Abstract
Estimation of kidney function in patients with cancer directly affects drug dosing, agent selection, and eligibility for clinical trials of novel agents. Overestimation of kidney function may lead to overdosing or inappropriate agent selection and corresponding toxicity. Conversely, underestimation of kidney function may lead to underdosing or inappropriate agent exclusion and subsequent therapeutic failure. It would seem obvious that the most accurate estimates of kidney function should be used to reduce variability in decision making and ultimately, the therapeutic outcomes of toxicity and clinical benefit. However, clinical decision making is often more complex. The Cockcroft–Gault formula remains the most universally implemented estimator of kidney function in patients with cancer, despite its relative inaccuracy compared with the Chronic Kidney Disease Epidemiology Collaboration equation. The Chronic Kidney Disease Epidemiology Collaboration equation is a more precise estimator of kidney function; however, many currently used kidney function cutoff values were determined before the development of the Chronic Kidney Disease Epidemiology Collaboration equation and creatinine assay standardization using Cockcroft–Gault estimates. There is a need for additional studies investigating the validity of currently used estimates of kidney function in patients with cancer and the applicability of traditional anticancer dosing and eligibility guidelines to modern and more accurate estimates of kidney function. In this review, we consider contemporary calculation methods used to estimate kidney function in patients with cancer. We discuss the clinical implications of using these various methods, including the potential influence on drug dosing, drug selection, and clinical trial eligibility, using carboplatin and cisplatin as case studies.
Keywords: cancer, chemotherapy, cisplatin, clinical trial, Cockcroft-Gault, creatinine clearance, creatinine, glomerular filtration rate, kidney dysfunction, pharmacokinetics, renal dysfunction
Introduction
Patients with cancer often receive multiple narrow therapeutic index drugs, many of which are eliminated by the kidneys and therefore, exhibit decreased clearance in patients with impaired kidney function. Anticancer drugs are no exception; these drugs are highly toxic with narrow therapeutic indices. Their adverse effects are often severe, but they are typically manageable when patient exposure to the drug is prospectively estimated and doses are adjusted accordingly. Thus, accurate patient-specific dosing and agent selection on the basis of drug clearance and exposure are vital to ensure safety while maintaining anticancer activity.
Quantitative estimates of kidney function have been used for decades to guide patient suitability, drug selection, and dose adjustments for anticancer agents cleared by the kidney. Kidney function estimates are also used to determine eligibility for clinical trials of novel agents. This process is not without risk. Overestimation of kidney function may lead to overdosing or inappropriate agent selection, lower than expected clearance of the drug, and an unanticipated increase in systemic exposure, leading to a corresponding increase in toxicity. Conversely, underestimation of kidney function may lead to underdosing or inappropriate agent exclusion, higher than expected clearance of the drug, and an unanticipated decrease in systemic exposure, leading to therapeutic failure. Therefore, accurate and clinically practical estimates of kidney function are required to optimize clinical outcomes in all patients but especially those receiving anticancer agents for which the adverse effect profile can be severe and maximal dosing may be important to optimize anticancer response.
In this review, we present contemporary bedside calculation methods used to estimate kidney function in the population of patients with cancer. The clinical implications of using various estimates of kidney function in these patients, including the potential influence on drug dosing decisions, agent suitability, and eligibility for clinical trial enrollment, are discussed. Finally, the effect of the most recent Food and Drug Administration (FDA) draft guidance regarding pharmacokinetic studies in patients with impaired kidney function is explored.
Kidney Function Estimates in the General Population
GFR is routinely used to quantify kidney function and diagnose CKD. GFR may be measured (measured GFR [mGFR]) directly by determining clearance of exogenous markers, such as inulin, radioactive agents (51Cr-EDTA), or radiocontrast agents (iothalamate and iohexol), although this is not clinically practical due to time, cost, and convenience. More commonly, GFR is estimated (eGFR) on the basis of endogenous serum creatinine (SCr) values (1–3). Implementation of isotope dilution mass spectrometry-traceable standardization of SCr assays in 2010 has led to reduced interlaboratory variability and improved consistency in SCr measurements in the United States (1). One method of determining kidney function has been to use creatinine clearance (CrCl), reported in milliliters per minute, as a surrogate for GFR. CrCl can be measured (measured creatinine clearance [mCrCl]) by 12- or 24-hour urine collections, but this method is time consuming and inconvenient. The Cockcroft–Gault (CG) formula was published in the 1970s as a bedside equation for estimated creatinine clearance (eCrCl). However, this equation is an imprecise estimate of true GFR in large part due to its failure to adequately compensate for several non-GFR determinants of SCr, including body composition, diet, age, sex, race, tubular secretion, and extrarenal elimination of creatinine, as well as the original study’s reliance on mCrCl by 24-hour urine collection as a surrogate for true GFR (1,4,5). Additionally, after the isotope dilution mass spectrometry standardization of SCr assays, eGFR values decreased by 10%–20% compared with nonstandardized values, further placing the accuracy of the CG formula into question in conjunction with the widespread modern use of standardized SCr values (1). Despite these limitations and its small and nondiverse study population (a subselection of mostly men and all white patients) (6), the CG formula has been widely adopted into clinical practice due to its convenience and perceived accuracy. Since its incorporation into the 1998 FDA guidance on pharmacokinetics for patients with impaired kidney function, the CG formula has become the most common measure by which recommendations for kidney function-based drug dosing and agent selection are made (1,7).
