvers E, Bettens K, Philtjens S, et al. ; BELNEU consortium . Investigating the role of rare heterozygous TREM2 variants in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging. 2014;35(3):726.e11-726.e19. doi: 10.1016/j.neurobiolaging.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 50.Jin SC, Benitez BA, Karch CM, et al. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet. 2014;23(21):5838-5846. doi: 10.1093/hmg/ddu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerreiro RJ, Lohmann E, Brás JM, et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol. 2013;70(1):78-84. doi: 10.1001/jamaneurol.2013.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerreiro R, Bilgic B, Guven G, et al. Novel compound heterozygous mutation in TREM2 found in a Turkish frontotemporal dementia-like family. Neurobiol Aging. 2013;34(12):2890.e1-2890.e5. doi: 10.1016/j.neurobiolaging.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coelho D, Kim JC, Miousse IR, et al. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat Genet. 2012;44(10):1152-1155. doi: 10.1038/ng.2386 [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Liu S, Ji L, et al. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: a case-control study. Curr Alzheimer Res. 2015;12(1):88-94. doi: 10.2174/1567205012666141218144035 [DOI] [PubMed] [Google Scholar]
- 55.Robinson A, Escuin S, Doudney K, et al. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33(2):440-447. doi: 10.1002/humu.21662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada Y, Fuku N, Tanaka M, et al. Identification of CELSR1 as a susceptibility gene for ischemic stroke in Japanese individuals by a genome-wide association study. Atherosclerosis. 2009;207(1):144-149. doi: 10.1016/j.atherosclerosis.2009.03.038 [DOI] [PubMed] [Google Scholar]
- 57.Boutin C, Goffinet AM, Tissir F. Celsr1–3 cadherins in PCP and brain development In: Yang Y, ed. Current Topics in Developmental Biology. Cambridge, MA: Academic Press; 2012:161-183. [DOI] [PubMed] [Google Scholar]
- 58.Raghavendra Prasad HS, Qi Z, Srinivasan KN, Gopalakrishnakone P. Potential effects of tetrodotoxin exposure to human glial cells postulated using microarray approach. Toxicon. 2004;44(6):597-608. doi: 10.1016/j.toxicon.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 59.Narindrasorasak S, Lowery DE, Altman RA, Gonzalez-DeWhitt PA, Greenberg BD, Kisilevsky R. Characterization of high affinity binding between laminin and Alzheimer’s disease amyloid precursor proteins. Lab Invest. 1992;67(5):643-652. [PubMed] [Google Scholar]
- 60.Palu E, Liesi P. Differential distribution of laminins in Alzheimer disease and normal human brain tissue. J Neurosci Res. 2002;69(2):243-256. doi: 10.1002/jnr.10292 [DOI] [PubMed] [Google Scholar]
- 61.Saad M, Brkanac Z, Wijsman EM. Family-based genome scan for age at onset of late-onset Alzheimer’s disease in whole exome sequencing data. Genes Brain Behav. 2015;14(8):607-617. doi: 10.1111/gbb.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy S, Das S, Kretzschmar W, et al. ; Haplotype Reference Consortium . A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279-1283. doi: 10.1038/ng.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartl D, May P, Gu W, et al. ; AESG . A rare loss-of-function variant of ADAM17 is associated with late-onset familial Alzheimer disease [published online July 9, 2018]. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freischmidt A, Wieland T, Richter B, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci. 2015;18(5):631-636. doi: 10.1038/nn.4000 [DOI] [PubMed] [Google Scholar]
- 65.Zhang B, Gaiteri C, Bodea LG, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707-720. doi: 10.1016/j.cell.2013.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methodology
eReferences
eTable 1. Characteristics of Subjects in the ADSP WES Case-Control Dataset
eTable 2. Previously Established Genes for AD, AD-Related Traits and Other Dementias
eTable 3. Filtering Pipeline of Rare Variants
eTable 4. High and Moderate Impact Rare Variants in Previously Established AD Genes Occurring In ≥ 4 Participants With AD and No Controls
eTable 5. Characteristics of AD Subjects in the ADSP WES Dataset With the NOTCH3 rs149307620 Mutation
eTable 6. Characteristics of Subjects in the WGS Replication Datasets
eTable 7. Characteristics of AD Subjects With the TREM2 rs104894002 Mutation (Q33X)
eTable 8. High and Moderate Impact Rare Variants Genome-Wide Occurring in > 10 Participants With AD and No Controls
eTable 9. Genes With ≥ 3 Distinct High/Moderate Disease Impact Rare Variants Each With a MAC ≥ 5 and Occurring Only in Cases
eTable 10. High Impact Rare Variants Genome-Wide With a MAC ≥ 7 and Occurring Only in AD Cases
eFigure 1. Study Design
eFigure 2. Haplotype Analysis of the Rare NOTCH3 rs149307620 Variant
eFigure 3. Population Substructure of the ADSP Discovery Sample
eFigure 4. Utah Pedigree Segregating rs141402160 and rs140914494 Mutations
eFigure 5. Utah Pedigree Segregating rs112197217 Mutation
eFigure 6. Protein-Protein Interaction Network Including NOTCH3 and JAG1
eFigure 7. Haplotype Analysis of ABCD4 and CELSR1/GTSE1