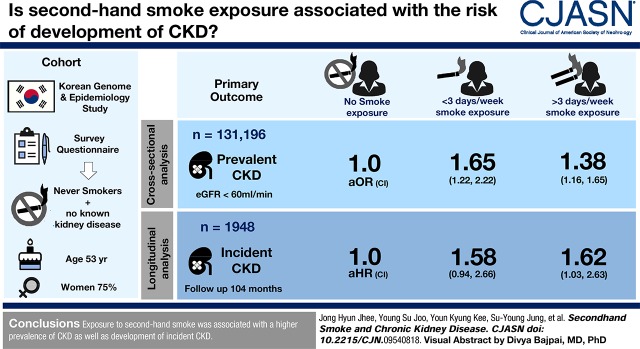
Visual Abstract
Keywords: chronic kidney disease; clinical epidemiology; glomerular filtration rate; risk factors; Tobacco Smoke Pollution; Odds Ratio; Cross-Sectional Studies; Prevalence; Confidence Intervals; Follow-Up Studies; Smokers; Smoking; Risk; Epidemiologic Studies; Renal Insufficiency, Chronic
Abstract
Background and objectives
Active smoking is associated with higher risk of various diseases. However, the risk of CKD development in nonsmokers exposed to secondhand smoke is not well elucidated. We aimed to investigate the association between secondhand smoke exposure and the risk of CKD development among never-smokers.
Design, setting, participants, & measurements
A total of 131,196 never-smokers with normal kidney function, who participated in the Korean Genome and Epidemiology Study from 2001 to 2014, were analyzed. The participants were classified into three groups on the basis of frequency of secondhand smoke exposure, assessed with survey questionnaires; no exposure, <3 days per week, and ≥3 days per week. The association between secondhand smoke and CKD, defined as eGFR<60 ml/min per 1.73 m2, was examined in the cross-sectional analysis. In addition, the risk of incident CKD development was analyzed in a longitudinal cohort of 1948 participants without CKD at baseline, which was a subset of the main cohort.
Results
The mean age of participants was 53 years, and 75% were women. Prevalent CKD was observed in 231 (1.8%), 64 (1.7%), and 2280 (2.0%) participants in the ≥3 days per week, <3 days per week, and no exposure groups. The odds ratio (OR) of prevalent CKD was significantly higher in the groups exposed to secondhand smoke than the no exposure group (<3 days per week: OR, 1.72; 95% confidence interval [95% CI], 1.30 to 2.27; and ≥3 days per week: OR, 1.44; 95% CI, 1.22 to 1.70). During a mean follow-up of 104 months, CKD occurred in 319 (16%) participants. Multivariable Cox analysis revealed that the risk for CKD development was higher in participants exposed to secondhand smoke than the no exposure group (<3 days per week: hazard ratio, 1.59; 95% CI, 0.96 to 2.65; and ≥3 days per week: hazard ratio, 1.66; 95% CI, 1.03 to 2.67).
Conclusions
Exposure to secondhand smoke was associated with a higher prevalence of CKD as well as development of incident CKD.
Introduction
The number of patients with CKD is rapidly increasing worldwide (1–3). CKD has become a global health problem because of its association with poorer quality of life and higher mortality risk for patients (4,5). Considering that established CKD is an irrecoverable condition, identifying modifiable factors and applying early interventions are crucial to reducing the burden of CKD.
Cigarette smoking is associated with a higher risk of various diseases, including cancer, lung diseases, and cardiovascular diseases (6–8). Reports on the association of smoking with CKD have shown mixed results. Several investigations failed to find a significant relationship between cigarette smoking and kidney function decline (9–11). However, a large population-based study of 65,589 participants showed that smoking was a significant risk factor for future kidney failure. In addition, a recent analysis of 15 cohort studies showed that cigarette smoking is independently associated with CKD (12). These recent investigations support the hypothesis that active smoking may be a notable risk factor for kidney function decline.
Because of these adverse effects, legislative actions have been taken to prohibit smoking in public areas and workplaces. Nonetheless, despite these measures, the prevalence of secondhand smoke exposure is still increasing in some countries. The Korea National Health and Nutrition Examination Survey reported that the prevalence of secondhand smoke exposure in the workplace has steadily increased between 2005 and 2013 (13). Exposure to secondhand smoke has been reported to be associated with a higher risk of several types of cancer and cardiovascular disease (14,15). In addition, recent data have shown that side-stream smoke, which is the main component of secondhand smoke, comprises more toxic substances than those found in mainstream smoke, postulating that secondhand smoke exposure could also cause serious harm (16).