Improved methods for determining eGFR have been developed in the last 20 years, notably the several iterations of the Modification of Diet in Renal Disease (MDRD) Study equation (MDRD-4 and MDRD-6) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and the CKD-EPI cystatin C equations (1,3,5,8,9). These equations report eGFR indexed for body surface area (BSA) in milliliters per minute per 1.73 m2. Importantly, when comparing kidney function estimates within individuals, the estimates must be expressed in equivalent units. Therefore, estimation of a patient’s absolute eGFR in units of milliliters per minute (nonindexed for BSA) must be performed by multiplying the indexed eGFR value by (patient’s BSA/1.73 m2). This allows for a direct comparison of absolute eGFR as calculated by the CKD-EPI equation or the MDRD equation, with CrCl by achieving congruent units (milliliters per minute) between the two measures. These equations were developed with standardized SCr values and iothalamate clearance as the reference, and they incorporate easily measured surrogates (age, sex, and race) to account for the effects of some non-GFR determinants of SCr. Use of these equations results in a value that is closer to the true GFR compared with the CG formula, especially for older patients (1,3). The CKD-EPI equation is recommended for use in routine clinical practice by the Kidney Disease Improving Global Outcomes and the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guideline groups, but this recommendation has not yet been fully adopted by many non-nephrology specialties, including oncology (10–12) (Table 1).
Table 1.
Comparison of bedside equations used to estimate kidney function
| Variables | Measures | Study Population Demographics | Study Population Kidney Function | Advantages | Limitations |
|---|---|---|---|---|---|
| CG (1976) | |||||
| Age, SCr, sex, weight | eCrCl, ml/min | n=236 (subpopulation of 534 patients on the basis of duplicate 24-h mCrCl being within 20%) | Average CrCl approximately 78 ml/min | Convenient to use | Estimates creatinine clearance as a surrogate for GFR |
| Mean age 53 yr, 24% >70 yr, 96% men | Model used for determining recommendations for drug dose adjustment for kidney function | Correlation between mCrCl and eCrCl R2=0.69 | |||
| Veterans Hospital patients | Uses 24-h urine collection as standard | ||||
| Does not use standardized SCr laboratory values, and eGFRs before standardization were 10%–20% higher | |||||
| Underestimates at severely reduced kidney function | |||||
| Less accurate in patients with extremes of age or body size | |||||
| Adjustment for sex is empirical | |||||
| MDRD (2006) | |||||
| Age, SCr, sex, race | eGFR, ml/min per 1.73 m2 | n=1628 | Average GFR 39.8 ml/min per 1.73 m2 | P30 values range 73%–93% | Underestimates at normal and mildly reduced kidney function (>60 ml/min) |
| Mean age 50.6 yr | Few patients with GFR>90 ml/min per 1.73 m2 | Uses iothalamate clearance as standard | Not used for determining most kidney drug-dosing recommendations | ||
| 60% men | MDRD-4 uses standardized SCr laboratory values | ||||
| 88% white | Improves on CG estimation at GFR<60 ml/min per 1.73 m2 | ||||
| 6% diabetic | |||||
| Patients with CKD | |||||
| CKD-EPI (2009) | |||||
| Age, SCr, sex, race | eGFR, ml/min per 1.73 m2 | n=5253 | Mean GFR 68 ml/min per 1.73 m2 | P30=91.5% with cystatin C | Not used for determining most kidney drug-dosing recommendations |
| Mean age 43 yr, 13% >65 yr | Uses iothalamate clearance as standard | ||||
| 58% men | Uses standardized SCr laboratory values | ||||
| 63% white, 32% black, 1% Asian | Improves on MDRD estimation at GFR>60 ml/min per 1.73 m2 | ||||
| Patients with CKD |
CG, Cockcroft–Gault; eCrCl, estimated creatinine clearance; mCrCl, measured creatinine clearance; CrCl, creatinine clearance; SCr, serum creatinine; MDRD, Modification of Diet in Renal Disease; P30, percentage of estimates that were within 30% of the reference value; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Kidney Function Estimates in Patients with Cancer
Patients with cancer commonly present with underlying impaired kidney function. Over one half and up to one fifth of patients with solid tumors have eCrCl (milliliters per minute) or eGFR (milliliters per minute per 1.73 m2) measures of <90 and <60, respectively (2,13). These numbers likely underestimate the true prevalence of decreased GFR in patients with cancer, because the studies from which they were derived excluded patients with hematologic malignancies, diseases that are associated with a high prevalence of kidney impairment. Importantly, use of SCr in isolation (i.e., assessment of eligibility for treatment defined as SCr<1 or 1.5 times the upper limit of normal) typically overestimates kidney function, with about 60% of patients with “normal” SCr values presenting with decreased kidney function. In fact, 5%–15% of patients with eCrCl (milliliters per minute) or eGFR (milliliters per minute per 1.73 m2) <60 present with “normal” SCr values (2,13). Moreover, the high toxicity of anticancer drugs and fatal consequences of the disease if treated ineffectively underscore the need for routine and accurate estimation of GFR to optimize drug safety and efficacy. The older age of patients with cancer portends additional risk, because it is associated with a normal age-related decline in kidney function as well as increased risks of developing a malignancy, suffering cancer-related death, and experiencing chemotherapy-related toxicity (4,14,15). Therefore, it is essential that estimates of kidney function can maintain accuracy in this older patient subpopulation.
Kidney function plays a large role in determining anticancer therapy, including anticancer agent selection, dosing, and eligibility for investigational drugs and clinical trials, and thereby, it affects clinical outcomes of patients with cancer. Although definition of the appropriate way to estimate kidney function is important to the dosing of many drugs within the general medical population, it is particularly crucial in patients with cancer due to the highly toxic adverse event profiles and often steep dose-therapeutic response relationships that characterize anticancer agents as a class. Typically, anticancer drug dosing is on the basis of the maximum tolerated dose, which is the highest dose that may be administered without unacceptable toxicity, to maximize anticancer efficacy. Dose reductions or alternative agent selection due to decreased eGFR may lead to reduced effectiveness, failure of therapy, use of less effective or more toxic second- or third-line agents, and ultimately, decreased survival. Investigational oncology drug clinical trials offer patients with advanced-stage, relapsed, and refractory cancer potentially effective novel therapeutics, but many require minimum kidney function thresholds for enrollment. Patients with cancer may benefit from aggressive anticancer regimens. Therefore, underestimation of true kidney function may unnecessarily preclude patients from more effective agents, higher doses, or clinical trial enrollment and thereby, potentially worsen outcomes. Conversely, overestimation of kidney function may put patients with cancer at unnecessary risk for major organ toxicity from narrow therapeutic index anticancer drugs cleared by the kidneys (4,14,15). Most patients with cancer and impaired kidney function have stage 2 or 3 (eGFR=60–89 and 30–59 ml/min per 1.73 m2, respectively) kidney disease (approximately 40%–50% and 15%–20% of all patients with cancer, respectively) according to NKF-KDOQI classification (2,11,13). These two stages straddle many important drug dose, drug selection, and clinical trial enrollment thresholds (16–18).
The process of deciding which kidney function estimate to implement in modern oncology practice remains complicated. Many anticancer drug cutoffs were determined before the development of the CKD-EPI equation using CG estimates of kidney function and before creatinine assay standardization in 2010. Although it has been firmly established that the CKD-EPI equation is superior to the CG formula in estimating GFR, the real clinical question that needs to be answered is the method of assessing kidney function that is best suited to dose adjust anticancer agents for kidney function. Important considerations include the accuracy of the model as it relates to estimation of kidney function in the individual patient and the kidney function model used to determine unacceptable toxicity when the chemotherapeutic agent was developed. Additionally, one must evaluate the risk-benefit scenario (i.e., the potential severity and complications of adverse effects when overdosing versus potential therapeutic failure when underdosing). There may be clinical scenarios in which the use of the CG formula may be preferable, despite its decreased precision and accuracy in the estimation of GFR. Admittedly, one of the weaknesses of using GFR to dose drugs is that it does not account for the contribution of tubular secretion to drug clearance, which can be significant for some drugs. Unless a drug has a secretion profile similar to that of creatinine, neither eCrCl- nor creatinine-based estimates of GFR are good representations of that drug’s net kidney clearance. However, the fact remains that the CKD-EPI equation provides an eGFR that is closer to true GFR than eCrCl. Accurate estimations of kidney function are imperative for optimizing anticancer efficacy while avoiding unacceptable toxicity in patients with cancer, especially the elderly, in whom both decreased kidney function and malignancies are more common.
Several clinical oncology groups, including the International Society of Geriatric Oncology (SIOG) and the National Comprehensive Cancer Network (NCCN), recommend an assessment of kidney function to adjust dose and reduce toxicity in patients before chemotherapy, even when SCr is within the normal range. However, there are currently no universal guidelines stating which method of estimating kidney function is preferred in patients with cancer. The NCCN vaguely recommends use of CrCl in their guidelines pertaining to elderly adults and “GFR calculations” in their guidelines related to adolescent and young adults, whereas the SIOG does not state a preferred estimation method (4,14,19,20). Most currently published models of estimating kidney function and all of those regularly used in clinical practice are derived from populations of patients without cancer (3,6,9). The CG formula is known to be markedly less accurate in the elderly and patients with extremes of body composition and decreased muscle mass, scenarios that are common to many patients with cancer (1). However, many oncology clinicians continue to use CG-based eCrCl to guide anticancer drug dosing for kidney function and selection, and some groups and investigators even use multiples of SCr upper limit of normal to determine enrollment into clinical trials. Despite its relative inaccuracy compared with the CKD-EPI equation, the CG formula continues to be the most universally implemented estimator of kidney function in patients with cancer (12,14,19).