Unfortunately, despite the potential toxic effect of secondhand smoke, the association between secondhand smoke exposure and the risk of CKD development has not been well elucidated. Therefore, this study aimed to investigate the association between secondhand smoke exposure and the risk of CKD development in never-smoking adults by using a large community-based cohort comprising participants with normal kidney function.
Materials and Methods
Study Design and Participants
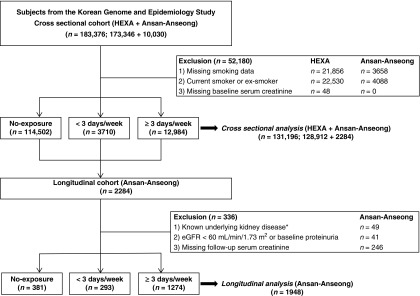
This study used data from the Korean Genome and Epidemiology Study (KoGES), a community-based cohort study. The detailed profile and methods concerning the development of KoGES has been described previously (17). The KoGES study is composed of two geographically distinctive cohorts, the KoGES_health examinees (KoGES_HEXA) study and the KoGES_Ansan-Anseong study (17). The KoGES_HEXA study includes participants residing in metropolitan cities of Korea. The participants of the KoGES_Ansan-Anseong study are from a medium-sized city (Ansan) and a rural area (Anseong) near Seoul, Korea. For the cross-sectional analysis, baseline data from the KoGES_HEXA study and the KoGES_Ansan-Anseong study were used. The KoGES_Ansan-Anseong study data were used for the longitudinal analysis. A total of 183,376 participants (173,326 from the KoGES_HEXA study and 10,030 from the KoGES_Ansan-Anseong study) aged 40–69 years were initially screened. For the cross-sectional analysis, participants with information on smoking history were initially screened. Those with missing smoking data or serum creatinine levels, and those who were current or ex-smokers were excluded. A total of 131,196 participants were included in the final analysis (Figure 1). Participants included in the KoGES_Ansan-Anseong study underwent serial health examinations and surveys biennially from 2001 to 2014. After excluding those whose baseline eGFR was <60 ml/min per 1.73 m2, those with underlying kidney disease or positive for proteinuria, and those without follow-up creatinine data, a total of 1948 individuals were analyzed for the longitudinal analysis. The presence of underlying kidney disease was surveyed by a questionnaire asking whether the participants had been previously diagnosed with CKD. All participants were enrolled in the study voluntarily, and informed consent was obtained in all cases. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Yonsei University Health System Clinical Trial Center (approval no. 4–2016–0900).
Figure 1.
Study participants were grouped according to secondhand smoke exposure frequency. *Presence of underlying kidney disease was surveyed through a questionnaire asking whether the participants had been previously diagnosed with CKD.
Smoking and Secondhand Smoke Assessments
Smoking status was assessed with the following questions: “Have you ever smoked?” and “Do you currently smoke?”. If a participant answered that they currently smoke, they were categorized as “current smokers.” If a participant answered that they do not currently smoke but had a history of ever smoking, they were classified as “ex-smokers.” If a participant answered that they do not currently smoke and have never had a history of smoking, they were classified as “never-smokers.”
Among never-smokers, exposure to secondhand smoke was surveyed with the question “Have you been exposed to cigarette smoke from other smokers?”. Never-smokers who were not exposed to secondhand smoke were allocated as the no exposure group. The frequency of secondhand smoke exposure was defined by the answers to the question “How many days per week have you been exposed to cigarette smoke?”. Participants were to select from the given choices of none, <3 days per week, ≥3 days per week, or every day. According to the answers, participants were classified into three groups: no exposure, <3 days per week, or ≥3 days per week. The overall response rate of the smoking status assessment survey was 86%.
Study Outcome
For the cross-sectional analysis, the association between secondhand smoke exposure and prevalent CKD was evaluated. For the longitudinal analysis, the primary end point was incident CKD development. CKD was defined as eGFR<60 ml/min per 1.73 m2. Serum creatinine level was measured by Jaffe assay. The samples were analyzed in two KoGES central laboratories and the intra- and interlaboratory reliability of the serological parameters have been previously confirmed (18). eGFR was calculated by the CKD Epidemiology Collaboration equation (19). In the longitudinal cohort, serum creatinine levels were measured biennially. The dipstick urine test was done, and proteinuria was classified on the basis of a color scale that quantified proteinuria as absent, trace, 1+, 2+, or 3+. This scale correlates approximately with urine protein levels of <10, 10–20, >30, >100, and >500 mg/dl, respectively (18). Proteinuria was determined as present if the results of the dipstick urine test were higher than trace levels. For detailed methods, see Supplemental Material.