Implications for Anticancer Drug Dosing
Many anticancer drugs have a narrow therapeutic index with potentially severe toxicity, and a large number of drugs are excreted predominantly as unchanged drug or active metabolite in the urine and therefore, may require dose adjustment for kidney function (16,17,21). For patients with decreased kidney function, this translates to diminished drug clearance and increased exposure, possibly leading to unacceptable toxicity. Underestimation of kidney function, however, can result in unintentional prescription of a subtherapeutic dose and diminished anticancer activity. Patient-specific dose adjustments of anticancer drugs cleared by the kidneys are, therefore, vital to ensure safety while maintaining anticancer drug efficacy (Table 2). Up to 50% of anticancer drugs either need dose adjustment for kidney function or do not have data on whether dose adjustments are required (2,13,21). Intensive pharmacotherapy and polypharmacy are common features of clinical oncology practice; as such, approximately 50% of kidney function–impaired patients with solid tumors receive at least one anticancer drug that requires dose adjustment for kidney function (2,13,21). However, many patients do not receive appropriate chemotherapy dosage adjustments on the basis of their kidney function. In one retrospective study, approximately one half of kidney function–impaired patients with solid tumors who were receiving a drug that necessitated dose adjustments for kidney function received almost 50% of their prescriptions at standard doses (i.e., without appropriate dose adjustment), potentially causing unacceptable toxicity to the patient. The most commonly implicated drugs included cisplatin, carboplatin, capecitabine, etoposide, and zoledronate. In fact, approximately 3% of patients received a drug for which a dose adjustment would be necessary in the setting of impaired kidney function without receiving any kidney function evaluation (13). This illustrates the lack of a universal approach to evaluating kidney function in patients with cancer and applying the clinical information to tailor pharmacotherapy for individual patients.
Table 2.
Selected drugs with kidney function cutoffs for eligibility and dose modifications
| Drug | Kidney Function Cutoff Below Which Not to Treat, ml/min | Kidney Function Ranges with Dose Modifications, ml/min | Reference |
|---|---|---|---|
| Bendamustine | 30 | — | 42 |
| Bleomycin | — | 5%–10% to 40% | 41 |
| 10%–20% to 45% | |||
| 20%–30% to 55% | |||
| 30%–40% to 60% | |||
| 40%–50% to 70% | |||
| Capecitabine | 30 | 30%–50% to 75% | 43 |
| Cisplatin | 60 | — | 37 |
| Etoposide | 15 | 15%–50% to 75% | 44 |
| Fludarabine | 30 | 30%–49% to 60% | 45 |
| 50%–79% to 80% | |||
| Methotrexate | 60 | — | 46 |
| Mitomycin | 30a | — | 47 |
| Oxaliplatin | — | <30%–75% | 48 |
| Pemetrexed | 45 | — | 49 |
| Pentostatin | — | 50%–60% to 50% | 50 |
| Topotecan | 10 | 20%–39% to 50% | 51 |
—, not applicable.
Related to hydroxypropyl-β-cyclodextrin excipient.
Many anticancer drugs are routinely dosed according to BSA in an effort to account for the effect of body size on pharmacokinetics, although this often does nothing to reduce variability in exposure (22). Despite many oncology drugs being dosed according to BSA, the most commonly used method of estimating kidney function in oncology remains the CG formula, which yields an absolute kidney function metric (milliliters per minute) that is not indexed to BSA. This is problematic, because the use of an absolute kidney function estimate to prescribe anticancer drugs that are dosed according to BSA will likely alter the dose assignment compared with dosing decisions on the basis of BSA-indexed kidney function estimates. Small patients will be penalized for having a low absolute kidney function, although their drug dose will already accommodate this size difference (Figure 1). In a post hoc analysis of a study using the CG formula (milliliters per minute) to dose stratify patients with impaired kidney function being administered oxaliplatin to develop dosing guidelines, it was revealed that BSA indexing of eCrCl (milliliters per minute per 1.73 m2) did alter dose classification of several patients versus absolute eCrCl classification. Although this reclassification did not alter the results of the dose guidelines for kidney function determined by the study, it does show that dose stratification of patients can be affected by whether measures of kidney function are indexed for BSA, and this can have potential clinical implications (23,24). The effect of these internal inconsistencies would be most pronounced in the dosing of patient groups with BSAs that differ significantly from 1.73 m2. Therefore, it would seem pertinent to use BSA-indexed estimates of kidney function for drugs dosed by BSA and absolute estimates of kidney function for drugs dosed absolutely so that the units are congruent (23,25). Notably, the output of the CKD-EPI equation can be easily converted to absolute values through multiplication by (patient’s BSA/1.73 m2).
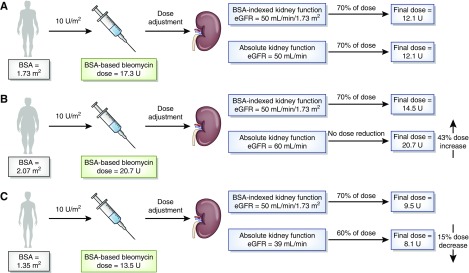
Figure 1.
Many anticancer drugs are dosed according to body surface area (BSA), but they are dose adjusted according to measures of absolute kidney function (i.e., estimated creatinine clearance as milliliters per minute) as opposed to BSA-indexed measures of kidney function (i.e., eGFR as milliliters per minute per 1.73 m2). (A) This practice will likely alter dose assignments of patients at the extremes of body size (patients who are obese and patients who are cachectic) compared to patients with BSA of 1.73 m2. (B) Larger patients (BSA>1.73 m2) are already receiving a larger dose of BSA-dosed drugs due to their increased BSA. Use of an absolute kidney function value may preclude necessary dose reduction, because the absolute kidney function value of patients with BSA>1.73 m2 will be greater than the BSA-indexed value and may be above a dose-adjustment breakpoint. (C) Smaller patients (BSA<1.73 m2) are already receiving a smaller dose of BSA-dosed drugs due to their decreased BSA. Use of an absolute kidney function value may lead to additional unnecessary dose reduction, because the absolute kidney function value of patients with BSA<1.73 m2 will be less than the BSA-indexed value and may be below a dose-adjustment breakpoint. Bleomycin dosing of 10 U/m2 was on the basis of manufacturer recommendations (41). Absolute eGFR values (milliliters per minute) were calculated by multiplying the BSA-indexed eGFR (milliliters per minute per 1.73 m2) by (patient’s BSA/1.73 m2).
Case Study: Carboplatin
Carboplatin is a platinum-based alkylating agent that is widely used in the treatment of lung, ovarian, testicular, bladder, breast, and head and neck cancers. Carboplatin exhibits an exposure-response relationship with increasing area under the curve (AUC), resulting in increased antitumor activity; the exposure-response relationship plateaus, and additional increases in exposure result in increased toxicity. An ultrafilterable carboplatin target AUC of 4–6 mg/ml per minute is suggested, because it seems to optimize anticancer efficacy within acceptable toxicities as shown in ovarian cancer (26,27). Even small changes in carboplatin dosing and exposure can have meaningful clinical consequences. For example, a carboplatin dose reduction as small as 10% may result in a doubling of the 5-year relapse rate (28). Currently, carboplatin is dosed on the basis of the Calvert equation (29), with carboplatin dose being directly related to the patient’s GFR as follows:
Carboplatin dosing varies significantly depending on the estimate of GFR incorporated into the Calvert formula, and there is poor concordance of carboplatin dose measured with eGFR or eCrCl versus mGFR. Up to three quarters of patients dosed by the CG formula and one quarter of patients dosed by the CKD-EPI equation receive a carboplatin dose over 10% and 20% different, respectively, than the dose that they should receive on the basis of mGFR (12,30). Similarly, only between one fifth and one third of patients prescribed carboplatin have a calculated eGFR or eCrCl within 10% of their mGFR (30). Differences in carboplatin dosing are dependent not only on the method used to calculate GFR (e.g., the CKD-EPI equation versus the CG formula) but also, on whether the BSA-indexed or absolute eGFR is incorporated into the Calvert formula. eGFR indexed for BSA as calculated by the CKD-EPI equation is less likely to overdose but more likely to underdose patients versus absolute eGFR calculated by the same method, further illustrating that the choice of using BSA-indexed versus absolute estimates of kidney function will significantly affect drug dosing and potential clinical efficacy and safety outcomes (Figure 2) (31). Importantly, no studies to date have documented exposure by measuring ultrafilterable carboplatin AUC or examined the differences in clinical safety and toxicity profiles of carboplatin dosing and exposure on the basis of GFR estimation method incorporated into the Calvert formula, an effort that is currently being pursued within the Cancer Therapy Evaluation Program of the National Cancer Institute.
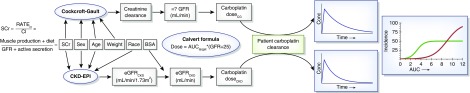
Figure 2.
Area under the curve (AUC)–targeted dosing of carboplatin using either the Cockcroft–Gault formula or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to inform kidney function may result in different doses and exposures. The CKD-EPI equation estimates a body surface area (BSA)–normalized GFR value from assumed steady-state serum creatinine (SCr) and anthropomorphic variables. This estimate is converted to an absolute value using the patient’s BSA. Similarly, the Cockcroft–Gault formula estimates an absolute value for creatinine clearance, which is then used as a surrogate of GFR. The GFR value is imputed into the Calvert equation with a target AUC (often 6 mg/ml per min), which results in a carboplatin dose to be administered to the patient. On the basis of the true carboplatin clearance of the patient, an exposure is observed. On the basis of a probability of response (green curve) or toxicity (red curve), the exposure and corresponding probability of response and toxicity can be quite different depending on the kidney function estimate used and the corresponding carboplatin dose administered.