Statistical Analyses
All statistical analyses were performed with IBM SPSS software for Windows, version 23.0 (IBM Corporation, Armonk, NY). Each variable was tested for normality before the statistical analysis, using the Kolmogorov–Smirnov test with Lilliefors correction and the Shapiro–Wilk test. Adjusted mean values of eGFR were produced by general linear models adjusted for age, sex, body mass index, systolic BP, history of hypertension, and diabetes. Multivariable logistic regressions were used to analyze the association of secondhand smoke exposure with CKD. The cumulative kidney survival rates were estimated with Kaplan–Meier analysis and a log-rank test. Survival time was defined as the interval between baseline and outcome or the last follow-up. Individuals who were lost to follow-up or death were censored at the date of the last examination. Cox proportional hazards analyses were performed to determine the independent association of secondhand smoke with the development of incident CKD. Proportional hazards assumptions were confirmed using Schoenfeld residuals (Supplemental Figure 1). Multivariable models were constructed with adjustment for confounding factors. Variables that showed statistical significance in univariable regression analyses were selected for the multivariable model. The causal diagram model included variables that have been selected from a “causal diagram,” which was used to choose variables with appropriate directional association (Supplemental Figure 2) (20). When dealing with missing covariate values, the pairwise deletion method was applied (21). Missing serum creatinine data in the longitudinal analysis were imputed with the values of the last observation. The missing data rates were <5% for the variables in the cross-sectional analysis. For the longitudinal analysis, expected and observed creatinine values were counted. For all analyses, a P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of the participants are described in Table 1. The mean age was 53±8 years and 75% were women. The average eGFR was 92±14 ml/min per 1.73 m2. A total of 114,502 (74%) participants were included in the no exposure group. Among those exposed to secondhand smoke, 12,984 (78%) participants were exposed to secondhand smoke ≥3 days per week, and 3710 (22%) participants were exposed <3 days per week. Participants were more often exposed to secondhand smoke at home (n=12,683, 76%) than at work (n=6674, 40%). Total duration of secondhand smoke exposure tended to be significantly longer in the secondhand smoke ≥3 days per week (22±13 years) than <3 days per week (17±12 years) group. The secondhand smoke exposure groups tended to be younger, include more female participants, and have higher body mass index than those in the no exposure group.
Table 1.
Baseline characteristics of participants in the Korean Genome and Epidemiology study
| Secondhand Smoke Frequency | ||||
|---|---|---|---|---|
| Characteristics | Total, n=131,196 | No Exposure, n=114,502 | <3 d/wk, n=3710 | ≥3 d/wk, n=12,984 |
| Secondhand smoke exposure | ||||
| Location, % | ||||
| At home only | N/A | 1924 (52) | 8096 (62) | |
| At work only | N/A | 1367 (37) | 2644 (21) | |
| At both home and work | 419 (11) | 2244 (17) | ||
| Total duration, yra | N/A | 17±12 | 22±13 | |
| Demographic data | ||||
| Age, yr | 53±8 | 54±8 | 51±8 | 52±8 |
| Women | 115,082 (75) | 86,790 (76) | 2963 (80) | 11,576 (89) |
| BMI, kg/m2 | 24±3 | 24±3 | 24±3 | 24±3 |
| Waist-to-hip ratio | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 |
| Alcohol status, ever | 67,464 (44) | 45,358 (40) | 1750 (48) | 5253 (41) |
| Physical activity, yes | 77,756 (52) | 58,586 (53) | 1801 (55) | 5426 (48) |
| Married, yes | 125,737 (88) | 93,783 (88) | 2871 (88) | 9936 (89) |
| Education status | ||||
| ≤Elementary | 30,355 (20) | 22,393 (20) | 817 (22) | 3985 (31) |
| Middle–high school | 84,137 (55) | 61,490 (55) | 2018 (55) | 7094 (55) |
| ≥College | 37,793 (25) | 28,964 (26) | 841 (23) | 1776 (14) |
| Income | ||||
| Low | 17,046 (13) | 13,152 (13) | 385 (12) | 1834 (17) |
| Intermediate | 57,227 (43) | 41,534 (42) | 1366 (44) | 4871 (46) |
| High | 57,828 (44) | 43,287 (44) | 1356 (44) | 3945 (37) |
| Systolic BP, mm Hg | 122±16 | 122±16 | 121±16 | 122±17 |
| Diastolic BP, mm Hg | 76±10 | 76±10 | 76±10 | 77±11 |
| Comorbidities | ||||
| Hypertension | 29,486 (19) | 22,534 (20) | 581 (16) | 2314 (18) |
| Diabetes | 9731 (6) | 7411 (7) | 204 (6) | 707 (6) |
| Dyslipidemia | 13,756 (9) | 10,339 (9) | 241 (7) | 828 (7) |
| Cardiovascular events | 3485 (2) | 2717 (2) | 34 (1) | 118 (1) |
| Laboratory data | ||||
| eGFR, ml/min per 1.73 m2 | 91±13 | 91±14 | 90±14 | 90±14 |
| Proteinuriab | 7866 (6) | 5994 (6) | 137 (5) | 447 (5) |
| Hemoglobin, g/dl | 13.6±1.5 | 13.6±1.4 | 13.3±1.5 | 13.2±1.3 |
| Albumin, g/dl | 4.6±0.3 | 4.6±0.2 | 4.6±0.3 | 4.6±0.3 |
| Glucose, mg/dl | 94±21 | 95±21 | 93±21 | 93±22 |
| Hemoglobin A1c, % | 5.7±0.7 | 5.7±0.7 | 5.7±0.8 | 5.7±0.9 |
| Total cholesterol, mg/dl | 198±36 | 198±36 | 197±35 | 198±36 |
| Triglyceride, mg/dl | 123±86 | 123±85 | 129±94 | 120±81 |
| HDL cholesterol, mg/dl | 54±13 | 54±13 | 54±13 | 55±13 |
| LDL cholesterol, mg/dl | 119±33 | 119±33 | 117±33 | 119±32 |
| hs-CRP, mg/dl | 0.1 [0.0–0.1] | 0.1 [0.0–0.1] | 0.1 [0.0–0.1] | 0.1 [0.0–0.1] |
Data are presented as mean±SD, median [interquartile range], or n (%). N/A, not applicable; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein.
The information of total duration of secondhand smoke exposure during lifetime was examined in 125,371 out of 131,196 (95%) participants.
Proteinuria was determined as present if the results of the dipstick urine test were higher than trace levels.
Baseline eGFR According to Frequency of Secondhand Smoke Exposure
When the mean baseline eGFR adjusted for confounding factors was compared among the secondhand smoke exposure frequency groups, the mean eGFR was 88 (95% CI, 77–88), 89 (95% CI, 88–89), and 91 (95% CI, 90–93) ml/min per 1.73 m2 in the <3 days per week, >3 days per week, and no exposure groups, respectively.
Association between Secondhand Smoke Frequency and Prevalent CKD
To examine the association between prevalent CKD and secondhand smoke exposure frequency, logistic regression analyses were performed. Prevalent CKD was observed in 231 (1.8%), 64 (1.7%), and 2280 (2.0%) cases in the ≥3 days per week, <3 days per week, and no exposure groups, respectively. The adjusted odds ratio (OR) for prevalent CKD was significantly higher in participants with secondhand smoke exposure than those without exposure (OR, 1.48; 95% confidence interval [95% CI], 1.25 to 1.74). When participants were stratified by frequency of secondhand smoke exposure, the <3 days per week (OR, 1.72; 95% CI, 1.30 to 2.27) group and ≥3 days per week (OR, 1.44; 95% CI, 1.22 to 1.70) group were associated with higher ORs compared with the no exposure group (Table 2). A similar association was found when CKD was defined as a composite of eGFR<60 ml/min per 1.73 m2 and/or development of proteinuria (Supplemental Table 1).
Table 2.
Relative risk for the prevalence of CKD at baseline according to frequency of secondhand smoke
| Variables | No. (%) with CKD | Multivariable Model | Causal Diagram Model |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Secondhand smoke (yes versus no) | |||
| No exposure | 2280 (2.0) | 1.00 (Reference) | 1 (Reference) |
| Exposure | 295 (1.8) | 1.44 (1.23 to 1.68) | 1.48 (1.25 to 1.74) |
| Secondhand smoke frequency | |||
| No exposure | 2280 (2.0) | 1.00 (Reference) | 1 (Reference) |
| <3 d/wk | 64 (1.7) | 1.65 (1.22 to 2.22) | 1.72 (1.30 to 2.27) |
| ≥3 d/wk | 231 (1.8) | 1.38 (1.16 to 1.65) | 1.44 (1.22 to 1.70) |
Multivariable model: adjustment for age, sex, body mass index, systolic BP, history of hypertension, history of diabetes, alcohol status, education levels, income levels, marital status, hemoglobin, and serum albumin. Causal diagram model: adjustment for age, sex, history of hypertension, history of diabetes, education levels, income levels, and marital status. OR, odds ratio; 95% CI, 95% confidence interval.
In an additional analysis of 125,371 participants for whom secondhand smoke exposure duration information was available, prevalent CKD was significantly associated with lifetime secondhand smoke exposure duration when the duration was longer than 10 years. In addition, a dose-response relationship was found with increasing exposure duration (Supplemental Table 2).