Implications for Anticancer Drug Selection
For many anticancer drugs, patients are placed into GFR stratifications, below which administration of the drug is not recommended due to reduced elimination or high potential for toxicity (Table 2). These drugs are generally dosed in a dichotomous fashion as opposed to continuous dosage adjustments, and therefore, accurate determination of patient GFR is critical, because small variations in GFR estimates may completely preclude patients from receiving potentially effective anticancer therapy. This is especially pertinent in stage 2/3 kidney disease, because these stages include the majority of patients with cancer and impaired kidney function and contain important dose adjustment thresholds for many drugs (e.g., 60, 45, and 30 ml/min) (2,13,14,16). Suitability of a drug is most often assessed depending on kidney function as estimated by the CG formula and reported in milliliters per minute; hence, there is considerable ambiguity in assessing drug suitability on the basis of eGFR estimates of kidney function in milliliters per minute per 1.73 m2. In fact, many studies investigating suitability of cisplatin therapy on the basis of the MDRD equation– or the CKD-EPI equation–derived eGFRs either make no mention of normalizing for patient-specific BSAs but report eGFR in milliliters per minute or use identical numerical cutoffs for both eCrCl (milliliters per minute) and eGFR (milliliters per minute per 1.73 m2) (32–36). In both the oncopharmacology and clinical oncology communities, more emphasis should be placed on the clinical implications of using BSA-indexed versus absolute estimates of GFR to minimize the likelihood of incorrectly assuming that estimates of kidney function are numerically equivalent across incongruent units.
Case Study: Cisplatin
Cisplatin is a platinum-based alkylating agent that is highly effective at treating many types of cancer. The drug is excreted predominantly unchanged in the urine, and unfortunately, it is extremely nephrotoxic. Although no universal guidelines exist, some recommendations and typical clinical practice discourage use of cisplatin in patients with GFR<60 ml/min (37). In fact, impaired kidney function is the reason for precluding anywhere from 20% to 83% of patients from receiving cisplatin therapy (32,33,35,36,38).
Cisplatin eligibility varies significantly depending on the kidney function estimate used. For example, CG formula–derived kidney function estimates exclude cisplatin therapy at an approximately 20% higher rate than the CKD-EPI equation–derived estimates (32,33,35,36,38). This bias is especially pronounced in women, the elderly, and whites (32). Patient eligibility on the basis of the CG formula versus eGFR has high discordance, with about 15% of patients changing eligibility status on the basis of the estimation used (32,33,36). Significantly more patients are deemed ineligible for cisplatin with any method of eCrCl or eGFR compared with mCrCl (38). Potentially inappropriate denial of cisplatin eligibility is again seen more prominently in the elderly, with 24%–53% of patients over 65 years old being denied by eCrCl or eGFR but not by mCrCl (34). Notably, the ability of a patient to complete three full cycles of chemotherapy has been correlated with mCrCl>60 ml/min (P=0.02) but not eCrCl>60 ml/min or eGFR>60 ml/min per 1.73 m2 (34). It is notable that there exists a correlation between a clinical outcome that may directly affect survival and mCrCl, a measured (although admittedly flawed) marker of kidney function, but not eGFR, an estimated and supposedly more accurate measure of kidney function. This calls into question the utility of current drug selection thresholds and their correlation to the various estimates and measures of kidney function in patients with cancer.
Implications for Oncology Clinical Trials
Historically, patients with impaired kidney function have tended to be excluded from phase 1 studies of anticancer drugs because of a perceived increased risk for major dose-limiting toxicity. Recently, however, there has been a call to be more inclusive of patients with mild to moderate kidney impairment in oncology clinical trials (17) as well as warnings to be cautious about sweeping changes (39). Current FDA classification of mild kidney impairment is defined as CrCl=50–79 ml/min, but typical phase 1 eligibility disqualifies patients from enrollment at CrCl<60 ml/min. Therefore, there is a proportion of patients with only mild kidney impairment according to FDA classification who are disqualified from potentially effective clinical trials due to their kidney function. However, a retrospective analysis of over 10,000 patients from 373 single-agent phase 1 clinical trials found that there was no clinically meaningful increase in grade 3 or 4 nonhematologic, grade 4 hematologic, or any clinically relevant toxicities in the approximately 36% of enrolled patients with mild kidney impairment compared with those with normal kidney function (18). Therefore, expanding inclusion of patients to the full FDA classification range of mild impairment (i.e., CrCl>50 ml/min) may increase eligibility of patients without any clinically meaningful difference in determination of the dose-limiting toxicity.
Current FDA guidelines recommend use of the CG formula to determine kidney function (7); however, the draft revision of the guidelines for assessing pharmacokinetics in kidney impairment suggests that the newer eGFR formula also should be used to estimate kidney function (40). Importantly, these draft guidelines do not state a preference as to which formula is used to estimate kidney function, although it is established that the CKD-EPI equation is a more accurate estimate of mGFR across a wider range of kidney function than the CG formula. This is of particular importance in patients with cancer, because at the border of mild to moderate kidney impairment for both FDA classification (50 ml/min) and the majority of phase 1 cancer trials (60 ml/min), the CG formula is known to underestimate kidney function at a higher degree than newer formulas. As such, this may unnecessarily preclude patients with mild kidney impairment from trial participation.
Additionally, there are significant inconsistencies regarding the use of BSA-indexed versus non–BSA-indexed estimates of kidney function to determine dosing and eligibility for anticancer drugs. Recognition of this problem, development of guidelines with the purpose of maintaining consistency in this regard (milliliters per minute for drugs dosed absolutely or on the basis of any non-BSA parameter versus milliliters per minute per 1.73 m2 for drugs dosed on the basis of BSA), completion of pharmacokinetic trials including patients with impaired kidney function, and development of corresponding dosing recommendations would significantly improve internal consistency in oncopharmacology.