Association of Secondhand Smoke Frequency with Incident CKD Development
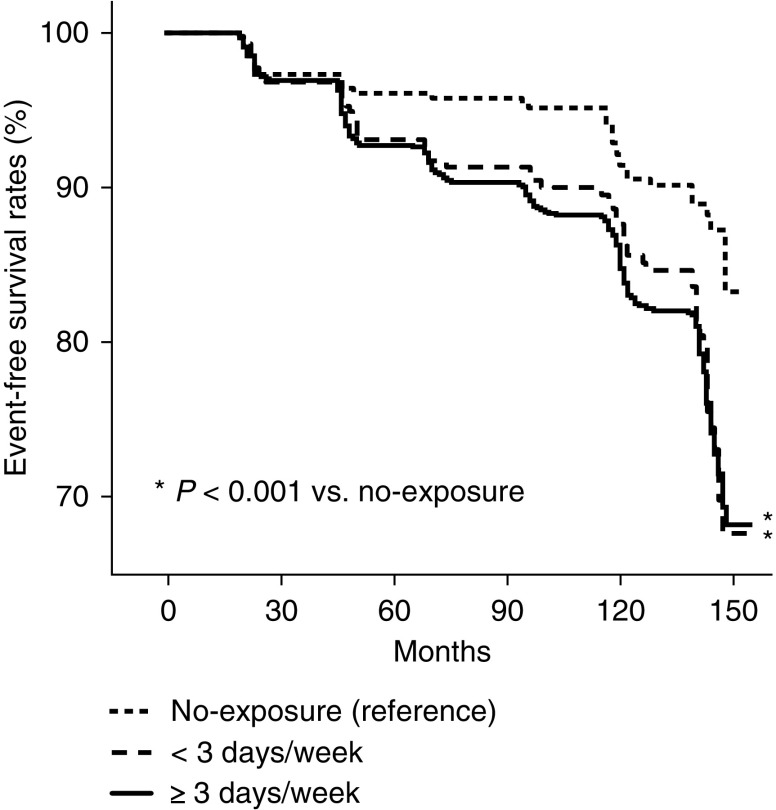
To further validate the association between kidney function and secondhand smoke exposure, the risk of secondhand smoke exposure on incident CKD development was evaluated in a longitudinal subgroup of 1948 individuals. In this subgroup, 1567 (80%) of participants were exposed to secondhand smoke. During a mean follow-up duration of 104±52 months, CKD newly developed in 319 (16%) individuals. The numbers of expected and observed serum creatinine values were 5.5 and 5.2 per participant, respectively. When multivariable Cox proportional hazards regression analysis was performed, the risk for incident CKD development was significantly higher in those exposed to secondhand smoke than those without exposure (hazard ratio [HR], 1.64; 95% CI, 1.03 to 2.60). Furthermore, those exposed to secondhand smoke <3 days per week (HR, 1.59; 95% CI, 0.96 to 2.65) or ≥3 days per week (HR, 1.66; 95% CI, 1.03 to 2.67) were associated with higher risk for incident CKD compared with those not exposed to secondhand smoke (Table 3). The proportional hazards assumption held reasonably for secondhand smoke exposure (Supplemental Figure 2). Factors associated with CKD in the univariable analyses are shown in Supplemental Table 3. Kaplan–Meier plots showed that the time to incident CKD development was significantly longer in the no exposure group than in groups exposed to secondhand smoke, regardless of exposure frequency (Figure 2).
Table 3.
Relative risk for incident CKD according to secondhand smoke exposure
| Variables | No. of Cases (%) | Number at Risk | Multivariable Model | Causal Diagram Model |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Secondhand smoke (yes versus no) | ||||
| No exposure | 35 (9) | 381 | 1.00 (Reference) | 1.00 (Reference) |
| Exposure | 319 (16) | 1948 | 1.61 (1.02 to 2.59) | 1.64 (1.03 to 2.60) |
| Secondhand smoke frequency | ||||
| No exposure | 35 (9) | 381 | 1.00 (Reference) | 1.00 (Reference) |
| <3 d/wk | 54 (18) | 293 | 1.58 (0.94 to 2.66) | 1.59 (0.96 to 2.65) |
| ≥3 d/wk | 230 (18) | 1274 | 1.62 (1.03 to 2.63) | 1.66 (1.03 to 2.67) |
Multivariable model: adjustment for age, sex, body mass index, systolic BP, history of hypertension, history of diabetes, alcohol status, education levels, income levels, marital status, hemoglobin, and serum albumin. Causal diagram model: adjustment for age, sex, history of hypertension, history of diabetes, education levels, income levels, and marital status. HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 2.
Time to CKD development was longer in secondhand smoke non-exposed than exposed participants. Kaplan–Meier plot of incident CKD development according to secondhand smoke exposure frequency in 1948 participants with follow-up data.
Discussion
In this study, the prevalence of secondhand smoke exposure among nonsmokers was surveyed and the association with CKD development was investigated in individuals with normal eGFR. Despite public health regulations prohibiting smoking in public places, a considerable proportion of the nonsmoking population was found to be exposed to secondhand smoke. Baseline eGFR was lower and the relative risk for the prevalence of CKD was significantly higher in those with secondhand smoke exposure. In addition, secondhand smoke was significantly associated with higher risk of incident CKD development, and this risk was evident even in individuals with less frequent secondhand smoke exposure.
To our knowledge, this is the first study to demonstrate a significant association between secondhand smoke and CKD development. Previously, in a cross-sectional analysis of 15,535 patients with hypertension, a history of secondhand smoke exposure was found to be associated with presence of albuminuria (22). In addition, another small investigation showed that serum cotinine, a nicotine metabolite, and urine albumin levels were higher in passive smokers compared with nonsmokers, suggesting that glomerular function could be affected by secondhand smoke exposure (23). Although these investigations have raised suspicion that exposure to secondhand smoke could have an influence on kidney function, the direct relationship has not been evaluated in a prospective observation of a large-scale cohort.
The proportion of participants who were exposed to secondhand smoke was considerably high (almost half of the nonsmoking individuals). Worldwide, the estimated proportions of men and women exposed to secondhand smoke in 2004 were 33% and 35%, respectively (24). The proportions of secondhand smoke exposure differ among countries because of different cultural background and policies regarding smoke-free areas (25–29). The regional variation of secondhand smoke exposure rate is known to range from <15% in Africa to >50% in the European and Western Pacific regions (24). As extensive legislative restrictions to prohibit smoking in public areas have been enacted in Korea since 2007, there is a possibility that the secondhand smoke exposure rate could have decreased recently. Nonetheless, the fact that most of the secondhand smoke exposure was household-oriented rather than from the workplace reduces the likelihood that these policies may not have had a large effect in lowering the rate of secondhand smoke exposure. In addition to legal restrictions, education and efforts to increase community consciousness should be provided to reduce household smoking rates.
In a previous meta-analysis of 15 cohort studies, the relative risk of CKD development in ever-smokers was 1.27 compared with nonsmokers (12). This higher risk in ever-smokers is comparable with the relative risk associated with secondhand smoke exposure found in this study. In addition, the OR for CKD prevalence in participants exposed to secondhand smoke in this study was also similar to that of ever-smokers (1.37; 95% CI, 1.22 to 1.53; Supplemental Table 4). These findings suggest that the association of CKD with secondhand smoke exposure could be at least similar to its relationship with active smoking. Although active smoking is an intermittent activity, nonsmokers are commonly exposed to secondhand smoke from multiple smokers. Therefore, some nonsmokers exposed to secondhand smoke could be exposed to a similar dose of cigarette smoke to active smokers. However, a subgroup analysis stratified by location of exposure showed a lack of association between secondhand smoke exposure and CKD in those exposed ≥3 days per week at work (Supplemental Table 5). The fact that those exposed at work were younger, had less comorbidities, and were exposed for a shorter total duration could account for this finding (Supplemental Table 6). Supporting this possibility, a clear dose-response relationship between CKD prevalence and lifetime secondhand smoke exposure duration was presented. These findings suggest that exposure duration than frequency could be a more considerable factor associated with kidney function. Nevertheless, further studies with more information on secondhand smoke exposure frequency or dose would be worthwhile.Interestingly, secondhand exposure rate was higher in the longitudinal analysis than in the cross-sectional analysis. Because of the geographic distinction, socioeconomic characteristics were also found to be different between the two cohorts (Supplemental Table 7). The participants from KoGES_Ansan-Anseong study were likely to be less educated and had lower income levels compared with the participants of the KoGES_HEXA study. Because the KoGES_HEXA study participants make up most of the cross-sectional cohort, the characteristics of the cross-section cohort are represented by those of the KoGES_HEXA study. Therefore, the geographic and demographic difference could have resulted in the observed secondhand smoke exposure difference between the cross-sectional and prospective cohort. This possibility is supported by a previous report showing that secondhand smoke exposure could vary depending on the characteristics of the society (30).