Summary
Estimation of kidney function in patients with cancer directly affects drug dosing, agent selection, and eligibility for clinical trials of novel agents. It would seem obvious that the most accurate estimates of kidney function should be used to reduce unexplained variability in decision making and ultimately, the therapeutic outcomes of toxicity and clinical benefit. There are many discrepancies between eGFR and true GFR and how these values correlate to absolute and BSA-indexed drug dosing, drug eligibility, and clinical trial enrollment. This illustrates the need for additional studies investigating the validity of currently used estimates of kidney function in patients with cancer, the applicability of traditional anticancer dosing and eligibility guidelines to modern and more accurate estimates of kidney function, and clinical harmonization of kidney function estimation across all patients with cancer.
Disclosures
T.D.N. reports personal fees from MediBeacon outside the submitted work. M.A.C. and J.H.B. have nothing to disclose.
Acknowledgments
J.H.B. received grants P30-CA47904 and UM1-CA186690 from the National Cancer Institute, National Institutes of Health during the conduct of the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hudson JQ, Nolin TD: Pragmatic use of kidney function estimates for drug dosing: The tide is turning. Adv Chronic Kidney Dis 25: 14–20, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, Morere JF, Beuzeboc P, Deray G; Renal Insufficiency and Cancer Medications (IRMA) Study Group: Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: The renal insufficiency and anticancer medications (IRMA) study. Cancer 110: 1376–1384, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Inker LA, Coresh J: GFR estimation: From physiology to public health. Am J Kidney Dis 63: 820–834, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launay-Vacher V, Chatelut E, Lichtman SM, Wildiers H, Steer C, Aapro M; International Society of Geriatric Oncology: Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol 18: 1314–1321, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Guidance for Industry: Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling, Rockville, MD, FDA, 1998, p 19 [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group KDIGO KCW: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Janowitz T, Williams EH, Marshall A, Ainsworth N, Thomas PB, Sammut SJ, Shepherd S, White J, Mark PB, Lynch AG, Jodrell DI, Tavaré S, Earl H: New model for estimating glomerular filtration rate in patients with cancer. J Clin Oncol 35: 2798–2805, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janus N, Launay-Vacher V, Byloos E, Machiels JP, Duck L, Kerger J, Wynendaele W, Canon JL, Lybaert W, Nortier J, Deray G, Wildiers H: Cancer and renal insufficiency results of the BIRMA study. Br J Cancer 103: 1815–1821, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtman SM, Wildiers H, Launay-Vacher V, Steer C, Chatelut E, Aapro M: International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer 43: 14–34, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Peterson LL, Hurria A, Feng T, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Glezerman I, Katheria V, Zavala L, Smith DD, Sun CL, Tew WP: Association between renal function and chemotherapy-related toxicity in older adults with cancer. J Geriatr Oncol 8: 96–101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kintzel PE, Dorr RT: Anticancer drug renal toxicity and elimination: Dosing guidelines for altered renal function. Cancer Treat Rev 21: 33–64, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Lichtman SM, Harvey RD, Damiette Smit MA, Rahman A, Thompson MA, Roach N, Schenkel C, Bruinooge SS, Cortazar P, Walker D, Fehrenbacher L: Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol 35: 3753–3759, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Beumer JH, Ding F, Tawbi H, Lin Y, Viluh D, Chatterjee I, Rinker M, Chow SL, Ivy SP: Effect of renal dysfunction on toxicity in three decades of cancer therapy evaluation program-sponsored single-agent phase I studies. J Clin Oncol 34: 110–116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NCCN: NCCN Clinical Practice Guidelines in Oncology: Older Adult Oncology, Version 1.2018, June 11, 2018 Ed., Plymouth Meeting, PA, NCCN, 2018 [Google Scholar]
- 20.NCCN: NCCN Clinical Practice Guidelines in Oncology: Adolescent and Young Adult (AYA) Oncology, Version 2.2018, October 11, 2017 Ed., Plymouth Meeting, PA, NCCN, 2017 [Google Scholar]
- 21.González J, Quiroga M, Escudero-Vilaplana V, Collado-Borrell R, Herranz-Alonso A, Sanjurjo Sáez M: Posology adjustments of oral antineoplastic agents for special populations: Patients with renal impairment, hepatic impairment and hematologic toxicities. Expert Opin Drug Saf 17: 553–572, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Beumer JH, Chu E, Salamone SJ: Body-surface area-based chemotherapy dosing: Appropriate in the 21st century? J Clin Oncol 30: 3896–3897, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Murray PT, Ratain MJ: Estimation of the glomerular filtration rate in cancer patients: A new formula for new drugs. J Clin Oncol 21: 2633–2635, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Takimoto CH, Remick SC, Sharma S, Mani S, Ramanathan RK, Doroshow J, Hamilton A, Mulkerin D, Graham M, Lockwood GF, Ivy P, Egorin M, Schuler B, Greenslade D, Goetz A, Knight R, Thomas R, Monahan BP, Dahut W, Grem JL; National Cancer Institute Organ Dysfunction Working Group Study: Dose-escalating and pharmacological study of oxaliplatin in adult cancer patients with impaired renal function: A National Cancer Institute Organ Dysfunction Working Group Study. J Clin Oncol 21: 2664–2672, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ratain MJ: Dear doctor: We really are not sure what dose of capecitabine you should prescribe for your patient. J Clin Oncol 20: 1434–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Jodrell DI, Egorin MJ, Canetta RM, Langenberg P, Goldbloom EP, Burroughs JN, Goodlow JL, Tan S, Wiltshaw E: Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 10: 520–528, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Bristol-Myers Squibb Company: Package Insert—PARAPLATIN® (Carboplatin) for Injection, USP, Princeton, NJ, Bristol-Myers Squibb Company, 2010 [Google Scholar]
- 28.Oliver RT, Mead GM, Rustin GJ, Joffe JK, Aass N, Coleman R, Gabe R, Pollock P, Stenning SP: Randomized trial of carboplatin versus radiotherapy for stage I seminoma: Mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol 29: 957–962, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748–1756, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Craig AJ, Samol J, Heenan SD, Irwin AG, Britten A: Overestimation of carboplatin doses is avoided by radionuclide GFR measurement. Br J Cancer 107: 1310–1316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd ST, Gillen G, Morrison P, Forte C, Macpherson IR, White JD, Mark PB: Performance of formulae based estimates of glomerular filtration rate for carboplatin dosing in stage 1 seminoma. Eur J Cancer 50: 944–952, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Tsao CK, Moshier E, Seng SM, Godbold J, Grossman S, Winston J, Oh WK, Galsky MD: Impact of the CKD-EPI equation for estimating renal function on eligibility for cisplatin-based chemotherapy in patients with urothelial cancer. Clin Genitourin Cancer 10: 15–20, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Horn T, Ladwein B, Maurer T, Redlin J, Seitz AK, Gschwend JE, Retz M, Kübler HR: The method of GFR determination impacts the estimation of cisplatin eligibility in patients with advanced urothelial cancer. World J Urol 32: 359–363, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Raj GV, Iasonos A, Herr H, Donat SM: Formulas calculating creatinine clearance are inadequate for determining eligibility for Cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 24: 3095–3100, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Dash A, Galsky MD, Vickers AJ, Serio AM, Koppie TM, Dalbagni G, Bochner BH: Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107: 506–513, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Canter D, Viterbo R, Kutikov A, Wong YN, Plimack E, Zhu F, Oblaczynski M, Berberian R, Chen DY, Greenberg RE, Uzzo RG, Boorjian SA: Baseline renal function status limits patient eligibility to receive perioperative chemotherapy for invasive bladder cancer and is minimally affected by radical cystectomy. Urology 77: 160–165, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, Dreicer R, Vogelzang N, Sternberg C, Bajorin DF, Bellmunt J: A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol 12: 211–214, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Niwa N, Kikuchi E, Masashi M, Tanaka N, Nishiyama T, Miyajima A, Saito S, Oya M: Are the formulas used to estimate renal function adequate for patients treated with cisplatin-based chemotherapy after nephroureterectomy for upper tract urothelial carcinoma? Clin Genitourin Cancer 14: e501–e507, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Masters JC, Wiernik PH: Are we Ready to include organ-impaired patients in oncology trials? A clinical pharmacology perspective on recent recommendations. J Clin Pharmacol 58: 701–703, 2018 [DOI] [PubMed] [Google Scholar]
- 40.US Department of Health and Human Services Food and Drug Administration: Draft Guidance for Industry—Pharmacokinetics in Patients with Impaired Renal Function, Rockville, MD, US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation, 2010 [Google Scholar]
- 41.Package Insert—BLENOXANE® Bleomycin Sulfate for Injection, 2010 [Google Scholar]
- 42.Package Insert—BENDEKA® (Bendamustine Hydrochloride) Injection, for Intravenous Use, 2018 [Google Scholar]
- 43.Package Insert—XELODA: (Capecitabine) Tablets, for Oral Use, 2015 [Google Scholar]
- 44.Package Insert—ETOPOPHOS: (Etoposide Phosphate) for Injection, for Intravenous Use, 2017 [Google Scholar]
- 45. Package Insert—Fludarabine Phosphate Injection for Intravenous Use Only, 2010.
- 46.Package Insert—Methotrexate Injection: USP, 2011 [Google Scholar]
- 47. Package Insert—Mitozytrex™ (Mitomycin for Injection), 2002.
- 48.Package Insert—ELOXATIN: (Oxaliplatin) Powder, for Solution for Intravenous Use, 2011 [Google Scholar]
- 49.Package Insert—ALIMTA: (Pemetrexed Disodium) Injection, Powder, Lyophilized, for Solution for Intravenous Use, 2004 [Google Scholar]
- 50.Package Insert—NIPENT: (Pentostatin for Injection), 2018 [Google Scholar]
- 51.Package Insert—HYCAMTIN® (Topotecan) for Injection, for Intravenous Use, 2018 [Google Scholar]