Several mechanisms are suspected to contribute to secondhand smoke–induced kidney function decline. There is evidence suggesting that exposure to environmental tobacco smoke decreases the serum antioxidant defense and impairs endothelial function in nonsmokers (31–34). Animal studies have shown that the amount of reactive oxygen species in kidney tissue significantly increased in mice exposed to cigarette smoke compared with mice not exposed to cigarette smoke (35). As inflammation and oxidative stress in the lungs have been found to affect the circulatory system, resulting in an increase in systemic oxidative stress (36), there is a possibility that secondhand smoke–induced respiratory reactive oxidative species could have resulted in kidney damage. In addition, a recent study showing that nicotine directly causes apoptosis of podocytes through the induction of oxidative stress suggested that the toxins in secondhand smoke could directly act on the kidney (34).
This study has a number of limitations. First, the exposure amount of secondhand smoke was not directly measured, but was on the basis of answers from questionnaires surveying the exposure frequency. Because most individuals are exposed to secondhand smoke at irregular intervals, unlike in active smoking, the amount of secondhand smoke exposure is difficult to quantify. Second, information regarding medication was not available. Relevant renoprotective medications that block the renin-angiotensin-aldosterone system, and potential harmful medications such as nonsteroidal anti-inflammatory drugs, would have had effect on kidney function. Future investigations including medication history would be necessary. Third, direct measurements of the accumulation of secondhand smoke–related toxic substances were not done. To more directly demonstrate the association between secondhand smoke and its adverse kidney effect, further investigations on serum cotinine or nicotine levels are needed.
In conclusion, exposure to secondhand smoke was significantly associated with higher risk of CKD development. Lowering the risk of secondhand smoke exposure by enhancing public smoking restriction policies and educating the public about the potential harm of household tobacco use could reduce the risk of CKD development in the nonsmoking population with normal kidney function.
Disclosures
None.
Supplementary Material
Acknowledgments
The epidemiologic data used in this study were obtained from the Korean Genome and Epidemiology Study (KoGES, 4851–302) of the Korea Centers for Disease Control and Prevention, Republic of Korea.
This research was supported by a grant from the Ministry for Health and Welfare, Republic of Korea. This study was also supported by a research grant from Inha University Hospital.
The funding source had no role in the conception of the study or the collection, analysis, and interpretation of the data, writing of the manuscript, or the decision to submit for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09540818/-/DCSupplemental.
Supplemental Table 1. The prevalence of CKD on the basis of eGFR and proteinuria definition.
Supplemental Table 2. Relative risk for the prevalence of CKD according to lifetime total duration of secondhand smoke.
Supplemental Table 3. Univariable associations between clinical factors and CKD in cross-sectional and prospective analysis.
Supplemental Table 4. Relative risk for the prevalence of CKD according to ever-smoker versus never-smoker.
Supplemental Table 5. Relative risk for the prevalence of CKD according to frequency of secondhand smoke exposure at home and work.
Supplemental Table 6. Baseline characteristics according to exposure location.
Supplemental Table 7. Baseline characteristics according to two different cohort datasets.
Supplemental Figure 1. Graphical evaluation of the proportional hazards assumption.
Supplemental Figure 2. Causal diagram showing assumed associations between baseline secondhand smoke, CKD, and baseline characteristics.
References
- 1.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Wildman RP, Gu D, Kusek JW, Spruill M, Reynolds K, Liu D, Hamm LL, Whelton PK, He J: Prevalence of decreased kidney function in Chinese adults aged 35 to 74 years. Kidney Int 68: 2837–2845, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC: Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol 14[7 Suppl 2]: S131–S138, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Yoon CY, Lee M, Kim SU, Lim H, Chang TI, Kee YK, Han SG, Han IM, Kwon YE, Park KS, Lee MJ, Park JT, Han SH, Ahn SH, Kang SW, Yoo TH: Fatty liver associated with metabolic derangement in patients with chronic kidney disease: A controlled attenuation parameter study. Kidney Res Clin Pract 36: 48–57, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staplin N, Haynes R, Herrington WG, Reith C, Cass A, Fellström B, Jiang L, Kasiske BL, Krane V, Levin A, Walker R, Wanner C, Wheeler DC, Landray MJ, Baigent C, Emberson J; SHARP Collaborative Group : Smoking and adverse outcomes in patients with CKD: The Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 68: 371–380, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orth SR, Hallan SI: Smoking: A risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients--absence of evidence or evidence of absence? Clin J Am Soc Nephrol 3: 226–236, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Jee SH, Ohrr H, Kim IS: Effects of husbands’ smoking on the incidence of lung cancer in Korean women. Int J Epidemiol 28: 824–828, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Webb L, Gilg J, Feest T, Fogarty D: UK Renal registry 13th annual report (december 2010): Chapter 4: Comorbidities and current smoking status amongst patients starting renal replacement therapy in England, Wales and Northern Ireland from 2008 to 2009. Nephron Clin Pract 119[Suppl 2]: c85–c96, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Goetz FC, Jacobs DR Jr., Chavers B, Roel J, Yelle M, Sprafka JM: Risk factors for kidney damage in the adult population of Wadena, Minnesota. A prospective study. Am J Epidemiol 145: 91–102, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Halimi JM, Philippon C, Mimran A: Contrasting renal effects of nicotine in smokers and non-smokers. Nephrol Dial Transplant 13: 940–944, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Xia J, Wang L, Ma Z, Zhong L, Wang Y, Gao Y, He L, Su X: Cigarette smoking and chronic kidney disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant 32: 475–487, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Kim Y, Park S, Lee J, Oh K: Trends in cigarette smoking among adolescents and adults in South Korea. Epidemiol Health 36: e2014023, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnoya J, Glantz SA: Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 111: 2684–2698, 2005 [DOI] [PubMed] [Google Scholar]
- 15.He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK: Passive smoking and the risk of coronary heart disease--a meta-analysis of epidemiologic studies. N Engl J Med 340: 920–926, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Schick S, Glantz S: Philip Morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob Control 14: 396–404, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Han BG; KoGES Group : Cohort profile: The Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol 46: e20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JJ, Yang JH, Kim J, Cho LY, Park B, Ma SH, Song SH, Min WK, Kim SS, Park MS, Park SK: Reliability of quadruplicated serological parameters in the korean genome and epidemiology study. Epidemiol Health 33: e2011004, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staplin N, Herrington WG, Judge PK, Reith CA, Haynes R, Landray MJ, Baigent C, Emberson J: Use of causal diagrams to inform the design and interpretation of observational studies: An example from the Study of Heart and Renal Protection (SHARP). Clin J Am Soc Nephrol 12: 546–552, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham JW: Missing data analysis: Making it work in the real world. Annu Rev Psychol 60: 549–576, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Hogan SL, Vupputuri S, Guo X, Cai J, Colindres RE, Heiss G, Coresh J: Association of cigarette smoking with albuminuria in the United States: The third National Health and Nutrition Examination Survey. Ren Fail 29: 133–142, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Dülger H, Dönder A, Sekeroğlu MR, Erkoç R, Ozbay B: Investigation of the relationship between serum levels of cotinine and the renal function in active and passive smokers. Ren Fail 33: 475–479, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A: Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 377: 139–146, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ; Comparative Risk Assessment Collaborating Group : Selected major risk factors and global and regional burden of disease. Lancet 360: 1347–1360, 2002 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization: WHO Report on the Global Tobacco Epidemic 2009: Implementing Smoke-Free Environments, 2009. Available at: http://www.who.int/tobacco/mpower/2009/en/. Accessed March 22, 2010
- 27.Eriksen MP, Cerak RL: The diffusion and impact of clean indoor air laws. Annu Rev Public Health 29: 171–185, 2008 [DOI] [PubMed] [Google Scholar]
- 28.McNabola A, Gill LW: The control of environmental tobacco smoke: A policy review. Int J Environ Res Public Health 6: 741–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sureda X, Fernández E, López MJ, Nebot M: Secondhand tobacco smoke exposure in open and semi-open settings: A systematic review. Environ Health Perspect 121: 766–773, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris JK, Geremakis C, Moreland-Russell S, Carothers BJ, Kariuki B, Shelton SC, Kuhlenbeck M: Demographic and geographic differences in exposure to secondhand smoke in Missouri workplaces, 2007-2008. Prev Chronic Dis 8: A135, 2011 [PMC free article] [PubMed] [Google Scholar]
- 31.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE: Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Holay MP, Paunikar NP, Joshi PP, Sahasrabhojney VS, Tankhiwale SR: Effect of passive smoking on endothelial function in: Healthy adults. J Assoc Physicians India 52: 114–117, 2004 [PubMed] [Google Scholar]
- 33.Kato T, Inoue T, Morooka T, Yoshimoto N, Node K: Short-term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Can J Physiol Pharmacol 84: 523–529, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, Danforth OM, Pinnamaneni K, Rodriguez HJ, Luu E, Sievers RE, Schick SF, Glantz SA, Springer ML: One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc 5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemmar A, Raza H, Subramaniyan D, Yasin J, John A, Ali BH, Kazzam EE: Short-term systemic effects of nose-only cigarette smoke exposure in mice: Role of oxidative stress. Cell Physiol Biochem 31: 15–24, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Sinden NJ, Stockley RA: Systemic inflammation and comorbidity in COPD: A result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 65: 930–936, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.